Abstract
Oral inoculation of 5-day-old gnotobiotic pigs with Salmonella enterica serovar Typhimurium strain F98 resulted in severe enteritis and invasive disease. Preinoculation 24 h earlier with an avirulent mutant of Salmonella enterica serovar Infantis (1326/28) completely prevented disease for up to 14 days (when the experiment was terminated). S. enterica serovar Infantis colonized the alimentary tract well, with high bacterial counts in the intestinal lumen but with almost no invasion into the tissues. Unprotected pigs had high S. enterica serovar Typhimurium counts in the intestines, blood, and major nonintestinal organs. Recovery of this strain from the blood and major organs in S. enterica serovar Infantis-protected pigs was substantially reduced despite the fact that intestinal counts were also very high. Protection against disease thus did not involve a colonization exclusion phenomenon. Significant (P < 0.05) infiltration of monocytes/macrophages was observed in the submucosal regions of the intestines of both S. enterica serovar Infantis-protected S. enterica serovar Typhimurium-challenged pigs and unprotected S. enterica serovar Typhimurium-challenged pigs. However, only polymorphonuclear neutrophils (PMNs) were observed throughout the villus, where significant (P < 0.05) numbers infiltrated the lamina propria and the subnuclear and supranuclear regions of the epithelia, indicating that PMN induction and positioning following S. enterica serovar Infantis inoculation was consistent with rapid protection against the challenge strain. Similarly, in vitro experiments using a human fetal intestinal epithelial cell line (INT 407) demonstrated that, although significantly (P < 0.05) fewer S. enterica serovar Infantis than S. enterica serovar Typhimurium organisms invaded the monolayers, S. enterica serovar Infantis induced an NF-κB response and significantly (P < 0.05) raised interleukin 8 levels and transmigration of porcine PMN. The results of this study suggest that attenuated Salmonella strains can protect the immature intestine against clinical salmonellosis by PMN induction. They also demonstrate that PMN induction is not necessarily associated with clinical symptoms and/or intestinal pathology.
Salmonella enterica serovar Typhimurium infection in pigs is a public and animal health problem of considerable significance. S. enterica serovar Typhimurium is the serovar most frequently isolated from humans and pigs in Europe (24), while in the United States alone it is estimated that up to 4 million human cases of salmonellosis occur annually, with about 500 fatalities, many of which are the result of infection with this serovar (19).
Entry into the human food chain results largely from disease-free carriage in the intestine. Clinical disease may occur in the postweaning period or, less frequently, in the neonatal pig, with the symptoms of diarrhea, vomiting, enterocolitis, and bacteremia (with major organ involvement in more severe infections). Therefore, the young pig is a desirable target for vaccination and is also a good model for human gastroenteritis (20, 48).
Preventative control of human and animal salmonellosis by the use of live oral vaccines is being increasingly contemplated for pigs (27, 39) and poultry (5, 11). In chickens, live vaccines not only induce typical immunity (1, 21) but, if given orally to newly hatched chicks, confer a high degree of specific colonization resistance within a few hours of administration, which is a microbiological phenomenon (4, 7). Live vaccines administered orally to very young animals may, therefore, confer rapid resistance to infection and should continue to colonize and invade sufficiently to induce immunity. In earlier experiments with chickens, a preliminary search was made to find a Salmonella strain which produced a high level of inhibition against colonization by a wide selection of field Salmonella strains. A strain of Salmonella enterica serovar Infantis (1326/28) which possessed these characteristics (7) and which was nonvirulent for chicks and mice was found (2). This study describes the protection of 5-day-old gnotobiotic pigs against fatal challenge with S. enterica serovar Typhimurium F98 by precolonization with avirulent S. enterica serovar Infantis. Furthermore, since microbiological exclusion did not seem to be involved, the immunological nature of this protection was studied in vivo (in the pig) and in vitro in a human fetal intestinal epithelial cell line (INT 407).
MATERIALS AND METHODS
Bacterial strains.
S. enterica serovar Typhimurium F98 (phage type 14) has been studied extensively (2, 4). S. enterica serovar Infantis 1326/28 is a nonvirulent poultry strain described previously (2). In addition, the virulence of this strain was reduced further by inducing roughness following lytic bacteriophage activity (1, 3).
In all cases, mutants of the above-mentioned strains were made resistant to either nalidixic acid (S. enterica serovar Typhimurium) or spectinomycin (S. enterica serovar Infantis) to facilitate enumeration (43). These mutations did not affect the virulence of the strains (2, 43).
All strains were cultured for 24 h in 10 ml of Luria-Bertani broth (Difco Laboratories, Detroit, Mich.) in a shaking incubator (150 rpm). They generally reached densities of 3 × 109 to 5 × 109 CFU/ml. In some in vitro assays, a sipB mutant of S. enterica serovar Typhimurium (a Cmr insertion mutant provided by E. Galyov, Institute for Animal Health, Compton Laboratory, Compton, Newbury, United Kingdom) was used as a measure of reduced capacity to invade (17).
Experimental animals.
Gnotobiotic Large-White pigs were obtained by hysterotomy and were reared in metal-floored cages or in large glass fiber tanks in positive-pressure isolators (45, 47). They were reared at an ambient temperature of 25 to 28°C and were given increasing amounts of a mixture of equal volumes of sterilized condensed milk and water with a mineral supplement. They were checked for absence of bacterial contamination and were used at 5 days of age.
Infection protocol and quantitative bacteriological analysis.
The pigs were infected at 5 days of age. Each experiment consisted of four groups each containing three pigs, and each experiment was replicated on at least four separate occasions. The pigs in the first group were uninfected controls, while the pigs in the second group were infected with 103 CFU of S. enterica serovar Typhimurium F98, delivered in 1 ml of phosphate-buffered saline (PBS) to the back of the throat with a syringe. In the third group, the pigs were orally dosed with rough S. enterica serovar Infantis 1326/28 (106 CFU) as previously stated, and in the fourth group, the pigs were dosed with S. enterica serovar Infantis (106 CFU) followed by S. enterica serovar Typhimurium (103 CFU) 24 h later. All of the pigs were given access to sterilized milk, and their clinical condition, rectal temperature, and fecal counts were monitored daily.
In one experiment, 48 h after challenge with S. enterica serovar Typhimurium (groups 2 and 4) or S. enterica serovar Infantis (group 3), organ samples were taken under deep anesthesia with halothane (Merial Animal Health Ltd., Essex, United Kingdom), after which the animals were killed by lethal injection administered directly into the heart (5 ml of sodium pentabarbitone; Rhone-Merieux Ltd., Essex, United Kingdom). The uninfected control pigs were also killed 48 h after the beginning of the experiment.
Tissue examination.
While the pigs were under halothane anesthesia, the skin over the abdominal wall was cleaned with alcohol. Following laparotomy, a 3- to 4-cm section of the terminal ileum (not including the ileocecal Peyer's patch) was removed, washed in PBS, and immediately immersed in paraformaldehyde (5% [wt/vol]) for myeloid cell counts. Postmortem removal of tissues for microbiological examination was done aseptically in the order heart blood, kidney, spleen, and liver. Regions of the gastrointestinal tract were removed in the order duodenum, jejunum, ileum, cecum, colon, and stomach. The lymph nodes corresponding to each section of gut were removed prior to removal of the section. The samples were mixed with PBS and homogenized by vigorous mixing (gut contents) or by Griffiths tubes or pestle and mortar with sand (tissues). The numbers of viable bacteria in the homogenates were estimated by plating serial dilutions on Luria-Bertani agar containing spectinomycin (50 μg/ml) or sodium nalidixate (20 μg/ml).
Innate immune-cell counts in porcine intestine.
To examine innate cell infiltration following inoculation of pigs with Salmonella, excised pieces of the terminal ileum (3- to 4-cm thickness) were fixed in paraformaldehyde, embedded in wax, and sectioned on a Leica cm 1900 microtome. Fifteen sections (6 to 8 μm thick) per sample were stained with hematoxylin and eosin and examined using a Nikon eclipse E400 light microscope. For each sample, monocytes/macrophages, polymorphonuclear leukocytes (PMNs), eosinophils, and total cells in five fields of view from 15 sections were counted and compared with those in uninfected (control) tissue. Five pigs per test were used for comparison (n = 300 fields of view per test). The cell types were verified by an independent observer.
Pathological index.
A pathological index (10) was used to estimate Salmonella-associated intestinal pathology. A scoring system of 0 to 3 determined the extent of flattening of the plica circularis and whether epithelial cell exfoliation had occurred at the tips or at the sides of the villi as follows: 0, no flattening of plica and long slender villi with no exfoliation of tips or sides of villi; 1, flattened plica with stunted villi; 2, flattened plica with stunted villi with exfoliation of tips; 3, flattened plica with stunted villi and exfoliation of tips and sides of villi. To further investigate the micropathology of protected and unprotected S. enterica serovar Typhimurium-infected animals, scanning electron microscopy was employed using standard methods.
Cell culture.
Human fetal intestinal epithelial INT 407 cells were grown to confluence at 37°C in CO2 (5%) on 5.0-μm-pore-size filter supports in 24-well plates (Nunc, Naperville, Ill.) containing 1 ml of Eagle minimal essential medium (EMEM) supplemented with fetal calf serum (5% [vol/vol]), nonessential amino acids (1% [wt/vol]), glutamine (1% [wt/vol]), and NaHCO3 (7.5% [wt/vol]). Prior to use, the monolayers were washed three times with PBS and left at room or ambient temperature in PBS for 30 min to restore transcellular resistance.
Electrical measurement.
To assess the ability of INT 407 cells to form resistant monolayers, an evometer (WPI, Sarasota, Fla.) was used. The electrodes were equilibrated with KCl solution, and resistance was measured three times across five monolayers, as described previously (29).
Adherence and invasion studies in cultured human epithelium.
Adherence and invasion of human epithelial INT 407 monolayers by S. enterica serovar Typhimurium and S. enterica serovar Infantis were studied using a confocal laser scanning microscope equipped with an argon laser (Leica) by the method of Jepson et al. (22). Briefly, filters containing cells were incubated with bacteria (multiplicity of infection [MOI] = 10) for 60 min at 37°C in CO2 (5%). The filters were washed six times in ice-cold PBS to remove nonadherent bacteria and then placed on ice to prevent further invasion. After 30 min, the filters were placed in wells containing PBS and goat anti-Salmonella immunoglobulin G (IgG) (1/200) (Dynex, Ashford, United Kingdom) for a further 30 min to detect adherent bacteria. The monolayers were then washed three times in cold PBS prior to incubation in PBS containing rabbit anti-goat IgG conjugated to fluorescein isothiocyanate (FITC) (1/100; Sigma, Poole, United Kingdom). The cells were washed again in PBS before being placed in ice-cold methanol for 30 min, and further steps were performed at room or ambient temperature. To detect total bacterial numbers (adherent and invading), the cells were incubated in PBS containing anti-Salmonella antibodies (1/200) for 30 min, washed three times in PBS, and then incubated for a further 30 min in tetramethyl rhodamine isothiocyanate-conjugated rabbit anti-goat IgG (1/50 dilution) (Sigma).
The monolayers were then washed in PBS and stained with rabbit anti-mouse tubulin IgG (1/200) (Sigma) for 60 min, followed by FITC-conjugated goat anti-rabbit IgG (Sigma) for 60 min. After a further washing step, the filters were cut out and mounted on microscope slides using Vectashield antiquenchant (Vector Laboratories, Burlingame, Calif.). Five fields of view in five different samples were counted, each sample being replicated 10 times. The number of bacteria that adhered to the cell membrane (FITC conjugated) was compared to the total bacterial count (tetramethyl rhodamine isothiocyanate conjugated). The numbers of internalized bacteria were estimated by subtracting the number of adhered bacteria from the total number. Adherence and invasion of the S. enterica serovar Typhimurium sipB mutant were also measured as a comparison for reduced invasion of epithelial cells. Further controls included primary antibodies or secondary antibodies only or primary and secondary antibodies incubated with uninfected monolayers. All controls tested were negative.
Assessment of CE on INT 407 monolayers.
To assess competitive exclusion (CE) of S. enterica serovar Typhimurium by the rough S. enterica serovar Infantis on INT 407 monolayers, cells were incubated for 60 min at 37°C and 5% CO2 with different MOIs (10, 100, and 1,000) of S. enterica serovar Infantis in 100 μl of PBS. After 60 min, the PBS was removed and replaced with 100 μl of PBS containing S. enterica serovar Typhimurium F98 (MOI = 10). The monolayers were then washed three times to remove nonadherent bacteria and placed in EMEM containing 100 μg of gentamicin/ml for 90 min at 37°C. After the monolayers were washed with PBS, the cells were lysed with Triton X prior to counting of the S. enterica serovar Typhimurium and S. enterica serovar Infantis organisms on plates containing the appropriate antibiotic.
Detection of NF-κB induction in infected INT 407 monolayers.
Induction of interleukin 8 (IL-8) requires the degradation of inhibitory κB (IκB) and subsequent release of the IL-8 transcription factor nuclear factor κB (NF-κB). A Western blot analysis (46) was used to detect NF-κB activity in S. enterica serovar Typhimurium- and S. enterica serovar Infantis-infected monolayers. A bicinchoninic acid (Pierce, Cheshire, United Kingdom) assay was used to measure the total protein concentrations of infected or uninfected INT 407 monolayers incubated with 1 μg of phorbol myristate acetate (PMA) (Sigma)/ml or uninfected monolayers which were not incubated with PMA. The protein concentration loaded into each lane of a sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel was standardized to 10 μg/ml. The proteins were then separated by electrophoresis (23). After the proteins were transferred to Hybond-C nitrocellulose paper (Amersham, Little Chalfont, United Kingdom) by a Trans-blot SD semidry transfer cell (Bio-Rad, Hemel Hemstead, Herts, United Kingdom), the nitrocellulose was blocked overnight at 4°C with PBS-Tween containing bovine serum albumin (5% [wt/vol]; Sigma). After being blocked, the nitrocellulose was washed three times in PBS-Tween 20 (5 min per wash) on an end-to-end shaker at room or ambient temperature. The nitrocellulose was then incubated with rabbit anti-mouse NF-κB (p50) (1:200) (Autogenbioclear; Calne, Wilts, United Kingdom) for 60 min at room or ambient temperature on an end-to-end shaker. After being washed three times as before, the nitrocellulose was incubated for 60 min with goat anti-rabbit IgG (1:16,000) (Sigma). After being washed, the nitrocellulose was then developed using an enhanced 3′,3′-diaminobenzidine kit (Vector Laboratories).
IL-8 assays.
The amount of IL-8 secreted by the basolateral membrane of infected INT 407 cells was determined with an IL-8 enzyme-linked immunosorbent assay kit (Boehringer, Bracknell, Berks, United Kingdom). Confluent INT 407 monolayers were grown on 5.0-μl filter supports (Nunc) in 24-well plates (Corning, High Wickham, Bucks, United Kingdom) containing EMEM as stated above. The monolayers were washed to remove the medium and then infected apically with bacteria (MOI = 10) in PBS for 60 min at 37°C in CO2 (5%) by a standard method (C. C. Schurer-Maly, F. E. Maly, and M. F. Kagnoff, abstract from Gastroenterology 102(Suppl.):A692, 1992). The monolayers were washed in Hanks balanced salt solution without Mg2+ or Ca2+ (HBSS−) (Gibco, Paisley, United Kingdom) and placed in wells containing 100 μl of HBSS− basolaterally and apically. After 120 min, 0.05% Tween was added to the basolateral medium for a further 60 min. The basolateral medium was then removed from each of four replicates per strain for analysis. The optical densities of test solutions and controls were then read at 450 nm on an enzyme-linked immunosorbent assay plate reader (Anthos Labtech Instruments, Hamburg, Germany). As a negative control, uninfected INT 407 cells were used, and as a positive control, PMA (1 μg/ml; Sigma) was used to stimulate IL-8. The assay was repeated three times, and the mean for each group was analyzed.
PMN transepithelial-migration studies.
Physiologically directed PMN transepithelial migration in response to Salmonella was measured by a standard method (36). Confluent INT 407 monolayers were grown on inverted filter supports (5.0-μm pore size; 25-mm diameter; Nunc). The cells were then washed three times by immersion in HBSS containing Ca2+ and Mg2+ (HBSS+) (Gibco) prior to the addition of bacteria (MOI = 10) to the apical surface in 50-μl aliquots. The filters were then placed in a closed container which had a PBS-soaked towel at its base and incubated at 37°C for 60 min, after which the cells were washed three times in HBSS+ and everted back into 1 ml of fresh HBSS+ buffer. One hundred sixty microliters of HBSS+ was then aliquoted into the upper (basolateral) chamber, and the cells were maintained at 37°C for a further 2 h prior to the addition of PMNs.
Porcine blood was isolated by venipuncture, and 50 ml was spun at 400 × g for 20 min at room or ambient temperature to obtain a buffy coat. Serum was removed just below the buffy coat by pipette and then mixed with a gelatin solution (2%) as previously described (36). Further removal of erythrocytes was achieved by lysis in cold NH4Cl buffer (in 1 liter of H2O, 8.29 g of NH4Cl, 1.0 g of NaHCO3, and 0.038 g of EDTA). The PMNs were then washed and stored on ice in HBSS for up to 60 min before use. Purity was assessed by hemocytometer and was estimated at 87%, while viability (as assessed by trypan blue exclusion) was estimated to be >90%.
The PMNs (106) were then aliquoted into the basolateral chambers of the infected filters and incubated at 37°C for 2 h. Transepithelial migration was assessed by a myeloperoxidase assay, which detects PMN azurophil activity, as previously reported (35). As a positive control, N-formyl-Met-Leu-Phe (fMLP) (1 μM; Sigma) was used in the lower (apical) chamber of uninfected cells, while the negative controls were neither infected nor had fMLP in their apical chambers.
Statistical analysis.
To determine statistical significance at the 95% confidence limit in IL-8 assays, PMN transepithelial-migration studies, and invasion studies and in vivo cell migration, a Mann-Whitney analysis (Minitab) was used.
RESULTS
Clinical disease and fecal excretion.
Within 1 day of infection, the pigs inoculated with S. enterica serovar Typhimurium had developed diarrhea and anorexia. As the disease progressed, they became dehydrated and occasionally vomited, and they were then killed to prevent further suffering (48 h postinfection). When the challenge S. enterica serovar Typhimurium strains were inoculated alone, the fecal counts reflected the acute course of the infection, increasing sharply to 109 CFU/g of feces in the first 24 h postinfection (Fig. 1). The pigs infected with S. enterica serovar Infantis alone or preinfected with the S. enterica serovar Infantis strain and challenged with S. enterica serovar Typhimurium F98 remained perfectly healthy throughout the 14-day experimental period.
FIG. 1.
Colonization of the intestines of gnotobiotic pigs by Salmonella strains as assessed by counting viable bacteria in feces. The effect of precolonization with the rough S. enterica serovar Infantis 1326/28 on fecal counts of S. enterica serovar Typhimurium F98 Nalr is shown. Piglets inoculated with S. enterica serovar Typhimurium F98 Nalr only (Control; n = 12) are also shown. The error bars indicate standard deviations.
The fecal counts of the rough S. enterica serovar Infantis were very high in all the pigs and usually exceeded 1010 CFU/g of feces 1 to 2 days after infection (Fig. 1), although all the pigs remained healthy. This high level of S. enterica serovar Infantis continued for 2 weeks without any ill effects (results not shown). The fecal counts of S. enterica serovar Typhimurium F98 in the pigs challenged with the organism after preinfection with the S. enterica serovar Infantis strain increased at a lower rate than in the control pigs and did not reach 109 CFU/g until 5 days after challenge (Fig. 1). Thereafter, the counts continued to increase and were in excess of 1010 CFU/g for 4 of the remaining 6 days. During this period, the counts were higher than those of the S. enterica serovar Infantis strain. S. enterica serovar Typhimurium F98 was recovered from the intestines of pigs which had been protected by preinoculation with the S. enterica serovar Infantis strain and used for infection. The rate of onset and the severity of the subsequent infection was comparable to that in pigs which had been inoculated with the stock strain of S. enterica serovar Typhimurium F98 (data not shown). This indicated that S. enterica serovar Typhimurium had not become attenuated in the presence of larger numbers of the attenuated S. enterica serovar Infantis organisms.
Examination of tissue samples.
The intestines of all animals infected with S. enterica serovar Typhimurium only which became ill were inflamed, whereas the intestines of pigs which had been protected with the rough S. enterica serovar Infantis strain, which remained clinically healthy, also remained healthy in appearance.
The pigs infected with the challenge strain only demonstrated large numbers of S. enterica serovar Typhimurium organisms in the liver and spleen and, to a lesser extent, in the blood (Fig. 2). Extensive colonization of the alimentary tract had also occurred, with viable numbers in excess of 109 CFU per g of colon contents. In contrast, no quantifiable organisms of the challenge strains were isolated from the tissues of most of the pigs which had been infected with the protective S. enterica serovar Infantis strain. Reduced numbers were also isolated from the lymph node samples despite extensive colonization of the gut, with the densities of the challenge strain in the lower sections of the gut in protected and control pigs being very similar (Fig. 2).
FIG. 2.
Recovery of Salmonella from porcine organs. The effect of precolonization with rough S. enterica serovar Infantis 1326/28 on counts of S. enterica serovar Typhimurium F98 Nalr is shown. Control, piglets inoculated with 103 CFU of S. enterica serovar Typhimurium F98 Nalr only (n = 12). The error bars indicate standard deviations.
Pathological index and microscopic investigation of pathology.
Results obtained for a pathological index are shown in Table 1. Pigs which had been protected with S. enterica serovar Infantis showed no lesions in intestinal plicae or villi, even after challenge with S. enterica serovar Typhimurium F98. In comparison, pigs which had been challenged with S. enterica serovar Typhimurium F98 but had not been protected with S. enterica serovar Infantis had a marked flattening of plicae, with stunted villi which had exfoliated at the tips (Table 1 and Fig. 3C). Further investigation by electron microscopy also demonstrated destruction of villi in diseased S. enterica serovar Typhimurium-challenged pigs compared with no villus damage in S. enterica serovar Infantis-protected pigs (Fig.4).
TABLE 1.
Pathological indices of porcine intestinea
| Oral inoculum (pathological) | Plica circularis and villus morphology | Exfoliation of enterocytes atb:
|
Score | |
|---|---|---|---|---|
| Villus tips | Villus sides | |||
| F98 only | Stunted; villus flattened | + | − | 2 |
| 1326/28 only | Pointed; villus long | − | − | 0 |
| 1326/28 + F98 | Pointed; villus long | − | − | 0 |
| Uninfected control | Pointed; villus long | − | − | 0 |
Three days postinoculation with S. enterica serovar Typhimurium F98 Nalr (103 CFU) (F98), rough S. enterica serovar Infantis 1326/28 Spcr (106 CFU) (1326/28), or rough S. enterica serovar Infantis 1326/28 Spcr followed by S. enterica serovar Typhimurium F98 NaIr 24 h later (1326/28 + F98).
+, present; −, absent.
FIG.3.
Hematoxylin and eosin staining of porcine terminal ileum. (A) High-power (autofluorescence) image of S. enterica serovar Infantis-protected ileum 24 h after S. enterica serovar Typhimurium challenge showing PMN infiltration into villus tips (arrows). (B) High-power (autofluorescence) image of S. enterica serovar Infantis-protected ileum 24 h after S. enterica serovar Typhimurium challenge showing substantial PMN migration into submucosa (arrows). (C) Low-power (light microscope) image of S. enterica serovar Infantis-protected ileum following S. enterica serovar Typhimurium challenge showing long slender villi (arrow) and fully formed plica (arrowhead). (D) High-power (autofluorescence) image of unprotected ileum 24 h after S. enterica serovar Typhimurium challenge showing PMN migration into villus tips (arrows). (E) High-power (autofluorescence) image of unprotected ileum 24 h after S. enterica serovar Typhimurium challenge showing substantial PMN migration into the submucosa (arrows). (F) Low-power (light micrscope) image of unprotected ileum 24 h after S. enterica serovar Typhimurium challenge showing stunted villi (solid arrows), exfoliation at villus tips (open arrow), and flattened plica (arrowhead). (G) High-power (autofluorescence) image of uninfected control ileum with innate cell infiltration absent. (H) Low-power image of uninfected control intestine showing long slender villi (arrow) and fully formed plica (arrowhead). Bars, 90 μm (C, F, and H) and 9 μm (all others).
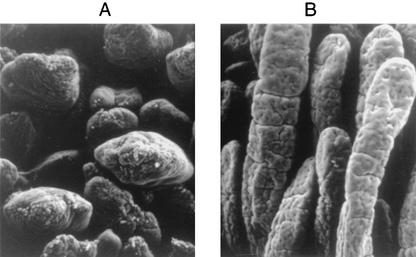
FIG. 4.
Scanning electron micrograph of porcine ileum 3 days postinoculation with S. enterica serovar Typhimurium F98 Nalr (103 CFU) (A) or rough S. enterica serovar Infantis followed by S. enterica serovar Typhimurium F98 Nalr (B) showing normal villus structure (B) and shortening and rounding of villi (A).
Induction of innate cell migration in vivo by S. enterica serovar Typhimurium F98 or S. enterica serovar Infantis.
Inoculation of gnotobiotic pigs with either of the experimental strains investigated during this study induced a significant migration of macrophages into the intestinal submucosa (Fig. 3D and F and Fig. 5A). Although significantly (P < 0.05) fewer macrophages were recorded in the villi of infected and/or challenged pigs compared with PMN numbers, macrophage infiltration might have increased further if the duration of the experiment had been extended. However, significant PMN migration into both the submucosa and villi (lamina propria and epithelium) was recorded in these infected and/or protected pigs, but in contrast to macrophage migration, PMN numbers were significantly (P < 0.05) higher in the villi (Fig. 3D and F and 5B). In other experiments, PMN migration was also recorded in the villi after 12 h, and very significant increases were observed in older pigs in response to oral inoculation with S. enterica serovar Infantis (data not shown).
FIG. 5.
Innate cell infiltration into porcine intestinal submucosa and villi 3 days postinfection with S. enterica serovar Typhimurium F98 Nalr (F98), rough S. enterica serovar Infantis (1326/28), or S. enterica serovar Infantis followed by S. enterica serovar Typhimurium 24 h later (1326/28+F98). Control, uninoculated porcine intestine. (A) Macrophage infiltration; (B) changes in percentage of PMNs; (C) changes in percentage of eosinophils. The results represent the means of five pigs (n = 300 fields) plus standard deviations. *, significant difference (P < 0.05) from uninfected-control value.
Eosinophil numbers were also increased in the submucosae of infected animals compared with those in uninfected controls (Fig. 5C). However, populations of eosinophils in villi were unchanged or higher in the controls and there was not a significant increase in induction with age (data not shown).
In vitro CE assay.
CE assays showed that preincubation of INT 407 monolayers with S. enterica serovar Infantis 1326/28 (MOI = 10) significantly (P < 0.05) reduced S. enterica serovar Typhimurium F98 invasion. However, further reduction did not occur when the monolayers were preincubated with S. enterica serovar Infantis at a higher MOI (100 or 1,000) (Fig. 6).
FIG. 6.
Effect of increasing MOIs of rough S. enterica serovar Infantis (⧫) on invasion of INT 407 monolayers by S. enterica serovar Typhimurium F98 Nalr (▪). Each point represents the mean (plus standard deviation) of three experiments each replicated three times.
Invasion of human INT 407 cells and IL-8 secretion.
There was not a significant (P> 0.05) difference among the abilities of S. enterica serovar Typhimurium F98 wild type, S. enterica serovar Typhimurium F98 sipB, or rough S. enterica serovar Infantis to adhere to INT 407 cells, as assessed by confocal laser scanning microscope. Between 16 and 18% of cells had adherent bacteria (Fig. 7A). However, both the parental S. enterica serovar Typhimurium F98 and the rough S. enterica serovar Infantis invaded monolayers in significantly greater numbers (P < 0.05) than did the S. enterica serovar Typhimurium F98 sipB mutant. The most significant amount of invasion was measured for the S. enterica serovar Typhimurium F98 wild type at almost 10% of cells infected compared with 4% infected by S. enterica serovar Infantis; this difference was significant (P < 0.05).
FIG. 7.
Adherence and invasion and IL-8 secretion from INT 407 monolayers incubated with Salmonella (MOI = 10). (A) Adherence and invasion by S. enterica serovar Typhimurium F98 wild type (F98), S. enterica serovar Typhimurium F98 sipB mutant (sip B), and S. enterica serovar Infantis (1326/28). The results represent the means (plus standard deviations) of five fields of view taken from five samples and replicated 10 times (n = 250). *, significant difference (P < 0.05) from the largest value (F98). (B) Concentrations of IL-8 secreted from INT 407 basolateral membranes following incubation with S. enterica serovar Typhimurium F98 Nalr (F98), rough S. enterica serovar Infantis (1326/28), or S. enterica serovar Typhimurium F98 sipB mutant (sip B). PMA, 1 μg of PMA/ml; uninfected control, INT 407 monolayers incubated in RPMI 1640 only; *, significant difference (P < 0.05) from largest value (PMA); +, significant decrease (P < 0.05) from F98 value.
On invasion, all of the test strains induced the production by INT 407 basolateral membranes of IL-8 concentrations higher than basal levels (cells only) (Fig. 7B). However, only S. enterica serovar Typhimurium F98 and the S. enterica serovar Infantis mutant stimulated significant (P = 0.05) IL-8 production.
NF-κB release in INT 407 monolayers infected with S. enterica serovar Typhimurium F98 and S. enterica serovar Infantis.
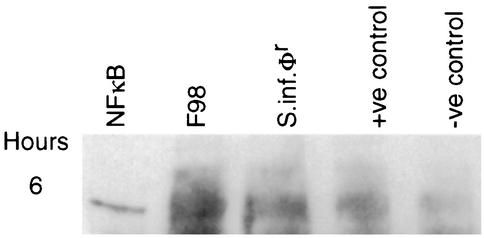
When either S. enterica serovar Typhimurium F98 or the S. enterica serovar Infantis mutant invaded INT 407 monolayers, NF-κB was detected by Western blotting of whole-cell lysates 180 min after invasion (Fig. 8). NF-κB was also detected in monolayers which had been incubated with PMA (1 μg/ml) as a positive inducer of NF-κB, but it was only very faintly detected in uninfected control monolayers which were also not exposed to PMA (Fig. 8).
FIG. 8.
Western blotting analysis of INT 407 whole-cell lysates probed with anti-NF-κB p50 antibody following incubation of monolayers with S. enterica serovar Typhimurium F98 Nalr (F98) or rough S. enterica serovar Infantis (S.inf.φr) at 37°C for 6 h (MOI = 10). +ve control, 1 μg of PMA/ml; −ve control, INT 407 cells incubated in RPMI 1640 medium without Salmonella or PMA; NFκB, p50 protein.
PMN transepithelial-migration assays.
Prior to transepithelial-migration studies, electrical resistance was measured at 230 Ω cm−2 across confluent INT 407 monolayers (data not shown). Two hours after the addition of PMNs to the basolateral bath, a myeloperoxidase assay was used to detect both filter-associated and fully migrated PMNs (Fig. 9). As a positive control, fMLP was used to attract PMNs, and although the migration of PMNs through the INT 407 zona occludens was not significantly different (P > 0.05) from that of S. enterica serovar Typhimurium F98 or S. enterica serovar Infantis, total migration (filter associated plus transmigrated) due to fMLP was significantly greater (P < 0.05). Both S. enterica serovar Typhimurium and S. enterica serovar Infantis induced PMN migration significantly (P < 0.05) greater than that through unstimulated monolayers (Fig. 9).
FIG. 9.
Porcine PMN transepithelial migration across INT 407 monolayers in response to Salmonella invasion (MOI = 10). F98, S. enterica serovar Typhimurium F98 Nalr; 1326/28, rough S. enterica serovar Infantis; fMLP, 1 μM fMLP; Uninfected control, INT407 monolayers incubated in RPMI 1640 medium only; *, significant difference (P < 0.05) compared to highest value (migration due to fMLP). The error bars indicate standard deviations.
DISCUSSION
These results have shown that, under experimental conditions, enteritis and systemic infection produced in highly sensitive gnotobiotic pigs by virulent S. enterica serovar Typhimurium strains can be prevented by precolonization of the gut, at a high density, with a nonvirulent S. enterica serovar Infantis strain without the elimination of the challenge strain from the gut. As far as we are aware, this is the first report of this phenomenon. Similar results were obtained using challenge with a second S. enterica serovar Typhimurium strain and with a strain of S. enterica serovar Choleraesuis (results not shown).
Duval-Iflah and his colleagues (13-15) have described the inhibition of intestinal colonization of gnotobiotic pigs with pathogenic Escherichia coli by precolonization with an avirulent nonisogenic E. coli strain, and similar studies have been carried out on Salmonella strains in chickens and pigs (4, 7, 26). However, in those cases, the inhibition effect appeared to involve the prevention of establishment of the second strain in the gut, resulting in poor colonization. Their results were similar to those regarding the interference between Salmonella strains in the gut of newly hatched chickens (4, 7). In the latter case, the inhibition of colonization of the second strain was thought to be related to the extensive colonization observed following oral inoculation of the alimentary tract, which at that age was devoid of a complex microflora. The present results more closely resemble those of Dlabac et al. (12), in which pigs were infected orally with a rough strain of S. enterica serovar Minnesota followed 1 week later by a virulent S. enterica serovar Typhimurium strain. In that case, however, the response of the pig appeared to be the early stages of an adaptive immune response which was protective, although it was not possible to determine, in pigs not treated orally with antibiotic, how far multiplication and colonization of the challenge strain were suppressed (12). Similarly neutrophil infiltration was not studied. In the present experiments, the S. enterica serovar Infantis strain colonized the gut of the gnotobiotic swine extremely well. This was perhaps not surprising, since in these animals there was neither microflora nor maternal antibody. The second (challenge) strains colonized extensively but less rapidly than the first strain. The reasons for this are unclear, since cessation of growth at early stationary phase in broth cultures containing very low concentrations of fermentable carbohydrate is a complex phenomenon, only now being studied in detail (18, 26, 32, 51). That complete inhibition of colonization was not obtained was surprising, since in newly hatched chickens, the S. enterica serovar Infantis strain was found to be highly inhibitory for S. enterica serovar Typhimurium F98. However, no inhibition between the strains occurred in vitro (26) in milk, suggesting that the nutritional content is very different from that of chicken food. The protection against S. enterica serovar Typhimurium F98 obtained by oral inoculation with S. enterica serovar Infantis became apparent 3 days after the inoculation of the latter strain. Large numbers of the challenge strain remained in the intestine throughout the experiment, which suggested that the protection had an immunological basis rather than a microbiological exclusion effect (26). Further in vitro analyses using INT 407 cell monolayers supported this supposition.
Extensive systemic invasion had occurred in the pigs infected with S. enterica serovar Typhimurium only. In contrast, very few challenge organisms were found in the systemic tissues of the protected pigs, indicating that either the organisms had been prevented from invading or once small numbers had invaded they had been prevented from multiplying. Lower numbers of challenge organisms were also found in the lymph nodes examined, suggesting that some degree of protection may have occurred at the level of the intestinal lymph node or mucosa. Previous authors have suggested that a considerable degree of invasion in pigs occurs at the palatine tonsil (42). However, in that work, the authors used an exceedingly large inoculum size (1010 CFU), while we used much smaller ones (103 CFU). In addition, in pigs protected against S. enterica serovar Typhimurium F98, the counts of this strain at the proximal regions of the gut were lower than counts obtained in unprotected pigs infected with S. enterica serovar Typhimurium, indicating that invasion was in fact occurring at more distal regions of the gut. The S. enterica serovar Typhimurium organisms that had been reisolated from protected pigs were not attenuated as a result of in vivo culture in the presence of S. enterica serovar Infantis.
The rapidity of the onset of immunity suggested that it was independent of an adaptive immune response. There have been numerous examples where a rapid onset of nonspecific immunity to bacterial infection may be induced by parenteral administration of killed or live bacteria or lipopolysaccharide (50). Parenteral immunization of poultry with a live attenuated S. enterica serovar Gallinarum vaccine induced a significant degree of protection against the virulent parent strain within only a few hours (41). This is associated with stimulation of the monocyte/macrophage series and is apparently at its strongest ∼1 day after administration of the protective antigen or strain (16). Similarly, studies have also shown that macrophages induce the initial growth suppression of Salmonella associated with infections in mice (30). The present study has shown that, although very quick activation of macrophages was apparent from influx into the submucosa, this did not extend to the villi, and since no pathology or clinical symptoms were associated with protected and challenged pigs, this would suggest that rapid protection occurred within the villi. In contrast, PMN induction was recorded in both the submucosae and the villi, with the latter being most significant, suggesting that the innate protection against S. enterica serovar Typhimurium is stimulated by S. enterica serovar Infantis and is probably PMN driven. Further in vitro analysis showed that S. enterica serovar Infantis was able to stimulate PMN transepithelial migrations via IL-8 induction, the level of which was in fact slightly higher than reported previously (31). This may be explained by the lower resistance recorded in INT 407 monolayers compared with that in the T84 monolayers used by these authors. The importance of IL-8 as a chemoattractant for PMNs has now been comprehensively studied (6, 9). The upregulation of IL-8 secretion by infected cells is due to the degradation of IκB, which releases the IL-8 transcription factor NF-κB (6).
Significantly elevated IL-8 and NF-κB responses were detected in human INT 407 cells infected with both S. enterica serovar Typhimurium F98 and the rough S. enterica serovar Infantis strains. This indicates that NF-κB-transcribed IL-8 upregulation was the stimulus for PMN transepithelial migration in vitro and presumably also PMN migration in vivo in porcine intestine. Significant intestinal emigration of PMNs in response to E. coli K88 has been shown to occur in neonatal pigs which have been suckled by immune or resistant dams (40), possibly indicating that the interaction between opsonic colostrum-derived antibodies and K88 antigen may stimulate the PMN influx. Studies have also suggested that sows susceptible to E. coli mastitis are neutropenic and that the PMNs, which are induced to migrate, have reduced function (25). In humans, the importance of PMNs in clearing gram-negative and gram-positive bacterial infections and inducing clinical immunopathology is well studied (9). It is perhaps significant that although PMN induction was apparent both in vitro and in vivo, S. enterica serovar Infantis-protected pigs survived for up to 2 weeks after F98 challenge without the inflammation usually associated with intestinal PMN infiltration (28). This phenomenon needs further investigation, since the pathological index, clinical symptoms, and both light and electron microscopy revealed no pathological changes in the intestines of pigs protected with S. enterica serovar Infantis and subsequently challenged with S. enterica serovar Typhimurium. PMN migration into the intestine per se did not cause the clinical signs and pathological lesions observed when unprotected pigs were inoculated with S. enterica serovar Typhimurium F98. A similar effect has also been observed in rabbits infected with S. enterica serovar Typhimurium (49). Factors which may cause the different pathologies observed in the intestines of diseased and protected pigs may be due to differential ileal cytokine expression. Gnotobiotic piglets inoculated orally with virulent S. enterica serovar Typhimurium LT2 have elevated levels of ileal gamma interferon, tumor necrosis factor alpha, and IL-1β compared to those in gnotobiotic piglets inoculated with an avirulent rough mutant of S. enterica serovar Typhimurium SF1591 (44). These Th1 cytokines have been shown to cause severe intestinal inflammation and pathology in murine intestinal epithelia (34). Another possible cause of the destructive ileal pathology associated with S. enterica serovar Typhimurium infection in gnotobiotic piglets may be the production of cellular metalloproteinases. These have previously been shown to destroy fetal ileal mesenchymal tissue in response to tumor necrosis factor alpha (37). The factors involved in S. enterica serovar Typhimurium pathology or the inhibition of pathological lesions when protected piglets are infected with S. enterica serovar Typhimurium will be investigated in future experiments. The migration of eosinophils into the submucosa of Salmonella-infected pigs was also measured in this study. Uninfected porcine intestine contains large numbers of eosinophils, which we clearly observed in the villi. This indicates that eosinophils are probably not directly involved in the rapid bacterial clearing we have observed but may play some role in later immunological events.
In summary, these results demonstrate that under circumstances in which high intestinal counts of bacteria are attainable, oral inoculation using live attenuated Salmonella strains may provide protection against gastroenteritis and systemic salmonellosis within a matter of a few hours. Previous work looking at the colonization interference between live Salmonella vaccines and wild-type strains suggested that for early protection to occur it was necessary to use a vaccine strain, which produced the genus-specific colonization inhibition described previously (4, 32). The results that we now report indicate that, at least to protect against systemic and diarrheal disease, this may not be necessary. Oral inoculation with the S. enterica serovar Infantis 1326/28 rough mutant may, therefore, prove to be a useful preventative immunotherapy for patients with recurrent bacterial infections. In this regard, it may have an immunostimulating effect comparable to that of oral RU41740 (Biostim; Aventis Pharmaceuticals, Paris, France), which increases PMN migration, phagocytosis, and respiratory burst (8, 33, 38) and is a currently available antibacterial human preventative therapy.
Acknowledgments
We thank Elaine Bennett for assistance with cell culture and Helen Mathews for histological preparations.
This work was funded by a European Union grant (Fair 98-4006) and the Department of Food, the Environment and Rural Affairs, United Kingdom.
Editor: A. D. O'Brien
REFERENCES
- 1.Barrow, P. A., J. O. Hassan, and A. Berchieri. 1990. Reduction in faecal excretion of Salmonella typhimurium F98 in chickens vaccinated with live and killed S. typhimurium organisms. Epidemiol. Infect. 104:413-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrow, P. A., M. B. Huggins, and M. A. Lovell. 1994. Host specificity of Salmonella infection in chickens and mice is expressed in vivo primarily at the level of the reticuloendothelial system. Infect. Immun. 62:4602-4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrow, P. A., J. M. Simpson, and M. A. Lovell. 1988. Intestinal colonisation in the chicken by food-poisoning Salmonella serotypes; microbiological characteristics associated with faecal excretion. Avian Pathol. 17:571-588. [DOI] [PubMed] [Google Scholar]
- 4.Barrow, P. A., J. F. Tucker, and J. M. Simpson. 1987. Inhibition of colonisation of the chicken alimentary tract with Salmonella typhimurium by Gram-negative facultatively anaerobic bacteria. Epidemiol. Infect. 98:311-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrow, P. A, and T. S. Wallis. 2000. Vaccination against Salmonella infections in food animals: rationale, theoretical basis and practical approach, p. 323-339. In C. Wray and A. Wray (ed.), Salmonella in domestic animals. CABI Publishing, Wallingford, Oxfordshire, United Kingdom.
- 6.Ben-Baruch, A., D. F. Michiel, and J. J. Oppenheim. 1995. Signals and receptors involved in recruitment of inflammatory cells. J. Biol. Chem. 270:11703-11706. [DOI] [PubMed] [Google Scholar]
- 7.Berchieri, A., and P. A. Barrow. 1990. Further studies on the inhibition of colonisation of the chicken alimentary tract with Salmonella typhimurium by pre-colonisation with an avirulent mutant. Epidemiol. Infect. 104:427-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Capsoni, F., F. Minonzio, E. Venegoni, A. M. Ongari, P. L. Meroni, G. Guidi, and C. Zanussi. 1988. In vitro and ex vivo effect of RU41740 on human polymorphonuclear leukocyte function. Int. J. Immunopharmacol. 10:121-133. [DOI] [PubMed] [Google Scholar]
- 9.Cassatella, M. A. 1999. Neutrophil derived proteins: selling cytokines by the pound. Adv. Immunol. 73:369-509. [DOI] [PubMed] [Google Scholar]
- 10.Clark, R. C., and C. L. Gyles. 1987. Virulence of wild and mutant strains of Salmonella typhimurium in ligated intestinal segments of calves, pigs and rabbits. Am. J. Vet. Res. 48:504-510. [PubMed] [Google Scholar]
- 11.Cooper, G. L. 1994. Salmonellosis-infections in man and the chicken: pathogenesis and the development of live vaccines—a review. Vet. Bull. 64:123-143. [Google Scholar]
- 12.Dlabac, V., I. Trebichavsky, Z. Rehakova, B. Hofman, I. Splinchal, and B. Cukrowska. 1997. Pathogenicity and protective effect of rough mutants of Salmonella species in germ-free pigs. Infect. Immun. 65:5238-5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duval-Iflah, Y., J. P. Chappuis, R. Ducluzeau, et al. 1983. Intraspecific interactions between Escherichia coli strains in human newborns and in gnotobiotic piglets. Prog. Food Nutr. Sci. 7:107-116. [PubMed] [Google Scholar]
- 14.Duval-Iflah, Y., M. F. Ovriet, M. C. Moreau, et al. 1982. Implantation precoce d'une souche de Escherichia coli dans l'intestin de nouveau-nes humains: effet de barriere vis-a-vis de souches de E. coli antibioresistantes. Ann. Microbiol. 133A:393-408. [PubMed]
- 15.Duval-Iflah, Y., P. Raibaud, and M. Rousseau. 1981. Antagonisms among isogenic strains of Escherichia coli in the digestive tracts of gnotobiotic mice. Infect. Immun. 34:957-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Field, T. E., J. G. Howard, and J. L. Whitby. 1955. Studies on the rapid production of a non-specific type of immunity to Salmonella typhi infection in mice. J. R. Army Med. Corps 101:324-344. [PubMed] [Google Scholar]
- 17.Galan, J. E. 1996. Molecular genetic basis of Salmonella entry into cells. Mol. Microbiol. 20:263-271. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Lara, J., L. H. Shang, and L. I. Rothfield. 1996. An extracellular factor regulates expression of sdiA, a transcriptional activator of cell division genes in Escherichia coli. Infect. Immun. 178:2742-2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glynn, M. K., C. Bopp, W. Dewitt, P. Dabney, M. Mokhtar, and F. J. Angulo. 1992. Emergence of multidrug resistant Salmonella enterica serotype Typhimurium DT104 infections in the United states. N. Engl. J. Med. 338:1331-1338. [DOI] [PubMed] [Google Scholar]
- 20.Gomez, G. C. 1997. The colostrum-deprived, artificially reared, neonatal pig as a model animal for studying rotavirus gastroenteritis. Front. Biosci. 2:471-481. [DOI] [PubMed] [Google Scholar]
- 21.Hassan, J. O., and R. Curtiss. 1994. Development and evaluation of an experimental vaccination program using a live avirulent Salmonella typhimurium strain to protect immunized chickens against challenge with homologous and heterologous Salmonella serotypes. Infect. Immun. 62:5519-5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jepson, M. A., T. F. Lang, K. A. Reed, and N. L. Simmons. 1996. Evidence for a rapid, direct effect on epithelial integrity and transepithelial transport in response to Salmonella invasion. Eur. J. Physiol. 432:225-233. [DOI] [PubMed] [Google Scholar]
- 23.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 24.Laval, A., H. Morvan, G. Desprez, and B. Corbion. 1989. Salmonellosis in swine, p. 164-175. In Proceedings of the International Symposium on Salmonella and Salmonellosis, Ploufragan, St. Brievc, Brittany, France. ISPAIA-Zoopole Development, St. Brievc, France.
- 25.Lofstedt, J., J. A. Roth, R. F. Ross, and W. C. Wagner. 1983. Depression of polymorphonuclear leukocyte function associated with experimentally induced Escherichia coli mastitis in sows. Am. J. Vet. Res. 44:1224-1228. [PubMed] [Google Scholar]
- 26.Lovell, M. A, and P. A. Barrow. 1999. Intestinal colonisation of gnotobiotic pigs by Salmonella organisms; interaction between isogenic and unrelated strains. J. Med. Microbiol. 48:1-10. [DOI] [PubMed] [Google Scholar]
- 27.Lumsden, S., B. N. Wilkie, and R. C. Clarke. 1991. Resistance to fecal shedding in pigs and chickens vaccinated with an aromatic-dependent mutant of Salmonella typhimurium. Am. J. Vet. Res. 52:1784-1787. [PubMed] [Google Scholar]
- 28.Madara, J. L. 1994. Migration of neutrophils through epithelial monolayers. Trends Cell. Biol. 4:4-7. [DOI] [PubMed] [Google Scholar]
- 29.Madara, J. L., C. A. Parkos, S. P. Colgan, R. J. MacLeod, S. Nash, J. Mathews, C. Delp, and W. P. Lencer. 1992. Cl-secretion in a model intestinal epithelium induced by a neutrophil-derived secretagogue. J. Clin. Investig. 89:1938-1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maskell, D. J., C. E. Hormaeche, K. A. Harrington, H. S. Joysey, and F. Y. Liew. 1987. The initial suppression of bacterial growth in a salmonella infection is mediated by a localized rather than a systemic response. Microb. Pathog. 2:295-305. [DOI] [PubMed]
- 31.McCormick, B., S. Miller, D. Carnes, and J. L. Madara. 1995. Transepithelial signalling to neutrophils by Salmonella: a novel virulence mechanism for gastroenteritis. Infect. Immun. 63:2302-2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Methner, U., P. A. Barrow, G. Martin, and H. Meyer. 1997. Comparative study of the protective effect against Salmonella colonization in newly hatched SPF chickens of using live, attenuated Salmonella vaccine strains, wild-type Salmonella strains or a competitive exclusion product. Int. J. Food Prot. 35:223-230. [DOI] [PubMed] [Google Scholar]
- 33.Minonzio, F., A. M. Ongar, R. Palmieri, D. Bochicchio, G. Guidi, and F. Capsoni. 1991. Immunostimulation of neutrophil phagocytic function by (RU41740) (Biostim) in elderly subjects. Allergol. Immunopathol. 19:58-62. [PubMed] [Google Scholar]
- 34.Ohta, N., T. Hiroi, M.-N. Kweon, N. Kinoshita, M. Ho Jang, T. Mashimo, J.-I. Miyazaki, and H. Kiyono. 2002. IL-15 dependent activation-induced cell-death resistant th1 type CD8αβ+ NK1.1+ T cells for the development of small intestinal inflammation. J. Immunol. 169:460-468. [DOI] [PubMed] [Google Scholar]
- 35.Parkos, C. A., C. Delp, M. A. Arnaout, and J. L. Madara. 1991. Neutrophil migration across a cultured intestinal epithelium: dependence on a CD11b/CD-18-mediated event and enhanced efficiency in the physiologic direction. J. Clin. Investig. 88:1605-1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parkos, C. A., S. P. Colgan, C. Delp, and J. L. Madara. 1992. Neutrophil migration across a cultured epithelial monolayer elicits a biphasic resistance response representing sequential effects on transcellular and paracellular pathways. J. Cell Biol. 117:757-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pender, S. L., J. M. Fell, S. M. C. Hamour, A. Ashkenazi, and T. M. MacDonald. 1998. A p55 TNF receptor immunoadhesin prevents T cell-mediated intestinal injury by inhibiting matrix metalloproteinase production. J. Immunol. 160:4098-4101. [PubMed] [Google Scholar]
- 38.Roch-Arveiller, M., A. el Abbouyi, J. L. Paul, P. Smets, D. Raichvrag, and J. P. Giroud. 1987. Effects exerted by RU 41740 on oxidative metabolism and migration of rat polymorphonuclear leukocytes collected after induction of one acute nonspecific inflammatory reaction. Int. J. Immunopharmacol. 9:417-424. [DOI] [PubMed] [Google Scholar]
- 39.Roof, M. B., and D. D. Doitchinoff. 1995. Safety, efficacy and duration of immunity induced in swine by use of an avirulent live Salmonella cholerae-suis-containing vaccine. Am. J. Vet. Res. 56:39-44. [PubMed] [Google Scholar]
- 40.Sellwood, R., G. Hall, and H. Anger. 1986. Emigration of polymorphonuclear leucocytes into the intestinal lumen of the neonatal piglet in response to challenge with K88-positive Escherichia coli. Res. Vet. Sci. 40:128-135. [PubMed] [Google Scholar]
- 41.Smith, H. W. 1956. The use of live vaccines in experimental Salmonella gallinarum infection in chickens with observations on their interference effect. J. Hyg. 54:419-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith, H. W., and S. Halls. 1968. The simultaneous administration of Salmonella dublin, S. typhimurium and S. cholerae-suis to calves and other animals. J. Med Microbiol. 1:203-209. [DOI] [PubMed] [Google Scholar]
- 43.Smith, H. W., and J. F. Tucker. 1980. The virulence of Salmonella strains for chickens; their excretion by infected chickens. J. Hyg. 84:479-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Splichal, I., I. Trebichavsky, Y. Munetab, and Y. Mori. 2002. Early cytokine response of gnotobiotic piglets to Salmonella enterica serotype Typhimurium. Vet. Res. 33:291-297. [DOI] [PubMed] [Google Scholar]
- 45.Tavernor, W. D., P. C. Trexler, L. C. Vaughan, J. E. Cox, and D. G. C. Jones. 1971. The production of gnotobiotic piglets and calves by hysterectomy under general anaesthesia. Vet. Rec. 88:10-14. [DOI] [PubMed] [Google Scholar]
- 46.Towbin, H., T. Staehlin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:43500-43504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trexler, P. C. 1971. Microbiological isolation of large animals. Vet. Rec. 88:15-20. [DOI] [PubMed] [Google Scholar]
- 48.Tzipori, S., J. Montanaro, R. M. Robins-Browne, P. Vial, R. Gibson, and M. M. Levine. 1992. Studies with enteroaggregtive Escherichia coli in the gnotobiotic piglet gastroenteritis model. Infect. Immun. 60:5302-5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wallis, T. S., R. J. H. Hawker, D. C. A. Candy, G.-M. Qi, G. J. Clarke, K. J. Worton, M. P. Osborne, and J. Stephen. 1989. Quantification of the leucocyte influx into rabbit ileal loops induced by strains of Salmonella typhimurium of different virulence. J. Med. Microbiol. 30:149-156. [DOI] [PubMed] [Google Scholar]
- 50.Wilson, G. S., and A. A. Miles. 1964. Topley and Wilson's principles of bacteriology and immunity, 5th ed. Edward Arnold, London, United Kingdom.
- 51.Zambrano, M. M., D. A. Siegele, M. Almiron, A. Tormo, and R. Kolter. 1993. Microbial competition: Escherichia coli mutants that take over stationary-phase cultures. Science 259:1757-1759. [DOI] [PubMed] [Google Scholar]