Abstract
We examined the role of the cytokines gamma interferon (IFN-γ) and interleukin-12 (IL-12) in the model of acute babesiosis with the WA1 Babesia. Mice genetically deficient in IFN-γ-mediated responses (IFNGR2KO mice) and IL-12-mediated responses (Stat4KO mice) were infected with the WA1 Babesia, and observations were made on the course of infection and cytokine responses. Levels of IFN-γ and IL-12 in serum increased 24 h after parasite inoculation. The augmented susceptibility observed in IFNGR2KO and Stat-4KO mice suggests that the early IL-12- and IFN-γ-mediated responses are involved in protection against acute babesiosis. Resistance appears to correlate with an increase in nitric oxide (NO) production. In order to assess the contribution of different cell subsets to resistance against the parasite, we also studied mice lacking B cells, CD4+ T cells, NK cells, and macrophages. Mice genetically deficient in B lymphocytes or CD4+ T lymphocytes were able to mount protective responses comparable to those of immunosufficient mice. In contrast, in vivo depletion of macrophages or NK cells resulted in elevated susceptibility to the infection. Our observations suggest that a crucial part of the response that protects from the pathogenic Babesia WA1 is mediated by macrophages and NK cells, probably through early production of IL-12 and IFN-γ, and induction of macrophage-derived effector molecules like NO.
Despite years of effort, our understanding of the immune mechanisms mediating protection against Babesia is still incomplete. A serious attempt has been undertaken to identify cells and molecules that could be useful for the development of an effective vaccine against Babesia in cattle (7), but conclusive data still need to be gathered. The laboratory models of this infection in mice are amenable to more experimental manipulation than the infection in cattle and can contribute useful information about the protective strategies used by mammalian hosts. Here, we report observations made in the murine model of acute babesiosis with the recently described WA1 Babesia, a human pathogen that also causes severe acute infection in laboratory mice.
Babesia and Plasmodium are phylogenetically similar (8) and exhibit similarities in life cycle, developmental processes, and parasitic strategies. In fact, it has been proposed that malaria and babesiosis are conceptually identical diseases (10). The experimental murine model of acute babesiosis with the WA1 Babesia and the murine malaria model with Plasmodium chabaudi show many similarities as well. The kinetics of the infections and their pathological consequences are comparable, and the same inbred strains are genetically susceptible to both parasites (29, 37, 38). Given these similarities, we set out to investigate whether the immune mechanisms implicated in protection against malaria were correspondingly important in resistance against babesiosis.
Resistance to P. chabaudi has been shown to involve production of interleukin-12 (IL-12), gamma interferon (IFN-γ), and tumor necrosis factor alpha (TNF-α) during the acute phase, which result in protective immunity through a NO-dependent mechanism (40-43). Specifically, IFN-γ produced during innate and acquired immune responses plays a central role in protective immunity (28, 40). IL-12 production and expression of IL-12 receptors correlate with the differential susceptibility of mouse strains to the parasite (33, 40). Macrophage activation (41) and NK cell cytokine production (28) have also been identified as important players in resistance against P. chabaudi.
In order to determine if the immunological pathways activated in response to P. chabaudi were involved in resistance to the WA1 Babesia we performed experiments with mice genetically deficient in the β subunit of the IFN-γ receptor (IFNGR2) and the IL-12-specific signal transduction molecule Stat4 (signal transduction and activator of transcription-4). The β-subunit of the receptor for IFN-γ (IFN-γ-R-β) is responsible for coupling the binding of the ligand to the signal transduction pathway (2). In the IFNGR2KO mouse, which carries a targeted disruption of the gene encoding the IFN-γ-R-β molecule, there are no IFN-γ-mediated responses (27). Stat4 is the central protein in the cellular response triggered by the binding of IL-12 to its receptor (12, 26). Mice genetically deficient in Stat4 (Stat4KO) exhibit a disruption of IL-12-dependent functions, including the induction of IFN-γ by NK and T cells in response to IL-12 (25, 45).
In addition, to identify the cells involved in the protective response, we analyzed the course of infection in genetically resistant C57BL mice in which different immune cell populations (B cells, CD4+ T cells, NK cells, and macrophages) had been eliminated by either targeted mutation or pharmacological depletion in vivo.
MATERIALS AND METHODS
Mice.
Inbred C57BL/6, C3H/HeJ, BALB/c, and 129/SvS3ImJ mice were purchased from the Jackson Laboratory (Bar Harbor, Maine). Immunodeficient mice of the strains B10 μMT [B10.129S2(B6)Igh-6tm1Cgn] and B10 CD4− [B10.129S2(B6)-Cd4tm1Litt] were originally purchased from the Jackson Laboratory and maintained at the Mouse Immunogenetics Colony at the Mayo Clinic. Mice lacking the ifngr2 gene (IFNGR2KO) on the 129 background were generously provided by Paul B. Rothman, Columbia University (New York, N.Y.). Mice lacking the Stat4 gene (Stat4KO) on the BALB/c background were generously provided by Michael J. Grusby, Harvard Medical School (Cambridge, Mass.).
Parasites and infection.
Mice were 5 to 7 weeks old at the time of infection. Acute babesiosis was induced with WA1 Babesia parasites (31) that had been maintained by cryopreservation in liquid nitrogen and assayed for pathogenicity by passage in female Syrian golden hamsters prior to experiments with mice. Total erythrocytes in hamster blood were counted in a clinical counter (Coulter Corporation, Miami, Fla.), and the percent parasitemia was determined by microscopic examination of Giemsa-stained thin blood smears. The same Babesia inoculum was used to infect all mice included in an experiment, with each mouse receiving 107 parasitized erythrocytes intraperitoneally in 100 μl of saline. As previously reported (29), mice of the C57BL/6 background were highly resistant, BALB/c and 129/SvS3ImJ were moderately resistant, and C3H/HeJ were highly susceptible to the parasite.
Assessment of infection course.
Mice were monitored daily for mortality. Peripheral blood from 4 to 7 mice was sampled every 3 to 4 days by tail bleeding. Parasitemia was determined by microscopic examination of at least 10 high-magnification fields of Giemsa-stained thin blood smears.
Macrophage depletion.
Splenic macrophages were depleted by the van Rooijen liposome-mediated suicide technique (46, 48). Mice were injected intravenously with 100 μl of a liposome suspension containing 5 mg of clodronate (dichloromethylene diphosphonate, Cl2MDP; Sigma Chemical Co. St. Louis, Mo.)/ml 48 h prior to inoculation with the parasite. The clodronate liposome technique has been shown to deplete macrophages in the marginal zone and red pulp of the spleen for at least 1 week starting 24 h after treatment (46, 47). Phosphatidylcholine-cholesterol multilamellar liposomes containing clodronate as well as control phosphate-buffered saline-containing liposomes were prepared as described previously (46). Macrophage depletion was confirmed by immunohistochemistry of the spleen with the macrophage-specific F4/80 monoclonal antibody at days 7 and 14 after clodronate liposome treatment.
NK cell depletion.
Natural killer cells were immunodepleted in vivo with anti-asialo GM1 rabbit antiserum (Wako Bioproducts, Richmond, Va.) by using 50 μl/mouse (titer, ∼1:1,000). Antiserum was administered intravenously 48 h before infection with Babesia and then at days 2, 6, and 10 postinfection. Analysis by flow cytometry with the anti-NK1.1 monoclonal antibody showed removal of more than 85% of NK cells at days 3 and 7 postinfection (data not shown).
Determination of NO levels in serum.
Blood was obtained by cardiac puncture of anesthetized mice at the indicated times, and serum samples were stored at −70°C until assay. NO levels in serum were determined by the Griess reaction (18) after reduction of nitrite by enzymatic methods (32).
Enzyme-linked immunosorbent assay.
Serum cytokines were quantitated by two-site enzyme-linked immunosorbent assay by using OptEIA sets (Pharmingen, San Diego, Calif.) specific for mouse IL-12 p70 and IFN-γ.
Statistics.
Survival was analyzed by the log-rank and Wilcoxon tests with the Kaplan-Meier nonparametric model. Parasitemia was evaluated by mixed-model analysis of variance, as implemented in PROC MIXED (SAS Institute, Cary, N.C.). Data were analyzed on the square-root scale in order to meet modeling assumptions. Mean parasitemia levels and 95% confidence intervals were computed from data from all mice in the groups after adjusting for repeated measurements.
RESULTS
Resistance is impaired in IFNGR2KO mice.
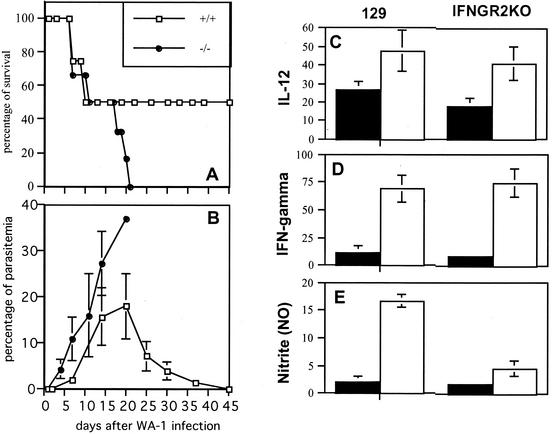
Preliminary experiments revealed an early induction of IL-12 and IFN-γ upon Babesia inoculation in genetically resistant C57BL/6 mice but not in genetically susceptible C3H and BALB/c mice (1; data not shown), which led us to believe that these cytokines could be involved in the protective mechanism against acute babesiosis. To directly assess the role of IFN-γ during Babesia infection, we analyzed the infection course and cytokine response in IFNGR2KO mice (in the 129/Sv background) that are genetically deficient in IFN-γ-R-β. IFN-γ-R-β is responsible for coupling the binding of the ligand to the signal transduction pathway (2). In the IFNGR2KO mouse, which carries a targeted disruption of the gene encoding the IFN-γ-R-β molecule, all IFN-γ-mediated responses are absent (27).
Following infection with the WA1 Babesia, IFNGR2KO mice exhibited an increased mortality rate compared to wild-type 129/SvS3ImJ mice (P ≤ 0.004) (Fig. 1A) and developed significantly higher levels of parasitemia during acute infection compared to control animals (P ≤ 0.02) (Fig. 1B). Twenty-four hours after the infectious challenge, a substantial induction of IL-12 and IFN-γ in serum was observed in the experimental animals. Both 129/SvS3ImJ mice and IFNGR2KO mice exhibited comparable levels of these cytokines (Fig. 1C and D). However, wild-type mice exhibited a notable increase in NO levels in serum, which remained at basal levels in IFNGR2KO mice (Fig. 1E). NO is produced in macrophages by inducible nitric oxide synthase, which is activated upon stimulation by IFN-γ and a microbial stimulus. The decreased resistance of IFNGR2KO mice thus appeared to correlate with their inability to respond to IFN-γ by triggering NO production.
FIG. 1.
Infection course in IFN-γ-R2-deficient mice. (A) Survival in IFNGR2KO mice and 129 controls. (B) Parasitemia levels in IFNGR2KO mice and 129 controls. (C through E) Levels of cytokines and NO in serum after infection with the WA1 Babesia. Filled bars represent uninfected controls; white bars represent infected animals. Data shown are the averages of 3 to 5 individual plasma samples. (C) Levels of IL-12 24 h after infection. (D) Levels of IFN-γ 24 h after infection. (E) Nitrite levels in serum at day 7 after infection. Data shown are pooled from two replicate experiments.
Resistance is impaired in Stat4KO mice.
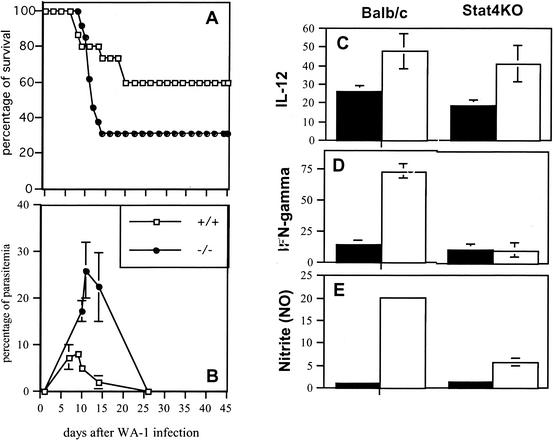
Stat4, the signal transduction and activator of transcription-4, is the central protein in the cellular response triggered by IL-12 after binding to its receptor (12, 26). Mice genetically deficient in Stat4 (Stat4KO) exhibit a disruption of IL-12-dependent functions, including the induction of IFN-γ by NK and T cells in response to IL-12 (25, 45).
We found that Stat4KO mice developed severe babesiosis with a higher mortality rate following infection with the WA1 Babesia than wild-type BALB/c mice (P ≤ 0.001) (Fig. 2A) and levels of parasitemia that were also significantly higher than those observed in the controls (P ≤ 0.003) (Fig. 2B). WA1 infection triggered an early production of IL-12 and IFN-γ in BALB/c mice, and a comparable increase occurred in infected Stat4KO mice (Fig. 2C). In contrast, serum IFN-γ in Stat4KO mice was significantly reduced compared to the levels observed in the control animals (Fig. 2D). In addition, NO levels were increased in BALB/c controls after infection but remained at basal levels in the Stat4-deficient animals (Fig. 2E). The decreased resistance of Stat4KO mice thus appeared to correlate with their inability to induce early production of IFN-γ in response to IL-12 and the subsequent failure to induce NO production.
FIG. 2.
Infection course in Stat4-deficient mice. (A) Survival in Stat-4KO mice and BALB/c controls. (B) Parasitemia levels in Stat-4KO mice and BALB/c controls. (C through E) Levels of cytokines and NO in serum after infection with the WA1 Babesia. Filled bars represent uninfected controls; white bars represent infected animals. Data shown are the averages of 3 to 5 individual plasma samples. (C) Levels of IL-12 24 h after infection. (D) Levels of IFN-γ 24 h after infection. (E) Levels of nitrite in serum at day 7 after infection. Data shown are pooled from two replicate experiments.
Resistance to acute babesiosis requires macrophages and NK cells but not CD4+ T or B cells.
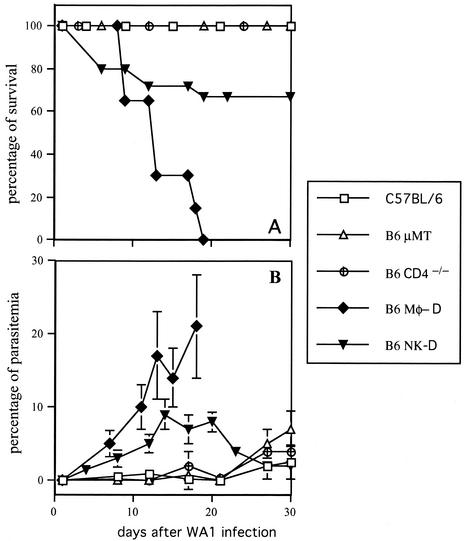
Mice of the C57BL lineage are notably resistant to the WA1 Babesia, undergoing an infection course with parasitemias lower than 5% and survival rates above 90% (1, 29). We followed the infection course of C57BL/6 mice lacking different immune cell populations in order to observe their relative contributions to host resistance. In B-cell-deficient B10-μMT mice, parasitemia levels showed a moderate increase (up to 9%) around day 30 after infection (Fig. 3A), but the animals exhibited few signs of disease and no deaths occurred during the acute infection (Fig. 3B). CD4+-T-cell-deficient B10.CD4− mice followed a very similar course (Fig. 3). Survival rates and parasitemia levels in both B10.μMT and B10-CD4−/− mice exhibited no statistical differences from those observed in control C57BL/6 mice (P > 0.05).
FIG. 3.
Immune cells involved in protection to acute babesiosis in mice. Intact C57BL/6 controls as well as C57BL/6 mice lacking B cells (B6 μMT mice), CD4+ T cells (B6 CD4−/− mice), macrophages (B6-Mφ-D mice), and NK cells (B6-NK-D mice) were inoculated with the WA1 Babesia. Each experimental group included 10 to 16 mice, pooled from two independent experiments. (A) Survival during the course of the infection. (B) Parasitemia levels during infection.
When we tested C57BL/6 mice that were depleted of macrophages by infusion of clodronate-containing liposomes, a severe impairment of resistance was observed. Parasitemia reached up to 27% in macrophage-depleted mice, appearing to be significantly elevated in comparison to intact C57BL/6 controls (P ≤ 0.007) (Fig. 3A). Mortality in macrophage-depleted mice was highly increased compared to that of control animals either untreated or treated with phosphate-buffered saline-containing liposomes (P ≤ 0.008) (Fig. 3B). The fatal outcome generally occurred within 14 days after infection. Accordingly, macrophage-depleted mice exhibited highly increased levels of parasitemia compared to controls (P ≤ 0.007). Splenic macrophage depletion up to day 14 was confirmed by immunohistochemistry (data not shown).
Finally, we observed that anti-asialo 6M1-mediated removal of NK cells during the course of the infection also impaired resistance to WA1 in C57BL/6 mice, although not as severely as macrophage depletion. NK-cell-depleted mice exhibited rising parasite levels that peaked around day 14, reaching up to 9% of total circulating erythrocytes, an apparent increase compared with intact mice, but the result was not statistically significant (P > 0.05) (Fig. 3A). However, infection of NK-cell-depleted mice resulted in a moderate course of babesiosis with mild pathological manifestations (piloerection and weakness) and an overall survival rate of 70% that was significantly lower than the one exhibited by intact C57BL/6 mice (P ≤ 0.020) (Fig. 3B).
DISCUSSION
The immune response that develops in the face of infectious challenge with Babesia has evolved to facilitate the rapid and efficient clearance of the parasites. However, as with many infectious processes a misdirected or exacerbated response might directly contribute to pathogenesis in the host. It has been experimentally demonstrated that TNF-α and CD8+ T lymphocytes are involved in WA1-induced disease in mice (22). The immune mechanisms responsible for protection against the parasite are not well characterized as yet. Recently, Babesia bovis was shown to stimulate the production of IL-1β, IL-12, and TNF-α by bovine macrophages in vitro (36). Furthermore, age-related immunity appears to correlate with increased splenic production of IFN-γ, IL-12, and inducible nitric oxide synthase during B. bovis infection in cattle (17). These immune mediators are thought to be associated with the babesiacidal mechanisms that allow recovery of the infected host.
Here we show the involvement of cytokines and specific cellular subjects in the response of mice infected with the WA1 Babesia and offer evidence suggesting their direct involvement in protection against the parasite in vivo.
We demonstrate that infection with the WA1 Babesia in mice induces production of the cytokines IL-12 and IFN-γ. Mice genetically modified for unresponsiveness to IL-12 fail to stimulate protective levels of IFN-γ and NO and exhibit a higher susceptibility to the infection. Mice genetically modified for unresponsiveness to IFN-γ show an early increase in both IL-12 and IFN-γ but fail to increase NO in serum from basal levels. Lower levels of NO in plasma correlate with a higher susceptibility to the infection. It thus appears that early induction of IL-12 and IFN-γ is associated with resistance to the WA1 Babesia, which might be mediated through a NO-mediated mechanism. The activation of NO production in response to Babesia infection has been documented extensively for B. bovis (36, 42). The involvement of NO in protection, however, would have to be confirmed by direct manipulation of NO levels. The induction of IL-12 and IFN-γ expression, with similar kinetics, has been shown in the experimental model of murine malaria with P. chabaudi (43). Both cytokines are induced at the acute infection stage, during which parasitemia can be prevented from escalating to overwhelming levels.
It has been observed that C57BL/6, C3H, and BALB/c macrophages exhibit intrinsic differences in their abilities to produce IL-12 (5). Also, genetically encoded differences between BALB/c and C57BL have been described in relation to IL-12 responsiveness (20, 21). Interestingly, the differential susceptibility to the WA1 Babesia exhibited by these strains is consistent with their reported abilities to produce and respond to IL-12. Increased production and higher responsiveness to IL-12 is observed in C57BL/6, which exhibits the highest resistance to the infection, whereas diminished IL-12 production and lower responsiveness to it occur in the more-susceptible BALB/c and C3H mouse strains. The possibility of a direct relationship between IL-12 production/responsiveness and resistance to infection is supported by our demonstration that Stat4KO mice are susceptible to the WA1 Babesia.
The role of IFN-γ in the protective mechanism is substantiated by experiments with the IFNGR2KO mice, demonstrating that the ability to respond to IFN-γ-mediated signals is necessary for an effective defense against the WA1 Babesia. The important role of IFN-γ in protection to murine babesiosis is supported also by the finding that IFN-γ-deficient mice (IFN-γ−/− BALB/c) are unable to resolve primary infection with Babesia microti (24). More recently, it has been found that IFN-γ is crucial for controlling parasitemia at early stages of infection in the model of KR1 B. microti in BALB/c mice (11).
Our experiments with mice in which specific immune cell populations were either genetically absent or experimentally depleted provided information on the immune cell subsets involved in protection against acute babesiosis. We found that the absence of B lymphocytes does not significantly impair the resistance of C57BL/6 mice to the WA1 Babesia. This finding is in agreement with studies indicating that the crucial mechanisms during the early stages of infection in both babesiosis and malaria are antibody independent (7, 19, 23, 44). The recrudescence observed in B-cell-deficient mice at 30 days after WA1 infection support the view that B lymphocytes and antibodies are important for final clearance of residual parasitemia (50).
Our data indicate that the absence of CD4+ T cells does not necessarily prevent the development of a protective response against WA1 infection. We previously reported that B6 SCID mice, which possess neither B nor T lymphocytes, are nearly as resistant to WA1 Babesia as healthy B6 mice (1). Our results with the B10 CD4−/− mice suggest that resistance to acute babesiosis in C57 mice is at least partially mediated by a CD4+-T-cell-independent pathway, at least during the acute phase of the infection, when control of parasitemia is a critical determinant of survival. These and earlier results with C57BL/6 SCID mice support the possibility of a T-cell-independent mechanism of resistance to babesiosis (4). Nevertheless, involvement of CD4+ T lymphocytes in resistant mice cannot be entirely discounted since it has been observed that CD4+ T cells can contribute to parasite clearance under circumstances of high parasite load (22). Also, genetic determinants can alter the ability of the host to mount an effective CD4+-T-cell-independent response. It has been demonstrated that CD4+ T cells are important for defense against B. microti in BALB/c mice (24) and that αβ-T lymphocytes are involved in protection against KR1 B. microti in the BALB/c model (11). A CD4+-T-cell-independent response could be sufficient for protection to babesiosis in resistant C57BL/6 mice but not in more-susceptible strains.
Macrophage activation is known to occur during babesiosis (51), and a protective role for macrophages has been documented during infection with several Babesia species (23). Macrophage-derived soluble factors have been postulated to be responsible for killing these parasites (10). Our data indicate that resistance to the WA1 Babesia is impaired after macrophage depletion in vivo, thereby providing a strong indication that macrophages play a critical role in the protective mechanism.
Natural killer cells have also been proposed as mediators of anti-Babesia activity (23). Our observations of an increased susceptibility following depletion of NK cells support the hypothesis that this immune population is involved in the protective response to the WA1 Babesia. The identification of macrophages and NK cells as components in resistance to WA1 appear relevant in the light of recent evidence of Babesia bigemina and B. bovis infection in cattle, indicating that protective immunity involves activated macrophages and natural killer cells (7). These findings suggest a critical role for cells of the innate immune system in protection against Babesia. Involvement of innate immunity is also supported in the WA1 model by studies of mice carrying the scid mutation (1), which constitutes a powerful system for assessing the relative contributions of both types of immunity (3).
The absence of IFN-γ induction observed in infected IL-12-unresponsive mice (Stat4KO), suggests that IFN-γ is produced in response to IL-12. Target cells that activate IFN-γ expression in response to IL-12 include CD4+ Th1 cells, NK cells, and, in some cases, macrophages (16, 25, 30, 45). The kinetics of the response and the results with mice lacking CD4+ lymphocytes argue against a CD4+-T-cell-mediated IFN-γ response. Cytokine production by NK cells has been shown to contribute to resistance in the P. chabaudi model (28). It is possible, then, that NK cells might be the main population responsible for IFN-γ production in response to Babesia infection. Macrophage contribution to IFN-γ levels cannot be ruled out, however, particularly considering the moderate impairment in resistance caused by NK cell depletion. Overall, the involvement of IFN-γ, IL-12, macrophages, and NK cells in resistance to babesiosis is consistent with the findings for murine malaria, supporting the view that a common set of immune mechanisms is responsible for protection against Babesia and Plasmodium (10).
The involvement of macrophages and NK cells in IL-12- and IFN-γ-mediated responses could suggest an innate immunity-mediated protective mechanism, similar to the T-cell-independent macrophage activation described originally for Toxoplasma gondii infection. In this pathway, macrophages and NK cells establish a positive feedback loop of IL-12 and IFN-γ production that stimulates cellular activation before the development of T-cell-mediated responses (13, 14, 15, 34). This mechanism seems to be an important early barrier against infection with intracellular parasites. Early after infection, the presence of the parasites triggers the induction of IL-12, which then stimulates IFN-γ production by NK cells. Protection during acute infection results from IFN-γ-mediated macrophage activation and the production of metabolites that are toxic to the pathogens (6, 35). Our findings might add Babesia to the list of microbial agents that can activate such a defensive strategy.
Acknowledgments
We thank Paul B. Rothman and Michael J. Grusby at Columbia University and Harvard Medical School, respectively, for kindly providing breeders for the IFNGR2KO and Stat4KO mice used in these studies. We also thank Joseph Standing for technical expertise on clodronate liposome preparation and V. Shane Pankratz for valuable assistance with statistical analysis.
This work was supported by grant AI 41497 from the National Institutes of Health and by a gift contributed by the Leia Heuer Charitable Trust. I.A.-D. was supported by a fellowship from Consejo Nacional de Ciencia y Tecnología (CONACYT), Mexico City, México.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Aguilar-Delfin, I., M. J. Homer, P. J. Wettstein, and D. H. Persing. 2001. Innate resistance to Babesia infection is influenced by genetic background and gender. Infect. Immun. 69:7955-7958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bach, E. A., M. Aguet, and R. D. Schreiber. 1997. The IFN-γ receptor: a paradigm for cytokine receptor signaling. Annu. Rev. Immunol. 15:563-591. [DOI] [PubMed] [Google Scholar]
- 3.Bancroft, G. J., J. P. Kelly, P. M. Kaye, V. McDonald, and C. Cross. 1994. Pathways of macrophage activation and innate immunity. Immunol. Lett. 43:67-70. [DOI] [PubMed] [Google Scholar]
- 4.Bancroft, G. J., R. D. Schreiber, and E. R. Unanue. 1989. T cell-dependent macrophage activation in scid mice. Curr. Top. Microbiol. Immunol. 152:235-242. [DOI] [PubMed] [Google Scholar]
- 5.Belkaid, Y., B. Butcher, and D. L. Sacks. 1998. Analysis of cytokine production by inflammatory mouse macrophages at the single-cell level: selective impairment of IL-12 induction in Leishmania-infected cells. Eur. J. Immunol. 28:1389-1400. [DOI] [PubMed] [Google Scholar]
- 6.Biron, C. A., and R. T. Gazzinelli. 1995. Effects of IL-12 on immune responses to microbial infections: a key mediator in regulating disease outcome. Curr. Opin. Immunol. 7:485-496. [DOI] [PubMed] [Google Scholar]
- 7.Brown, W. C., and G. H. Palmer. 1999. Designing blood-stage vaccines against Babesia bovis and B. bigemina. Parasitol. Today 15:275-281. [DOI] [PubMed] [Google Scholar]
- 8.Cavalier-Smith, T. 1993. Kingdom protozoa and its 18 phyla. Microbiol. Rev. 57:953-994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark, I. A., and L. S. Jacobson. 1998. Do babesiosis and malaria share a common disease process? Ann. Trop. Med. Parasitol. 92:483-488. [DOI] [PubMed] [Google Scholar]
- 10.Clark, I. A., J. E. Richmond, E. J. Wills, and A. C. Allison. 1975. Immunity to intra-erythrocytic protozoa. Lancet ii:1128-1129. [DOI] [PubMed]
- 11.Clawson, M. L., N. Paciorkowski, T. V. Rajan, C. La Vake, C. Pope, M. La Vake, S. K. Wikel, P. J. Krause, and J. D. Radolf. 2002. Cellular immunity, but not gamma interferon, is essential for resolution of Babesia microti infection in BALB/c mice. Infect. Immun. 70:5304-5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gately, M. K., L. M. Renzetti, L. M. Magram, A. S. Stern, L. Adorini, U. Gubler, and D. H. Presky. 1998. The interleukin 12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu. Rev. Immunol. 16:495-521. [DOI] [PubMed] [Google Scholar]
- 13.Gazzinelli, R. T. 1996. Molecular and cellular basis of interleukin 12 activity in prophylaxis and therapy against infectious diseases. Mol. Med. Today 2:258-267. [DOI] [PubMed] [Google Scholar]
- 14.Gazzinelli, R. T., M. Wysocka, S. Hayashi, E. Y. Denkers, S. Hieny, P. Caspar, G. Trinchieri, and A. Sher. 1994. Parasite-induced IL-12 stimulates early IFN-γ synthesis and resistance during acute infection with Toxoplasma gondii. J. Immunol. 153:2533-2543. [PubMed] [Google Scholar]
- 15.Gazzinelli, R. T., S. Hieny, T. A. Wynn, S. Wolf, and A. Sher. 1993. Interleukin 12 is required for the T-lymphocyte-independent induction of interferon γ by an intracellular parasite and induces resistance in T-cell deficient hosts. Proc. Natl. Acad. Sci. USA 90:6115-6119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gessani, S., and F. Belardelli. 1998. IFN-γ expression in macrophages and its possible biological significance. Cytokine Growth Factor Rev. 9:117-123. [DOI] [PubMed] [Google Scholar]
- 17.Goff, W. L., W. C. Johnson, S. M. Parish, G. M. Barrington, W. Tuo, and R. A. Valdez. 2001. The age-related immunity in cattle to Babesia bovis infection involves the rapid induction of interleukin-12, interferon-gamma and inducible nitric oxide synthase mRNA expression in the spleen. Parasite Immunol. 23:463-471. [DOI] [PubMed] [Google Scholar]
- 18.Green, L. C., D. A. Wagner, J. Glogowski, P. L. Skipper, J. S. Wishnok, and S. R. Tannenbaum. 1982. Analysis of nitrate, nitrite and [15N]nitrate in biological fluids. Anal. Biochem. 126:131-134. [DOI] [PubMed] [Google Scholar]
- 19.Grun, J. L., and W. P. Weidanz. 1981. Immunity to Plasmodium chabaudi adami in the B-cell deficient mouse. Nature 290:143-145. [DOI] [PubMed] [Google Scholar]
- 20.Guler, M. L., J. D. Gorham, C.-S. Hsieh, A. J. Mackey, R. G. Steen, W. F. Detrich, and K. M. Murphy. 1996. Genetic susceptibility to Leishmania: IL-12 responsiveness in Th1 cell development. Science 271:984-987. [DOI] [PubMed] [Google Scholar]
- 21.Guler, M. L., N. G. Jacobson, U. Gubler, and K. M. Murphy. 1997. T cell genetic background determines maintenance of IL-12 signaling. Effects on Balb/c and B10.D2 T helper cell type-1 phenotype development. J. Immunol. 159:1767-1774. [PubMed] [Google Scholar]
- 22.Hemmer, R. M., D. A. Ferrick, and P. A. Conrad. 2000. Role of T cells and cytokines in fatal and resolving experimental babesiosis: protection in TNFRp55−/− mice infected with the human Babesia WA1 parasite. J. Parasitol. 86:736-742. [DOI] [PubMed] [Google Scholar]
- 23.Homer, M. J., I. Aguilar-Delfin, S. R. Telford III, P. J. Krause, and D. H. Persing. 2000. Babesiosis. Clin. Microbiol. Rev. 13:451-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Igarashi, I., R. Suzuki, S. Waki, Y.-I. Tagawa, S. Seng, S. Tum, Y. Omata, A. Saito, H. Nagasawa, Y. Iwakura, N. Suzuki, T. Mirami, and Y. Toyoda. 1999. Roles of CD4+ T cells and gamma interferon in protective immunity against Babesia microti infection in mice. Infect. Immun. 67:4142-4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaplan, M. H., Y.-L. Sun, T. Hoey, and M. J. Grusby. 1996. Impaired IL-12 responses and enhanced development of Th2 cells in Stat-4 deficient mice. Nature 382:174-177. [DOI] [PubMed] [Google Scholar]
- 26.Leonard, W. J., and J. J. O'Shea. 1998. Jaks and STATs: biological implications. Annu. Rev. Immunol. 16:293-322. [DOI] [PubMed] [Google Scholar]
- 27.Lu, B., C. Ebensperger, Z. Dembie, Y. Wang, M. Kvatyuk, T. Lu, R. L. Coffmann, S. Pestka, and P. B. Rothmann. 1998. Targeted disruption of the interferon-γ receptor 2 gene results in severe immune defects in mice. Proc. Natl. Acad. Sci. USA 95:8233-8238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohan, K., P. Moulin, and M. M. Stevenson. 1997. Natural killer cell cytokine production, not cytotoxicity, contributes to resistance against blood-stage Plasmodium chabaudi AS infection. J. Immunol. 159:4990-4998. [PubMed] [Google Scholar]
- 29.Moro, M. H., C. S. David, J. M. Magera, P. J. Wettstein, S. W. Barthold, and D. H. Persing. 1998. Differential effects of infection with a Babesia-like piroplasm, WA1, in inbred mice. Infect. Immun. 66:492-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Puddu, P., L. Fantuzzi, P. Borghi, B. Varano, G. Rainaldi, E. Guillemard, W. Mallorni, P. Nicaise, S. F. Wolf, F. Bellardelli, and S. Gessani. 1997. IL-12 induces IFN-γ expression and secretion in mouse peritoneal macrophages. J. Immunol. 159:3490-3497. [PubMed] [Google Scholar]
- 31.Quick, R. E., B. L. Herwaldt, J. W. Thomford, M. E. Garnett, M. L. Eberhard, M. Wilson, D. H. Spach, J. W. Dickerson, S. R. Telford III, K. R. Steingart, R. Pollock, D. H. Persing, J. M. Kobayashi, D. D. Juranek, and P. A. Conrad. 1993. Babesiosis in Washington State: a new species of Babesia? Ann. Intern. Med. 119:284-290. [DOI] [PubMed] [Google Scholar]
- 32.Rockett, K. A., M. M. Awburn, E. J. Rockett, W. B. Cowden, and I. A. Clark. 1994. Possible role of nitric oxide in malarial immunosuppresion. Parasite Immunol. 16:243-246. [DOI] [PubMed] [Google Scholar]
- 33.Sam, H., and M. M. Stevenson. 1999. In vivo IL-12 production and IL-12 receptors β1 and β2 mRNA expression in the spleen are differentially up-regulated in resistant B6 and susceptible A/J mice during early blood-stage Plasmodium chabaudi AS malaria. J. Immunol. 162:1582-1589. [PubMed] [Google Scholar]
- 34.Scharton-Kersten, T. M., and A. Sher. 1997. Role of natural killer cells in innate resistance to protozoan infections. Curr. Opin. Immunol. 9:44-51. [DOI] [PubMed] [Google Scholar]
- 35.Sher, A., T. A. Wynn, E. Y. Denkers, I. P. Oswald, D. Jankovic, M. Stevenson, R. Seder, S. Vogel, A. W. Cheever, and R. T. Gazzinelli. 1995. IL-12 dependent IFN-γ synthesis: a major pathway of innate resistance and immunoregulation in parasitic disease, p. 117-126. In S. Romagnani, G. del Prete, and A. K. Abbas (ed.), Cytokines: basic principles and practical applications. Springer Publications Co., New York, N.Y.
- 36.Shoda, L. K. M., G. Y. Palmer, J. Florin-Christensen, M. Florin-Christensen, D. L. Godson, and W. C. Brown. 2000. Babesia bovis-stimulated macrophages express interleukin-1β, interleukin-12, tumor necrosis factor alpha, and nitric oxide and inhibit parasite replication in vitro. Infect. Immun. 68:5139-5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stevenson, M. M. 1985. Genetic control of resistance in mice to Plasmodium chabaudi. Prog. Leukoc. Biol. 3:531-543. [Google Scholar]
- 38.Stevenson, M. M., and M. F. Tam. 1993. Differential induction of helper T cell subsets during blood-stage Plasmodium chabaudi AS infection in resistant and susceptible mice. Clin. Exp. Immunol. 92:77-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stevenson, M. M., J. J. Lyanga, and E. Skamene. 1982. Murine malaria: genetic control of resistance to Plasmodium chabaudi. Infect. Immun. 38:80-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stevenson, M. M., M. F. Tam, S. F. Wolf, and A. Sher. 1995. IL-12-induced protection against blood-stage Plasmodium chabaudi AS requires IFN-γ and TNF-α and occurs via a nitric oxide-dependent mechanism. J. Immunol. 155:2545-2556. [PubMed] [Google Scholar]
- 41.Stevenson, M. M., D. Y. Huang, J. E. Podoba, and M. E. Nowotarski. 1992. Macrophage activation during Plasmodium chabaudi AS infection in resistant C57BL/6 and susceptible A/J mice. Infect. Immun. 60:1193-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stich, R. W., L. K. M. Shoda, M. Dreewers, B. Adler, T. W. Jungi, and W. C. Brown. 1998. Stimulation of nitric oxide production in macrophages by Babesia bovis. Infect. Immun. 66:4130-4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Su, Z., and M. M. Stevenson. 2000. Central role of endogenous gamma interferon in protective immunity against blood-stage Plasmodium chabaudi AS infection. Infect. Immun. 68:4399-4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taylor-Robinson, A. W. 1999. Immunity to asexual blood stages of Plasmodium: is resistance to acute malaria adaptive or innate? - a response. Parasitol. Today 15:208.. [DOI] [PubMed] [Google Scholar]
- 45.Thierfelder, W. E., J. M. van Deursen, K. Yamamoto, R. A. Tripp, S. R. Sarawar, R. T. Carson, M. Y. Sangster, D. A. A. Vignali, P. C. Doherty, G. C. Grosveld, and J. N. Ihle. 1996. Requirement for Stat4 in interleukin-12-mediated responses of natural killer and T cells. Nature 382:171-177. [DOI] [PubMed] [Google Scholar]
- 46.van Rooijen, N., and A. Sanders. 1994. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J. Immunol. Methods 174:83-93. [DOI] [PubMed] [Google Scholar]
- 47.van Rooijen, N., and R. van Nieuwmegen. 1984. Elimination of phagocytic cells in the spleen after intravenous injection of liposome-encapsulated dichloromethylene diphosphonate. An enzyme-histochemical study. Cell Tissue Res. 238:355-358. [DOI] [PubMed] [Google Scholar]
- 48.van Rooijen, N., A. Sanders, and T. K. van den Berg. 1996. Apoptosis of macrophages induced by liposome-mediated intracellular delivery of clodronate and propamidine. J. Immunol. Methods 193:93-99. [DOI] [PubMed] [Google Scholar]
- 49.van Rooijen, N., N. Kors, and G. Kraal. 1989. Macrophage subset repopulation in the spleen: differential kinetics after liposome-mediated elimination. J. Leukoc. Biol. 45:97-104. [DOI] [PubMed] [Google Scholar]
- 50.Von der Weid, T., N. Honarvar, and J. Langhorne. 1996. Gene-targeted mice lacking B cells are unable to eliminate a blood stage malaria infection. J. Immunol. 156:2510-2516. [PubMed] [Google Scholar]
- 51.Wood, P. R., and I. A. Clark. 1984. Macrophages from Babesia and malaria infected mice are primed for monokine release. Parasite Immunol. 6:309-317. [DOI] [PubMed] [Google Scholar]