Abstract
MPB70 is a secreted protein of Mycobacterium bovis and Mycobacterium tuberculosis which stimulates both cellular and humoral immune responses during infection with bovine and human tubercle bacilli. In addition, vaccination with MPB70 has been shown to induce Th1 cell responses and protection in animal models of tuberculosis. The present study was carried out to map the dominant human Th1 cell epitopes of MPB70 in relation to major histocompatibility complex (MHC) class II restriction in healthy subjects showing strong T-cell responses to complex mycobacterial antigens. Peripheral blood mononuclear cells (PBMC) from HLA-DR-typed donors were tested with complex mycobacterial antigens (whole-cell M. tuberculosis and M. tuberculosis culture filtrates), with MPB70 purified from the culture filtrate of M. bovis BCG Tokyo, and with 13 synthetic peptides (25-mers overlapping by 10 residues) covering the sequence of MPB70. The donors that responded to the complex antigens and MPB70 also responded to the cocktail of synthetic MPB70 peptides. Testing of PBMC with individual peptides showed that peptides p5 (amino acids [aa] 61 to 85), p6 (aa 76 to 100), p8 (aa 106 to 130), p12 (aa 166 to 190), and p13 (aa 181 to 193) were most frequently recognized in proliferation and gamma interferon (IFN-γ) assays. Testing of antigen-specific CD4+ T-cell lines with the individual peptides of MPB70 confirmed that peptides p8, p12, and p13 contain immunodominant Th1 cell epitopes of MPB70. MHC restriction analysis with HLA-typed donors showed that MPB70 and its immunodominant peptides were presented to T cells promiscuously. The T-cell lines responding to MPB70 and peptides p8, p12, and p13 in IFN-γ assays mediated antigen-peptide-specific cytotoxic activity against monocytes/macrophages pulsed with the whole-protein antigen or the peptides. In conclusion, the promiscuous recognition of MPB70 and its immunodominant peptide defined epitopes (aa 106 to 130 and 166 to 193) by IFN-γ-producing Th1 cells supports possible application of this secreted antigen to subunit vaccine design.
Tuberculosis (TB) is a chronic infectious disease of worldwide importance. In 1993 the World Health Organization declared TB a global emergency, and according to the latest survey conducted by this organization TB still exists at an alarming level, with about one-third of the world population infected with Mycobacterium tuberculosis, 8 million people developing the disease, and 2 million to 3 million people dying of TB each year (6). Control and eventual eradication of TB require protective vaccines. However, the protection induced by the currently available Mycobacterium bovis BCG vaccine against pulmonary TB varies tremendously in different parts of the world, and the efficacy ranges from 0 to 80% (7). In addition, BCG vaccination induces a positive response to the purified protein derivative (PPD) of M. tuberculosis, thereby compromising the diagnostic value of PPD. Moreover, use of the BCG vaccine, which consists of attenuated live organisms, is contraindicated in AIDS patients, who are at high risk of developing TB. The present situation calls for identification and characterization of M. tuberculosis antigens and peptides that might be used to develop universally efficacious and safer vaccines against TB.
Although infection with M. tuberculosis induces both humoral immunity and cell-mediated immunity, only cell-mediated immunity responses mediated primarily by gamma interferon (IFN-γ)-producing Th1 cells are relevant to protection (4, 16). Thus, identification of antigens and peptides that induce Th1 cell responses could be useful for designing new vaccines to protect against TB. Secreted proteins of M. tuberculosis have been the focus of extensive research on the development of subunit vaccines because these proteins are considered to be important targets of recognition by the immune system (1, 31).
MPB70 is a major secreted protein of M. bovis, and small quantities of this protein are expressed by M. tuberculosis. M. bovis BCG strains can be divided into strains that produce high levels of MPB70 and strains that produce low levels of MPB70 (9, 18, 34, 58). There are no differences between M. bovis and M. tuberculosis (H37Rv and CDC1551) in terms of the sequences of the expressed proteins encoded by the mpb70 and mpt70 genes, respectively (17; http://www.ncbi.nlm.nih.gov/BLAST). MPB70 is an M. tuberculosis complex-specific nonglycosylated protein with a signal peptide consisting of 30 amino acids (aa) and a mature protein sequence consisting of 163 aa with a deduced molecular mass of 16.3 kDa (53). The mature MPB70 protein stimulates both cellular and humoral immune responses during infection with bovine and human tubercle bacilli (4, 10, 16, 28, 47, 49, 36).
The present work was carried out to identify dominant and natural Th1 cell epitopes of MPB70 which can be recognized by humans in the context of multiple HLA molecules. Peripheral blood mononuclear cells (PBMC) from healthy donors showing strong responses to complex M. tuberculosis antigens and MPB70 in proliferation and IFN-γ assays were tested for reactivity against synthetic peptides (25-mers with 10-residue overlaps) covering the entire MPB70 sequence, including the signal peptide. In addition, antigen-specific T-cell lines were established from HLA-DR-typed antigen-responding donors and were screened for proliferation and IFN-γ secretion in response to the peptides of MPB70. Finally, selected T-cell lines were also tested for HLA restriction and cytotoxic activity to confirm the promiscuous presentation and Th1 cell reactivity of MPB70 and its immunodominant peptides.
MATERIALS AND METHODS
Study population.
BCG-vaccinated healthy subjects were randomly selected from the blood donors at the Central Blood Bank, Kuwait. The donors were all PPD skin test positive (as determined with tuberculin PPD RT 23SSI from Statens Serum Institute, Copenhagen, Denmark) and were both Kuwaiti and non-Kuwaiti citizens.
Complex and purified mycobacterial antigens.
The complex antigens used in this study were killed whole cells of M. tuberculosis H37Rv (20) and M. tuberculosis culture filtrate (CF) (provided by P. J. Brennan, Colorado State University, Fort Collins) and were obtained through the repository of TB research materials at the National Institute of Allergy and Infectious Diseases (contract AI-25147). The secreted antigen, MPB70 from BCG Tokyo, was purified as previously described (34, 35).
Synthetic peptides.
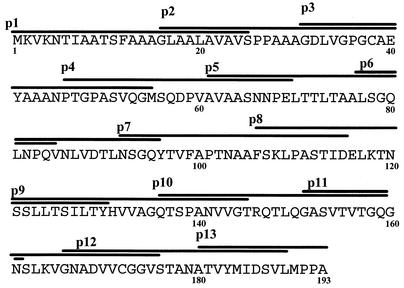
Thirteen synthetic peptides (25-mers overlapping neighboring peptides by 10 aa) spanning the sequence of MPB70 (Fig. 1) were purchased from Genemed Synthesis Inc., San Francisco, Calif. Pools of synthetic peptides covering the sequences of ESAT-6 and CFP-10 were synthesized by using fluorenylmethoxy carbonyl chemistry, and sequence fidelity and purity (>90%) were confirmed by mass spectrometry and analytical high-pressure liquid chromatography, respectively (32). The stock concentrations (5 mg/ml) of the peptides were prepared in normal saline (0.9%) by vigorous pipetting, and the working concentrations were prepared by further dilution in complete tissue culture medium (RPMI 1640 containing 10% human AB serum, 100 U of penicillin per ml, 100 μg of streptomycin per ml, 40 μg of gentamicin per ml, and 2.5 μg of amphotericin B per ml).
FIG. 1.
Thirteen 25-mer synthetic peptides covering the entire amino acid sequence of MPB70. The peptides overlap each other by 10 aa. The single-letter designations for amino acids are used.
Isolation of PBMC.
PBMC were isolated from buffy coats of healthy subjects by using standard procedures (19). In brief, each buffy coat was diluted with warm tissue culture medium (RPMI 1640) at a ratio of 1:2 and gently mixed. Two volumes of the buffy coat was loaded very slowly on top of 1 volume of a Lymphoprep gradient. After centrifugation, the white ring of PBMC between the plasma and the Lymphoprep was removed and washed three times with RPMI 1640. The cells were finally suspended in complete tissue culture medium and counted with a Coulter Counter (Coulter Electronics Ltd., Luton, Beds, England).
Antigen- and peptide-induced proliferation of PBMC.
Antigen- and peptide-induced proliferation of PBMC was performed by using standard procedures (21, 24). In brief, PBMC (2 × 105 cells/well) suspended in 50 μl of complete tissue culture medium were seeded into the wells of 96-well tissue culture plates (Nunc, Roskilde, Denmark). Antigen or peptide in 50 μl of complete medium was added to the wells in triplicate at an optimal concentration of 5 μg/ml. Whole bacilli were used at a concentration of 10 μg (wet weight) per ml (28). The final volume of the culture in each well was adjusted to 200 μl. The plates were incubated at 37°C in a humidified atmosphere containing 5% CO2 and 95% air. The cultures were pulsed for 4 h on day 6 with 1 μCi of [3H]thymidine (Amersham Life Sciences, Little Chalfont, United Kingdom) and harvested on filter mats with a Skatron harvester (Skatron Instruments AS, Oslo, Norway), and the amount of radioactivity incorporated was measured by liquid scintillation counting as described previously.
HLA typing of PBMC.
PBMC were HLA typed genomically by isolating the high-molecular-weight genomic DNA from each PBMC by treatment of the cells with proteinase K and salting out in miniscale as described by Olerup and Zetterquist (43). The amount of DNA obtained was quantified by spectrophotometry. The localization, sequences, lengths, and specificities of the sequence-specific primers used for typing the DRB1, DRB3, DRB4, and DRB5 alleles were described previously by Olerup and Zetterquist (43). An HLA-DR low-resolution kit containing all the primers was purchased from Dynal AS (Oslo, Norway) and was used in PCR as specified by the manufacturer. DNA amplification was carried out with a Gene Amp 2400 PCR system (Perkin-Elmer Cetus), and the amplified products were analyzed by gel electrophoresis by using standard procedures. Serologically defined HLA-DR specificities were determined from the genotypes by following the guidelines provided by Dynal AS.
Establishment of antigen-specific T-cell lines.
Antigen-specific T-cell lines were established from the donors by stimulating PBMC with CF or purified MPB70 by using procedures described previously (22, 26). In brief, PBMC (2 × 105 cells/well) were stimulated with the antigen (5 μg/ml) in triplicate in 96-well plates and incubated at 37°C in an atmosphere containing 5% CO2 and 95% air. After 6 days, interleukin-2 (100 U/well; Amersham Life Sciences) was added twice a week until the cell culture density allowed transfer to 24-well tissue culture plates (Nunc). The growing T-cell lines were expanded in 24-well plates with addition of interleukin-2 twice a week until they were tested for antigen reactivity.
Antigen- and peptide-induced proliferation of T-cell lines.
The T-cell lines were tested for antigen- and peptide-induced proliferation in the presence of autologous and allogeneic HLA-typed antigen-presenting cells (APC) by using the procedures described previously (20, 41). In brief, irradiated (2,400 rads) PBMC were seeded into the wells of 96-well plates at a concentration of 1 × 105 cells/well. The plates were incubated for 1 h at 37°C in a humidified atmosphere containing 5% CO2 and 95% air. Nonadherent cells were removed, and adherent cells were washed three times with tissue culture medium (RPMI 1640) and used as APC. Antigen-specific T-cell lines were harvested, washed three times, and added to the APC-containing wells at a concentration of 5 × 104 cells/well. Antigen and peptides were each added in triplicate at a final concentration of 5 μg/ml, and the culture volume in the wells was adjusted to 200 μl with complete tissue culture medium. The plates were incubated at 37°C in an atmosphere containing 5% CO2 and 95% air. On day 3, the cultures were pulsed with 1 μCi of [3H]thymidine and harvested on filter mats, and the amount of radioactivity incorporated was determined by liquid scintillation counting as described previously (37).
Interpretation of proliferation results.
The radioactivity incorporated was obtained as counts per minute. The average radioactivity was calculated from triplicate cultures stimulated with each antigen or peptide, as well as from triplicate wells of negative control cultures lacking antigen. Cellular proliferation results are expressed below by using the stimulation index (SI), which is defined as follows: SI = counts per minute in antigen-stimulated cultures/counts per minute in cultures without antigen. An SI of ≥3 was considered a positive proliferative response (55, 57).
IFN-γ assay.
Supernatants (100 μl) were collected from antigen-stimulated cultures of PBMC and T-cell lines (96-well plates) before they were pulsed with [3H]thymidine. The supernatants were kept frozen at −70°C until they were assayed for IFN-γ activity. The amounts of IFN-γ in the supernatants were quantified by using PREDICTA immunoassay kits (Genzyme Co., Cambridge, Mass.) as specified by the manufacturer. The detection limit of the IFN-γ assay kits was 8 pg/ml. Secretion of IFN-γ in response to a given antigen or peptide was considered positive when the IFN-γ concentration in cultures stimulated with antigen minus the IFN-γ concentration in cultures without antigen was ≥500 pg/ml (30).
Inhibition assays with monoclonal anti-HLA antibodies.
Inhibition of antigen-induced T-cell proliferation was studied as described previously (25, 27) in the presence of monoclonal antibody L243 (anti-HLA-DR) purchased from the American Type Culture Collection, Rockville, Md., and monoclonal antibody FN81 (anti-HLA-DQ), a gift from S. Funderud, Oslo, Norway. In brief, adherent APC in the wells of 96-well flat-bottom plates were preincubated with the antibodies for 30 min at 37°C in an atmosphere containing 5% CO2 and 95% air. After preincubation, antigen-induced proliferation of T-cell lines was assayed as described above. The results were expressed as percentages of inhibition, calculated as follows: [1 − (counts per minute in antigen-stimulated cultures in the presence of antibodies/counts per minute in antigen-stimulated cultures in the absence of antibodies)] × 100.
Cytotoxicity assay.
Cytotoxicity of T-cell lines against antigen- and peptide-pulsed monocytes/macrophages was assessed by the neutral red release assay as previously described (25, 29). In brief, adherent monocytes/macrophages from 1 × 106 autologous irradiated PBMC in 24-well Costar plates were pulsed with MPB70 or peptide. The T-cell lines were added at a concentration of 2 × 105 cells/well. After 7 days of incubation at 37°C, the wells were washed to remove nonadherent T cells, and the macrophages were allowed to take up neutral red for 30 min. The dye taken up by macrophages was released by adding 0.5 ml of 0.05 M acetic acid in 50% ethanol. The results are expressed below as percentages of cytotoxicity, which were calculated from spectrophotometric measurement of optical density at 540 nm (OD540) by using the following equation: percentage of cytotoxicity = [(OD540 of the control − OD540 of the experimental preparation)/(OD540 of the control)] × 100, where OD540 of the control is the OD540 of cultures containing adherent cells plus T cells and OD540 of the experimental preparation is the OD540 of cultures containing adherent cells plus T cells plus antigen or peptide.
RESULTS
Identification of MPB70 responding donors.
To identify donors suitable for testing reactivity against the peptides of MPB70 and establishing antigen-specific T-cell lines, PBMC from 53 healthy subjects were screened for proliferation and IFN-γ secretion in response to M. tuberculosis, CF, MPB70, and pools of synthetic peptides of ESAT-6 and CFP-10. The results showed that PBMC from a majority of the donors (≥92%) responded to complex M. tuberculosis and CF antigens in both assays (Table 1). When single antigens were used, 64 and 71% of the donors responded to MPB70 in the proliferation and IFN-γ assays, respectively, whereas only 28 and 28% of the donors responded to ESAT-6, respectively, and 49 and 28% of the donors responded to CFP-10, respectively (Table 1). In addition, the results obtained when PBMC were tested with a pool of MPB70 peptides in the same assays were comparable to the results obtained with the complete antigen (results not shown). The donors responding to MPB70 were then selected to identify the individual MPB70 peptides recognized by PBMC and to establish antigen-specific T-cell lines.
TABLE 1.
Antigen-induced proliferation and secretion of IFN-γ from PBMC of healthy blood donors in response to CF, M. tuberculosis, MPB70, ESAT-6, and CFP-10
| Antigen | Response (no. positive/no. tested) of PBMC ina:
|
|
|---|---|---|
| Proliferation assaya | IFN-γ assayb | |
| M. tuberculosis | 47/51 (92)c | 51/53 (96) |
| CF | 50/51 (98) | 50/53 (94) |
| MPB70 | 32/50 (64) | 36/51 (71) |
| ESAT-6 | 11/39 (28) | 11/39 (28) |
| CFP-10 | 19/39 (49) | 11/39 (28) |
A positive response was defined as antigen-induced proliferation with an SI of ≥3.
A response was considered positive if the IFN-γ concentration in a culture stimulated with antigen minus the IFN-γ concentration in a culture without antigen was ≥500 pg/ml.
The values in parentheses are percentages.
Peptides of MPB70 recognized by PBMC.
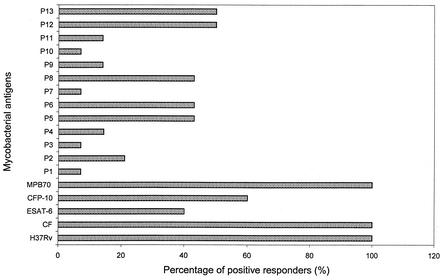
To map the T-cell epitopes recognized by healthy subjects, PBMC from 14 of 34 MPB70-responding donors were chosen at random and tested for reactivity against 13 overlapping peptides covering the entire MPB70 sequence, including the signal sequence (Fig. 1). Although positive responses were obtained with all the peptides in one or more donors, the results showed that the most frequent responses (ranging from 43 to 50%) in the proliferation assays were obtained with peptides p5, p6, p8, p12, and p13, whereas the remaining peptides showed low responder frequencies (7 to 21%) (Fig. 2). The peptides that showed strong proliferation responses also showed strong IFN-γ responses (data not shown). HLA-DR typing of the subjects responding to MPB70 and the peptides demonstrated that they represented a heterogeneous group of donors with respect to HLA expressing DR1, DR2, DR3, DR5, DR6, DR7, DR51, DR52, and DR53 molecules (data not shown), thus indicating the permissive nature of MPB70 and its immunodominant peptides in recognition by human PBMC.
FIG. 2.
Proliferation of PBMC of healthy donors in response to complex mycobacterial antigens, including ESAT-6, CFP-10, MPB70, and synthetic peptides of MPB70. Healthy donors (n = 14) were randomly chosen from the group of 34 positive responders to MPB70 shown in Table 1. Antigen- or peptide-induced proliferation of PBMC from each donor was determined as described in Materials and Methods. The results are expressed as the percentage of positive responders, which was defined as follows: [number of positive responders (SI, >3)/number of tested donors] × 100.
Proliferation and IFN-γ secretion of antigen-specific T-cell lines in response to mycobacterial antigens and synthetic MPB70 peptides.
To confirm that Th1 cells were the major cells responding to MPB70 and its peptides in PBMC assays, antigen-specific T-cell lines were established from 15 positive donors after primary stimulation of PBMC with CF or purified MPB70. Surface phenotyping revealed that all of the established T-cell lines had the CD4+ CD8− phenotype. Furthermore, all of the T-cell lines responded to MPB70 in proliferation and/or IFN-γ assays (Table 2). When tested with the peptides most frequently recognized by PBMC (peptides p5, p6, p8, p12, and p13), the responses to p8 (27 and 60% in proliferation and IFN-γ assays, respectively), p12 (40 and 73%), and p13 (47 and 87%) confirmed the dominant recognition of these peptides by CD4+ Th1 cells (Table 2). Compared to the responses to p8, p12, and p13, the responses of the T-cell lines to peptides p5 and p6 were weaker in both assays (Table 2). HLA-DR typing of these donors further showed that they covered a large spectrum of HLA-DR types (DR1, DR2, DR3, DR4, DR5, DR6, DR7, DR51, DR52, and DR53), thus reinforcing the finding that MPB70 and its dominant peptides were presented to T cells promiscuously (Table 2).
TABLE 2.
Proliferation and IFN-γ secretion by specific T-cell lines in response to MPB70 and its synthetic peptides
| T-cell line | HLA-DR | Response to:a
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MPB 70
|
Peptide p5
|
Peptide p6
|
Peptide p8
|
Peptide p12
|
Peptide p13
|
||||||||
| Proliferation assay (SI) | IFN-γ assay (ΔIFN-γ [102 pg/ml]) | Proliferation assay (SI) | IFN-γ assay (ΔIFN-γ [102 pg/ml]) | Proliferation assay (SI) | IFN-γ assay (ΔIFN-γ [102 pg/ml]) | Proliferation assay (SI) | IFN-γ assay (ΔIFN-γ [102 pg/ml]) | Proliferation assay (SI) | IFN-γ assay (ΔIFN-γ [102 pg/ml]) | Proliferation assay (SI) | IFN-γ assay (ΔIFN-γ [102 pg/ml]) | ||
| SF1 | 4,6,52,53 | 27 | 29 | 0.9 | 1.0 | 1.1 | 2.0 | 0.7 | <0.08 | 24.8 | 31 | 32 | 35 |
| C1 | 1,6,52 | 3.2 | 36 | 0.6 | <0.08 | 0.6 | <0.08 | 3.0 | 46 | 0.8 | <0.08 | 0.8 | <0.08 |
| C2 | 7,53 | 21 | 49 | 1.0 | <0.08 | 1.0 | <0.08 | 1.0 | <0.08 | 19 | 105 | 18 | 110 |
| C3 | NDb | 1.8 | 12 | 1.1 | 5.0 | 0.9 | 1.5 | 0.7 | <0.08 | 0.9 | 1.5 | 0.5 | 11 |
| SF9 | 4,6,52,53 | 18 | 54 | 1.5 | 0.8 | 1.5 | 0.7 | 22 | 52.5 | 25 | 56 | 4.0 | 195 |
| SF10 | ND | 5 | 43 | 0.7 | <0.08 | 0.7 | <0.08 | 0.7 | <0.08 | 3.4 | 23.9 | 4.7 | 41 |
| SF11 | 4,53 | 1.7 | 36.5 | 0.8 | 1.6 | 0.8 | 2.5 | 0.8 | 6.7 | 1.5 | 20.4 | 2.2 | 29 |
| SF14 | 3,52 | 1.2 | 42.5 | 0.9 | 27 | 0.8 | 20.7 | 1.1 | 62 | 0.9 | 35 | 0.8 | 24.3 |
| SF15 | 4,5,52,53 | 18 | 100 | 0.8 | 6.9 | 0.9 | 7.9 | 1.8 | 100 | 14 | 100 | 13.5 | 100 |
| SF16 | 3,52 | 3.6 | 24.1 | 0.9 | 1.9 | 1.0 | 2.3 | 0.9 | 2.9 | 1.8 | 32 | 4.0 | 49 |
| SF17 | 3,52 | 6.2 | 50 | 1.7 | 3.6 | 3.3 | 6.9 | 19 | 67.5 | 1.8 | 5.5 | 1.7 | 5.0 |
| SH8 | 4,53 | 7.8 | 35.5 | 3.5 | 38.6 | 1.9 | 32.2 | 2.1 | 38 | 1.1 | 24.2 | 1.0 | 10.5 |
| SH15 | 2,5,51,52 | 12.2 | 18.7 | 1.7 | 6.1 | 2.2 | 3.2 | 4.8 | 8.1 | 1.2 | 2.3 | 0.9 | 8.4 |
| SF49 | ND | 0.8 | 5.3 | 0.7 | 2.9 | 0.6 | 3.6 | 2.5 | 26 | 0.6 | 8.0 | 0.7 | 5.4 |
| SF16 | 3,6,52 | 13 | 11 | 0.9 | 0.6 | 0.9 | <0.08 | 1.0 | 1.1 | 4.2 | 2.2 | 3.7 | 3.0 |
In proliferation assays a positive response was defined as an SI of ≥3. In IFN-γ assays a response was considered positive if the IFN-γ concentration in a culture stimulated with antigen or peptide minus the IFN-γ concentration in a culture without antigen or peptide (ΔIFN-γ) was ≥500 pg/ml. Positive responses are indicated by boldface type. For MPB70 11 of 15 (73%) and all 15 (100%) of the cell lines were positive in the proliferation and IFN-γ assays, respectively; for peptide p5 1 of 15 (7%) and 5 of 15 (33%) of the cell lines were positive in the proliferation and IFN-γ assays, respectively; for peptide p6 1 of 15 (7%) and 4 of 15 (27%) of the cell lines were positive in the proliferation and IFN-γ assays, respectively; for peptide p8 4 of 15 (27%) and 9 of 15 (60%) of the cell lines were positive in the proliferation and IFN-γ assays, respectively; for peptide p12 6 of 15 (40%) and 11 of 15 (73%) of the cell lines were positive in the proliferation and IFN-γ assays, respectively; and for peptide p13 7 of 15 (47%) and 13 of 15 (87%) of the cell lines were positive in the proliferation and IFN-γ assays, respectively.
ND, not determined.
HLA restriction analysis of MPB70 and peptide presentation to antigen-specific T-cell lines.
HLA restriction analysis of antigen-specific T-cell lines was performed to confirm the promiscuous presentation of MPB70 and its dominant peptides, as indicated by the HLA typing of the donors. These studies were carried out by combining information from antibody blocking assays and panel studies with HLA-DR-typed autologous and allogeneic APC.
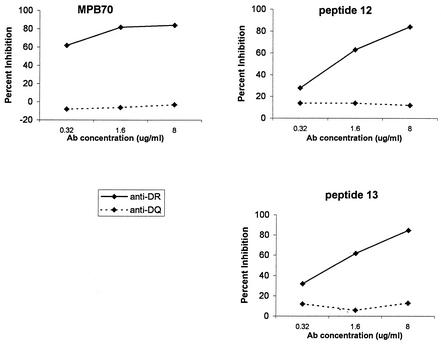
In antibody blocking assays, the use of well-defined monoclonal antibodies against HLA-DR and -DQ molecules showed that MPB70 and the peptides were presented to T cells in the context of HLA-DR molecules. The results of representative experiments demonstrating anti-HLA-DR blocking of antigen-and peptide-induced proliferation in a dose-dependent manner are shown in Fig. 3.
FIG. 3.
Inhibition of the proliferative responses of T-cell line SF1 in the presence of anti-HLA class II monoclonal antibodies. T-cell line SF1 was stimulated with MPB70, peptide p12, or peptide p13. Ab, antibody.
To identify the HLA-DR molecules responsible for presentation of MPB70 and its peptides to T cells, a selected T-cell line was tested for IFN-γ secretion in the presence of a panel of autologous and allogeneic HLA-DR-typed APC. The T-cell line (C2), which was established by using the CF antigens tested for HLA restriction with a panel of HLA-DR-typed APC, demonstrated that the presentation of MPB70 and peptides p12 and p13 in IFN-γ assays was DR53 restricted (Table 3).
TABLE 3.
IFN-γ secretion by T-cell line C2 (HLA-DR7,53) in response to MPB70 and its peptides in the presence of HLA class II-typed APC
| APC | ΔIFN-γ (102 pg/ml) in response toa:
|
||
|---|---|---|---|
| MPB70 mixture | Peptide p12 | Peptide p13 | |
| HLA-DR7,53 | 25.5 | 15.7 | 30.2 |
| HLA-DR4,53 | 51.6 | 25.4 | 43 |
| HLA-DR5,52,53 | 36 | 18.1 | 20.7 |
| HLA-DR5,7,52,53 | 33.8 | 22.7 | 21.7 |
| HLA-DR6,7,52 | <5 | <5 | <5 |
| HLA-DR3,7,52,53 | 35.2 | 35.6 | 22.8 |
In IFN-γ assays a response was considered positive if the IFN-γ concentration in a culture stimulated with antigen or peptide minus the IFN-γ concentration in a culture without antigen or peptide (ΔIFN-γ) was ≥500 pg/ml. Positive responses are indicated by boldface type. For the MPB70 mixture, peptide p12, and peptide p13 the HLA-DR restriction was DR53.
Cytotoxic activity of MPB70-specific T-cell lines.
T-cell lines from three HLA-DR heterogeneous donors (SF15 [HLA-DR4,5,52,53], SF17 [HLA-DR3,52], and C2 [HLA-DR7,53]), which responded to MPB70 in IFN-γ assays, were tested for cytotoxic activity against autologous monocytes/macrophages pulsed with the complete protein antigen and synthetic peptides. The results revealed that all three T-cell lines were cytotoxic for monocytes/macrophages pulsed with MPB70 (Table 4). When individual peptides were examined, cytotoxic activity seemed to be associated with IFN-γ secretion. Peptides p5 and p7 induced neither IFN-γ secretion nor cytotoxic activity in any of the T-cell lines tested. However, peptide p8 induced IFN-γ secretion and cytotoxic activity in T-cell lines SF15 and SF17, whereas peptides p12 and p13 induced both IFN-γ secretion and cytotoxic activity in T-cell lines SF15 and C2 (Table 4). This could indicate that in the absence of DR53, SF17 (HLA-DR3,52) could respond only to p8.
TABLE 4.
Cytotoxic activities of MPB70-specific T-cell lines against monocytes/macrophages pulsed with MPB70 and MPB70 peptides
| Antigen or peptide | Activities of MPB70-specific T-cell linesa
|
|||||
|---|---|---|---|---|---|---|
| SF15 (HLA-DR4,5,52,53)
|
SF17 (HLA-DR3,52)
|
C2 (HLA-DR7,53)
|
||||
| % Cytotoxicity | ΔIFN-γ (102 pg/ml) | % Cytotoxicity | ΔIFN-γ (102 pg/ml) | % Cytotoxicity | ΔIFN-γ (102 pg/ml) | |
| MPB70 | 87.5 | 105 | 55 | 54 | 90 | 69 |
| p5 | 19 | <5 | <5 | <5 | NDb | <5 |
| p7 | <5 | <5 | <5 | 8.5 | ND | <5 |
| p8 | 77.6 | 110 | 56 | 65 | ND | <5 |
| p9 | 69 | 49 | 36 | 29 | 20 | <5 |
| p12 | 74.5 | 98 | <5 | <5 | 80 | 69 |
| p13 | 58.6 | 82 | <5 | <5 | 80 | 75 |
In cytotoxicity assays a level of cytotoxicity of ≥30% was considered positive. In IFN-γ assays a response was considered positive if the IFN-γ concentration in a culture stimulated with antigen or peptide minus the IFN-γ concentration in a culture without antigen or peptide (ΔIFN-γ) was ≥500 pg/ml. Positive responses are indicated by boldface type.
ND, not determined.
DISCUSSION
In the present study, PBMC from a large number of healthy donors (n = 53) were tested for T-cell responses (i.e., antigen-induced proliferation and IFN-γ secretion) to complex and secreted single mycobacterial antigens. Most of the donors (>90%) responded to the complex mycobacterial antigens, suggesting that these donors were sensitized to mycobacteria. When the same donors were tested for responses to single mycobacterial antigens, about two-thirds, one-half, and one-third of the donors responded to MPB70, CFP-10, and ESAT-6, respectively (Table 1). MPB70 is M. tuberculosis complex specific; small amounts of this protein are secreted by M. tuberculosis, and various amounts are secreted by different BCG strains (9, 18, 34, 58). ESAT-6 and CFP-10 are M. tuberculosis-specific proteins, and the genes encoding them are absent in all BCG strains (2). The PBMC responses to MPB70 could have been due to either exposure to or latent infection with M. tuberculosis or vaccination with BCG. However, about 50% of the donors responded to the M. tuberculosis-specific antigen ESAT-6 or the M. tuberculosis-specific antigen CFP-10 or both, which suggests that a significant proportion of the donors that responded to MPB70 could have been infected with M. tuberculosis.
Several studies have demonstrated that TB patients exposed to healthy household contacts and individuals with inactive self-healed pulmonary TB show strong T-cell responses to ESAT-6 and/or CFP-10 (2, 14, 46, 55), whereas BCG-vaccinated and nonvaccinated healthy individuals from countries with low levels of TB endemicity show either low-level responses (The Netherelands) or no responses (Germany and United Kingdom) to ESAT-6 or CFP-10 (2, 15, 55). In contrast, the majority of healthy adults (80%) from an area where TB is endemic (Bombay, India) showed positive responses to ESAT-6 and CFP-10, thereby suggesting that the prevalence of latent TB in urban India is 80% (15). The positive responses in about one-half of the healthy donors to ESAT-6 and/or CFP-10 in proliferation assays in this study suggest that about 50% of our donors were latently infected with M. tuberculosis.
It has recently been shown in the mouse model that immunization with a synthetic peptide of ESAT-6 along with a proper adjuvant induced IFN-γ secretion from T cells and provided protection against challenge with M. tuberculosis (44). Interestingly, the level of protection afforded by the peptide vaccine was equivalent to the level of protection observed after immunization with the complete ESAT-6 antigen (44). These results suggest that some of the IFN-γ-inducing peptides could replace complete antigens in subunit vaccines. To identify the T-cell epitopes of MPB70 that induce strong IFN-γ responses, in this study we used a synthetic peptide approach. As the T-cell epitopes are usually 8 to 10 aa long (31), the MPB70 peptides used in this study overlapped each other by 10 aa to minimize the possibility of missing the T-cell epitopes of the protein. Moreover, in the cattle model it has previously been shown that in addition to the mature protein, the peptides belonging to the 30-aa signal sequence of MPB70 also induced IFN-γ secretion (56). We therefore used peptides covering the entire sequence of MPB70, including the signal sequence. A total of 13 overlapping peptides were tested for recognition by human T cells by using PBMC and antigen-specific CD4+ T-cell lines. The results showed that the T-cell epitopes were scattered throughout the sequence of MPB70, but some peptides were recognized more frequently than others. The peptides that were frequently recognized by PBMC in both proliferation and IFN-γ assays were p5 (aa 61 to 85), p6 (aa 76 to 100), p8 (aa 106 to 130), p12 (aa 166 to 190), and p13 (aa 181 to 193). Interestingly, peptides p8, p12, and p13 were also frequently recognized in both the assay systems (proliferation and IFN-γ secretion) by the antigen-specific CD4+ T-cell lines. The frequent recognition of these peptides by PBMC and by antigen-specific T-cell lines described in this study demonstrated that the corresponding epitopes are not cryptic but are relevant to natural processing and presentation of the MPB70 antigen to CD4+ Th1 cells. Such knowledge is required for application of synthetic peptides in vaccine design.
We used both proliferation and IFN-γ assays to obtain readouts for antigen-specific responses of PBMC and the T-cell lines. The importance of the Th1 cytokine IFN-γ as the primary mediator of protective immunity to mycobacterial infections has been well demonstrated in animal models (8, 45, 52). In addition, the strong IFN-γ responses in PPD-positive healthy subjects and TB patients with minimal disease (3, 11, 48, 54, 59) compared to the weak responses in TB patients with advanced disease (11, 54, 59) suggest that IFN-γ plays an important role in mediating protective immunity in humans. This is further emphasized by the observation that the growth of mycobacteria was inhibited in macrophages (blood-derived as well as lung macrophages) stimulated with IFN-γ (12, 13). The fact that there were peptide-specific Th1 cell responses, as judged by IFN-γ secretion, allowed us to suggest that the corresponding MPB70 peptides are relevant to protective immune responses in humans.
Although MPB70 has previously been tested for responses in TB patients and healthy donors (49), this is the first study in which the T-cell epitopes of MPB70 recognized by humans were mapped. However, MPB70 has been extensively studied for T-cell epitope mapping in cattle (16, 47, 56, 57). Like our results obtained with a population that is heterogeneous with respect to HLA, previous results obtained with PBMC from experimentally infected cattle of three different breeds showed the immunodominance of the MPB70 peptides at aa 88 to 105 and aa 144 to 163 (47), which overlap peptide p8 and peptides p12 and p13 used in this study. Pollock et al. (47) showed that these peptides were not recognized by noninfected control animals but showed reactivity with PBMC from field animals that responded to intradermal tuberculin testing, suggesting that they had been exposed to mycobacteria. This was further confirmed by Lightbody et al. (16), who observed strong T-cell responses to peptides overlapping peptides p12 and p13 in both immunized cattle and cattle experimentally infected with M. bovis. Recently, it has also been shown that the C-terminal end of MPB70 is highly immunogenic and induces production of a high level of IFN-γ by spleen cells of mice immunized with MPB70 DNA (53a). The frequent T-cell recognition of similar MPB70 peptides in humans, cattle, and mice might have benefits for development of a common vaccine.
Recognition of mycobacterial antigens and peptides by CD4+ T cells circulating in peripheral blood is mostly restricted by HLA-DR molecules (22-25, 27, 29, 37-40, 42). Therefore, during development of a universally efficacious vaccine against TB in humans, the antigens and peptides selected as vaccine candidates should have Th1 cell epitopes recognized in association with multiple allelic products of HLA-DR. HLA-DR typing of the donors showed that MPB70 and its immunodominant peptides were recognized by T cells obtained from donors having various HLA backgrounds. These results are encouraging and suggest that within a human population there are genetically promiscuous MPB70 epitopes recognized by a high proportion of donors. This observation supports the conclusion that MPB70 or some of its peptides should be included as candidate antigens in experimental subunit vaccines.
In addition to IFN-γ secretion, we also tested the T-cell lines for cytotoxic activity against antigen- and peptide-pulsed monocytes. Usually, CD4+ T cells are considered to be helper cells; however, it has been shown that the Th1 subset of CD4+ T cells also has cytotoxic activity that may play a role in killing intracellular pathogens directly (5, 33, 51), as well as enhancing the effector function of the classical CD8+ cytotoxic T cells (50). Our results demonstrate that IFN-γ secretion by antigen-specific T-cell lines in response to MPB70 and its peptides is associated with cytotoxic activity. Thus, the antigen- and peptide-specific Th1 cells may provide protection through direct killing mechanisms, as well as activation of macrophages through IFN-γ secretion (12, 13).
Acknowledgments
This study was supported by KFAS grant 97-07-05 and by Kuwait University Research Administration grant MI 114.
The supply of buffy coats by the Central Blood Bank, Kuwait, is gratefully acknowledged.
Editor: J. M. Mansfield
REFERENCES
- 1.Andersen, P. 1997. Host responses and antigens involved in protective immunity to Mycobacterium tuberculosis. Scand. J. Immunol. 45:115-131. [DOI] [PubMed] [Google Scholar]
- 2.Arend, S. M., P. Andersen, E. van Meijgaarden, R. L. V. Skjot, Y. W. Subronto, J. T. van Dissel, and T. H. M. Ottenhoff. 2000. Detection of active tuberculosis infection by T cell responses to early-secreted antigenic target 6-kDa protein and culture filtrate protein 10. J. Infect. Dis. 181:1850-1854. [DOI] [PubMed] [Google Scholar]
- 3.Barnes, P. F., S. Lu, J. S. Abrams, E. Wang, M. Yamamura, and R. L. Modlin. 1993. Cytokine production at the site of disease in human tuberculosis. Infect. Immun. 61:3482-3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Billman-Jacobe, H., A. J. Radford, J. S. Rothel, and P. R. Wood. 1990. Mapping of the T and B cell epitopes of the Mycobacterium bovis protein, MPB70. Immunol. Cell Biol. 68:359-365. [DOI] [PubMed] [Google Scholar]
- 5.Dieli, F., M. Troye-Blomberg, J. Ivanyi, J. J. Founie, M. Bonneville, M. A. Peyrat, G. Sireci, and A. Salerno. 2000. Vγ9/Vδ2 lymphocytes reduce the viability of intracellular Mycobacterium tuberculosis. Eur. J. Immunol. 30:1512-1519. [DOI] [PubMed] [Google Scholar]
- 6.Dye, C., S. Scheele, P. Dolin, V. Pathania, and M. C. Raviglione. 1999. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence and mortality by country. W. H. O. Global Surveillance and Monitoring Project. JAMA 282:677-686. [DOI] [PubMed]
- 7.Fine, P. E. 1995. Variation in protection by BCG: implications of and for heterologous immunity. Lancet 346:1339-1345. [DOI] [PubMed] [Google Scholar]
- 8.Flynn, J. L., J. Chan, K. J. Triebold, D. K. Dalton, T. A. Stewart, and B. R. Bloom. 1993. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249-2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harboe, M., and S. Nagai. 1984. MPB70, a unique antigen of Mycobacterium bovis BCG. Am. Rev. Respir. Dis. 129:444-452. [DOI] [PubMed] [Google Scholar]
- 10.Harboe, M., H. G. Wiker, J. R. Duncan, M. M. Garcia, T. W. Dukes, B. W. Brooks, C. Turcotte, and S. Nagai. 1990. Protein G-based enzyme-linked immunosorbent assay for anti-MPB70 antibodies in bovine tuberculosis. J. Clin. Microbiol. 28:913-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huygen, K., J. P. Van Vooren, M. Turneer, R. Bosmans, P. Dierckx, and J. De Bruyn. 1988. Specific lymphoproliferation, gamma interferon production and serum immunoglobulin G directed against a purified 32 kDa mycobacterial protein antigen (P32) in patients with active tuberculosis. Scand. J. Immunol. 27:187-194. [DOI] [PubMed] [Google Scholar]
- 12.Kampmann, B., P. O'Gaora, V. A. Snewin, M.-P. Gares, D. B. Young, and M. Levin. 2000. Evaluation of human anti-mycobacterial immunity using recombinant reporter mycobacteria. J. Infect. Dis. 182:895-901. [DOI] [PubMed] [Google Scholar]
- 13.Kawakami, K., K. Teruya, M. Tohyama, N. Kudeken, and A. Saito. 1994. A therapeutic trial of experimental tuberculosis with γ-interferon in an immunocompromised mouse model. Kekkaku 69:607-613. [PubMed] [Google Scholar]
- 14.Lalvani, A., A. A. Pathan, H. Durkan, K. A. Wilkinson, A. Whelan, J. J. Deeks, W. H. H. Reece, M. Latif, G. Rasvol, and A. V. S. Hill. 2001. Enhanced contact tracing and special tracking of Mycobacterium tuberculosis infection by enumeration of antigen-specific T cells. Lancet 357:2017-2021. [DOI] [PubMed] [Google Scholar]
- 15.Lalvani, A., P. Nagvenkar, Z. Udwadia, A. A. Pathan, K. A. Wilkinson, J. S. Shastri, K. Ewer, A. V. Hill, A. Mehta, and C. Rodrigues. 2001. Enumeration of T cells specific for RD1-encoded antigens suggests a high prevalence of latent Mycobacterium tuberculosis infection in healthy urban Indians. J. Infect. Dis. 183:469-477. [DOI] [PubMed] [Google Scholar]
- 16.Lightbody, K. A., R. M. Girvin, D. P. Mackie, S. D. Neill, and J. M. Pollock. 1998. T cell recognition of mycobacterial proteins MPB70 and MPB64 in cattle immunized with antigen and infected with Mycobacterium bovis. Scand. J. Immunol. 48:44-51. [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto, S., T. Matsuo, N. Ohara, H. Hotokezaka, M. Naito, J. Minami, and T. Yamada. 1995. Cloning and sequencing of a unique antigen MPT70 from Mycobacterium tuberculosis H37Rv and expression in BCG using E. coli-mycobacteria shuttle vector. Scand. J. Immunol. 41:281-287. [DOI] [PubMed] [Google Scholar]
- 18.Miura, K., S. Nagai, M. Kinomoto, S. Haga, and T. Tokunaga. 1983. Comparative studies with various substrains of Mycobacterium bovis BCG on the production of an antigenic protein, MPB70. Infect. Immun. 39:540-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mustafa, A. S., and T. Godal. 1983. In vitro induction of human suppressor T cells by mycobacterial antigens. BCG activated OKT4+ cells mediate suppression of antigen-induced T cell proliferation. Clin. Exp. Immunol. 52:29-37. [PMC free article] [PubMed] [Google Scholar]
- 20.Mustafa, A. S., F. Oftung, H. K. Gill, and I. Natvig. 1986. Characteristics of human T cell clones from BCG and killed M. leprae vaccinated subjects and tuberculosis patients. Lepr. Rev. 57(Suppl. 2):123-130. [PubMed] [Google Scholar]
- 21.Mustafa, A. S., and T. Godal. 1987. BCG induced CD4+ cytotoxic T cells from BCG vaccinated healthy subjects: relation between cytotoxicity and suppression in vitro. Clin. Exp. Immunol. 69:255-262. [PMC free article] [PubMed] [Google Scholar]
- 22.Mustafa, A. S. 1988. Identification of T-cell activating recombinant antigens shared among three candidate antileprosy vaccines, killed M. leprae, M. bovis BCG and Mycobacterium w. Int. J. Lepr. 50:265-273. [PubMed] [Google Scholar]
- 23.Mustafa, A. S., and E. Qvigstad. 1988. HLA-DR restricted antigen induced proliferation and cytotoxicity mediated by CD4+ T-cell clones from subjects vaccinated with killed M. leprae. Int. J. Lepr. 57:1-11. [PubMed] [Google Scholar]
- 24.Mustafa, A. S., K. E. A. Lundin, and F. Oftung. 1993. Human T cells recognize mycobacterial heat shock proteins in the context of multiple HLA-DR molecules: studies with healthy subjects vaccinated with Mycobacterium bovis BCG and Mycobacterium leprae. Infect. Immun. 61:5294-5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mustafa, A. S., A. Deggerdal, K. E. A. Lundin, R. H. Meloen, T. M. Shinnick, and F. Oftung. 1994. An HLA-DRw53-restricted T-cell epitope from a novel Mycobacterium leprae protein antigen important to the memory T-cell repertoire against M. leprae. Infect. Immun. 62:5595-5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mustafa, A. S. 1996. Restoration of proliferative response to M. leprae antigens in lepromatous T cells against candidate antileprosy vaccines. Int. J. Lepr. 64:257-267. [PubMed] [Google Scholar]
- 27.Mustafa, A. S., K. E. A. Lundin, R. H. Meloen, T. M. Shinnick, A. F. Coulson, and F., Oftung. 1996. HLA DR4-restricted T-cell epitopes from the mycobacterial 60 000 MW heat shock protein (hsp 60) do not map to the sequence homology regions with the human hsp 60. Immunology 87:421-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mustafa, A. S., H. A. Amoudy, H. G. Wiker, A. T. Abal, P. Ravn, F. Oftung, and P. Andersen. 1998. Comparison of antigen specific T-cell responses of tuberculosis patients using complex or single antigens of Mycobacterium tuberculosis. Scand. J. Immunol. 48:535-543. [DOI] [PubMed] [Google Scholar]
- 29.Mustafa, A. S., K. E. A. Lundin, R. H. Meloen, T. M. Shinnick, and F. Oftung, F. 1999. Identification of promiscuous epitopes from the mycobacterial 65-kilodalton heat shock protein recognized by human CD4+ T cells of the Mycobacterium leprae memory repertoire. Infect. Immun. 67:5683-5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mustafa, A. S., F. Shaban, A. T. Abal, R. Al-Attiyah, H. G. Wiker, K. E. A. Lundin, F. Oftung, and K. Huygen. 2000. Identification and HLA restriction of naturally derived Th1-cell epitopes from the secreted Mycobacterium tuberculosis antigen 85B recognized by antigen-specific human CD4+ T-cell lines. Infect. Immun. 68:3933-3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mustafa, A. S. 2001. Bio/technology in the development of new vaccines and diagnostic reagents against tuberculosis. Curr. Pharm. Bio/Technol. 2:157-173. [DOI] [PubMed] [Google Scholar]
- 32.Mustafa, A. S., P. J. Cockle, F. Shaban, R. G. Hewinson, and H. M. Vordermeier. 2002. Immunogenicity of Mycobacterium tuberculosis RD1 region gene products in infected cattle. Clin. Exp. Immunol. 130:37-42. [DOI] [PMC free article] [PubMed]
- 33.Mutis, T., Y. E. Cornelisse, and T. H. Ottenhoff. 1993. Mycobacteria induce CD4+ T cells that are cytotoxic and display Th1-like cytokine secretion profile: heterogeneity in cytotoxic activity and cytokine secretion levels. Eur. J. Immunol. 23:2189-2195. [DOI] [PubMed] [Google Scholar]
- 34.Nagai, S., J. Matsumoto, and T. Nagasuga. 1981. Specific skin-reactive protein from culture filtrate of Mycobacterium bovis BCG. Infect. Immun. 31:1152-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagai, S., H. G. Wiker, M. Harboe, and M. Kinomoto. 1991. Isolation and partial characterization of main protein antigens in the culture fluid of Mycobacterium tuberculosis. Infect. Immun. 59:372-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oettinger, T., M. Jorgensen, A. Ladefoged, K. Haslov, and P. Andersen. 1999. Development of the Mycobacterium bovis BCG vaccine: review of the historical and biochemical evidence for a genealogical tree. Tuber. Lung Dis. 79:243-250. [DOI] [PubMed] [Google Scholar]
- 37.Oftung, F., A. S. Mustafa, T. M. Shinnick, R. A. Houghton, G. Kvalheim, M. Degre, K. E. A. Lundin, and T. Godal. 1988. Epitopes of the Mycobacterium tuberculosis 65-kilodalton protein antigen as recognized by human T cells. J. Immunol. 141:2749-2754. [PubMed] [Google Scholar]
- 38.Oftung, F., T. M. Shinnick, A. S. Mustafa, K. E. A. Lundin, T. Godal, and A. H. Nerland. 1990. Heterogeneity among human T cell clones recognizing an HLA-DR4, Dw4-restricted epitope from the 18 kDa antigen of Mycobacterium leprae defined by synthetic peptides. J. Immunol. 144:1478-1483. [PubMed] [Google Scholar]
- 39.Oftung, F., A. Geluk, K. E. A. Lundin, R. H. Meloen, J. E. R. Thole, A. S. Mustafa, and T. H. Ottenhoff. M. 1994. Mapping of multiple HLA class II restricted T-cell epitopes of the mycobacterial 70-kilodalton heat shock protein. Infect. Immun. 62:5411-5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oftung, F., K. E. A. Lundin, A. Geluk, T. H. M. Ottenhoff, T. M. Shinnick, R. Meloen, and A. S. Mustafa. 1997. Primary structure and MHC restriction of peptide defined T-cell epitopes from recombinantly expressed mycobacterial protein antigens. Med. Princ. Pract. 6:66-73. [Google Scholar]
- 41.Oftung, F., E. Borka, G. Kvalheim, and A. S. Mustafa. 1998. Mycobacterial crossreactivity of M. tuberculosis reactive T cell clones from naturally converted PPD positive healthy subjects. FEMS Immunol. Med. Microbiol. 20:231-238. [DOI] [PubMed] [Google Scholar]
- 42.Oftung, F., K. E. A. Lundin, R. Meloen, and A. S. Mustafa. 1999. Human T cell recognition of the Mycobacterium leprae LSR antigen: epitopes and HLA restriction. FEMS Immunol. Med. Microbiol. 24:151-159. [DOI] [PubMed] [Google Scholar]
- 43.Olerup, O., and H. Zetterquist. 1993. DR “low resolution” PCR-SSP typing—a correction and update. Tissue Antigens 41:55-56. [DOI] [PubMed] [Google Scholar]
- 44.Olsen, A. W., P. R. Hansen, A. Holm, and P. Andersen. 2000. Efficient protection against Mycobacterium tuberculosis by vaccination with a single subdominant epitope from the ESAT-6 antigen. Eur. J. Immunol. 30:1724-1732. [DOI] [PubMed] [Google Scholar]
- 45.Orme, I. M., A. D. Roberts, J. P. Griffin, and J. S. Abrams. 1993. Cytokine secretion by CD4 T lymphocytes acquired in response to Mycobacterium tuberculosis infection. J. Immunol. 151:518-526. [PubMed] [Google Scholar]
- 46.Pathan, A. A., K. A. Wilkinson, R. J. Wilkinson, M. Latif, H. McShane, G. Pasvol, A. V. S. Hill, and A. Lavlani. 2000. High frequencies of circulating IFN-γ-secreting CD8 cytotoxic T cells specific for a novel MHC class-I-restricted Mycobacterium tuberculosis-infected subjects without disease. Eur. J. Immunol. 30:2713-2721. [DOI] [PubMed] [Google Scholar]
- 47.Pollock, J. M., A. J. Douglas, D. P. Mackie, and S. D. Neil. 1994. Identification of bovine T cell epitopes for three Mycobacterium bovis antigens: MPB70, 19,000 MW and MPB57. Immunology 82:9-15. [PMC free article] [PubMed] [Google Scholar]
- 48.Ravn, P., A. Demissie, T. Eguale, H. Wondwosson, D. Lein, H. A. Amoudy, A. S. Mustafa, A. K. Jensen, A. Holm, I. Rosenkrands, F. Oftung, J. Olobo, F. von Reyn, and P. Andersen. 1999. Human T cell responses to the ESAT-6 antigen from Mycobacterium tuberculosis. J. Infect. Dis. 179:637-645. [DOI] [PubMed] [Google Scholar]
- 49.Roche, P. W., J. A. Ticcas, D. T. Avery, T. Fifis, H. Billman-Jacobe, and W. J. Britton. 1994. Differential T cell responses to mycobacteria-secreted proteins distinguish vaccination with Bacille Calmette-Guerin from infection with Mycobacterium tuberculosis. J. Infect. Dis. 170:1326-1330. [DOI] [PubMed] [Google Scholar]
- 50.Serbina, N. V., V. Lazarevic, and J. L. Flynn. 2001. CD4(+) T cells are required for the development of cytotoxic CD8(+) T cells during Mycobacterium tuberculosis infection. J. Immunol. 167:6991-7000. [DOI] [PubMed] [Google Scholar]
- 51.Stenger, S., R. J. Mazzaccaro, K. Uyemura, S. Cho, P. F. Barnes, J. P. Rosat, A. Sette, M. B. Brenner, S. A. Porcelli, B. R. Bloom, and R. L. Modlin. 1997. Differential effects of cytolytic T cell subsets on intracellular infection. Science 276:1684-1687. [DOI] [PubMed] [Google Scholar]
- 52.Tascon, R. E., E. Stavropoulos, K. V. Lukacs, and M. J. Colston. 1998. Protection against Mycobacterium tuberculosis infection by CD8+ T cells requires the production of gamma interferon. Infect. Immun. 66:830-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Terasaka, K., R. Yamaguchi, K. Matsuo, A. Yamazaki, S. Nagai, and T. Yamada. 1989. Complete nucleotide sequence of immunogenic protein MPB70 from Mycobacterium bovis BCG. FEMS Microbiol. Lett. 49:273-276. [DOI] [PubMed] [Google Scholar]
- 53a.Tollefsen, S., H. Pollock, T. Lea, M. Harboe, and H. Wiker. Mouse T- and B-cell epitopes in the secreted Mycobacterium bovis antigen MPB70. Scand. J. Immunol., in press. [DOI] [PubMed]
- 54.Torres, M., T. Herrera, H. Villareal, E. A. Rich, and E. Sada. 1998. Cytokine profiles for peripheral blood lymphocytes from patients with active pulmonary tuberculosis and healthy household contacts in response to the 30-kilodalton antigen of Mycobacterium tuberculosis. Infect. Immun. 66:176-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ulrich, T., M. E. Munk, H. Mollenkopf, S. Behr-Perst, R. Colanggeli, M. L. Gennaro, and H. E. Kaufmann. 1998. Differential T cell responses to Mycobacterium tuberculosis ESAT-6 in tuberculosis patients and healthy donors. Eur. J. Immunol. 28:3949-3958. [DOI] [PubMed] [Google Scholar]
- 56.Vordermeier, H. M., P. C. Cockle, A. Whelan, S. Rhodes, N. Palmer, D. Bakker, and R. G. Hewinson. 1999. Development of diagnostic reagents to differentiate between Mycobacterium bovis BCG vaccination and M. bovis infection in cattle. Clin. Diagn. Lab. Immunol. 6:675-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vordermeier, H. M., P. J. Cockle, A. O. Whelan, S. Rhodes, and R. G. Hewinson. 2000. Toward the development of diagnostic assays to discriminate between Mycobacterium bovis infection and Bacille Calmette-Guerin vaccination in cattle. Clin. Infect. Dis. 30:S291-S298. [DOI] [PubMed]
- 58.Wiker, H. G., P. Konstantin, A. Lyashchenko, A. Murat, K. A. Lightbody, J. M. Pollock, S. V. Komissarenko, S. O. Bobrovink, I. N. Kolesnikova, L. O. Mykhalsky, M. L. Gennaro, and M. Harboe. 1998. Immunochemical characterization of the MPB70/80 and MPB83 proteins of Mycobacterium bovis. Infect. Immun. 66:1445-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilkinson, R. J., X. Zhu, K. A. Wilkinson, A. Lalvani, J. Ivanyi, G. Pasvol, and H. M. Vordermeir. 1998. 38 000 MW antigen-specific major histocompatibility complex class I restricted interferon-gamma-secreting CD8+ T cells in healthy contacts of tuberculosis. Immunology 95:585-590. [DOI] [PMC free article] [PubMed] [Google Scholar]