Abstract
Genes of the major histocompatibility complex (MHC) play a critical role in immune recognition, and many alleles confer susceptibility to infectious and autoimmune diseases. How these deleterious alleles persist in populations is controversial. One hypothesis postulates that MHC heterozygote superiority emerges over multiple infections because MHC-mediated resistance is generally dominant and many allele-specific susceptibilities to pathogens will be masked by the resistant allele in heterozygotes. We tested this hypothesis by using experimental coinfections with Salmonella enterica (serovar Typhimurium C5TS) and Theiler's murine encephalomyelitis virus (TMEV) in MHC-congenic mouse strains where one haplotype was resistant to Salmonella and the other was resistant to TMEV. MHC heterozygotes were superior to both homozygotes in 7 out of 8 comparisons (P = 0.0024), and the mean standardized pathogen load of heterozygotes was reduced by 41% over that of homozygotes (P = 0.01). In contrast, no heterozygote superiority was observed when the MHC haplotype combinations had similar susceptibility profiles to the two pathogens. This is the first experimental evidence for MHC heterozygote superiority against multiple pathogens, a mechanism that would contribute to the evolution of MHC diversity and explain the persistence of alleles conferring susceptibility to disease.
Major histocompatibility complex (MHC) genes are the most diverse genes known. One of the postulated mechanisms for maintaining this diversity is MHC heterozygote superiority, or overdominance. The phrase heterozygote advantage (7, 12) is often used as a synonym for heterozygote superiority but is also used for other meanings (36, 38). In population studies, it is used in the general sense to imply that the mean fitness of heterozygotes is higher than the mean fitness of all homozygotes (8, 47). In laboratory studies and evolutionary models, it is often used in the narrow sense to imply that heterozygotes are superior to both parental homozygotes (46). To avoid this confusion, we have defined heterozygote advantage to mean that the heterozygote is better than the average of both homozygotes and we have defined heterozygote superiority to mean that the heterozygote is better than both homozygotes (overdominance) (36). This distinction is important because heterozygote advantage by itself cannot contribute to MHC diversity, whereas heterozygote superiority can (46).
For any given autoimmune or infectious disease, some MHC alleles are usually found that confer susceptibility (3, 47). It is unclear what keeps these demonstrably harmful genes from being eliminated by natural selection. Some form of balancing and diversifying selection must be operating consistently over long periods of evolutionary time to explain the relatively unique and extreme features of MHC diversity (2). This MHC diversity is generally thought to result from pathogen-mediated selection, through either heterozygote superiority or antagonistic host-pathogen coevolution (7, 17). Both mechanisms favor rare and new alleles (selection for divergence) and also maintain large numbers of alleles because allelic extinction rates are reduced. Moreover, both mechanisms predict the existence of some MHC alleles (or genotypes) that confer susceptibility to certain diseases. In the case of heterozygote superiority, low-fitness homozygotes will express a susceptible phenotype. In the case of antagonistic coevolution, MHC alleles that have been the target of recent pathogen evasion (evolution) will be susceptible.
MHC heterozygotes are expected to be superior to both homozygotes because they can present a wider variety of peptide antigens to T lymphocytes (12), the triggering event of the adaptive immune response (51). Although this is the mechanistic explanation generally given for heterozygote superiority, single-infection experiments do not support this hypothesis: heterozygotes are usually similar to the resistant homozygote but are seldom better (2). This is not surprising, since most immune responses are dominated by T-cell recognition of one or a few MHC-presented peptides (immunodominance) (54) rather than all or most of the presented peptides. Therefore, presentation of more peptide antigens by MHC heterozygotes may have little effect on the immune recognition of pathogens.
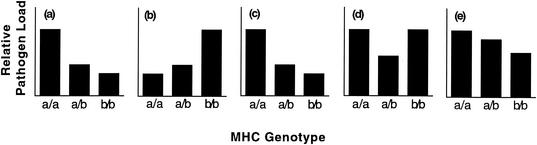
Still, heterozygote superiority is expected to emerge after serial or simultaneous coinfections (12, 24) when resistance is dominant and the susceptibility profiles of two pathogens are opposite or reciprocal (39). We will refer to this as the dual-infection heterozygote superiority (DIHS) hypothesis. Heterozygotes should be superior under these two conditions because, for any pathogen, the susceptibility of the worst allele would be masked by the resistant allele (Fig. 1). In fact, the literature does indicate that MHC-mediated resistance to infections is generally dominant (63% of 49 haplotype-pathogen combinations tested and 79% of 13 pathogens tested) (Table 1). Resistance being dominant provides a mechanistic basis for how MHC heterozygote superiority can occur during dual infections but not during single infections. It also suggests that immunodominance generally favors T-cell responses associated with the more-resistant allele (though this mechanism is not yet characterized) (54).
FIG. 1.
Hypothetical MHC-associated pathogen loads for three pathogens and the predicted combined pathogen loads during different dual infections. MHC genotypes infected with single pathogens (a, b, and c) show different patterns of susceptibility. Combined pathogen loads during dual infections (d and e) are obtained by adding the single pathogen loads for each genotype and standardizing the axes. When animals are infected with two pathogens that show opposite susceptibility profiles (a and b), heterozygotes are predicted to be superior (d). When animals are infected with two pathogens that show similar susceptibility profiles (a and c), heterozygotes are predicted to be intermediate (e). MHC-mediated resistance is dominant in all cases and is required for the predicted patterns (2).
TABLE 1.
Survey of published literature to determine if MHC-dependent resistance is dominant or recessivea
| Infectious agent | Assay | MHC heterozygote(s) | Resistance | Reference(s) |
|---|---|---|---|---|
| Mouse hepatitis virus | Paralysis | b/f | Dominant | 27 |
| Cytomegalovirus | Survival | b/d, b/k, d/k | Dominant | 16 |
| Theiler's virus | Spinal cord viral titer | b/q, f/k | Dominant | This study |
| d/k, d/q females | Dominant | |||
| d/k, d/q males | Recessive | |||
| Demyelination | b/f, b/r, d/f, f/k | Dominant | 35 | |
| Bacteriophage fd | Antibody response | Db/d | Recessive | 25 |
| d/k, b/k | Dominant | |||
| Murine AIDS retrovirus | Disease severity | a/b, b/d, b/k | Recessive | 29 |
| Friend virus | Spleen focus formation | a/b, a/i5, b/d, h4/i5 | Recessive | 50 |
| Recovery | ||||
| High dose | b/k | Recessive | 33 | |
| Low dose | b/k | Dominant | ||
| Vaccinated | b/k | Dominant | ||
| Murine leukemia virus | Virus titer | a/b | Recessive | 48 |
| a/b | Dominant | 31 | ||
| b/pa | Dominant | 11 | ||
| Antibody response | b/d, b/a | Dominant | 49 | |
| Survival | Dd/q | Dominant | 32 | |
| Salmonella enterica (serovar Typhimurium C5TS) | Bacterial titer | b/q | Dominant | This study |
| d/k, d/q, f/k males | Dominant | |||
| d/k, d/q, f/k females | Recessive | |||
| Streptococcus pyogenes | Hepatic granuloma formation | b/d, d/k | Dominant | 10 |
| b/k | Recessive | |||
| Leishmania donovani | Parasitemia | b/d | Recessive | 6 |
| Schistosoma mansoni | Parasitemia | b/k | Dominant | 45 |
| Trypanosoma cruzi | Parasitemia | b/s females | Dominant | 52, 53 |
| b/s males | Recessive | |||
| Trichuris muris | Worm burden | k/q | Dominant | 13 |
| Heligmosomoides polygyrus | Worm burden | b/k, d/k, s/k | Dominant | 4 |
This table provides a survey of the literature to evaluate whether MHC-dependent resistance shows dominant or recessive patterns of inheritance. The literature was accumulated by using previous reviews (2, 36) as well as MedLine searches with the following keywords in various combinations: MHC, H-2∗, congenic, F1, resistance, susceptibility, dominan∗, and susceptib∗ (where ∗ returns all possible endings). To be included, a study had to use MHC-congenic animals (a necessary condition not completely followed in previous results [2, 36]) in an infection model where heterozygotes and both homozygotes were evaluated. Resistance of a particular MHC haplotypic combination was scored as dominant when the heterozygote was more like the best than the worst homozygote. If the converse was true, it was scored as recessive. If an MHC heterozygote was assayed more than once with the same result, it was only counted once for summary statistics (secondary accounts are underlined). When each haplotype was counted, resistance was dominant in 63% of the 49 haplotype-pathogen combinations tested. If each pathogen was scored as either dominant or recessive, dependent on the majority pattern (ties are excluded), then resistance was dominant 79% of the time (10 out of 13 pathogens). Lowercase italicized letters indicate MHC haplotypes. Capital letters indicate MHC genes, with superscript allelic designations.
Therefore, the critical empirical test of the DIHS hypothesis requires resistance to be dominant in experimental dual infections with pathogens and MHC combinations that show both opposite (Fig. 1d) and similar susceptibility profiles (Fig. 1e, negative control). If MHC haplotypes confer opposite patterns of susceptibility (Fig. 1a and b), then heterozygotes should be superior when infected by both pathogens (Fig. 1d). However, if MHC haplotypes confer similar patterns of susceptibility (Fig. 1a and c), then heterozygotes should be intermediate between the two homozygotes (Fig. 1e).
In this study, we provide the first experimental test of the DIHS hypothesis by coinfection with the pathogens Salmonella enterica (serovar Typhimurium C5TS) and Theiler's murine encephalomyelitis virus (TMEV). Although serial infections are generally more common in nature, coinfections represent a potentially important case in which MHC-dependent escape variants emerge (causing different susceptibility profiles) during the course of single infections, as seen for diversifying pathogens such as human immunodeficiency virus (HIV) (30), simian immunodeficiency virus (1), hepatitis B (5), hepatitis C (14), and human T-cell leukemia virus (15). We demonstrate that MHC heterozygote superiority emerges when resistance is dominant and MHC haplotypic combinations have susceptibility profiles that are opposite. These results provide a mechanism that could help explain the unprecedented diversity of MHC genes and the persistence of MHC alleles which confer disease susceptibility.
MATERIALS AND METHODS
Mice.
MHC-congenic mice (C57BL/10SnJ-H2b, B10.D2-H2d, B10.M-H2f, B10.BR-H2k, and B10.Q-H2q) were obtained from Jackson Laboratories and bred thereafter under specific-pathogen-free conditions. In order to randomize any genetic mutations that have become differentially fixed in these strains of mice (9), the F1 heterozygotes (b/q, d/q, d/k, and f/k) were intercrossed to produce F2 segregants, which were used for all infections. To control for potential variation among cages, mice were housed by sex in groups of six individuals representing the three genotypes to be compared (e.g., b/b, b/q, and q/q or d/d, d/q, and q/q). All q/q and k/k mice were analyzed according to their housing conditions and thus not pooled for analysis (e.g., q/q mice housed with b/b and b/q mice were analyzed only with the b/q haplotypic combination). All cagemates were unrelated and had not been previously housed together when infected. The original coinfection experiment was repeated (see in Fig. 5, experiment 2) in an independent experiment in which the F2 mice were housed with F1 and P0 mice of the same genotype to evaluate any differences between the generations (data not shown for F1 and P0). All animal use complied with federal regulations and the University of Utah's Institutional Animal Care and Use Committee guidelines.
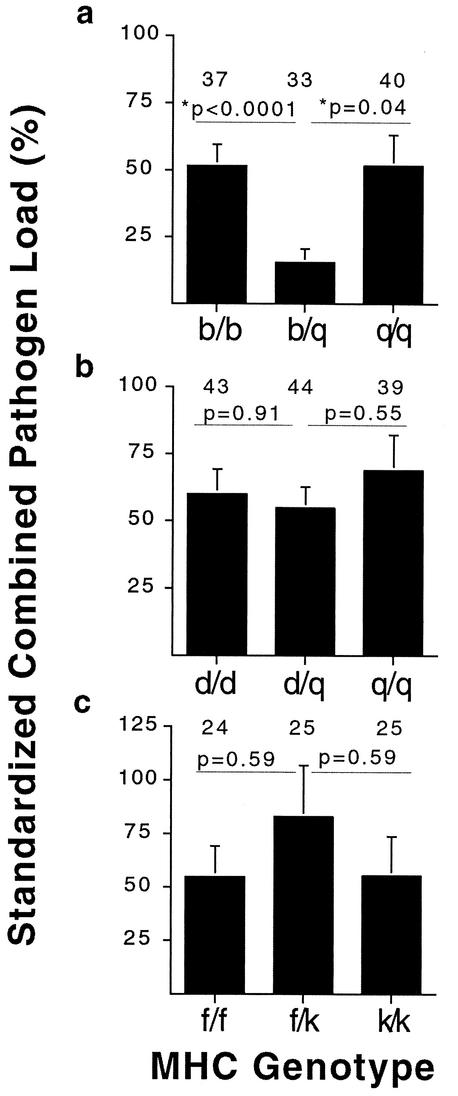
FIG. 5.
Pooled standardized combined pathogen loads for genotypic combinations showing opposite (a and b) and similar (c) susceptibility profiles. The sexes and both experiments were pooled for the b/q and d/q combinations (a and b). All P values shown are from a Wilcoxon rank-sum test. Sample sizes are indicated above each bar.
Pathogens.
Mice were anesthetized with metofane and simultaneously infected retro-orbitally with 1 × 106 CFU of the C5TS strain of S. enterica (serovar Typhimurium)/ml and intracerebrally with 2 × 105 PFU of the DA strain of TMEV/ml. The C5TS strain is a temperature-sensitive mutant strain of Salmonella (22). As a control, the single infections also received a sham infection of phosphate-buffered saline to control for the effects of two injections. Both agents are natural pathogens of mice.
Salmonella loads.
Mice were sacrificed 4 weeks postinfection. Salmonella loads were assayed from platings of homogenized spleens. Briefly, spleens were collected under antiseptic conditions, immediately homogenized, and plated on Shigella-Salmonella agar, which was then incubated overnight at 30°C. When this strain was created in the early 1970s (22) with a UV screen, the authors found that a small (unspecified) percentage reverted to the wild-type C5 virulent strain. This revertant phenotype was seen in 8.7% of the mice infected in this study. To check for possible wild-type revertants, the spleen homogenate was also plated on Shigella-Salmonella agar and incubated overnight at 37°C. If more than 50% of the colonies of an infected mouse also grew at 37°C, then the infection was deemed to be dominated by a revertant and the data point was excluded on the basis that the revertant was more lethal (i.e., faster replication rate than C5TS). Thus, comparing mortality or pathogen loads between animals that did or did not contain a revertant would be inappropriate. A total of 205 (out of 1,061) animals were excluded either because they died or were sacrificed prior to day 28 postinfection or because they contained Salmonella revertants. Of the excluded mice, 78 died early, 94 were sacrificed prior to day 28 postinfection due to severe illness, 31 contained revertants, and 2 were excluded due to unknown genotype. Of the 94 mice (8.9%) sacrificed early, 61 contained revertants. The other 33 mice that were sacrificed early were ill due to high TMEV loads or high C5TS loads. For the 78 that died early (7.4%), the cause of death was probably due to a reversion in C5TS or high TMEV or C5TS loads. There was no association with MHC genotype in the excluded animals that died (or were sacrificed) early (χ2 = 4.914, P = 0.77). However, there was a significant association with MHC genotype in animals that contained revertants versus those that did not (χ2 =20.515, P = 0.0086). The genotype with the highest number of revertants was the most susceptible (b/b) while the genotype with the lowest number of revertants was the most resistant (q/q), suggesting that the pattern may simply be due to mutation rate (i.e., more revertants would occur in hosts with faster pathogen replication). We believe this makes our results more conservative because the animals with the high pathogen loads were disproportionately thrown out, making differences smaller.
TMEV loads.
TMEV loads were assayed by conducting viral plaque assays on brain and spinal cord homogenates (26). Briefly, brain and spinal cords were collected and stored in 0.5 ml of TMEV diluent (phosphate-buffered saline, 1% antibiotics, 1% fetal calf serum) at −70°C until assayed. Brain and spinal cords were homogenized and freeze-thawed twice. Samples were then centrifuged at 450 × g for 10 min, and the supernatant was collected. Supernatant fluid was then added to baby hamster kidney-21 (BHK) cells and incubated for 1 h at 37°C, with hand rocking every 10 to 15 min. The fluid was then aspirated off the BHK cells, and a 1:1 mixture of 1% agarose-2× 199 medium was added to the wells. Plates were incubated at 37°C for 4 days before fixation with 2.5% formalin followed by staining with 0.1% crystal violet and enumeration of plaques.
Standardized combined pathogen loads.
Because the actual pathogen loads of Salmonella and TMEV differed by at least 1 order of magnitude, we standardized the loads to make them comparable and to obtain an overall pathogen load for every mouse. Thus, the standardized combined pathogen load was calculated as follows. The mean Salmonella and TMEV loads were determined for each MHC genotype. For example, the Salmonella loads of each b/b, b/q, and q/q mouse in the group were divided by the mean of the worst genotype and multiplied by 100. All other genotypic combinations were calculated in the same way. This was repeated for the TMEV loads, and then the Salmonella and TMEV percents were averaged for every mouse. Thus, the overall pathogen load for every mouse was standardized by sex and experiment, allowing all of the data to be pooled. This standardized combined pathogen load gives equal weight to both pathogens. This may not be true in relation to actual fitness impacts on the host, but at this point we have no way to test for possible fitness differences.
Statistics.
Male and female animals were analyzed separately because they had significantly different pathogen loads (Fig. 2 and 3). Because sex, genotype, and experiment were independent (Fig. 4), a binomial distribution was used to test whether the results of these independent experiments showed a significant pattern of heterozygote superiority. Since there were three possible patterns, (i) overdominance (the heterozygote was better than both homozygotes), (ii) underdominance (the heterozygote was worse than both homozygotes), or (iii) codominance (the heterozygote was intermediate between the two homozygotes), a value of 1/3 was used for P (the probability of obtaining any one of the three patterns). All other comparisons were analyzed with the Wilcoxon rank-sum test, unless otherwise stated. All pathogen loads were determined without knowledge of the MHC genotype.
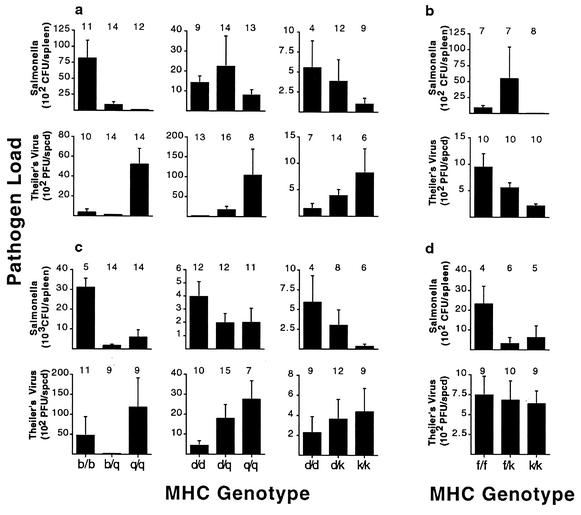
FIG. 2.
Salmonella and TMEV loads for single infections. Pathogen loads of female (a) and male (c) animals of different MHC heterozygote combinations showing opposite susceptibility profiles. Pathogen loads of female (b) and male (d) animals of different MHC heterozygote combinations showing similar susceptibility profiles. In the case of opposite susceptibility profiles (a and c), all homozygotes were significantly different (P < 0.05 by t test), except the following: Salmonella loads for the d/d and q/q genotypic combinations in females (P = 0.08), TMEV loads for the d/d and k/k genotypic combinations in females (P = 0.17), and TMEV loads for the d/d and k/k genotypic combinations in males (P = 0.08). Sample sizes are indicated above each bar. spcd, spinal cord.
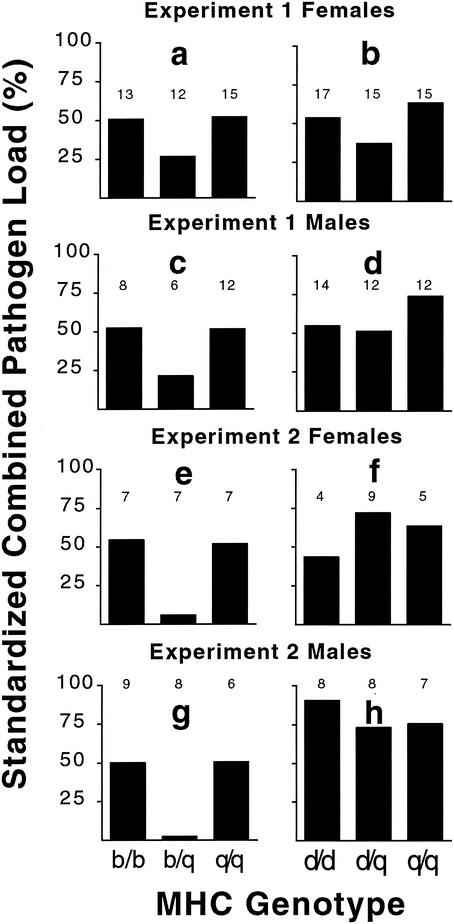
FIG. 4.
Standardized combined pathogen loads for animals showing opposite susceptibility profiles. We calculated the combined pathogen load from the individual Salmonella and TMEV loads for the two different genotypic combinations showing opposite susceptibility profiles, b/q (a, c, e, and g) and d/q (b, d, f, and h). This was done for two independent experiments and for each sex. Sample sizes are indicated above each bar.
RESULTS
To verify that the MHC haplotypic combinations that we chose from the literature indeed showed opposite (or similar) susceptibility profiles, we evaluated these profiles in both single infections and coinfections. The results of single-infection experiments with the pathogen Salmonella or TMEV are indicated in Fig. 2. MHC had a significant effect on resistance, as predicted from the published literature (34, 43). MHC haplotypic combinations (b with q, d with q, and d with k) showed opposite susceptibility profiles, whereas the MHC haplotypic combination (f with k) showed similar susceptibility profiles when comparing these two pathogens. Resistance was usually dominant (11 out of 16 comparisons), with some exceptions. These haplotypic combinations were then coinfected, and pathogen loads were determined 4 weeks postinfection.
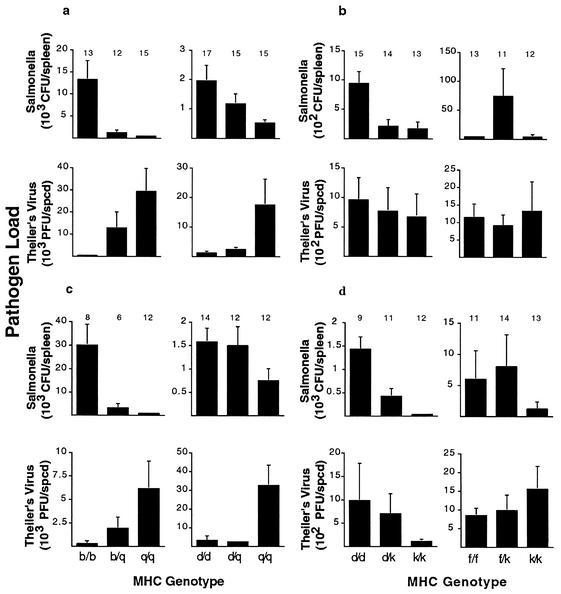
Figure 3 illustrates the pathogen loads for MHC genotypes of coinfected mice showing either opposite (Fig. 3a and c) or similar (Fig. 3b and d) patterns of susceptibility. Female data are depicted in Fig. 3a and b, and male data are shown in Fig. 3c and d. As expected, the MHC b/b and q/q genotypes show opposite patterns of susceptibility to Salmonella and TMEV, as do the MHC d/d and q/q genotypes (Fig. 3a and c). Also as expected, the MHC f/f and k/k genotypes show similar patterns of susceptibility to these two pathogens. However, the MHC genotypes d/d and k/k (Fig. 3b and d) showed similar patterns of susceptibility to Salmonella and TMEV, the reverse of what we found in the single-infection experiments (Fig. 2).
FIG. 3.
Salmonella and TMEV loads for coinfected mice of either opposite or similar susceptibility profiles. Pathogen loads are given for coinfected female (a) and male (c) animals of different MHC heterozygote combinations showing opposite susceptibility profiles. Pathogen loads are given for coinfected female (b) and male (d) animals of different MHC heterozygote combinations showing similar susceptibility profiles. In the case of opposite susceptibility profiles (a and c), all homozygotes were significantly different (P < 0.05 by t test), except TMEV loads for the b/b and q/q genotypic combinations in males (P = 0.07), and resistance was dominant (pathogen loads of heterozygotes were more like the resistant than the susceptible homozygote). In the case of similar susceptibility profiles, all homozygotes were significantly different (P < 0.05 by t test) for the Salmonella infection but not for the TMEV infection (P > 0.60). Sample sizes are indicated above each bar.
This apparent reversal of susceptibility patterns between single infections and coinfections suggests there is an interaction between these two pathogens during coinfection and these specific MHC genotypes, as seen in other coinfections such as HIV and many parasitic diseases (19). When we tested this hypothesis, there was not a significant difference in the overall pattern of susceptibility (opposite or similar) between single infections and coinfections (data not shown). However, there was no significant difference between the d/d and k/k homozygotes for either the single infections (Fig. 2) or coinfections (Fig. 3). Thus, for this genotypic combination, we cannot determine whether the TMEV loads show an opposite or similar pattern of susceptibility, and so we excluded them from further analysis. All patterns seen in single infections and coinfections (Fig. 2 and 3) are similar to those seen in previous studies (34, 43), except the d/k genotypic combination.
Figure 4 compares the results of eight coinfection groups of MHC genotypic combinations showing opposite (reciprocal) patterns of susceptibility. These eight experiments represent comparisons of two haplotype combinations (b with q plus d with q), both sexes, and two independent experiments. The pattern of heterozygote superiority was observed for standardized combined pathogen loads in seven of the eight groups (P = 0.0024 by binomial distribution, where P = 1/3). Even if we conservatively excluded the two groups where the heterozygote load was similar to the best homozygote (Fig. 4d and h), the pattern of heterozygote superiority is still significant (five out of six groups, P = 0.01 by binomial distribution, where P = 1/3). Analyzing all eight groups, the standardized combined pathogen load in heterozygotes was 41% lower than the load in homozygotes (P = 0.01, by the Wilcoxon rank-sum test).
To estimate the relative strength that each genotypic combination contributed to the overall significant pattern of heterozygote superiority (Fig. 4), we pooled the standardized data across sex and experiments for each genotype. Figure 5 illustrates the standardized combined pathogen loads for the genotypic combinations showing opposite (Fig. 5a and b) and similar (Fig. 5c) susceptibility profiles. For opposite susceptibility profiles, the b with q haplotypic combinations showed the largest effect, with pathogen loads of the b/q heterozygotes approximately 71% lower than those of both the b/b and q/q homozygotes (P < 0.0001 and P = 0.04, respectively, by the Wilcoxon rank-sum test) (Fig. 5a). The d and q genotypic combination (Fig. 5b) showed a nonsignificant pattern of heterozygote superiority, and the relative strength was smaller than that of b and q, with pathogen loads of the d/q heterozygote being 9% lower than those of d/d homozygotes and 20% lower than those of q/q homozygotes. When the MHC genotypic combination showing similar patterns of susceptibility was examined, MHC f/k heterozygotes were worse than both homozygotes (Fig. 5c). This nonsignificant pattern may be due to the susceptibility being dominant for the Salmonella infection (Fig. 3b and d).
We used pathogen load as a measure of host immunocompetence due to its strong inverse correlation with overall host fitness (e.g., HIV) (41). We tested the effects of MHC genotype on death and weight, but no significant differences were found (data not shown). These infections were generally benign, with these young, growing animals gaining weight during the infection, although at a slower rate than their uninfected siblings (data not shown).
DISCUSSION
This is the first experimental evidence for MHC heterozygote superiority, a pathogen-mediated mechanism that could contribute to the evolution of MHC diversity. A pattern of MHC heterozygote superiority was seen in 7 out of 8 comparisons (P = 0.0024) (Fig. 4), and the mean standardized load of the heterozygotes was 41% lower than the mean of the homozygotes (P = 0.01). The pooled data also showed a significant pattern of heterozygote superiority for the b/q genotypic combination (Fig. 5). The negative-control combination of f and k haplotypes did not show a heterozygote superiority pattern. This control excludes the alternative hypothesis that MHC heterozygotes have a general advantage under all coinfections, as would be the case if the basis of MHC heterozygote superiority were the presentation of more peptide antigens.
For the DIHS hypothesis to be a general explanation (at least in part) for MHC diversity, the infections experienced over the lifetimes of individuals must in general lead to heterozygote superiority. This requires two relationships. First, MHC heterozygotes must be superior in dealing with infections separated in time, as this will be more common than coinfections. While our experiments used simultaneous dual infections, there are no a priori reasons that multiple infections separated in time would not also lead to heterozygote superiority. The combined pathogen load, although occurring over a longer time period, would still be expected to be lower for heterozygotes. Second, the susceptibility and resistance patterns of host MHC alleles to natural pathogens must be somewhat balanced or the worst alleles will become extinct (28). A recent survey of MHC-dependent susceptibility and resistance patterns across a number of pathogens shows that any given haplotype tends to be resistant to some infectious agents and susceptible to others (37). Whether these two relationships are generally true is an empirical question and remains to be tested.
Simulation models demonstrate that symmetrical heterozygote superiority (where fitness of heterozygotes is greater than that of homozygotes and all homozygotes and heterozygotes have equal lifetime fitnesses within these two genotypic classes) is a powerful diversity-maintaining force that can account for MHC diversity (46). The degree of asymmetry that can be tolerated and still maintain MHC diversity is more controversial (19, 28). This study provides evidence for the hypothesis that heterozygote superiority can emerge under conditions where resistance is dominant and susceptibility profiles are opposite, which are commonly observed (37) (Table 1). However, determining whether heterozygote superiority emerges under the variety of pathogens experienced in the wild will be difficult, as it will require measuring lifetime fitness.
Observational evidence for heterozygote advantage (which may or may not be due to heterozygote superiority) (38) has been reported for HIV (8) and hepatitis B virus (47) in humans. Although these studies found heterozygote advantage for single infections, the specific pathogens involved can emulate a multiple infection. Both HIV (40, 42) and hepatitis B virus (23) are polymorphic infections due to mutations that accumulate rapidly during chronic infection. Also, both HIV (30) and hepatitis B virus (5) have been shown to escape MHC-dependent immune recognition, so it is possible that some of these polymorphisms could contain viral variants that would create opposite susceptibility profiles in MHC-heterozygous hosts. Both studies propose that MHC heterozygotes had an advantage because they present more viral epitopes, or as found in the HIV study, that MHC heterozygotes may prevent the escape of MHC-dependent immune recognition. The DIHS hypothesis provides a new way to explain both the HIV and hepatitis B results. However, since both studies only compared heterozygotes to the means of homozygotes, their results may simply be heterozygote advantage and not heterozygote superiority (36, 38), a result expected simply by MHC resistance being dominant over susceptibility (Table 1).
Simulation models demonstrate that small levels of (symmetrical) heterozygote superiority (approximately 1%) are sufficient to explain the observed levels of MHC genetic diversity (44, 46). It is generally impossible to obtain sample sizes with vertebrates to statistically demonstrate a 1% effect, which may explain why MHC heterozygote superiority has been so hard to find in previous studies (21). The 9 to 20% effect found for the d and q genotypic combination was not significant by itself (Fig. 5), but when combined with the b and q genotypic combination, it contributed to the significance of overall heterozygote superiority (Fig. 4). The fact that both genotypic combinations showing opposite susceptibility profiles in this study were considerably larger than 1% is not too surprising because the 1% requirement applies to the combined effects of all pathogens over a lifetime. Any pathogen pair and genotypic combination that might show a large effect will be countered by other pathogen or genotypic combinations showing small or opposite effects.
This is the first experimental evidence for MHC heterozygote superiority under coinfection, a mechanism that could explain (at least in part) the divergence of MHC genes and the maintenance of high levels of allelic diversity found in nature. The excess of MHC heterozygotes found in some natural populations is consistent with such a mechanism (20). However, these results do not exclude other proposed mechanisms of selection for MHC diversity such as pathogen evasion, autoimmunity, mating preferences or other reproductive mechanisms (2, 19). Any or all of them may be more important than heterozygote superiority. Future studies will be required to evaluate the relative importance of these proposed evolutionary forces for diversifying MHC genes and the functional significance these polymorphisms have on vertebrate immunity.
Acknowledgments
We thank Linda Morrison, Jane Libbey, and Elena Enioutina for excellent technical assistance; Lara Carroll, Andrew Pacejka, Bradley Demarest, Sarah Zala, Patricia Slev, David Witherspoon, and Robert Fujinami for helpful comments; Carlos Hormaeche for donation of the Salmonella strain C5TS; Jay Renolds, Maureen Wilkinson, and numerous undergraduates for help with mouse care; and Fred Adler, Jon Seger, Pat Corneli, and Paul Bernhardt for statistical advice.
This work was supported by an NIH grant to W.K.P. (GM39578) and partially by an NIH grant to Robert S. Fujinami (NS34497).
Editor: A. D. O'Brien
REFERENCES
- 1.Allen, T. M., D. H. O'Connor, P. Jing, J. L. Dzuris, B. R. Mothe, T. U. Vogel, E. Dunphy, M. E. Liebl, C. Emerson, N. Wilson, K. J. Kunstman, X. Wang, D. B. Allison, A. L. Hughes, R. C. Desrosiers, J. D. Altman, S. M. Wolinsky, A. Sette, and D. I. Watkins. 2000. Tat-specific cytotoxic T lymphocytes select for SIV escape variants during resolution of primary viraemia. Nature 407:386-390. [DOI] [PubMed] [Google Scholar]
- 2.Apanius, V., D. Penn, P. Slev, L. R. Ruff, and W. K. Potts. 1997. The nature of selection on the major histocompatibility complex. Crit. Rev. Immunol. 17:179-224. [DOI] [PubMed] [Google Scholar]
- 3.Baum, H., and N. A. Staines. 1997. MHC-derived peptides and the CD4+ T-cell repertoire: implications for autoimmune disease. Cytokines Cell. Mol. Ther. 3:115-125. [PubMed] [Google Scholar]
- 4.Behnke, J. M., and F. N. Wahid. 1991. Immunological relationships during primary infection with Heligmosomoides polygyrus (Nematospiroides dubius): H-2 linked genes determine worm survival. Parasitology 103:157-164. [DOI] [PubMed] [Google Scholar]
- 5.Bertoletti, A., A. Sette, F. V. Chisari, A. Penna, M. Levrero, M. De Carli, F. Fiaccadori, and C. Ferrari. 1994. Natural variants of cytotoxic epitopes are T-cell receptor antagonists for antiviral cytotoxic T cells. Nature 369:407-410. [DOI] [PubMed] [Google Scholar]
- 6.Blackwell, J., J. Freeman, and D. Bradley. 1980. Influence of H-2 complex on acquired resistance to Leishmania donovani infection in mice. Nature 283:72-74. [DOI] [PubMed] [Google Scholar]
- 7.Bodmer, W. F. 1972. Evolutionary significance of the HL-A system. Nature 237:139-145. [DOI] [PubMed] [Google Scholar]
- 8.Carrington, M., G. W. Nelson, M. P. Martin, T. Kissner, D. Vlahov, J. J. Goedert, R. Kaslow, S. Buchbinder, K. Hoots, and S. J. O'Brien. 1999. HLA and HIV-1: heterozygote advantage and B∗35-Cw∗04 disadvantage. Science 283:1748-1752. [DOI] [PubMed] [Google Scholar]
- 9.Carroll, L. S., and W. K. Potts. 2001. Accumulated background variation among H2 mutant congenic strains: elimination through PCR-based genotyping of F(2) segregants. J. Immunol. Methods 257:137-143. [DOI] [PubMed] [Google Scholar]
- 10.Chen, C. Y., S. A. Cohen, M. B. Zaleski, and B. Albini. 1992. Genetic control of streptococcus-induced hepatic granulomatous lesions in mice. Immunogenetics 36:28-32. [DOI] [PubMed] [Google Scholar]
- 11.Colombatti, A., A. Dux, A. Berns, P. Demant, and J. Hilgers. 1979. H-2-dependent regulation of the high level of expression of ecotropic murine leukemia virus. J. Natl. Cancer Inst. 63:869-873. [DOI] [PubMed] [Google Scholar]
- 12.Doherty, P. C., and R. M. Zinkernagel. 1975. A biological role for the major histocompatibility antigens. Lancet i:1406.. [DOI] [PubMed] [Google Scholar]
- 13.Else, K. J., D. Wakelin, D. L. Wassom, and K. M. Hauda. 1990. The influence of genes mapping within the major histocompatibility complex on resistance to Trichuris muris infections in mice. Parasitology 101:61-67. [DOI] [PubMed] [Google Scholar]
- 14.Erickson, A. L., Y. Kimura, S. Igarashi, J. Eichelberger, M. Houghton, J. Sidney, D. McKinney, A. Sette, A. L. Hughes, and C. M. Walker. 2001. The outcome of hepatitis C virus infection is predicted by escape mutations in epitopes targeted by cytotoxic T lymphocytes. Immunity 15:883-895. [DOI] [PubMed] [Google Scholar]
- 15.Furukawa, Y., R. Kubota, M. Tara, S. Izumo, and M. Osame. 2001. Existence of escape mutant in HTLV-I tax during the development of adult T-cell leukemia. Blood 97:987-993. [DOI] [PubMed] [Google Scholar]
- 16.Grundy, J. E., J. S. Mackenzie, and N. F. Stanley. 1981. Influence of H-2 and non-H-2 genes on resistance to murine cytomegalovirus infection. Infect. Immun. 32:277-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haldane, J. B. S. 1949. Disease and evolution. Ric. Sci. 19:68-75. [Google Scholar]
- 18.Harms, G., and H. Feldmeier. 2002. HIV infection and tropical parasitic diseases-deleterious interactions in both directions? Trop. Med. Int. Health 7:479-488. [DOI] [PubMed] [Google Scholar]
- 19.Hedrick, P. W. 1999. Balancing selection and MHC. Genetica 104:207-214. [DOI] [PubMed] [Google Scholar]
- 20.Hedrick, P. W., and F. L. Black. 1997. Random mating and selection in families against homozygotes for HLA in south Amerindians. Hereditas 127:51-58. [DOI] [PubMed] [Google Scholar]
- 21.Hill, A. V., C. E. Allsopp, D. Kwiatkowski, N. M. Anstey, P. Twumasi, P. A. Rowe, S. Bennett, D. Brewster, A. J. McMichael, and B. M. Greenwood. 1991. Common west African HLA antigens are associated with protection from severe malaria. Nature 352:595-600. [DOI] [PubMed] [Google Scholar]
- 22.Hormaeche, C. E., R. A. Pettifor, and J. Brock. 1981. The fate of temperature-sensitive Salmonella mutants in vivo in naturally resistant and susceptible mice. Immunology 42:569-576. [PMC free article] [PubMed] [Google Scholar]
- 23.Hosono, S., P. C. Tai, W. Wang, M. Ambrose, D. G. Hwang, T. T. Yuan, B. H. Peng, C. S. Yang, C. S. Lee, and C. Shih. 1995. Core antigen mutations of human hepatitis B virus in hepatomas accumulate in MHC class II-restricted T cell epitopes. Virology 212:151-162. [DOI] [PubMed] [Google Scholar]
- 24.Hughes, A. L., and M. Nei. 1992. Models of host-parasite interaction and MHC polymorphism. Genetics 132:863-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kolsch, E., and F. W. Falkenberg. 1977. Genetic control of the immune response to phage fd. IV. Complementation between H-2-linked Ir genes. J. Immunol. 119:2114-2119. [PubMed] [Google Scholar]
- 26.Kurtz, C. I., X. M. Sun, and R. S. Fujinami. 1995. Protection of SJL/J mice from demyelinating disease mediated by Theiler's murine encephalomyelitis virus. Microb. Pathog. 18:11-27. [DOI] [PubMed] [Google Scholar]
- 27.Levy-Leblond, E., D. Oth, and J. M. Dupuy. 1979. Genetic study of mouse sensitivity to MHV3 infection: influence of the H-2 complex. J. Immunol. 122:1359-1362. [PubMed] [Google Scholar]
- 28.Lewontin, R. C., L. R. Ginzburg, and S. Tuljapurkar. 1978. Heterosis as an explanation for large amounts of genic polymorphism. Genetics 88:149-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Makino, M., H. C. Morse III, T. N. Fredrickson, and J. W. Hartley. 1990. H-2-associated and background genes influence the development of a murine retrovirus-induced immunodeficiency syndrome. J. Immunol. 144:4347-4355. [PubMed] [Google Scholar]
- 30.McMichael, A. J., and R. E. Phillips. 1997. Escape of human immunodeficiency virus from immune control. Annu. Rev. Immunol. 15:271-296. [DOI] [PubMed] [Google Scholar]
- 31.Melief, C. J., A. Vlug, R. E. de Goede, C. de Bruyne, W. Barendsen, and P. de Greeve. 1980. Naturally occurring leukemia viruses in H-2 congenic C57BL mice. I. High lymphoma incidence following milk-borne transmission of virus. J. Natl. Cancer Inst. 64:1179-1189. [PubMed] [Google Scholar]
- 32.Meruelo, D., M. Leiberman, N. Ginzton, B. Deak, and H. O. McDevitt. 1977. Genetic control of radiation leukemia virus-induced tumorigenesis. I. Role of the major murine histocompatibility complex, H-2. J. Exp. Med. 146:1079-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyazawa, M., J. Nishio, and B. Chesebro. 1988. Genetic control of T cell responsiveness to the Friend murine leukemia virus envelope antigen. Identification of class II loci of the H-2 as immune response genes. J. Exp. Med. 168:1587-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nauciel, C., E. Ronco, J. L. Guenet, and M. Pla. 1988. Role of H-2 and non-H-2 genes in control of bacterial clearance from the spleen in Salmonella typhimurium-infected mice. Infect. Immun. 56:2407-2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patick, A. K., L. R. Pease, C. S. David, and M. Rodriguez. 1990. Major histocompatibility complex-conferred resistance to Theiler's virus-induced demyelinating disease is inherited as a dominant trait in B10 congenic mice. J. Virol. 64:5570-5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Penn, D. 2002. The scent of genetic compatibility: sexual selection and the major histocompatibility complex. Ethology 108:1-21. [Google Scholar]
- 37.Penn, D., and W. Potts. 1999. The evolution of mating preferences and major histocompatibility genes. Am. Nat. 153:145-164. [DOI] [PubMed] [Google Scholar]
- 38.Penn, D. J., K. Damjanovich, and W. K. Potts. 2002. MHC heterozygosity confers a selective advantage against multiple-strain infections. Proc. Natl. Acad. Sci. USA 99:11260-11264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Potts, W. K., and P. R. Slev. 1995. Pathogen-based models favoring MHC genetic diversity. Immunol. Rev. 143:181-197. [DOI] [PubMed] [Google Scholar]
- 40.Preston, B. D., B. J. Poiesz, and L. A. Loeb. 1988. Fidelity of HIV-1 reverse transcriptase. Science 242:1168-1171. [DOI] [PubMed] [Google Scholar]
- 41.Regoes, R. R., S. I. Staprans, M. B. Feinberg, and S. Bonhoeffer. 2002. Contribution of peaks of virus load to simian immunodeficiency virus pathogenesis. J. Virol. 76:2573-2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roberts, J. D., K. Bebenek, and T. A. Kunkel. 1988. The accuracy of reverse transcriptase from HIV-1. Science 242:1171-1173. [DOI] [PubMed] [Google Scholar]
- 43.Rodriguez, M., and C. S. David. 1985. Demyelination induced by Theiler's virus: influence of the H-2 haplotype. J. Immunol. 135:2145-2148. [PubMed] [Google Scholar]
- 44.Satta, Y., C. O'Huigin, N. Takahata, and J. Klein. 1994. Intensity of natural selection at the major histocompatibility complex loci. Proc. Natl. Acad. Sci. USA 91:7184-7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sher, A., S. Hieny, and S. James. 1984. Mechanisms of protective immunity against S. mansoni infection in mice vaccinated with irradiated cercariae. VI. Influence of the major histocompatibility complex. Parasite Immunol. 6:319-328. [DOI] [PubMed] [Google Scholar]
- 46.Takahata, N., and M. Nei. 1990. Allelic genealogy under overdominant and frequency-dependent selection and polymorphism of major histocompatibility complex loci. Genetics 124:967-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thursz, M. R., H. C. Thomas, B. M. Greenwood, and A. V. Hill. 1997. Heterozygote advantage for HLA class-II type in hepatitis B virus infection. Nat. Genet. 17:11-12. [DOI] [PubMed] [Google Scholar]
- 48.Tucker, H., J. Weens, P. Tsichlis, R. S. Schwartz, R. Khiroya, and J. Donnelly. 1977. Influence of H-2 complex on susceptibility to infection by murine leukemia virus. J. Immunol. 118:1239-1243. [PubMed] [Google Scholar]
- 49.Vasmel, W. L., M. Zijlstra, T. Radaszkiewicz, C. J. Leupers, R. E. de Goede, and C. J. Melief. 1988. Major histocompatibility complex class II-regulated immunity to murine leukemia virus protects against early T- but not late B-cell lymphomas. J. Virol. 62:3156-3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wettstein, P. J., and K. J. Blank. 1982. Use of H-2:H-7 congenic mice to study H-2-mediated resistance to Friend leukemia virus. J. Immunol. 129:358-361. [PubMed] [Google Scholar]
- 51.Whitton, J. L., and M. B. Oldstone. 1989. Class I MHC can present an endogenous peptide to cytotoxic T lymphocytes. J. Exp. Med. 170:1033-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wrightsman, R., S. Krassner, and J. Watson. 1982. Genetic control of responses to Trypanosoma cruzi in mice: multiple genes influencing parasitemia and survival. Infect. Immun. 36:637-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wrightsman, R. A., S. M. Krassner, J. D. Watson, and J. E. Manning. 1984. Role of the H-2s haplotype in survival of mice after infection with Trypanosoma cruzi. Infect. Immun. 44:351-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yewdell, J. W., and J. R. Bennink. 1999. Immunodominance in major histocompatibility complex class I-restricted T lymphocyte responses. Annu. Rev. Immunol. 17:51-88. [DOI] [PubMed] [Google Scholar]