Abstract
Of the proteins encoded by the pcrGVH-popBD operon of the Pseudomonas aeruginosa type III secretion system, PcrG bound to PcrV and PcrH bound to PopB/PopD. In addition, Yersinia LcrG bound to PcrV, and Yersinia LcrH bound to PopD. The results imply a highly functional conservation of type III secretion between P. aeruginosa and Yersinia species.
Pseudomonas aeruginosa possesses a type III secretion system that is highly homologous to that of Yersinia species (39, 40). In type III secretion, bacteria inject their effector proteins directly into adjacent host cells (15, 20). In P. aeruginosa infections, exoenzyme S (ExoS) and its coregulated type III secreted toxins (ExoT, ExoU, and ExoY) are responsible for causing acute lung injury and sepsis (8, 9, 12, 21, 33, 38). In type III secretion of Yersinia species, translocation, a process of toxin transfer directly into the eukaryotic cytosol across the eukaryotic plasma membrane, involves LcrG, LcrV, LcrH, YopB, and YopD. These proteins are encoded by the lcrGVH-yopBD operon in the Yop regulon of Yersinia pathogenic plasmids (5, 6). In P. aeruginosa, a chromosomal operon, pcrGVH-popBD, encodes five proteins, PcrG, PcrV, PcrH, PopB, and PopD, that are homologous to Yersinia LcrG, LcrV, LcrH, YopB, and YopD, respectively. For Yersinia pestis, protective antigenic characteristics of LcrV were reported previously as a V antigen (1, 3, 4, 18, 22, 23, 25, 35). LcrV likely forms the translocation pore in eukaryotic cell membranes in conjunction with YopB and YopD (16, 17, 19, 27, 29, 30, 31, 34). LcrG, which forms a stable complex with LcrV, acts as a negative regulator that blocks secretion of Yops (7, 24, 28, 37). LcrH was reported previously as a cognate chaperone of YopD (26, 32) and was found to be necessary for YopD stabilization before secretion (10). Recently, an active role of LcrH in Yop regulation was also reported (2, 10, 11).
The importance of V antigen in cytotoxicity has been well established. Isogenic mutants of P. aeruginosa lacking the genes for pcrV or popD were unable to intoxicate eukaryotic cells (36). Active and passive immunization against PcrV in animal models of P. aeruginosa-induced lung injury greatly increased survival (36). Functional conservation from PopB and PopD of P. aeruginosa to YopB and YopD of Yersinia pseudotuberculosis was previously reported (14). However, there have been fewer studies analyzing the proteins encoded by the pcrGVH-popBD operon of the P. aeruginosa type III secretion system. In this study, we examined the interactions among the proteins encoded by the pcrGVH-popBD operon to investigate the functional homology between the type III secretion systems of P. aeruginosa and Yersinia.
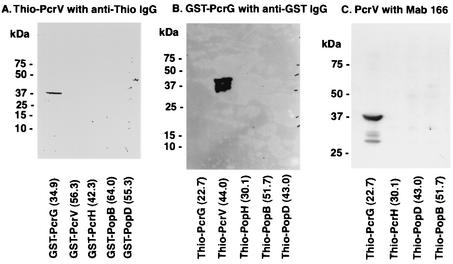
In Escherichia coli, we induced the expression of glutathione S-transferase (GST) fusion PcrG, PcrV, PcrH, PopB, and PopD proteins whose genes were subcloned in pGEX plasmids under the lac promoter. We also induced the expression of the thioredoxin (Thio) fusion PcrG, PcrV, PcrH, PopB, and PopD proteins from genes subcloned into the pThio plasmid under the lac promoter. Induction of PopB fusion proteins appeared to decrease E. coli density after isopropyl-β-d-thiogalactopyranoside (IPTG) induction, suggesting bactericidal activity. We performed affinity immunoblotting to examine the interaction between PcrV and other proteins encoded by the pcrGVH-popBD operon. We applied E. coli lysate containing Thio-PcrV to a membrane blotted with the lysates of E. coli expressing a series of GST tag-fused proteins. From this experiment, only the GST-PcrG band was visualized (Fig. 1A). Next, we applied GST-PcrG to a membrane blotted with the lysates of E. coli expressing Thio tag-fused proteins. From this experiment, only the Thio-PcrV band was intensely visualized (Fig. 1B). Next, we performed affinity immunoblotting with purified recombinant nontagged PcrV and applied it to membrane-bound Thio fusion proteins to determine whether PcrV-blocking antibodies could detect the PcrV-PcrG complex. Both rabbit polyclonal anti-PcrV antibody (data not shown) and murine anti-PcrV monoclonal antibody (MAb) 166 detected PcrV bound to Thio-PcrG (13) (Fig. 1C). All affinity immunoblotting resulted in the detection of a PcrV-PcrG interaction.
FIG. 1.
Affinity immunoblot analysis. (A) Binding of Thio-PcrV to GST-PcrG. The protein samples from induced E. coli clones carrying pGEX plasmids were electrophoresed onto a sodium dodecyl sulfate-4 to 12% bis-Tris polyacrylamide gel, electroblotted onto a nitrocellulose membrane, and incubated with E. coli lysate including expressed Thio-PcrV. The membrane was developed with anti-Thio immunoglobulin G (IgG) and secondary anti-mouse IgG conjugated with horseradish peroxidase and a chemiluminescent substrate. An intense isolated band represents binding of GST-PcrG to Thio-PcrV. (B) Binding of GST-PcrG to Thio-PcrV. The protein samples were from induced E. coli clones carrying pThio plasmids. The blotted membrane was incubated with recombinant GST-PcrG (10 μg/ml) and then developed with anti-GST IgG conjugated with horseradish peroxidase and a chemiluminescent substrate. An intense isolated band represents binding of GST-PcrG to Thio-PcrV. (C) Binding of PcrV to Thio-PcrG. The protein samples were from induced E. coli clones carrying pThio plasmids. The membrane was incubated with recombinant PcrV (10 μg/ml), then developed with murine anti-PcrV MAb 166, and developed with secondary anti-mouse antibodies conjugated with horseradish peroxidase and a chemiluminescent substrate. An intense isolated band represents binding of PcrV to Thio-PcrG.
Because LcrH, a Yersinia homolog of PcrH, was reported as a chaperone protein for Yersinia YopD, we purified recombinant GST-PcrH from E. coli transformed with pGEX-pcrH and examined the interaction between PcrH and other proteins in the same format as that previously used to find the PcrV-PcrG interaction. Affinity immunoblotting was performed with recombinant purified GST-PcrH to a membrane blotted with the lysates of E. coli expressing Thio tag-fused proteins. GST-PcrH bound to both Thio-PopB and Thio-PopD in this affinity immunoblot assay (Fig. 2). In order to verify protein interactions, a GST pull-down assay was performed on PA103 lysates with recombinant GST-PcrG and GST-PcrH. As a result, GST-PcrG coprecipitated with native PcrV, and GST-PcrH coprecipitated with PopD (data not shown).
FIG. 2.
Affinity immunoblot analysis demonstrates the binding of GST-PcrH to Thio-PopD and Thio-PopB. The protein samples from E. coli expressing Thio-tagged fusion proteins were loaded onto a sodium dodecyl sulfate-4 to 12% bis-Tris polyacrylamide gel electrophoresis gel for electrophoresis. Proteins were then blotted onto a nitrocellulose membrane for subsequent affinity immunoblot analysis. Affinity immunoblotting demonstrates the interaction between GST-PcrH and Thio-PopD. The blotted membrane was incubated in a solution with purified recombinant GST-PcrH. After the membrane was washed several times, immunostaining against the GST tag was performed with anti-GST antibody conjugated with horseradish peroxidase and chemiluminescent substrate. The intense bands demonstrating the interaction with GST-PcrH were recognized in Thio-tagged PopB and PopD lanes (arrowheads). IgG, immunoglobulin G.
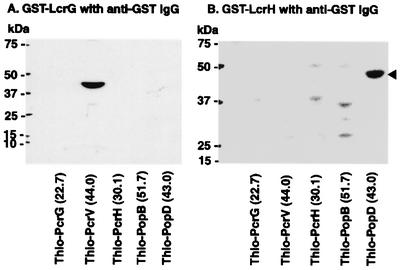
We performed affinity immunoblotting to examine the cross-species interaction between Yersinia and P. aeruginosa type III proteins. From this experiment, we found that GST-LcrG binds to Thio-PcrV (Fig. 3A) and GST-LcrH binds to Thio-PopD (Fig. 3B). Therefore, the protein binding between LcrG and PcrV and between LcrH and PopD occurred in a cross-species manner between Yersinia and P. aeruginosa.
FIG. 3.
Affinity immunoblot analysis demonstrates the binding of GST-LcrG to Thio-PcrV and of GST-LcrH to Thio-PopD. The protein samples from induced E. coli clones carrying the expression plasmids were loaded onto sodium dodecyl sulfate-4 to 12% bis-Tris polyacrylamide gels for electrophoresis. Protein was then electroblotted onto nitrocellulose membranes and incubated with each recombinant protein (8 to 10 μg/ml). The membranes were then developed with anti-GST antibodies conjugated with horseradish peroxidase and a chemiluminescent substrate. (A) An intense isolated band represents binding of GST-LcrG to Thio-PcrV. (B) An intense isolated band represents binding of GST-LcrH to Thio-PopD (arrowhead). IgG, immunoglobulin G.
These findings imply high functional and structural homology among these proteins despite the fact that their amino acid sequence similarities range from 56 to 57%. Our results suggest that PcrG serves the role of a potential negative regulator of PcrV. The neutralizing epitope on PcrV appears to be different from the PcrG binding site, given that the blocking anti-PcrV MAb 166 clearly detected the PcrV-PcrG complex in our study. Since P. aeruginosa PcrH and PopD are homolog equivalents of Yersinia LcrH and YopD, respectively, our findings suggest that PcrH is a chaperone for PopD secretion. Although PcrH binds to PopB in the immunoblot that we made, a similar interaction between LcrH and PopB was not found. The experimental conditions that we tested may have been affected by the fact that the expression of recombinant PopB in E. coli was bactericidal. This phenomenon has been reported elsewhere for Yersinia YopB expression in E. coli without coexpression of LcrH (26). LcrV, YopB, and YopD are thought to form a pore complex involved in the translocation of type III secreted proteins across the eukaryotic plasma membrane, but interactions with LcrV among these proteins have not been experimentally elucidated. We tested PcrV binding to PopB and PopD, but no interaction was found.
In conclusion, PcrG binds to PcrV and PcrH binds to PopB and PopD. From interactions between Pcr and Lcr proteins, a highly functional conservation of type III system translocators was also confirmed between P. aeruginosa and Yersinia. Thus, investigations of the roles and mechanisms of PcrV secretion and anti-PcrV blockade and of LcrV secretion and anti-LcrV blockade may complement each other.
Acknowledgments
This research was supported by National Institutes of Health grants RO1 HL59239 and AI44101 to J.P.W.-K. and HL067600 to T.S.; a supplement to AI44101 to L.R.A.; a Faculty Development Award, University of California, San Francisco, and the American Lung Association, RG004N, to T.S.; and grants from National Medical Fellowships and the AΩA Medical Society to L.R.A.
Editor: V. J. DiRita
REFERENCES
- 1.Anderson, G. W., Jr., S. E. C. Leary, E. D. Williamson, R. W. Titball, S. L. Welkos, P. L. Worsham, and A. M. Friedlander. 1996. Recombinant V antigen protects mice against pneumonic and bubonic plague caused by F1-capsule-positive and -negative strains of Yersinia pestis. Infect. Immun. 64:4580-4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergman, T., S. Håkansson, Å. Forsberg, L. Norlander, A. Macellaro, A. Bäckman, I. Bölin, and H. Wolf-Watz. 1991. Analysis of the V antigen lcrGVH-yopBD operon of Yersinia pseudotuberculosis: evidence for a regulatory role of LcrH and LcrV. J. Bacteriol. 173:1607-1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burrows, T. W., and G. A. Bacon. 1956. The basis of virulence in Pasteurella pestis: antigen determining virulence. Br. J. Exp. Pathol. 37:481-493. [PMC free article] [PubMed] [Google Scholar]
- 4.Burrows, T. W., and G. A. Bacon. 1958. The effects of loss of different virulence determinants on the virulence and immunogenicity of strains of Pasteurella pestis. Br. J. Exp. Pathol. 39:278-291. [PMC free article] [PubMed] [Google Scholar]
- 5.Cornelis, G. R., and F. Van Gijsegem. 2000. Assembly and function of type III secretory systems. Annu. Rev. Microbiol. 54:735-774. [DOI] [PubMed] [Google Scholar]
- 6.Cornelis, G. R., and H. Wolf-Watz. 1997. The Yersinia Yop virulon: a bacterial system for subverting eukaryotic cells. Mol. Microbiol. 23:861-867. [DOI] [PubMed] [Google Scholar]
- 7.DeBord, K. L., V. T. Lee, and O. Schneewind. 2001. Roles of LcrG and LcrV during type III targeting of effector Yops by Yersinia enterocolitica. J. Bacteriol. 183:4588-4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finck-Barbancon, V., J. Goranson, L. Zhu, T. Sawa, J. P. Wiener-Kronish, S. M. Fleiszig, C. Wu, L. Mende-Mueller, and D. W. Frank. 1997. ExoU expression by Pseudomonas aeruginosa correlates with acute cytotoxicity and epithelial injury. Mol. Microbiol. 25:547-557. [DOI] [PubMed] [Google Scholar]
- 9.Fleiszig, S. M., J. P. Wiener-Kronish, H. Miyazaki, V. Vallas, K. E. Mostov, D. Kanada, T. Sawa, T. S. Yen, and D. W. Frank. 1997. Pseudomonas aeruginosa-mediated cytotoxicity and invasion correlate with distinct genotypes at the loci encoding exoenzyme S. Infect. Immun. 65:579-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Francis, M. S., M. Aili, M.-L. Wiklund, and H. Wolf-Watz. 2000. A study of the YopD-LcrH interaction from Yersinia pseudotuberculosis reveals a role for hydrophobic residues within the amphipathic domain of YopD. Mol. Microbiol. 38:85-102. [DOI] [PubMed] [Google Scholar]
- 11.Francis, M. S., S. A. Lloyd, and H. Wolf-Watz. 2001. The type III secretion chaperone LcrH co-operates with YopD to establish a negative, regulatory loop for control of Yop synthesis in Yersinia pseudotuberculosis. Mol. Microbiol. 42:1075-1093. [DOI] [PubMed] [Google Scholar]
- 12.Frank, D. W. 1997. The exoenzyme S regulon of Pseudomonas aeruginosa. Mol. Microbiol. 26:621-629. [DOI] [PubMed] [Google Scholar]
- 13.Frank, D. W., A. Vallis, J. P. Wiener-Kronish, A. Roy-Burman, E. G. Spack, B. P. Mullaney, M. Megdoud, J. D. Marks, R. Fritz, and T. Sawa. 2002. Generation and characterization of a protective monoclonal antibody to Pseudomonas aeruginosa PcrV. J. Infect. Dis. 186:64-73. [DOI] [PubMed] [Google Scholar]
- 14.Frithz-Lindsten, E., A. Hölmstrom, L. Jacobsson, M. Soltani, J. Olsson, R. Rosqvist, and Å. Forsberg. 1998. Functional conservation of the effector protein translocators PopB/YopB and PopD/YopD of Pseudomonas aeruginosa and Yersinia pseudotuberculosis. Mol. Microbiol 29:1155-1165. [DOI] [PubMed] [Google Scholar]
- 15.Galan, J. E., and A. Collmer. 1999. Type III secretion machines: bacterial devices for protein delivery into host cells. Science 284:1322-1328. [DOI] [PubMed] [Google Scholar]
- 16.Håkansson, S., K. Schesser, C. Persson, E. E. Galyov, R. Rosqvist, F. Homblé, and H. Wolf-Watz. 1996. The YopB protein of Yersinia pseudotuberculosis is essential for the translocation of Yop effector proteins across the target cell plasma membrane and displays a contact-dependent membrane disrupting activity. EMBO J. 15:5812-5823. [PMC free article] [PubMed] [Google Scholar]
- 17.Håkansson, S., T. Bergman, J.-C. Vanooteghem, G. Cornelis, and H. Wolf-Watz. 1993. YopB and YopD constitute a novel class of Yersinia Yop proteins. Infect. Immun. 61:71-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hill, J., S. E. C. Leary, K. Griffin, E. D. Williamson, and R. W. Titball. 1997. Regions of Yersinia pestis V antigen that contribute to protection against plague identified by passive and active immunization. Infect. Immun. 65:4476-4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holmström, A., J. Olsson, P. Cherepanov, E. Maier, R. Nordfelth, J. Pettersson, R. Benz, H. Wolf-Watz, and Å. Forsberg. 2001. LcrV is a channel size-determining component of the Yop effector translocon of Yersinia. Mol. Microbiol. 39:620-632. [DOI] [PubMed] [Google Scholar]
- 20.Hueck, C. J. 1998. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev. 62:379-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurahashi, K., O. Kajikawa, T. Sawa, M. Ohara, M. A. Gropper, D. W. Frank, T. R. Martin, and J. P. Wiener-Kronish. 1999. Pathogenesis of septic shock in Pseudomonas aeruginosa pneumonia. J. Clin. Investig. 104:743-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lawton, W. D., R. I. Erdman, and M. J. Surgalla. 1963. Biosynthesis and purification of V and W antigens in Pasteurella pestis. J. Immunol. 91:179-184. [DOI] [PubMed] [Google Scholar]
- 23.Leary, S. E. C., E. D. Williamson, K. F. Griffin, P. Russell, S. M. Eley, and R. W. Titball. 1995. Active immunization with recombinant V antigen from Yersinia pestis protects mice against plague. Infect. Immun. 63:2854-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matson, J. S., and M. L. Nilles. 2001. LcrG-LcrV interaction is required for control of Yops secretion in Yersinia pestis. J. Bacteriol. 183:5082-5091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Motin, V. L., R. Nakajima, G. B. Smirnov, and R. R. Brubaker. 1994. Passive immunity to yersiniae mediated by anti-recombinant V antigen and protein A-V antigen fusion peptide. Infect. Immun. 62:4192-4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neyt, C., and G. R. Cornelis. 1999. Role of SycD, the chaperone of the Yersinia Yop translocators YopB and YopD. Mol. Microbiol. 31:143-156. [DOI] [PubMed] [Google Scholar]
- 27.Neyt, C., and G. R. Cornelis. 1999. Insertion of a Yop translocation pore into the macrophage plasma membrane by Yersinia enterocolitica: requirement for translocators YopB and YopD, but not LcrG. Mol. Microbiol. 33:971-981. [DOI] [PubMed] [Google Scholar]
- 28.Nilles, M. L., A. W. Williams, E. Skrzypek, and S. C. Straley. 1997. Yersinia pestis LcrV forms a stable complex with LcrG and may have a secretion-related regulatory role in the low-Ca2+ response. J. Bacteriol. 179:1307-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nilles, M. L., K. A. Fields, and S. C. Straley. 1998. The V antigen of Yersinia pestis regulates Yop vectorial targeting as well as Yop secretion through effects on YopB and LcrG. J. Bacteriol. 180:3410-3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nordfelth, R., and H. Wolf-Watz. 2001. YopB of Yersinia enterocolitica is essential for YopE translocation. Infect. Immun. 69:3516-3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pettersson, J., A. Holmström, J. Hill, S. Leary, E. Frithz-Lindsten, A. von Euler-Matell, E. Carlsson, R. Titball, Å. Forsberg, and H. Wolf-Watz. 1999. The V-antigen of Yersinia is surface exposed before target cell contact and involved in virulence protein translocation. Mol. Microbiol. 32:961-976. [DOI] [PubMed] [Google Scholar]
- 32.Price, S. B., C. Cowan, and S. C. Straley. 1989. LcrH, a gene necessary for virulence of Yersinia pestis and for the normal response of Y. pestis to ATP and calcium. Infect. Immun. 57:1491-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roy-Burman, A., R. Savel, S. Racine, B. L. Swanson, N. S. Ravadigar, J. Fujimoto, T. Sawa, D. W. Frank, and J. P. Wiener-Kronish. 2001. Type III protein secretion is associated with death in lower respiratory and systemic Pseudomonas aeruginosa infections. J. Infect. Dis. 183:1767-1774. [DOI] [PubMed] [Google Scholar]
- 34.Sarker, M. R., C. Neyt, I. Stainer, and G. R. Cornelis. 1998. The Yersinia Yop virulon: LcrV is required for extrusion of the translocators YopB and YopD. J. Bacteriol. 180:1207-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sato, K., R. Nakajima, F. Hara, T. Une, and Y. Osada. 1991. Preparation of monoclonal antibody to V antigen from Yersinia pestis. Contrib. Microbiol. Immunol. 12:225-229. [PubMed] [Google Scholar]
- 36.Sawa, T., T. L. Yahr, M. Ohara, K. Kurahashi, M. A. Gropper, J. P. Wiener-Kronish, and D. W. Frank. 1999. Active and passive immunization with the Pseudomonas V antigen protects against type III intoxication and lung injury. Nat. Med. 5:392-398. [DOI] [PubMed] [Google Scholar]
- 37.Skrzypek, E., and S. C. Straley. 1993. LcrG, a secreted protein involved in negative regulation of the low-calcium response in Yersinia pestis. J. Bacteriol. 175:3520-3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yahr, T. L., A. J. Vallis, M. K. Hancock, J. T. Barbieri, and D. W. Frank. 1998. ExoY, an adenylate cyclase secreted by the Pseudomonas aeruginosa type III system. Proc. Natl. Acad. Sci. USA 95:13899-13904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yahr, T. L., J. Goranson, and D. W. Frank. 1996. Exoenzyme S of Pseudomonas aeruginosa is secreted by a type III pathway. Mol. Microbiol. 22:991-1003. [DOI] [PubMed] [Google Scholar]
- 40.Yahr, T. L., L. M. Mende-Mueller, M. B. Friese, and D. W. Frank. 1998. Identification of type III secreted products of the Pseudomonas aeruginosa exoenzyme S regulon. J. Bacteriol. 179:7165-7168. [DOI] [PMC free article] [PubMed] [Google Scholar]