Abstract
Reactive oxygen and nitrogen intermediates are important antimicrobial defense mechanisms of macrophages and other phagocytic cells. While reactive nitrogen intermediates have been shown to play an important role in tuberculosis control in the murine system, their role in human disease is not clearly established. Glutathione, a tripeptide and antioxidant, is synthesized at high levels by cells during reactive oxygen intermediate and nitrogen intermediate production. Glutathione has been recently shown to play an important role in apoptosis and to regulate antigen-presenting-cell functions. Glutathione also serves as a carrier molecule for nitric oxide, in the form of S-nitrosoglutathione. Previous work from this laboratory has shown that glutathione and S-nitrosoglutathione are directly toxic to mycobacteria. A mutant strain of Mycobacterium bovis BCG, defective in the transport of small peptides such as glutathione, is resistant to the toxic effect of glutathione and S-nitrosoglutathione. Using the peptide transport mutant as a tool, we investigated the role of glutathione and S-nitrosoglutathione in animal and human macrophages in controlling intracellular mycobacterial growth.
Tuberculosis remains a leading cause of mortality worldwide due to the ability of Mycobacterium tuberculosis to adapt to a wide range of conditions both inside and outside the human host. The resurgence of tuberculosis worldwide has intensified research efforts directed at host defense and pathogenic mechanisms operative in M. tuberculosis infection. Despite advances in diagnosis and treatment, we still do not know the mechanism by which M. tuberculosis is eliminated by the immune system in healthy subjects (28). A more complete understanding of the roles that each component of the immune system plays in protection against or exacerbation of tuberculosis, as well as of the bacterium's weapons to evade those components, will enhance development of preventive and therapeutic strategies against this enormously successful pathogen (13).
The major phagocytic cell involved in protection against M. tuberculosis is the activated macrophage. Macrophages acquire the ability to kill the intracellular pathogen following exposure to cytokines released by antigen-sensitized T lymphocytes (10, 28). This event triggers antimicrobial mechanisms such as generation of reactive oxygen intermediates (ROI), generation of reactive nitrogen intermediates (RNI), phagolysosome fusion, etc. (3, 12, 25, 27). This pathway is the essence of cell-mediated immunity to intracellular infection.
Glutathione (GSH) is a tripeptide comprised of glutamate, cysteine, and glycine. GSH is present in most cells, where it functions as an antioxidant protecting cells from toxic effects of ROI and RNI (29). In addition to its antioxidant role, GSH plays a vital role in maintenance of cell viability, DNA replication, and thiolation of proteins (9). GSH has also been reported to regulate immune cell functions. GSH regulates the antigen-processing machinery in antigen-presenting cells and also plays a major role in regulating the Th1 and Th2 types of immunity (24).
ROI and RNI generated by phagocytic cells are thought to play an important role in inhibiting growth of intracellular pathogens (3, 12, 25, 32). However, the direct role of ROI in growth inhibition of M. tuberculosis is controversial. During phagocytosis, infection, or cytokine stimulus, phagocytes release ROI and RNI, which are known to inhibit the growth of intracellular pathogens and parasites. When host cells generate ROI and RNI, there is also simultaneous synthesis of GSH in order to protect the host cells from the toxic effects of ROI and RNI. Nitric oxide (NO) has been shown to inhibit the growth of M. tuberculosis in the murine system (3), but the role of NO in human tuberculoimmunity has not been resolved (1). NO also reacts with GSH to form S-nitrosoglutathione (GSNO), and thus, GSNO is considered an NO donor (2, 30, 31). Formation of GSNO is likely to increase the activity of NO, and release of NO from the GSNO complex causes death of the pathogen (8, 17, 18, 20, 22).
Our lab has demonstrated that unlike most cells (both prokaryotic and eukaryotic), mycobacteria are sensitive to GSH at concentrations above 5 mM (15). Further, a mutant strain of BCG that lacks peptide permease is defective in GSH transport and is partially resistant to the toxic effects of GSH. It has also been shown that GSNO is cytocidal to BCG and that the peptide permease mutant is partially resistant to this cytotoxicity (15).
These observations led us to the hypothesis that the growth of intracellular mycobacteria, including M. tuberculosis, may be controlled at least in part by increases in GSH and/or GSNO generated by the macrophages during oxidative or nitrosative stress. We predicted that the BCG peptide permease mutant would be resistant to GSH- or GSNO-based control mechanisms in the macrophage and would exhibit a “hypersurvival” phenotype. This prediction was borne out, as we describe in this paper. We performed experiments to study the survival of BCG and an oligopeptide permease mutant of BCG (DPP) in the murine J774.1 cell line, in primary peritoneal macrophages from wild-type and inducible NO synthase (iNOS) knockout mice, and in human monocyte-derived macrophages (HMDM). It is our conclusion that GSH levels play a pivotal role in regulating, directly or indirectly, antimicrobial activity in immune cells.
MATERIALS AND METHODS
Maintenance of J774 cell line.
The J774.1 cell line was maintained in Dulbecco's modified Eagle medium (DMEM; Sigma) containing 10% fetal bovine serum (Sigma), 2 mM glutamine, and essential amino acids (Gibco BRL).
Bacterial strains.
BCG (Pasteur), DPP, and complemented DPP (CDPP) were maintained as described by Green et al. (15). DPP was constructed by homologous recombination. A wild-type copy of the dpp operon was provided on pMV305, as described by Green et al. (15).
Isolation of peritoneal macrophages from wild-type and iNOS knockout mice.
Eight-week-old C57BL/6 mice were used. Five milliliters of sterile 3% thioglycolate (Sigma) solution was injected into the peritoneal cavities of the animals. Four days after injection, animals were euthanized with isoflurane, and ice-cold phosphate-buffered saline (PBS) (Gibco BRL) was injected into the peritoneal cavity. The abdomens of the animals were gently massaged several times, and peritoneal lavage fluid was recovered.
Separation of monocytes from human blood.
Use of human cells was approved by the institutional review board of UMDNJ-New Jersey Medical School. Buffy coat prepared from blood of healthy volunteers was supplied by the New Jersey Blood Center (West Orange, N.J.). Blood was diluted in an equal volume of PBS. The diluted blood was layered on an equal volume of Ficoll Hypaque (Sigma) and centrifuged at 800 × g for 30 min at room temperature. Peripheral blood mononuclear cells (PBMC) at the interface were aspirated, diluted to 50 ml with PBS, and washed three times at 1,600 rpm for 10 min.
After the final wash, PBMC were suspended in 60 ml of RPMI (Sigma) containing 5% AB serum (Sigma). The total number of viable PBMC in the suspension was determined by trypan blue dye exclusion counts in a Neubauer counting chamber. PBMC (10 × 106/well) were distributed into poly-dl-lysine (Sigma)-coated 12-well plates and incubated overnight at 37°C in 5% CO2, in a humidified atmosphere, to allow monocytes to adhere to the plate. Nonadherent cells were removed by gentle washing, and the adherent monocytes were cultured in RPMI containing 5% AB serum for 7 days before being used for infection experiments to allow differentiation to macrophages. The total number of macrophages per well (on day 7) was quantitated by detaching the macrophages from a single well by the addition of ice-cold 1 mM EDTA in PBS. Viable detached macrophages were counted in a Neubauer counting chamber by trypan blue dye exclusion. The average number of macrophages per well on day 7 was 105.
Processing of mycobacteria for infection.
BCG strains were grown in 7H9 with albumin-dextrose complex (ADC) and appropriate antibiotics (kanamycin, 20 μg/ml; streptomycin, 20 μg/ml; hygromycin, 50 μg/ml). Static cultures of mycobacteria at peak logarithmic phase of growth (between 0.5 and 0.8 at A600) were used for infection of macrophages. The bacterial suspension was diluted to 50 ml in PBS and centrifuged at 3,000 rpm for 15 min at room temperature. The bacterial pellet was resuspended in RPMI containing AB serum. Bacterial clumps were disaggregated by vortexing five times with 3-mm sterile glass beads (Fisher Scientific). The duration of each cycle was approximately 2 min. The bacterial suspension was passed through a 5-μm filter (Micron Separation Inc.) to remove any further clumps. The total number of organisms in the suspension was ascertained by counting in a Thoma counting chamber.
Infection.
Human monocytes were allowed to differentiate to macrophages by maintaining them in culture for 7 days before infection. Macrophages (HMDM, J774, and murine peritoneal macrophages) were infected with mycobacteria at a multiplicity of infection (MOI) of 10:1. Macrophages were incubated with mycobacteria for 2 h (to allow phagocytosis), after which extracellular organisms were removed by washing with serum-free medium. Infected macrophages were maintained in culture medium with and without gamma interferon (IFN-γ) (100 U/ml) or lipopolysaccharide (LPS) (1 μg/ml), for different periods (72 h for J774 and 7 and 5 days for HMDM and murine peritoneal macrophages, respectively). LPS and human and mouse recombinant IFN-γ were purchased from Sigma.
Assay of GSH by spectrophotometry.
GSH was assayed spectrophotometrically from the macrophage lysate. J774.1 cells were infected and treated as described previously. A GSH kit was procured from Calbiochem. Macrophages were detached from the 12-well plate with a cell scraper. The cell suspension was pelleted by centrifugation at 10,000 × g for 10 min at 4°C; an equal volume of ice-cold 10% metaphosphoric acid was added to the pellet. Supernatants were collected after centrifugation (10,000 × g at 4°C, 8 min) and analyzed for total GSH per the manufacturer's instructions (Calbiochem).
Inhibition of intracellular GSH in J774.1 and iNOS−/− macrophages by BSO.
J774.1 cells and peritoneal macrophages from the iNOS knockout mice were infected with BCG as described previously. At 2 h after infection, unphagocytosed mycobacteria were removed by washing the macrophage cultures three times in PBS. Infected macrophages were cultured in medium alone, in medium containing IFN-γ and LPS, or in medium containing IFN-γ, LPS, and 500 μM buthionine sulfoximine (BSO) (Sigma). BSO specifically inhibits the activity of γ-glutamyl-cysteinyl synthetase, the first step in the synthesis of GSH. Infected macrophage cultures were terminated at different times to determine the intracellular viability of BCG.
HMDM and NAC treatment.
HMDM were infected with BCG, and the infected cultures were maintained in medium alone, in medium containing IFN-γ and LPS, or in medium containing 5 mM N-acetyl cysteine (NAC) (Sigma). NAC addition is known to increase intracellular GSH levels in macrophages (9, 26). Infected HMDM cultures were terminated at 1 h and 7 days to determine the intracellular viability of BCG. In separate experiments, HMDM were cultured in 12-well tissue culture plates and treated with IFN-γ, LPS, or NAC. Macrophage cultures were terminated at 1 h and 7 days to determine the intracellular GSH levels.
Immunofluorescence staining and FACS detection of iNOS.
J774.1 cells were cultured on 18-mm circular coverslips (Fisher Scientific) positioned in 12-well tissue culture plates. Macrophages were infected and treated as described previously. At 72 h after infection or treatment, J774.1 cells were detached from the plates with a cell scraper. Cells were fixed with freshly prepared 3.8% paraformaldehyde (Sigma) in PBS for 30 min at room temperature. Fixed macrophages were permeabilized with 0.1% Triton X-100 for 1 min and blocked for 15 min in blocking solution (PBS containing 5% sucrose and 2% goat serum). Cells were incubated with rabbit anti-mouse immunoglobulin primary antibody (Transduction Laboratories) for 1 h at 37°C, washed three times in blocking solution, and incubated with goat anti-rabbit immunoglobulin secondary antibody conjugated with fluorescein isothiocyanate (Molecular Probes) for 1 h at 37°C. Cells were analyzed for iNOS staining by fluorescence-activated cell sorting (FACS) using a FACSCalibur (Becton Dickinson) equipped with a 488-nm argon ion laser and a 530-nm band-pass filter. The mean fluorescence intensity for each sample was determined and analyzed with CellQuest software.
Detection of nitrite in macrophages by spectrophotometry.
Nitrite levels in macrophage supernatants were determined spectrophotometrically by the Griess reaction. Briefly, 100 μl of sample, an equal volume of Griess reagent, 1% sulfanilamide, and 0.1% naphthalene-ethylene diamine dihydrochloride in 5% H3PO4 (Molecular Probes) were added and immediately mixed. After 5 min, the product of the reaction was read at 550 nm.
Statistical analysis.
The data are presented as means plus standard errors. Statistical analysis of the data was carried out with the StatView program, and the statistical significance was determined with an unpaired t test. Differences were considered significant at a P level of <0.05.
RESULTS
Intracellular survival of BCG in J774.1 murine macrophages.
We first looked at survival of BCG in J774.1 cells under conditions known to induce NO production. Mycobacterium-infected J774.1 macrophages were treated with IFN-γ (100 U/ml) and LPS (1 μg/ml). Treatment of murine macrophages with IFN-γ alone does not bring about enhanced synthesis of iNOS and generation of NO. J774.1 cells require tumor necrosis factor alpha as a second signal; addition of LPS to macrophage cultures results in tumor necrosis factor alpha production (3). Macrophage cultures were terminated at 1 h and 72 h after infection and treatment to analyze the growth of mycobacteria inside activated and nonactivated macrophages. At each time point, supernatants were collected and macrophages were lysed with sterile distilled water. Both supernatant and macrophage lysate were plated on 7H11 with ADC for extracellular and intracellular bacteria. No significant levels of extracellular bacteria were detected in the macrophage supernatant. No significant increase in the intracellular numbers of BCG in J774.1 cells between 1 h and 72 h was observed. Treatment of BCG-infected J774 cells with IFN-γ and LPS resulted in 50% reduction in BCG colony counts recovered 72 h after infection (Table 1). This reduction was statistically significant (P = 0.02).
TABLE 1.
Survival of BCG in J774.1 cells with and without IFN-γ plus LPSa
| Bacterial strain and treatmentb | Colony countc
|
Pd | |||
|---|---|---|---|---|---|
| Mean
|
SE
|
||||
| 1 h | 72 h | 1 h | 72 h | ||
| BCG | |||||
| None | 592 | 907 | 119.7 | 212 | 0.2067 |
| IFN-γ + LPS | 430 | 224 | 120.2 | 46.9 | 0.018 |
| DPP | |||||
| None | 283 | 518 | 53.09 | 76.94 | 0.0197 |
| IFN-γ + LPS | 279 | 266 | 37.47 | 55.25 | 0.851 |
| CDPP | |||||
| None | 930 | 1,380 | 70 | 85 | 0.02 |
| IFNγ + LPS | 946 | 460 | 53 | 30 | 0.0014 |
J774.1 cells were maintained in DMEM containing 10% FBS.
Macrophages were infected with mycobacteria at a 10:1 ratio and incubated for 2 h. Unphagocytosed organisms were removed by washing, and the infected macrophages were maintained in DMEM containing 10% FBS or in medium containing IFNγ (100 U/ml) and LPS (1 μg/ml).
Infected macrophage cultures were terminated at 1 and 72 h to determine the intracellular viability of BCG, DPP, and CDPP in unstimulated and stimulated cultures. Data were averaged from four experiments performed in triplicate. Intracellular viability of mycobacteria was determined by colony counts.
Calculated with the Statview program.
iNOS and nitrite levels in J774.1 cells.
To determine whether the killing or growth inhibition of BCG that we observe in IFN-γ-LPS-treated J774.1 macrophages is accompanied by NO production, iNOS levels and nitrite levels were assayed. Stimulation of J774.1 cells with IFN-γ plus LPS caused enhanced levels of iNOS and a statistically significant increase in the levels of nitrite detected by spectrophotometry (Griess reaction), as shown in Table 2.
TABLE 2.
iNOS, NO, and GSH levels in J774 cellsa
| Infection and treatment | Nitrite production (nmol/mg of protein)b
|
Pc | iNOS level (% fluorescent)d | GSH level (nmol/mg of protein)e
|
Pc | ||
|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | ||||
| No infection | 0.162 | 0.034 | 2.4 | 3.99 | 0.284 | ||
| BCG | |||||||
| None | 0.128 | 0.020 | 11.06 | 4.127 | 0.068 | ||
| IFN-γ + LPS | 1.889 | 0.147 | 0.003 | 26.22 | 0.434 | 0.041 | 0.0127f |
| DPP | |||||||
| None | 0.136 | 0.018 | 6.82 | 3.93 | 0.585 | ||
| IFN-γ + LPS | 2.241 | 0.041 | 0.0001 | 24.15 | 0.748 | 0.100 | 0.033f |
J774.1 cells were maintained in DMEM containing 10% fetal bovine serum. Macrophages were infected with mycobacteria at a 10:1 ratio and incubated for 2 h. Unphagocytosed organisms were removed by washing, and the infected macrophages were maintained in DMEM containing 10% fetal bovine serum or in medium containing IFN-γ (100 U/ml) and LPS (1 μg/ml). Infected macrophage cultures were terminated at 72 h to determine nitrite production and iNOS and GSH levels.
Determined by the Griess reaction.
Calculated with the Statview program.
iNOS levels were studied by immunocytochemical staining and analysed by flow cytometry.
Determined by spectrophotometry.
Compared with the value for untreated cells.
Survival of peptide permease mutant of BCG in J774.1 macrophage cell line.
In contrast to wild-type BCG, significant growth of the dpp mutant was observed in unstimulated J774.1 cells; in fact, there was an 83% increase in DPP colony counts (Table 1). The increase was statistically significant, with a P value of 0.019. Treatment of DPP-infected J774.1 cells with IFN-γ plus LPS caused increased iNOS synthesis and significantly increased nitrite production (Table 2) but no significant reduction in DPP colony counts (Table 1). The data suggest that GSH or GSNO generated in J774.1 macrophages during nitrosative stress plays a major role in controlling intracellular growth of mycobacteria, because the peptide permease mutant, defective in the transport of GSH and GSNO, is resistant to the toxic effects of GSH and GSNO. Importantly, CDPP showed wild-type levels of survival in J774.1 macrophages (Table 1). This result indicates that the peptide permease mutation is solely responsible for the altered phenotype.
Assay of GSH levels in J774 cells.
Intracellular GSH in J774.1 cells under various conditions was assayed spectrophotometrically, as described in Materials and Methods. Infection of J774.1 cells with BCG or DPP and treatment with IFN-γ plus LPS resulted in a decrease in intracellular GSH levels (Table 2). In separate experiments, GSH levels in the cytosolic fractions of the J774.1 macrophages were determined by high-pressure liquid chromatography. Treatment of J774 cells with IFN-γ plus LPS resulted in decreases in intracellular GSH levels, as determined by high-pressure liquid chromatography (data not shown). GSH assayed by two different techniques showed the same trend, i.e., treatment of macrophages with IFN-γ plus LPS resulted in decreases in intracellular GSH levels. Since NO production is significantly increased in IFN-γ-LPS- treated J774.1 cells (Table 2), with a simultaneous significant decrease in GSH levels, we propose that intracellular GSH in J774.1 cells treated with IFN-γ plus LPS is complexed with NO and is present as GSNO. Quantitative assay of GSNO is difficult due to the extreme instability of the molecule.
Survival of BCG and DPP in macrophages from iNOS+/+ mice.
We can distinguish between the effects of GSH and GSNO in the macrophage killing of intracellular mycobacteria in the murine system by comparing the extent of survival of the dpp mutant in primary macrophages derived from iNOS+/+ and iNOS−/− mice. If GSNO is a primary species responsible for controlling mycobacterial growth in murine macrophages, we predict that in iNOS+/+ macrophages, the dpp mutant should demonstrate a hypersurvival phenotype compared with wild-type BCG. In contrast, in iNOS−/− macrophages, the dpp mutant and the wild-type BCG should survive to the same extent. Infection of peritoneal macrophages from wild-type mice (iNOS+/+) with BCG resulted in a reduction in the intracellular viability of BCG (Table 3). However, the reduction was not statistically significant. Treatment of peritoneal macrophages from iNOS+/+ mice with IFN-γ plus LPS resulted in a statistically significant reduction in the intracellular viability of BCG (Table 3). Infection of peritoneal macrophages from iNOS+/+ mice with DPP resulted in significant multiplication of DPP inside the macrophages (Table 3). Treatment of peritoneal macrophages from iNOS+/+mice with IFN-γ plus LPS resulted in a statistically significant increase in the intracellular viability of DPP (Table 3) between the initial and final time points of the experiment. However, the increase in the intracellular numbers of DPP in unstimulated peritoneal macrophages from wild-type mice was greater than in IFN-γ-LPS-treated macrophages.
TABLE 3.
In vitro survival of BCG and DPP in murine peritoneal macrophages from wild-type mice, with and without IFN-γ plus LPSa
| Bacterial strain and treatment | Colony countb
|
Pc | |||
|---|---|---|---|---|---|
| Mean
|
SE
|
||||
| 24 h | 5 days | 24 h | 5 days | ||
| BCG | |||||
| None | 1,196 | 407 | 285 | 52.76 | 0.088 |
| IFN-γ + LPS | 1,088 | 235 | 289 | 68.5 | 0.0064 |
| DPP | |||||
| None | 1,670 | 13,189 | 184.29 | 461.5 | 0.0001 |
| IFN-γ + LPS | 3,371 | 9,948 | 552 | 382 | 0.006 |
Thioglycolate (3%) was injected into the peritoneal cavities of wild-type C57BL/6 mice. Four days after injection, animals were anesthetized with isoflurane. Peritoneal cavities were washed with ice-cold RPMI to recover peritoneal macrophages. Peritoneal macrophages were infected with BCG or DPP at an MOI of 10:1. At 2 h after infection, unphagocytosed organisms were removed. Infected macrophages were maintained in RPMI containing 10% fetal bovine serum or in RPMI containing 10% fetal bovine serum, IFN-γ (100 U/ml), and LPS (1 μg/ml).
Intracellular colony counts were determined by plating lysed cultures on Middlebrook 7H11. Data are averages from three experiments.
Calculated with the Statview program.
IFN-γ-LPS-treated peritoneal macrophages from wild-type mice.
The results obtained with IFN-γ-LPS-treated peritoneal macrophages from wild-type mice were similar to those with J774.1 cells.
Survival of BCG and DPP in macrophages from iNOS−/− mice.
Infection of peritoneal macrophages from iNOS−/− mice with BCG or DPP resulted in growth of these strains inside the macrophages (Table 4). Treatment of BCG-infected iNOS−/− macrophages with IFN-γ plus LPS resulted in significant reduction in the intracellular viability of BCG. On the other hand, treatment of DPP-infected iNOS−/− macrophages with IFN-γ plus LPS did not prevent the increase in the intracellular viability of DPP.
TABLE 4.
In vitro survival of BCG and DPP in murine peritoneal macrophages from iNOS knockout mice, with and without IFN-γ plus LPSa
| Bacterial strain and treatment | Colony countb
|
Pc | |||
|---|---|---|---|---|---|
| Mean
|
SE
|
||||
| 24 h | 5 days | 24 h | 5 days | ||
| BCG | |||||
| None | 1,238 | 2,847 | 304.9 | 688.2 | 0.1800 |
| IFN-γ + LPS | 987 | 287 | 286.4 | 58.4 | 0.0459 |
| DPP | |||||
| None | 2,154 | 6,573 | 338.59 | 823 | 0.0003 |
| IFN-γ + LPS | 1,093 | 2,557 | 327.15 | 684.8 | 0.033 |
Thioglycolate (3%) was injected into the peritoneal cavities of iNOS−/− mice. Four days after injection, animals were anesthetized with Isoflurane. Peritoneal cavities were washed with ice-cold RPMI to recover peritoneal macrophages. Peritoneal macrophages were infected with BCG or DPP at an MOI of 10:1. At 2 h after infection, unphagocytosed organisms were removed. BCG- or DPP-infected macrophages were maintained in RPMI containing 10% fetal bovine serum or in RPMI containing 10% fetal bovine serum, IFN-γ (100 U/ml), and LPS (1 μg/ml).
Intracellular colony counts were determined by plating lysed cultures on Middlebrook 7H11. Results are averages from three experiments done in triplicate.
Calculated with the Statview program.
Survival of BCG in BSO-treated J774.1 cells.
As shown in Table 1, IFN-γ-LPS treatment of J774.1 macrophages resulted in significant killing of intracellular BCG and significant increase in NO production (Table 2). To show that GSH and GSNO mediate the killing of BCG in IFN-γ-LPS-treated J774.1 macrophages, GSH synthesis was inhibited with BSO (thereby decreasing GSNO levels inside the cells). BSO specifically inhibits the activity of γ-glutamyl-cysteinyl synthetase, catalyzing the first step in the synthesis of GSH. Treatment of J774.1 cells with IFN-γ plus LPS resulted in 55% killing of BCG (Table 5). Treatment of BCG-infected J774.1 cells with IFN-γ plus LPS and BSO resulted in the abolition of the intracellular killing of BCG.
TABLE 5.
Growth of BCG in cytokine (IFN-γ and LPS)- and BSO-treated J774.1 macrophagesa
| Treatment | Colony countb
|
Pc | |||
|---|---|---|---|---|---|
| Mean
|
SE
|
||||
| 1 h | 72 h | 1 h | 72 h | ||
| None | 52,400 | 64,800 | 6,539 | 1,600 | 0.1393 |
| IFN-γ + LPS | 44,800 | 20,066 | 3,555 | 2,034 | 0.0038 |
| IFN-γ + LPS + BSO | 62,000 | 80,266 | 1,006 | 3,235.9 | 0.0057 |
J774.1 cells were maintained in DMEM containing 10% FBS and then infected with BCG at an MOI of 10:1. At 2 h after infection, unphagocytosed organisms were removed. BCG-infected macrophages were maintained in DMEM containing 10% fetal bovine serum, containing 10% fetal bovine serum and IFN-γ (100 U/ml) plus LPS (1 μg/ml), or containing 10% fetal bovine serum, IFN-γ (100 U/ml), LPS (1 μg/ml), and BSO (500 μM).
Intracellular colony counts were determined by plating lysed cultures on Middlebrook 7H11.
Calculated with the Statview program.
Survival of BCG in BSO-treated macrophages from iNOS knockout mice.
iNOS knockout mice cannot generate NO upon cytokine stimulation. However, we observed significant killing of BCG in macrophages from iNOS knockout mice following IFN-γ-LPS stimulation (Table 4). To investigate whether the killing of BCG in IFN-γ-LPS-stimulated macrophages from iNOS knockout mice is GSH mediated, BSO was added to the macrophages with IFN-γ and LPS. Treatment of BCG-infected macrophages from iNOS knockout mice with IFN-γ plus LPS resulted in almost 75% killing of intracellular BCG (Table 6). Treatment of BCG-infected macrophages from iNOS knockout mice with IFN-γ plus LPS and BSO resulted in abolition of the intracellular killing of BCG, leading to multiplication of BCG (Table 7).
TABLE 6.
Growth of BCG in IFN-γ-, LPS-, and BSO-treated macrophages from iNOS−/− micea
| Treatment | Colony count (mean)b
|
Pc | |||
|---|---|---|---|---|---|
| Mean
|
SE
|
||||
| 1 h | 72 h | 1 h | 72 h | ||
| None | 1,238 | 2,847 | 304 | 688 | 0.1800 |
| IFN-γ + LPS | 1,403 | 355 | 286 | 54.47 | 0.0025 |
| IFN-γ + LPS + BSO | 616 | 1,011 | 194 | 213 | 0.1906 |
Thioglycolate (3%) was injected into the peritoneal cavities of iNOS−/− mice. Four days after injection, animals were anesthetized with isoflurane. Peritoneal cavities were washed with ice-cold RPMI to recover peritoneal macrophages. Peritoneal macrophages were infected with BCG at an MOI of 10:1. At 2 h after infection, unphagocytosed organisms were removed. BCG-infected macrophages were maintained in RPMI containing 10% fetal bovine serum, containing 10% fetal bovine serum plus IFN-γ (100 U/ml) and LPS (1 μg/ml), or containing IFN-γ (100 U/ml), LPS (1 μg/ml), and BSO (500 μM).
Infected macrophage cultures were terminated at 1 h and 5 days to determine the intracellular viability of BCG in unstimulated and stimulated cultures. Intracellular viability of BCG was determined by colony counts.
Calculated with the Statview program.
TABLE 7.
Survival of BCG and DPP in HMDMa
| Bacterial strain and treatment | Colony countb
|
Pc | |||
|---|---|---|---|---|---|
| Mean
|
SE
|
||||
| 1 h | 7 days | 1 h | 7 days | ||
| BCG | |||||
| None | 2,431 | 4,215 | 587.8 | 1,224 | 0.18 |
| IFN-γ + LPS | 4,297 | 6,550 | 103.8 | 116.4 | 0.42 |
| DPP | |||||
| None | 2,367 | 2,019 | 379.5 | 317.1 | 0.489 |
| IFN-γ + LPS | 3,040 | 3,076 | 44.66 | 49 | 0.965 |
Human monocytes from peripheral blood were maintained in vitro in RPMI containing 5% AB serum for 7 days for differentiation to macrophages. HMDM were infected with BCG or DPP at an MOI of 10:1 and maintained in media with or without IFN-γ and LPS (100 U/ml and 1 μg/ml, respectively), as described in Materials and Methods.
Intracellular colony counts were determined by plating lysed cultures on Middlebrook 7H11. Data are averages from five experiments done in triplicate.
Calculated with the Statview program.
Survival of the peptide permease mutant of BCG in HMDM.
We examined the survival of BCG and DPP in HMDM. Experiments performed with macrophages from five subjects in triplicate showed that BCG successfully replicates inside human macrophages, with a 73% increase in colony counts at 7 days after HMDM infection (Table 7). Unlike results with J774.1 cells and mouse macrophages, no significant growth of DPP inside HMDM was observed. Treatment of HMDM with LPS plus IFN-γ did not result in growth inhibition of BCG or enhanced NO production (data not shown).
HMDM and NAC treatment.
NAC treatment is known to increase intracellular GSH levels in macrophages (9). Infection of HMDM with BCG resulted in growth of BCG inside macrophages. Treatment of HMDM with IFN-γ plus LPS resulted in no killing of intracellular BCG. Treatment of HMDM with NAC resulted in significant killing of intracellular BCG (Table 8) as well as a significant increase in intracellular GSH levels (data not shown).
TABLE 8.
Growth of BCG in unstimulated, IFN-γ-LPS-stimulated, and NAC-treated HMDMa
| Treatment | Colony countb
|
Pc | |||
|---|---|---|---|---|---|
| Mean
|
SE
|
||||
| 1 h | 7 days | 1 h | 7 days | ||
| None | 2,431 | 4,214 | 587 | 1,224 | 0.1988 |
| IFN-γ + LPS | 2,920 | 3,978 | 964 | 1,249 | 0.5095 |
| NAC | 1,393 | 756 | 58.97 | 118.93 | 0.0087 |
Human monocytes from peripheral blood were maintained in vitro in RPMI containing 5% AB serum for 7 days for differentiation to macrophages. HMDM were infected with BCG at an MOI of 10:1 and maintained in medium alone, in medium containing IFN-γ and LPS (100 U/ml and 1 μg/ml, respectively), or in medium containing NAC (5 mM), as described in Materials and Methods.
Intracellular colony counts were determined by plating lysed cultures on Middlebrook 7H11.
Calculated with the Statview program.
DISCUSSION
In human tuberculosis, the cytokines and mechanisms operating in human macrophages and resulting in the killing of M. tuberculosis remain unknown (6, 11, 19, 21). Knowledge of T-lymphocyte subsets and their products that have key roles in activating the macrophages to fight M. tuberculosis infection (and the mechanism involved) will help us to better understand the disease.
In the murine system, extensive studies have been carried out by various laboratories to show that IFN-γ released by the Th1 subset of CD4+ T cells plays a major role in activating macrophages (7, 12, 14). IFN-γ-stimulated mouse macrophages generate NO, a process which is catalyzed by iNOS, which brings about killing and/or growth inhibition of M. tuberculosis. This was confirmed by treating infected macrophages with l-arginine analogues, which block the synthesis of NO, resulting in abolition of the killing of M. tuberculosis (3).
Most of the GSH present in cells is present in the cytosol in its reduced form, GSH. During oxidative stress, GSH is oxidized to GSH thiodisulfide. GSH thiodisulfide is converted back to GSH by GSH reductase; this reaction requires NADPH as a cofactor. Various groups have studied the role of GSH in human immunodeficiency virus infection. AIDS patients with CD4+-T-cell counts of less than 200/μl have very low levels of GSH in CD4+ CD8+ T cells, monocytes, and plasma compared to healthy individuals (16). The reason for the low levels of GSH in AIDS patients with low CD4+-T-cell counts is not known. According to one school of thought, patients with advanced stages of AIDS have defective mitochondrial function and cannot synthesize NADPH and hence cannot activate GSH reductase (16). Also, many of these patients have defective liver functions, due to infections or adverse effects of drugs, and the liver is a major site of GSH synthesis.
The direct toxicity of GSNO for bacteria has been extensively studied, primarily in Salmonella enterica serovar Typhimurium (8). Mutations leading to GSNO resistance in S. enterica serovar Typhimurium map to two loci: rpoS, encoding the stationary-phase and stress response sigma factor, and the dpp operon, encoding the dipeptide permease. Mutants unable to transport dipeptides are resistant to the toxic effects of GSNO, a tripeptide. GSNO is converted to a dipeptide by the action of γ-glutamyl transpeptidase, encoded by ggt. The M. tuberculosis database indicates that there are two copies of ggt in the genome. Mutants lacking this enzyme would also be expected to exhibit a GSNO-resistant phenotype. Thus, in Salmonella, GSNO, and presumably GSH, is transported by a dipeptide permease (8).
As described above, earlier work from our laboratory constructed and characterized a deletion mutant of BCG lacking the peptide permease encoded by the operon extending from Rv1280c to Rv1283c. This strain, DPP, exhibits decreased sensitivity to the toxic effects of both GSH and GSNO compared with the wild-type BCG strain (15). Further, if the toxicity that GSH and GSNO exhibit in vitro plays a role in limiting mycobacterial growth in vivo, we predicted that the dpp mutant should have a hypersurvival phenotype in macrophages.
To examine the effect of decreased bacterial peptide transport on survival in the macrophage, we compared the growth of wild-type BCG and DPP in J774.1 macrophages, in peritoneal macrophages from wild-type and iNOS knockout C57BL/6 mice, and in HMDM.
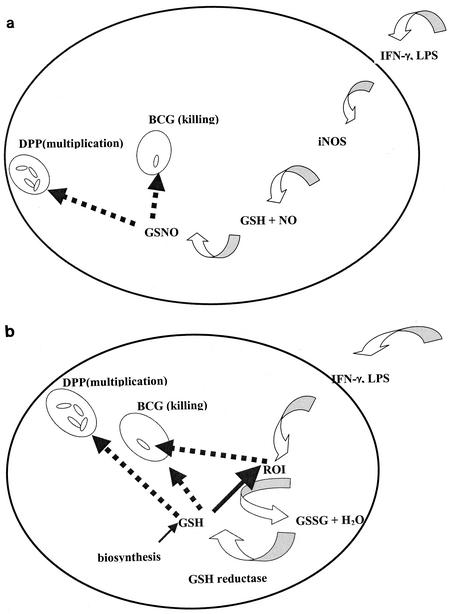
As shown above (Table 1), BCG does not replicate efficiently in J774.1 cells. Stimulation of J774.1 cells with IFN-γ plus LPS resulted in 50% reduction in intracellular BCG colony counts (Table 1). Stimulation of J774.1 cells with IFN-γ plus LPS resulted in enhanced NO generation. NO is highly unstable and it is rapidly detoxified to nitrite and nitrate; NO in the form of GSNO is slightly more stable. Unlike BCG, the DPP mutant significantly multiplied in J774.1 cells (Table 1). Since DPP is defective in the transport of peptides, it is resistant to the toxic effects of GSH and GSNO. Importantly, complementation of DPP mutant strain with a copy of the wild-type dpp gene resulted in loss of the mutant phenotype (i.e., increased survival) in murine macrophages (Table 1). The growth of BCG and DPP in resting and stimulated peritoneal macrophages from wild-type C57BL/6 mice was similar to that in resting and stimulated J774.1 cells. BCG did not multiply in stimulated macrophages from wild-type and iNOS knockout mice, whereas DPP multiplied inside the macrophages. Stimulation of peritoneal macrophages from wild-type C57BL/6 mice with IFN-γ plus LPS resulted significant killing of BCG. In contrast, the peptide permease mutant, defective in the transport of GSH and GSNO, multiplied in IFN-γ-LPS-stimulated macrophages. Based on our experiments with J774.1 and macrophages from wild-type C57BL/6 mice, we propose a model (Fig. 1a) describing the possible mechanism by which IFN-γ-LPS stimulation causes killing of intracellular BCG. IFN-γ-LPS stimulation of macrophages (J774.1 and primary murine macrophages) results in increased NO production and GSNO generation, leading to the killing of intracellular BCG. We demonstrated this phenomenon in macrophages by using the DPP mutant. When GSH synthesis is inhibited in J774.1 cells by BSO treatment, the killing effect of IFN-γ plus LPS is abolished, suggesting that GSH and GSNO play an important role in controlling mycobacterial growth in macrophages. Stimulation of macrophages (J774.1 and primary murine macrophages) with IFN-γ plus LPS may result in other significant antimycobacterial mechanisms independent of NO and GSH and/or GSNO, which may include generation of ROI or acidification of phagosomes. These alternative mechanisms are beyond the scope of this study.
FIG. 1.
(a) Model of GSNO-based control of BCG infection in J774 and primary macrophages from wild-type mice. (b) Model of GSH-based control of BCG infection in iNOS knockout mice. GSSG, GSH thiodisulfide.
We assayed intracellular GSH in J774.1 cells under different conditions by two different methods. Treatment of infected J774.1 cells with IFN-γ plus LPS caused significant reduction in intracellular GSH levels. We propose that GSH is associated with NO and available inside the cell as GSNO. The observation that treatment of J774.1 cells with cytokines caused an increase in NO production, leading to a decrease in the intracellular GSH levels, has been reported by others, using different in vitro conditions (4). For example, Clancy et al. reported that NO production in neutrophils caused depletion in the intracellular GSH levels due to the formation of intracellular GSNO as determined by sodium borohydride treatment (5).
Infection of iNOS−/− macrophages with BCG and DPP resulted in an insignificant increase in intracellular BCG counts but a statistically significant increase in intracellular DPP counts. Interestingly, treatment of macrophages from iNOS−/− mice with IFN-γ plus LPS resulted in significant killing of intracellular BCG but permitted significant multiplication of DPP in macrophages.
It is interesting that stimulation of macrophages from iNOS−/− mice resulted in significant killing of BCG. IFN-γ plus LPS stimulates macrophages to induce a respiratory burst and synthesis of ROI. This may lead to an increase in intracellular levels of GSH, in order to protect the macrophages from the toxic effects of reactive oxygen species. Since BCG is sensitive to GSH, an elevation in the intracellular levels of GSH in macrophages leads to growth inhibition of BCG. On the other hand, DPP, which is defective in the transport of GSH, is resistant to the toxic effects of GSH (Fig. 1b). We demonstrated that the killing of BCG in IFN-γ-LPS-treated iNOS−/− macrophages is mediated by GSH by treating the macrophages with BSO. BSO treatment of IFN-γ-LPS-treated iNOS−/− macrophages resulted in abolition of the intracellular killing of BCG. These observations suggest that in the murine system there are other significant antimycobacterial mechanisms independent of NO.
Since stimulation of HMDM with IFN-γ plus LPS did not result in growth inhibition of BCG or in generation of NO, we tested whether NAC treatment of HMDM enhances GSH synthesis (26) and reduces the intracellular viability of BCG. Results from our studies show that treatment of HMDM with NAC resulted in significant increase in the intracellular GSH and significant killing of intracellular BCG.
Attempts to demonstrate IFN-γ activation of HMDM in vitro and to generate NO and kill M. tuberculosis have failed (18, 23, 25). Knowledge of the mechanism(s) operating in human macrophages responsible for the killing of M. tuberculosis will help us to understand the disease and may pave the way for the development of vaccines or immunomodulatory agents against the disease. Nozaki et al. have shown NO production in alveolar macrophages from tuberculosis patients (23). However, to date there are no convincing reports of the successful demonstration of in vitro NO production by HMDM (1). The mechanism involved in killing and/or growth inhibition of M. tuberculosis by the human system may be independent of NO (V. Venketaraman, R. A. Fratti, J. C. Boucher, M. Mudd, J. Poschet, and V. Deretic, presented at the ASM Conference on Tuberculosis, 20 to 24 June 2000). Our methods will distinguish between direct and indirect effects of these important thiols in mycobacterial pathogenesis.
Acknowledgments
This work was supported by the New Jersey Medical School National Tuberculosis Center and by Public Health Service grant R01AI34436 to N. D. Connell.
We thank the New Jersey Blood Center for supplying blood samples.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Arias, M., J. Zabaleta, J. I. Rodriguez, M. Rojas, S. C. Paris, and L. F. Garcia. 1997. Failure to induce nitric oxide production by human monocyte-derived macrophages. Manipulation of biochemical pathways. Allergol. Immunopathol. (Madrid) 25:280-288. [PubMed] [Google Scholar]
- 2.Balazy, M., P. M. Kaminski, K. Mao, J. Tan, and M. S. Wolin. 1998. S-Nitroglutathione, a product of the reaction between peroxynitrite and glutathione that generates nitric oxide. J. Biol. Chem. 273:32009-32015. [DOI] [PubMed] [Google Scholar]
- 3.Chan, J., R. Zin, S. Magliozzo, and B. R. Bloom. 1992. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J. Exp. Med. 175:1111-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chatterjee, S., H. Noack, H. Possel, and G. Wolf. 2000. Induction of nitric oxide synthesis lowers intracellular glutathione in microglia of primary glial cultures. Glia 29:98-101. [PubMed] [Google Scholar]
- 5.Clancy, R. M., D. Levartovsky, J. Leszczynska-Piziak, J. Yegudin, and S. B. Abramson. 1994. Nitric oxide reacts with intracellular glutathione and activates the hexose monophosphate shunt in human neutrophils: evidence for S-nitrosoglutathione as a bioactive intermediary. Proc. Natl. Acad. Sci. USA 91:3680-3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clemens, D. L., and M. A. Horwitz. 1995. Characterization of Mycobacterium tuberculosis phagosome and evidence that phagosomal membrane is inhibited. J. Exp. Med. 181:257-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper, A. M., D. K. Dalton, T. A. Stewart, J. P. Griffin, D. G. Russell, and I. M. Orme. 1993. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J. Exp. Med. 178:2243-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Groote, M. A., D. Grange, Y. Xu, G. Campbell, R. Prince, and F. C. Fang. 1995. Genetic and redox determinants of nitric oxide cytotoxicity in a Salmonella typhimurium model. Proc. Natl. Acad. Sci. USA 92:6399-6403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deneke, S. M., and B. L. Fanburg. 1989. Regulation of cellular glutathione. Am. J. Physiol. 257:L163-L173. [DOI] [PubMed]
- 10.Denis, M. 1991. Killing of Mycobacterium tuberculosis within human monocytes: activation by cytokines and calcitriol. Clin. Exp. Immunol. 84:200-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Douvas, G. S., D. L. Looker, A. E. Vatter, and A. J. Crowle. 1985. Gamma interferon activates macrophages to become tumoricidal and leishmanicidal but enhances replication of macrophage-associated mycobacteria. Infect. Immun. 50:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flesch, I., and S. H. Kaufmann. 1987. Mycobacterial growth inhibition by interferon-γ activated bone marrow macrophages and differential susceptibility among strains of Mycobacterium tuberculosis. J. Immunol. 138:4408-4413. [PubMed] [Google Scholar]
- 13.Flynn, J. L., and J. Chan. 2001. Immunology of tuberculosis. Annu. Rev. Immunol. 19:93-129. [DOI] [PubMed] [Google Scholar]
- 14.Flynn, J. L., J. Chan, K. J. Triebold, D. K. Dalton, T. A. Stewart, and B. R. Bloom. 1993. An essential role for interferon-γ in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249-2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green, R. M., A. Seth, and N. D. Connell. 2000. A peptide permease mutant of Mycobacterium bovis BCG resistant to the toxic peptides glutathione and S-nitrosoglutathione. Infect. Immun. 68:429-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herzenberg, L. A., S. C. De Rosa, J. G. Dubs, M. Roederer, M. T. Anderson, S. W. Ela, S. C. Deresinski, and L. A. Herzenberg. 1997. Glutathione deficiency is associated with impaired survival in HIV disease. Proc. Natl. Acad. Sci. USA 94:1967-1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Incze, K., J. Farkas, V. Mihalyi, and E. Zukal. 1974. Antibacterial effect of cysteine-nitrosothiol and possible precursors thereof. Appl Microbiol. 27:202-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jagannath, C., J. K. Actor, and R. L. Hunter, Jr. 1998. Induction of nitric oxide in human monocytes and monocyte cell lines by Mycobacterium tuberculosis. Nitric Oxide 2:174-186. [DOI] [PubMed] [Google Scholar]
- 19.Koszycki, S. S., P. H. Schlesinger, P. Chakraborty, P. L. Haddix, H. L. Collins, A. K. Fok, R. D. Allen, S. L. Gluck, J. Heuser, and D. G. Russel. 1994. Lack of acidification of mycobacterium phagosomes produced by exclusion of vesicular proton ATPase. Science 263:678-681. [DOI] [PubMed] [Google Scholar]
- 20.Marzinzig, M., A. K. Nussler, J. Stadler, E. Marzinzig, W. Barthlen, N. C. Nussler, H. G. Beger, S. M. Morris, Jr., and U. B. Bruckner. 1997. Improved methods to measure end products of nitric oxide in biological fluids: nitrite, nitrate, and S-nitrosothiols. Nitric Oxide 1:177-189. [DOI] [PubMed] [Google Scholar]
- 21.McDonough, K. A., Y. Kress, and B. R. Bloom. 1993. Pathogenesis of tuberculosis: interaction of Mycobacterium tuberculosis with macrophages. Infect. Immun. 61:2763-2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morris, S. L., and J. N. Hansen. 1981. Inhibition of Bacillus cereus spore outgrowth by covalent modification of a sulfhydryl group by nitrosothiol and iodoacetate. J. Bacteriol. 148:465-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nozaki, Y., Y. Hasegawa, S. Ichiyama, I. Nakashima, and K. Shimokata. 1997. Mechanism of nitric oxide-dependent killing of Mycobacterium bovis BCG in human alveolar macrophages. Infect. Immun. 65:3644-3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peterson, J. D., L. A. Herzenberg, K. Vasquez, and C. Waltenbaugh. 1998. Glutathione levels in antigen presenting cells modulate Th1 versus Th2 response patterns. Proc. Natl. Acad. Sci. USA 95:3071-3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rockett, K. A., R. Brookes, I. Udalova, V. Vidal, A. V. S. Hill, and D. Kwiatkowski. 1998. 1,25-Dihydroxyvitamin-D3 induces nitric oxide and suppresses growth of Mycobacterium tuberculosis in a human macrophage-like cell line. Infect. Immun. 66:5314-5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sato, H., K. Kuriyama-Matsumura, T. Hashimoto, H. Sasaki, H. Wang, T. Ishii, G. E. Mann, and S. Bannai. 2001. Effect of oxygen on induction of the cystine transporter by bacterial lipopolysaccharide in mouse peritoneal macrophages. J. Biol. Chem. 276:10407-10412. [DOI] [PubMed] [Google Scholar]
- 27.Schaible, U. E., S. S. Koszycki, P. H. Schlesinger, and D. G. Russell. 1998. Cytokine activation leads to acidification and increases maturation of Mycobacterium avium-containing phagosomes in murine macrophages. J. Immunol. 160:1290-1296. [PubMed] [Google Scholar]
- 28.Schluger, N. W., and W. N. Rom. 1998. The host immune response to tuberculosis. Am. J. Respir. Crit. Care Med. 157:679-691. [DOI] [PubMed] [Google Scholar]
- 29.Seres, T., R. G. Knickelbein, J. B. Warshaw, and R. B. Johnston, Jr. 2000. The phagocytosis-associated respiratory burst in human monocytes is associated with increased uptake of glutathione. J. Immunol. 165:3333-3340. [DOI] [PubMed] [Google Scholar]
- 30.Stamler, J. S. 1994. Redox signaling: nitrosylation and related target interactions of nitric oxide. Cell 78:931-936. [DOI] [PubMed] [Google Scholar]
- 31.Stamler, J. S., D. I. Simon, O. Jaraki, J. A. Osborne, S. Francis, M. Mullins, D. Singel, and J. Loscalzo. 1992. S-nitrosylation of tissue-type plasminogen activator confers vasodilatory and antiplatelet properties on the enzyme. Proc. Natl. Acad. Sci. USA 89:8087-8091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walker, L., and D. B. Lowrie. 1981. Killing of Mycobacterium microti by immunologically activated macrophages. Nature 293:69-71. [DOI] [PubMed] [Google Scholar]