Abstract
Brucellae are gram-negative intracellular pathogens that survive and multiply within host phagocytic cells. Smooth organisms present O-polysaccharides (OPS) on their surface. The wboA gene, which codes for the enzyme glycosyl transferase, is essential for the assembly of O-chain in Brucella. Deletion of wboA in smooth, virulent B. melitensis 16M results in a rough mutant designated WRR51. Unlike B. abortus, both smooth and rough strains of B. melitensis are resistant to complement-mediated killing. To determine the role of surface OPS in the interactions of B. melitensis with monocytes/macrophages (M/M), 16M and WRR51 were transformed with the plasmid pBBR1MCS-6y encoding green fluorescent protein, and the transformants were used to infect human mononuclear phagocytes with and without fresh human serum as a source of complement. Human monocytes were cultured in the presence of macrophage colony-stimulating factor to allow their differentiation into macrophages during the course of infection. Intracellular bacteria were easily visualized using fluorescence microscopy. Infection in M/M, identified by surface staining and fate of infected phagocytes, was quantitated by flow cytometry. Rough bacteria were internalized, with no requirement for opsonization by serum, at a higher rate than smooth organisms. Smooth B. melitensis survived and multiplied for at least 6 days inside M/M, but rough organisms were eliminated by death of the infected cells. In human monocytes cultured for 1 day without serum in order to trigger the apoptotic pathway, infection by rough brucellae accelerated phagocyte death; smooth brucellae inhibited apoptosis. This study suggests that the presence of surface OPS on live B. melitensis benefits the bacterium by preventing the death of macrophages, Brucella's preferred target for intracellular replication.
Brucellae are gram-negative intracellular pathogens which can survive and multiply within the phagocytic cells of their hosts and are resistant to the bactericidal action of serum. Brucella melitensis is considered the principal cause of human brucellosis (40, 41) and is more virulent than B. abortus (42). These species may occur as either smooth or rough variants depending on the expression of O-polysaccharides (OPS) as a component of the bacterial outer membrane lipopolysaccharide (LPS). In rough strains expression of OPS is limited or absent and attenuation in virulence is generally observed (1, 4, 19, 26, 31, 35). Our previous studies demonstrated that smooth B. melitensis and B. abortus strains bind fewer complement components on their surface than their corresponding rough-mutant organisms (13) obtained by disruption or deletion of the wboA gene (13, 26, 37, 40). However, OPS-deficient strains derived from smooth, virulent B. melitensis 16M are as resistant as the wild type to the bactericidal action of nonimmune human serum (HS) and bind less complement than OPS-deficient strains derived from B. abortus 2308 (13). Since rough mutants of B. melitensis are protected from extracellular complement-mediated killing, we decided to investigate whether the attenuation in virulence previously observed in these strains was associated with differences in their interaction with macrophages.
The green fluorescent protein (GFP) from the jellyfish Aequorea victoria is a self-fluorescing protein that requires no substrates and emits bright green fluorescence at 509 nm (3). We took advantage of the properties of the GFP to study the interaction of fluorescent rough and smooth B. melitensis strains with human mononuclear phagocytes and to evaluate the importance of the presence of OPS in the pathogenesis of brucellosis. We introduced plasmid pBBR1MCS-6Y (29) expressing GFP into 16M, OPS-deficient ΔwboA B. melitensis strain WRR51, and WRR51 complemented with wboA and examined interactions of the fluorescent bacteria with human and murine macrophages by fluorescent and electron microscopy, flow cytometry, and release of lactate dehydrogenase (LDH). We found that infection of mononuclear phagocytes with WRR51 was followed by host cell apoptosis and bacterial death. In contrast, infection with either 16M or wboA-complemented WRR51 led to intracellular bacterial replication but not host cell apoptosis. Moreover, infection with the latter strains protected host cells from apoptosis induced by serum deprivation. These data indicate that surface OPS contribute to the antiapoptotic activity of B. melitensis and enhance the bacterium's ability to survive in macrophages.
MATERIALS AND METHODS
Bacterial strains.
Plasmid pBBR1MCS-6Y (29) is a GFP-expressing derivative of the broad-host-range cloning vector pBBR1MCS (24). This plasmid was electroporated into the wild-type 16M and an OPS-deficient ΔwboA strain, WRR51, to generate fluorescent smooth and rough bacteria that were designated 16M/GFP and WRR51/GFP, respectively. The plasmid pRFBUK11 containing the wboA gene with an antibiotic resistance cassette was also a derivative of the cloning vector pBBR1MCS (M. P. Nikolich, unpublished results) and was used in our previous studies to generate a complemented strain (13). The plasmid pRFBUK11 was modified to contain both the GFP and wboA genes (Nikolich, unpublished results). This new plasmid, pMNWG16, was electroporated into WRR51 to complement the wboA gene and restore the smooth phenotype. This complemented fluorescent strain was designated WRR51/GFP+wboA. The smooth and rough phenotypes of these strains have been confirmed by the crystal violet method. In this test, the smooth wild type and the complemented strains, 16M/GFP and WRR51/GFP+wboA, respectively, take up the dye, whereas the rough strain, WRR51/GFP, does not. Bacteria were grown to mid-logarithmic phase at 37°C with shaking in Brucella broth (Difco). Then, bacteria were washed by centrifugation and resuspended in 0.9% NaCl, recentrifuged, and suspended in RPMI 1640 medium (Gibco) at approximately 108 CFU/ml.
Sera for bacterial opsonization.
Normal nonimmune HS was obtained from members of the laboratory staff and stored at −70°C until required. Sera were negative for Brucella antibody by the standard tube agglutination test.
Cell culture and infection of monocytes/macrophages (M/M).
Monocytes were isolated from citrated peripheral venous blood from healthy volunteers by counterflow centrifugal elutriation; cultivated for their differentiation into macrophages in RPMI 1640 medium containing 10% heat-inactivated human AB serum (Sigma), 2 mM l-glutamine (Gibco), and macrophage colony-stimulating factor (10 ng/ml; Genetic Institute, Cambridge, Mass.); and incubated at 37°C in a humidified 5% CO2 atmosphere as described previously (10-12). The murine macrophage-like cell line J774 was cultured as previously described (10-12). One day before infection, human or murine phagocytes were suspended in fresh medium in either 24- or 6-well culture plates at a concentration of 106 cells/ml. The cells were infected with brucellae at a multiplicity of infection of 50:1 for 1 h. The cells were then washed three times with phosphate-buffered saline (PBS) and incubated again with fresh medium containing 5 μg of gentamicin per ml (9). At selected intervals between 0 and 6 days following infection, the medium was removed and the M/M were washed and lysed with 0.1% Triton X-100. The numbers of viable intracellular brucellae were determined by plating dilutions of the lysates on tryptic soy agar plates. Colonies were counted after 3 to 5 days incubation of the plates at 37°C.
Fluorescence microscopy of infected M/M.
Monocytes were seeded in 24-well culture plates containing glass coverslips as described before (12) using the medium described above for macrophages. Cells were incubated at 37°C in a humidified 5% CO2 atmosphere for 1 day. Cells were infected with B. melitensis strains as described before. At selected times, cells were washed with PBS and fixed with 4% formaldehyde for 1 h. Coverslips were washed twice with PBS and once with water before mounting in medium containing 0.1 M n-propyl gallate (to prevent photobleaching) in glycerol (59%, vol/vol) gelatin (0.9%, wt/vol). GFP-B. melitensis-infected phagocytes were visualized by fluorescent microscopy.
Flow cytometry.
Monocytes were seeded in six-well plates for 1 day and infected as previously described. At selected intervals after infection, the medium was removed and the cells were washed with PBS (Gibco) containing 1% bovine serum albumin (PBS-BSA) and fixed with 4% formaldehyde for 1 h. An aliquot of each sample was transferred to Brucella agar plates and incubated for 3 days at 37°C in a CO2 incubator as a check for sterility. Portions of the cells were incubated at 4°C with the appropriate monoclonal antibodies(1 μg/ml). Staining controls received isotypic monoclonal antibodies (for nonspecific binding) or PBS-BSA (unstained). The tubes were incubated at 4°C for 30 min. Finally, samples were washed as before and resuspended in PBS-BSA. Samples were then acquired on a Becton Dickinson FACSort flow cytometer and analyzed using CellQuest software (Becton Dickinson). For acquisition, forward and side scatter gates were adjusted to acquire macrophages. Unless indicated, 10,000 events were acquired for each sample. Compensation was adjusted. Positively fluorescent cells were defined as those with a fluorescence intensity greater than the fluorescence intensity of 97.5% of untreated control cells in the respective fluorescence channel.
Supravital exposure to propidium iodine (PI) to identify apoptotic adherent cells.
Thirty minutes before harvesting cells, infected and noninfected cells were incubated with propidium iodine (50 μg/ml) in the dark at room temperature (43). Cells were washed twice with PBS and fixed with 4% formaldehyde for 1 h. Then, the cells were prepared for flow or microscopic analysis as described above.
LDH assays for measuring cytotoxicity.
Monocytes were seeded in 24-well plates and infected as previously described. At selected times after infection, aliquots of the supernatants were collected and assayed for LDH release using a colorimetric Cytotox 96 kit (Promega Corp., Madison, Wis.) according to the manufacturer's instructions with some modifications as described (10, 11).
Transmission electron microscopic (TEM) analysis of M/M infection.
At selected intervals following infection, M/M monolayers were washed with HBSS three times and prefixed with 2% paraformaldehyde-1% glutaraldehyde in 0.2 M sodium cacodylate buffer (SCB), pH 7.2, for 1 h at room temperature. The cells were then scraped off the tissue culture plate surfaces and placed in fresh prefixative and stored at 4°C for further processing. The samples were again washed three times with SCB and postfixed with 1% osmium tetroxide in SCB for 2 h as described (10-12). The postfixed samples were further processed and embedded into EPON 812 (EPONATE 12, Ted Pella, Redding, Calif.). Ultrathin sections were prepared using a Leica Ultracut-S ultramicrotome. The sections were stained with uranyl acetate and lead citrate as described (10-12), and evaluated using a Leo 912 AB transmission electron microscope operating at an acceleration voltage of 100 kV.
Statistical analysis.
The Student's t test (INSTAT statistical analysis package; Graph Pad Software, Inc., San Diego, Calif.) was used for statistical analysis of the data as appropriate. P < 0.05 for comparisons between experimental groups was considered significant.
RESULTS
Evaluation of infection of rough and smooth B. melitensis strains in M/M.
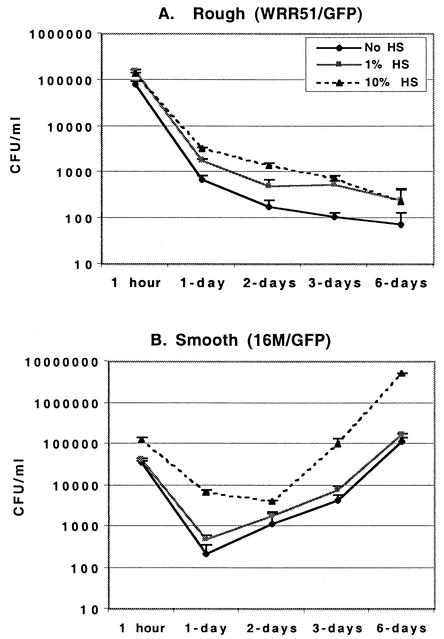
In the presence of medium alone, without addition of HS for opsonization, both rough (WRRR51/GFP) and smooth (16M/GFP and WRR51/GFP+wboA) organisms were internalized by phagocytic cells; however, the number of bacterial CFU recovered after 1 h exposure of M/M to rough WRR51/GFP was two to four times higher than the number of CFU recovered from phagocytes incubated with smooth bacteria (16M/GFP or WRR51/GFP+wboA, Fig. 1). Inclusion of 10% HS with bacteria and macrophages increased the recovery of rough bacteria 1.5- to 2-fold and recovery of smooth organisms 2- to 4-fold. As a result, opsonization with 10% HS led to recovery of identical numbers of rough or smooth bacteria. As little as 1% HS was equivalent to 10% HS for this enhanced recovery of rough bacteria, but 1% HS had little effect on smooth organisms. Preincubation of either rough or smooth bacteria with 1 or 10% HS for 1 h prior to addition of organisms to M/M, however, was equivalent to coincubation with 10% HS at the time of bacterial addition (data not shown). These data indicate that deposition of HS opsonic factors on the surface of Brucella is saturable and that saturation occurs faster or at lower HS concentrations on the surface of rough organisms.
FIG. 1.
Infection of human M/M by rough WRR51/GFP (A) and smooth 16M/GFP (B) B. melitensis. Bacteria were grown overnight at 37°C and either opsonized or not with 1 or 10% HS and left in contact with M/M for 1 h. M/M monolayers were then washed and further incubated in gentamicin-containing medium for 1 to 6 days. At selected times, M/M were washed and lysed, and the numbers of bacterial CFU were determined by serial dilution and plating on agar. Means + SD (error bars) are shown.
Infection of M/M with smooth or rough bacteria had profoundly different outcomes (Fig. 1). Bacterial CFU numbers decreased continuously from 1 h to 6 days following infection of M/M with WRR51/GFP. This led to an eventual 2- to 3-log decrease when the CFU counts from day 6 were compared to those from a 1-h infection (Fig. 1A). In contrast, infection of these phagocytic cells with 16M/GFP (Fig. 1B) or WRR51/GFP+wboA (data not shown) eventually resulted in a 1- to 2-log increase in bacterial CFU counts by 6 days after infection. Interestingly, recovery of both smooth and rough bacteria from M/M was reduced at least 10-fold between 1 and 24 h, suggesting that both were killed during or shortly after ingestion. Treatment with HS did not greatly change the shape of the bacterial growth curve for either rough or smooth organisms after the initial 1-h uptake period. Conditions that led to greater initial bacterial recovery (i.e., 1 or 10% HS for rough bacteria and 10% HS for smooth organisms) were associated with recovery of a consistently greater number of bacterial CFU throughout the 6-day course. These data suggested that opsonization with HS did not affect the intracellular survival of either rough or smooth brucellae.
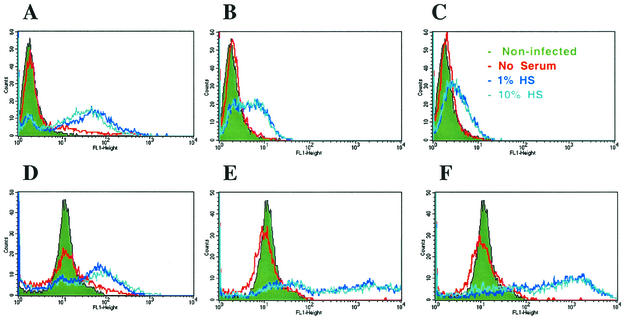
While these observations provided useful data on recovery of viable bacteria from M/M cultures, they provided little insight on the uptake of bacteria by individual cells. To address these issues, we used flow cytometry (Fig. 2) and fluorescence microscopy (Fig. 3) to follow the time course of infection in M/M exposed to opsonized and nonopsonized smooth and rough B. melitensis. In contrast to the CFU data, which indicated only a two- to fourfold difference in the number of rough and smooth bacteria recovered from M/M in the absence of HS, flow cytometry indicated that without opsonization, 21% of monocytes were infected with rough bacteria at 1 h after exposure to bacteria, while less than 1/10 that proportion of M/M was infected with either one of the two smooth strains used in this study (2% infection with 16M/GFP and 1.5% with WRR51/GFP+wboA [Fig. 2A to C]). Moreover, the fluorescence intensity (assumed to be directly proportional to the number of cell-associated bacteria) of WRR51/GFP-infected M/M was much greater than that of M/M infected with either of the smooth bacterial strains (Fig. 2A to C, red lines). The ratio of mean fluorescence intensity (MFI) of four different experiments of FL1-positive M/M from cultures exposed to nonopsonized WRR51/GFP versus 16M/GFP was 23.8 ± 11.2, and that of FL1-positive M/M from cultures exposed to nonopsonized WRR51/GFP versus WRR51/GFP+wboA was 27.6 ± 14.5 (data are means ± standard deviations [SD]) (Table 1). These flow cytometric data suggested that many more rough than smooth organisms associated with each infected M/M. To determine the total M/M burden of bacteria, we calculated a bacterial infection index by multiplying the percentage of positive M/M times the MFI of infected M/M (Table 1). This calculation indicated that M/M ingested 180 to 300 times as many nonopsonized rough bacteria as it did nonopsonized smooth bacteria. When compared to the culture data, these findings suggested that many of the rough bacteria associated with M/M were dead. Examination of M/M exposed to bacteria opsonized by treatment with HS led to similar conclusions (Table 1). Similar to the CFU recovery data, flow cytometry showed that 1 and 10% HS were equivalent for enhancing the association of M/M with Brucella, again suggesting that opsonins may reach saturating levels on the bacterial surface (Fig. 2A to C). The magnitude of the opsonic effect on both rough and smooth bacteria disclosed by flow cytometry, however, was much greater than suggested by the CFU data. Although there was no difference in the CFU counts obtained from M/M infected for 1 h with either rough or smooth Brucella when 10% HS was used as the source of opsonization (Fig. 1), flow cytometric (Table 1 and Fig. 2A to C, green lines) studies indicated that the proportion of fluorescent M/M was much higher when phagocytes were exposed to rough bacteria (82%) than when they were exposed to smooth bacteria (40% for 16M/GFP and 26% for WRR51/GFP+wboA). In addition, the intensity of infection per infected M/M was greater in cells exposed to WRR51/GFP (MFI of infected cells = 68) than it was in those exposed to 16M/GFP (MFI = 4.7) or WRR51/GFP+wboA (MFI = 3.4). As reflected by the bacterial infection index, M/M ingested 30 to 90 times as many rough opsonized as smooth opsonized bacteria. In addition, the total burden of smooth bacteria was increased 20- to 30-fold by opsonization (Table 1), not the 2- to 4-fold increase found by examination of CFU (Fig. 1), suggesting that many of the smooth GFP+ organisms were dead. This finding was supported by direct observation using fluorescence microscopy (Fig. 3) and by TEM analysis (data not shown). At 1 h after exposure of M/M to bacteria, both the proportion of infected phagocytes and the number of fluorescent bacteria per infected cell were much greater when M/M were exposed to the rough strain compared to M/M exposed to 16M (compare Fig. 3A and D). Since flow cytometry and fluorescence microscopy do not distinguish live, dead, or internalized versus attached bacteria, we determined the number of attached and internalized bacteria by TEM analysis of M/M cells at 1 h after exposure to opsonized bacteria. We examined 35 cells infected with smooth 16M/GFP and 53 cells infected with rough WRR51/GFP that had one or more bacteria associated with macrophages. Of these, all cells contained bacteria in internal vacuoles. Only one cell for each group had a bacterium attached to the outer surface. In addition, both intact and degenerating bacteria were observed inside of M/M infected with either rough or smooth Brucella. M/M exposed to rough organisms had 11 ± 8.1 internalized bacteria per cell, while those exposed to smooth organisms had only 3 ± 2.5 (means ± SD). These findings indicate that the MFI of infected M/M is overwhelmingly attributable to internalized live and dead bacteria with a minimal contribution of fluorescence generated by attached bacteria. In combination with the CFU data, these flow cytometry, fluorescence microscopic, and TEM observations showed that treatment with HS led to greatly enhanced uptake of both rough and smooth bacteria and also led to substantial bacterial killing within the first hour. At 6 days after infection, flow cytometry, in agreement with the CFU data, indicated that smooth B. melitensis had survived and multiplied inside M/M, since the intensity of fluorescence increased 40- to 250-fold over time in the case of M/M infected with wild-type or complemented strains and the proportion of infected M/M also greatly increased (compare Fig. 2E and B and Fig. 2F and C, respectively). In contrast, rough B. melitensis was eliminated inside M/M, since the intensity of fluorescence decreased with time of infection (compare Fig. 2D and A). Fluorescence microscopic observation of human M/M infected with rough and smooth B. melitensis confirmed these results: smooth organisms survived and multiplied inside phagocytic cells (Fig. 3D to F), while fewer and fewer bacteria were observed inside the rough-bacterium-infected M/M that remained at day 6 after infection (Fig. 3A to C). Taken together, these data indicated that surface OPS inhibits ingestion of B. melitensis in the absence of factors found in HS but that exposure to HS overcomes this inhibition. They also show that the presence of OPS protects bacteria from intracellular destruction and permits them to multiply intracellularly.
FIG. 2.
Flow cytometric analysis of human M/M exposed to rough and smooth GFP-B. melitensis strains. Bacteria were left in contact with monocytes for 1 h (A to C), washed, and further incubated in gentamicin-containing medium for 6 days (D to F). At selected times, M/M were washed and fixed with 4% formaldehyde as described in Materials and Methods. (A and D) Infection with WRR51/GFP; (B and E) infection with 16M/GFP; (C and F) infection with WRR51/GFP+wboA. In these cases, M/M exposed to bacteria were compared to nonexposed cells (shown in green). Nonopsonized bacteria are shown in red, while bacteria opsonized with 1% HS are shown in dark blue, and bacteria opsonized with 10% HS are shown in light blue. Cells were analyzed on a FACSort flow cytometer (Becton Dickinson). These data are from a representative experiment that was repeated with similar results.
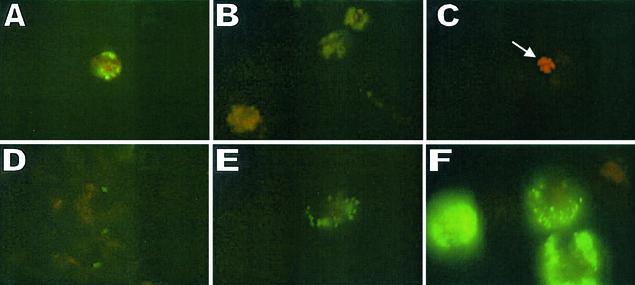
FIG. 3.
Fluorescence microscopic analysis of human M/M infected with rough WRR51 (A to C) and smooth 16M (D to F) GFP-B. melitensis strains. Bacteria were left in contact with M/M for 1 h, washed, and further incubated in gentamicin-containing medium for 1 to 6 days. At 1 h (A and D), 3 days (B and E), and 6 days (C and F) postinfection, M/M were washed and fixed with 4% formaldehyde as described in Materials and Methods. The arrow shows an apoptotic cell. Magnification, ×950.
TABLE 1.
Flow cytometric assessment of initial M/M infection by rough and smooth Brucellag
| Opsonic treatment | Strain | Mean ± SD
|
|||
|---|---|---|---|---|---|
| % M/M infecteda | MFI infectedc | Rough/smooth MFI ratiod | Bacterial infection indexe | ||
| Medium | WRR51/GFP | 21 ± 5 | 60.1 ± 9.8 | NAf | 903 ± 335 |
| Medium | 16M/GFP | 2.6 ± 0.8b | 2.5 ± 0.8b | 23.8 ± 11.2 | 5 ± 3b |
| Medium | WRR51/GFP+wboA | 1.9 ± 0.4b | 2.2 ± 0.8b | 27.6 ± 14.5 | 3 ± 2b |
| HS | WRR51/GFP | 82 ± 8.8 | 68.3 ± 12.2 | NA | 5,108 ± 1,295 |
| HS | 16M/GFP | 40.3 ± 11.9b | 4.7 ± 1.3b | 14.5 ± 3.5 | 153 ± 49b |
| HS | WRR51/GFP+wboA | 25.7 ± 9.4b | 3.4 ± 0.5b | 19.9 ± 3.8 | 58 ± 29b |
% infected = % cells positive in FL1 intensity.
P < 0.05 versus cells infected with WRR51/GFP.
MFI of FL1 positive cells − MFI of FL1 negative cells. This is an indicator of how many bacteria are present per infected M/M.
Ratio of MFI of infected rough to smooth strains.
% infected M/M × MFI of infected M/M.
NA, nonapplicable.
M/M monolayers were treated with bacteria at a multiplicity of infection of 50:1 in the presence of medium or medium supplemented with 1% HS. One hour later, monolayers were washed to remove nonadherent bacteria. M/M were scraped from the well, washed, fixed in formaldehyde, and acquired on a flow cytometer. FL1-positive cells were defined as those with a fluorescence intensity greater than the fluorescence intensity of 97.5% of untreated control cells. Data are means ± SD for four experiments.
Infection with rough B. melitensis strain WRR51 causes death of M/M.
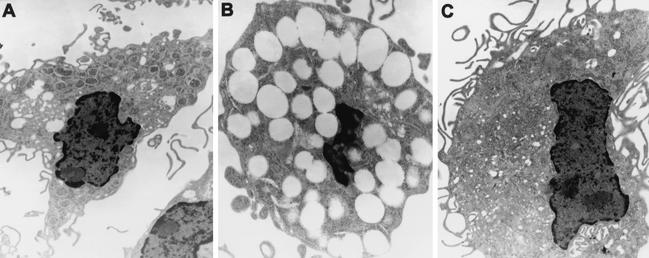
M/M infected 6 days previously with WRR51/GFP showed morphological evidence of apoptosis (Fig. 3C), while M/M infected with 16M remained healthy (Fig. 3F). These light-microscopic observations were confirmed by TEM (Fig. 4), which showed that WRR51/GFP-infected macrophages were smaller, were highly vacuolated with condensed nuclei, and contained few bacteria (Fig. 4B). On the other hand, 16M/GFP-infected macrophages (Fig. 4A) resembled noninfected phagocytes in size and nuclear morphology (Fig. 4C), even though numerous bacteria were observed inside the infected cells. Cell death or cytotoxicity was confirmed by measurement of LDH activity in the culture supernatants of M/M (Fig. 5). At day 6, cells infected with rough B. melitensis showed high release of LDH, indicating that these phagocytic cells were dying, but little LDH activity was detected in the supernatants of noninfected macrophages or macrophages infected with 16M/GFP (Fig. 5) or M/M infected with the complemented strain WRR51/GFP+wboA (data not shown).
FIG. 4.
TEM of Brucella-infected human M/M. Smooth 16M (A) and rough WRR51 (B) B. melitensis strains were left in contact with M/M for 1 h, washed, and further incubated in gentamicin-containing medium for 4 days. (C) Noninfected phagocyte. (A and C) Magnification, ×5,400; (B) magnification, ×8,500.
FIG. 5.
Evaluation of B. melitensis cytotoxicity for cultured human M/M. Rough (WRR51/GFP) and smooth (16M/GFP) B. melitensis strains were left in contact with M/M for 1 h, washed, and further incubated in gentamicin-containing medium for 3 days. Aliquots of the supernatant were collected and assayed for LDH release. Means ± SD (error bars) are shown. Abbreviations and symbols: NI, NI M/M (no exposure to bacteria); OD, optical density; ∗, P not significant compared to NI; ∗∗, P < 0.00004 when compared to NI.
Effect of B. melitensis infection on apoptosis induced by serum deprivation.
The cell shrinkage that occurs during the apoptotic process (23) may be observed by flow cytometry by a decrease in forward light scatter (6). Indeed, when we analyzed the cell pellet collected from centrifuged cell culture supernatants at 4 days after a 1-h exposure to WRR51/GFP in the presence of 10% HS, M/M showed profound reduction in forward scatter, while M/M treated with 16M/GFP had minimally reduced forward scatter compared to M/M treated with medium alone (Fig. 6). In fact, 78% of the M/M exposure to WRR51/GFP showed a reduced forward scatter and were infected with bacteria expressing the GFP (Fig. 6A). In contrast, only 10% of the cells from the cell culture supernatants of M/M exposed to 16M/GFP (Fig. 6B) showed a decreased forward light scatter and were green. These data indicated that both rough and smooth brucellae caused host cell shrinkage, but the effect of rough organisms was much greater.
FIG. 6.
Flow cytometric analysis of human M/M infected with rough (A) and smooth (B) GFP-B. melitensis strains. Rough (WRR51/GFP) and smooth (16M/GFP) B. melitensis strains were left in contact with eukaryotic cells for 1 h, washed, and further incubated in gentamicin-containing medium for 4 days. Supernatants were collected at this time and centrifuged to collect cells. Cells were washed and fixed with 4% formaldehyde as described in Materials and Methods. (C) Noninfected phagocyte. (D) Forward scatter histogram of noninfected (NI) M/M (green) or M/M infected with rough (pink) or smooth (blue) B. melitensis. Cells were analyzed on a FACSort flow cytometer. Green dots represent phagocytes infected with GFP-B. melitensis strains. These data are from a representative experiment that was repeated with similar results.
Our previous reports have indicated that a time-dependent differentiation of human monocytes into macrophages in in vitro studies is an important factor affecting the mode of cell death occurring after Shigella flexneri infection (10-12). To determine whether rough B. melitensis would have a similar effect on both 1-day-old human monocytes and the murine monocytic cell line J774 as it did on M/M in the present study, we infected both 1-day-old human monocytes and J774 cells with WRR51/GFP or 16M/GFP opsonized with 10% HS for 1 h, cultured them for 1 day, and examined them for indicators of cytotoxicity. One-day-old monocytes and J774 cells released substantial amounts of LDH when infected with rough, but not smooth bacteria (data not shown). As shown by flow cytometry, a peak of reduced forward scatter, corresponding to reduced cell size, was present in 1-day-old monocytes (Fig. 7) and J774 cells (data not shown) infected with rough, but not smooth, Brucella. These studies indicated that, like on M/M, J774 cells and 1-day-old human monocytes underwent apoptosis following infection by rough brucellae.
FIG. 7.
Flow cytometric analysis of 1-day-old human monocytes infected with rough WRR51 (A) and smooth 16M (B) GFP-B. melitensis strains. Bacteria were left in contact with eukaryotic cells for 1 h, washed, and further incubated in gentamicin-containing medium for 1 day. Monocytes were washed and fixed with 4% formaldehyde as described in Materials and Methods. (C) Noninfected phagocyte. (D) Forward scatter histogram of noninfected (NI) monocytes (green) or monocytes infected with rough (pink) or smooth (blue) B. melitensis. Cells were analyzed on a FACSort flow cytometer. These data are from a representative experiment that was repeated with similar results.
To ask whether infection with smooth B. melitensis protected cells from apoptosis we induced apoptosis in monocytes by culturing them without serum for 1 day. We then exposed these starving monocytes to WRR51/GFP or 16M/GFP opsonized with 10% HS for 1 h, cultured them for 1 day, and processed them for flow cytometry (Fig. 8). Forty-seven percent of serum-deprived monocytes without bacteria showed reduced forward scatter consistent with apoptosis. This pattern was greatly enhanced in monocytes exposed to WRR51/GFP, with essentially all cells dying from apoptosis (Fig. 8C and 8D). In contrast, monocytes exposed to 16M/GFP were much less affected. Even these cultures, however, contained a majority of cells with forward scatter less than that of cells not exposed to bacteria. Most of the monocytes with normal size were infected with 16M/GFP (Fig. 8B). These data suggested that exposure to either rough or smooth brucellae augmented serum starvation-induced apoptosis. This finding prevented analysis of a potential antiapoptotic effect of infection with smooth organisms.
FIG. 8.
Flow cytometric analysis of the effect of B. melitensis infection on apoptosis induced by serum deprivation. Human monocytes were cultured without serum for 1 day to induce apoptosis. Then, they were infected with rough WRR51 (A) and smooth 16M (B) GFP-B. melitensis strains. Bacteria were left in contact with eukaryotic cells for 1 h, washed, and further incubated in gentamicin-containing medium for 1 day. Monocytes were washed and fixed with 4% formaldehyde as described in Materials and Methods. (C) Noninfected phagocyte under starving conditions. (D) Forward scatter histogram of these starving monocytes that were either not exposed (NI) (green) or exposed to rough (pink) or smooth (blue) B. melitensis strains. Cells were analyzed on a FACSort flow cytometer. These data are from a representative experiment that was repeated with similar results.
DISCUSSION
It has been proposed that rough brucellae are less virulent due to their increased sensitivity to complement-mediated killing and inability to replicate intracellularly (4, 7, 19, 35). Whether loss of surface OPS affects intracellular killing of brucellae is a matter of some dispute. Discrepancies have even been reported using genetically defined rough Brucella (1, 19, 35). With respect to the complement issue, we recently reported that disruption of the wboA gene in B. abortus and B. melitensis results in rough mutants that bind more complement than their respective wild-type parent strains 2308 and 16M (13). However, while OPS-deficient strains derived from B. melitensis are as resistant to complement-mediated killing as their smooth parents, rough B. abortus strains with identical mutations are sensitive to the bactericidal action of serum (13). These species-specific differences in complement sensitivity may be a reflection of differences at the genetic level between B. abortus and B. melitensis (2, 38, 39). The present studies were designed to determine whether the differences in complement fixation to the surface of rough and smooth B. melitensis were associated with differences in uptake of bacteria and in bacterial survival inside human mononuclear phagocytes. We found that rough and smooth bacteria were internalized by human monocytes in the absence of opsonization by serum. However, both opsonized and nonopsonized rough bacteria were internalized at a higher rate than smooth organisms. Opsonization led to both increased uptake and higher final numbers of intracellular smooth Brucella. The initial step in the phagocytosis of microorganisms is their recognition by specific receptors on the surface membrane of the phagocytic cells (for a review, see references 8 and 30). M/M not only have receptors for serum-derived opsonins like immunoglobulin G Fc and complement but also express receptors that directly bind bacteria by recognizing molecules like LPS, lipid A, outer membrane proteins, lipoproteins, and mannosylated structures (5, 36) that are exposed on the surface of the microorganism. Examples of these receptors include the mannose receptor, CD14 and members of the Toll-like receptor (TLR) family (25, 27, 34). The presence of OPS on the surface of smooth B. melitensis may limit complement-independent ingestion by hiding several molecules that could be targets for their direct recognition by phagocytic cells.
We further found that not only did rough brucellae have impaired survival, but they were eliminated in association with apoptosis of their host cells. These findings with rough bacteria are consistent with studies of Freeman et al. (14), who reported 40 years ago that rough variants of B. abortus, B. suis, and B. melitensis are more cytopathogenic than smooth organisms for guinea pig macrophages. The destructive effect requires the presence of living bacteria and is not the result of the rupture of the phagocyte due to the ingestion of great quantities of rough Brucella (14, 15). Furthermore, the same authors found that the removal of surface components on Brucella to form spheroplast results in bacterial cells that are more cytopathogenic for cultured guinea pig monocytes (16). After these initial studies, there have been no more reports on the nature of this destructive effect of rough Brucella in macrophages.
Recently, however, Gross et al. reported that infection of human monocytes with smooth B. suis prevents apoptosis and demonstrated that Brucella LPS only partially protects against apoptosis (20). It is likely that the antiapoptotic effects of the brucellae occur in vivo. Galdiero and et al. (17) have shown delayed apoptosis of lymphocytes and monocytes from cattle with brucellosis compared to the same cells coming from healthy control or vaccinated animals. In our studies, a majority of monocytes died when cultured for 1 day without serum. Infection with rough B. melitensis exaggerated this process, but so did infection with smooth organisms, although the effect of smooth organisms was less (Fig. 8). It is possible that the rapidity of phagocyte death in our system prevented us from observing the antiapoptotic effect of smooth bacteria noted by Gross et al. (20). The enhancement of cell death we observed with both rough and smooth brucellae in this serum-free system may indicate that interactions of bacteria or a bacterial product with the host cell membrane promotes an apoptotic program, which can be blocked if brucellae survive long enough. Rough bacteria, whose outer membrane lipid A, lipoproteins, and outer membrane proteins may be more accessible to host cell receptors, may thus more efficiently trigger apoptosis. Moreover, the increased susceptibility of rough organisms to macrophage microbicidal mechanisms, as seen in this study and others, may prevent them from surviving long enough to protect the host cell from the apoptotic stimulus. This interpretation would be consistent with Gross et al.'s (20) further observation that an attenuated, isogenic, dnaK knockout mutant of B. suis fails to protect from apoptosis that occurs when phagocytes are cultured without addition of M-CSF or other trophic factors.
Our data are consistent with the hypothesis that the presence of OPS favors survival of B. melitensis by preventing the death of M/M, Brucella's preferred target for intracellular replication. In contrast, the absence of OPS on the bacterial surface will cause the death not only of rough organisms but also of the phagocytic cell by an altruistic mechanism: apoptosis. Apoptosis of infected phagocytes may be beneficial to avoid the onset of infection. In fact, apoptosis but not necrosis has been associated with killing of Mycobacterium tuberculosis (28); macrophages from mice resistant to mycobacterial infection are more susceptible to apoptosis (32), and attenuated strains of M. tuberculosis cause more apoptosis than virulent strains (22). Several obligate intracellular bacteria like Mycobacterium, Chlamydia, Rickettsia, and Coxiella species have been shown to inhibit apoptosis at the onset of the infection (for a review, see reference 18), and this seems to be the case for smooth B. suis (17, 20) and B. melitensis. Furthermore, recent evidence suggests that factors released by dead or dying host cells provide signals that alert the immune system to danger and induce the maturation of antigen-presenting cells, thus facilitating the immune response against foreign antigens (21, 33). In this way, apoptosis of M/M infected by rough bacteria will have a dual role: it not only will inhibit the bacterial growth and spread but also will alert and stimulate the immune system to control the infection. It is likely that a large part of the pathogenicity of smooth organisms derives from interference by surface OPS with host cell apoptosis, allowing intracellular survival and replication of bacteria to numbers sufficient to awaken the host immune response and cause disease.
Acknowledgments
We thank Eleni Antzoulatos, Chrysanthi Paranavitana, Adrian Ravizee, and Jennifer Steiner for their help in the elutriation of human monocytes.
Work in the laboratory of R.M.R. was supported by contracts DAMD17-94-C-4054 and DAMD17-98-C-8045 from the U.S. Army Medical Research and Materiel Command.
The views of the authors do not purport to reflect the position of the Department of the Army or the Department of Defense.
Editor: A. D. O'Brien
REFERENCES
- 1.Allen, C. A., L. G. Adams, and T. A. Ficht. 1998. Transposon-derived Brucella abortus rough mutants are attenuated and exhibit reduced intracellular survival. Infect. Immun. 66:1008-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boschiroli, M. L., V. Foulongne, and D. O'Callaghan. 2001. Brucellosis: a worldwide zoonosis. Curr. Opin. Microbiol. 4:58-64. [DOI] [PubMed] [Google Scholar]
- 3.Chalfie, M., Y. Tu, G. Euskirchen, W. W. Ward, and D. C. Prasher. 1994. Green fluorescent protein as a marker for gene expression. Science 263:802-805. [DOI] [PubMed] [Google Scholar]
- 4.Corbeil, L. B., K. Blau, T. J. Inzana, K. H. Nielsen, R. H. Jacobson, R. R. Corbeil, and A. J. Winter. 1988. Killing of Brucella abortus by bovine serum. Infect. Immun. 56:3251-3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darveau, R. P. 1998. Lipid A diversity and the innate host response to bacterial infection. Curr. Opin. Microbiol. 1:36-42. [DOI] [PubMed] [Google Scholar]
- 6.Darzynkiewicz, Z., E. Bedner, and P. Smolewski. 2001. Flow cytometry analysis of cell cycle and apoptosis. Semin. Hematol. 38:179-193. [DOI] [PubMed] [Google Scholar]
- 7.Eisenschenk, F. C., J. J. Houle, and E. M. Hoffman. 1999. Mechanism of serum resistance among Brucella abortus isolates. Vet. Microbiol. 68:235-244. [DOI] [PubMed] [Google Scholar]
- 8.Ernst, J. D. 2000. Bacterial inhibition of phagocytosis. Cell. Microbiol. 2:379-386. [DOI] [PubMed] [Google Scholar]
- 9.Eze, M. O., L. Yuan, R. M. Crawford, C. M. Paranavitana, T. L. Hadfield, A. K. Bhattacharjee, R. L. Warren, and D. L. Hoover. 2000. Effects of opsonization and gamma interferon on growth of Brucella melitensis 16M in mouse peritoneal macrophages in vitro. Infect. Immun. 68:257-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernandez-Prada, C. M., D. L. Hoover, B. Tall, and M. M. Venkatesan. 1997. Human monocyte-derived macrophages infected with virulent Shigella flexneri in vitro undergo a rapid cytolytic event similar to oncosis but not apoptosis. Infect. Immun. 65:1486-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandez-Prada, C. M., D. L. Hoover, B. Tall, S. Elliott, J. Nataro, and M. M. Venkatesan. 1998. Hemolysin-positive enteroaggregative and cell-detaching Escherichia coli strains cause oncosis of human monocyte derived macrophages and apoptosis of murine J774 cells. Infect. Immun. 66:3918-3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandez-Prada, C. M., D. L. Hoover, B. D. Tall, J. Kopelowitz, A. A. Hartman, and M. M. Venkatesan. 1999. Shigella flexneri ipaH7.8 facilitates escape of virulent bacteria from the endocytic vacuoles of mouse and human monocytes and macrophages. Infect. Immun. 68:3608-3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandez-Prada, C. M., M. Nikolich, R.Vemulapalli, N. Sriranganathan, S. M. Boyle, G. G. Schurig, T. L. Hadfield, and D. L. Hoover. 2001. Deletion of wboA enhances activation of the lectin pathway of complement in Brucella abortus and Brucella melitensis. Infect. Immun. 69:4407-4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freeman, B. A., D. J. Kross, and R. Circo. 1961. Host-parasite relationships in brucellosis. II. Destruction of macrophages cultures by Brucella of different virulence. J. Infect. Dis. 108:333-338. [DOI] [PubMed] [Google Scholar]
- 15.Freeman, B. A., G. R. Pearson, and W. D. Hines. 1964. Host-parasite relationships in brucellosis. III. Behavior of avirulent Brucella in tissue culture monocytes. J. Infect. Dis. 114:441-449. [DOI] [PubMed] [Google Scholar]
- 16.Freeman, B. A., and B. H. Rumack. 1964. Cytopathogenic effect of Brucella spheroplasts on monocytes in tissue culture. J. Bacteriol. 88:1310-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galdiero, E., C. Romano Carratelli, M. Vitiello, I. Nuzzo, E. Del Vecchio, C. Bentivoglio, G. Perillo, and F. Galdiero. 2000. HSP and apoptosis in leukocytes from infected or vaccinated animals by Brucella abortus. New Microbiol. 23:271.- [PubMed]
- 18.Gao, L.-Y., and Y. Abu Kwaik. 2000. The modulation of host cell apoptosis by intracellular bacterial pathogens. Trends Microbiol. 8:306-313. [DOI] [PubMed] [Google Scholar]
- 19.Godfroid, F., B. Taminiau, I. Danese, P. Denoel, A. Tibor, V. Weynants, A. Cloeckaert, J. Godfroid, and J. Letesson. 1998. Identification of the perosamine synthetase gene of Brucella melitensis 16M and involvement of lipopolysaccharide O side chain in Brucella survival in mice and in macrophages. Infect. Immun. 66:5485-5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gross, A., A. Terraza, S. Quahrani-Bettache, J. P. Liautard, and J. Dornand. 2000. In vitro Brucella suis infection prevents the programmed cell death of human monocytic cells. Infect. Immun. 68:342-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishii, K. J., K. Suzuki, C. Coban, F. Takeshita, Y. Itoh, H. Matoba, L. D. Kohn, and D. M. Klinman. 2001. Genomic DNA released by dying cells induces the maturation of APCs. J. Immunol. 167:2602-2607. [DOI] [PubMed] [Google Scholar]
- 22.Keane, J., M. K. Balcewicz-Sablinska, H. G. Remold, G. L. Chupp, B. B. Meek, M. J. Fenton, and H. Kornfeld. 1997. Infection by Mycobacterium tuberculosis promotes human alveolar macrophage apoptosis. Infect. Immun. 65:298-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kerr, J. F. R., and A. H. Wyllie. 1972. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 26:239-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kovach, M. E., R. W. Phillips, P. H. Eler, R. M. Roop, and K. M. Peterson. 1994. pBBR1MCS: a broad-host-range cloning vector. BioTechniques 16:800-802. [PubMed] [Google Scholar]
- 25.Krutzik, S. R., P. A. Sieling, and R. L. Modlin. 2001. The role of Toll-like receptors in host defense against microbial infection. Curr. Opin. Immunol. 13:104-108. [DOI] [PubMed] [Google Scholar]
- 26.McQuiston, J. R., R. Vemulapalli, T. J. Inzana, G. G. Shurig, N. Sriranganathan, D. Fritzinger, T. L. Hadfield, R. A. Warren, N. Snellings, D. Hoover, S. M. Halling, and S. M. Boyle. 1999. Genetic characterization of a Tn5-disrupted glycosyltransferase gene homolog in Brucella abortus and its effect on lipopolysaccharide composition and virulence. Infect. Immun. 67:3830-3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Medzgutiv, R. 2001. Toll-like receptors and innate immunity. Nat. Rev. 1:135-145. [DOI] [PubMed] [Google Scholar]
- 28.Molloy, A., P. Laochumroonvorapong, and G. Kaplan. 1994. Apoptosis, but not necrosis, of infected monocytes is coupled with killing of intracellular bacillus Calmette-Guerin. J. Exp. Med. 180:1499-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy, E., G. T. Robertson, M. Parent, S. D. Hagius, R. M. Roop II, P. H. Elzer, and C. L. Baldwin. 2002. Major histocompatibility complex class I and II expression on macrophages containing a virulent strain of Brucella abortus measured using green fluorescent protein-expressing brucellae and flow cytometry. FEMS Immunol. Med. Microbiol. 33:191-200. [DOI] [PubMed]
- 30.Pieters, J. 2001. Evasion of host cell defense mechanisms by pathogenic bacteria. Curr. Opin. Immunol. 13:37-44. [DOI] [PubMed] [Google Scholar]
- 31.Price, R. E., J. W. Templeton, and L. G. Adams. 1990. Survival of smooth, rough and transposon mutant strains of Brucella abortus in bovine mammary macrophages. Vet. Immunol. Immunopathol. 26:353-365. [DOI] [PubMed] [Google Scholar]
- 32.Rojas, M., Luis F. Barrera, G. Puzo, and L. F. Garcia. 1997. Differential Induction of apoptosis by virulent Mycobacterium tuberculosis in resistant and susceptible murine macrophages. J. Immunol. 159:1352-1361. [PubMed] [Google Scholar]
- 33.Shi, Y., W. Zheng, and K. L. Rock. 2000. Cell injury releases endogenous adjuvants that stimulate cytotoxic T cell responses. Proc. Natl. Acad. Sci. 97:14590-14595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sieling, P. A., and R. L. Modlin. 2001. Activation of toll-like receptors by microbial lipoproteins. Scand. J. Infect. Dis. 33:97-100. [DOI] [PubMed] [Google Scholar]
- 35.Ugalde, J. E., C. Czibener, M. F. Feldman, and R. A. Ugalde. 2000. Identification and characterization of the Brucella abortus phosphoglucomutase gene: role of lipopolysaccharide in virulence and intracellular multiplication. Infect. Immun. 68:5716-5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ulevich, R. J., and P. S. Tobias. 1999. Recognition of gram-negative bacteria and endotoxin by the innate immune system. Curr. Opin. Immunol. 11:19-22. [DOI] [PubMed] [Google Scholar]
- 37.Vemulapalli, R., J. R. McQuiston, G. G. Schurig, N. Sriranganathan, S. M. Halling, and S. M. Boyle. 1999. Identification of an IS711 element interrupting the wboA gene of Brucella abortus vaccine strain RB51 and a PCR assay to distinguish strain RB51 from other Brucella species and strains. Clin. Diagn. Lab. Immunol. 6:760-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vizcaino, N., J. M. Verger, M. Grayon, M. S. Zygmunt, and A. Cloeckaert. 1997. DNA polymorphism at the omp-31 locus of Brucella spp.: evidence for a large deletion in Brucella abortus, and other species-specific markers. Microbiology 143:2913-2921. [DOI] [PubMed] [Google Scholar]
- 39.Vizcaino, N., A. Cloeckaert, M. S. Zygmunt, and L. Fernandez-Lago. 1999. Molecular characterization of a Brucella abortus strains: evidence for a locus involved in the synthesis of a polysaccharide. Infect. Immun. 67:2700-2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Winter, A. J., G. G. Schurig, S. M. Boyle, N. Sriranganathan, J. S. Bevins, F. M. Enright, P. H. Elzer, and J. D. Kopec. 1996. Protection of BALB/c mice against homologous and heterologous species of Brucella by rough strain vaccines derived from Brucella melitensis and Brucella suis biovar 4. Am. J. Vet. Res. 57:677-683. [PubMed] [Google Scholar]
- 41.Young, E. J. 1983. Human brucellosis. Rev. Infect. Dis. 5:821-842. [DOI] [PubMed] [Google Scholar]
- 42.Young, E. J., M. Borchert, F. L. Kretzer, and D. M. Musher. 1985. Phagocytosis and killing of Brucella by human polymorphonuclear leukocytes. J. Infect. Dis. 151:682-690. [DOI] [PubMed] [Google Scholar]
- 43.Zamai, L., B. Canonico, F. Luchetti, P. Ferri, E. melloni, L. Guidotti, A. Cappellini, G. Cutronco, M. Vitale, and S. Papa. 2001. Supravital exposure to propidium iodine identifies apoptosis on adherent cells. Cytometry 44:57-64. [PubMed] [Google Scholar]