Abstract
T1/ST2 is a stable cell surface marker selectively expressed on type 2 T helper (Th2) effector cells. Since nonhealing Leishmania major infections in susceptible BALB/c mice have been ascribed to a polarized Th2 response, we used an anti-T1/ST2 monoclonal antibody (MAb) or a T1-Fc fusion protein to investigate the role of CD4+ T1/ST2+ Th2 cells in experimental leishmaniasis. We show that interfering with T1/ST2 signaling had no effect on lesion development or parasite replication; however, it induced a significantly higher type 1 response and an enhanced capacity of CD4+ T cells to respond to interleukin 12 (IL-12). Surprisingly, even in the presence of an elevated Th1 response, the production of antigen-specific type 2 cytokines was not altered in the group of mice treated with the anti-T1/ST2 MAb or the T1-Fc fusion protein. To characterize further this Th2 response, we assessed the cytokine profile of CD4+ T cells and found that interfering with T1/ST2 signaling did not alter the cytokine profile of CD4+ T1/ST2+ T cells. These results show that T1/ST2 signaling is not necessary for the differentiation of naive CD4+ T cells into antigen-specific CD4+ T1/ST2+ Th2 cells. In addition to CD4+ T1/ST2+ T cells, we detected another subpopulation of CD4+ Th2 cells, negative for the expression of T1/ST2, that could differentiate in vivo in response to L. major infection. Taken together, our results suggest that CD4+ T1/ST2+ Th2 cells but not CD4+ T1/ST2− Th2 cells can downregulate the Th1 response during the course of a nonhealing L. major infection through a mechanism that is independent of IL-4 or IL-10.
One major advance in the understanding of the immune system was the discovery that CD4+ T helper (Th) cells can differentiate from a common precursor cell into functionally distinct subsets (15, 16). The distinct Th-cell subsets can mediate different immunological effector functions in protection and pathology in many infectious and autoimmune diseases (19). Based on their cytokine profiles, at least three subsets can be distinguished: type 0 Th (Th0), Th1, and Th2. Th0 cells secrete interleukin 2 (IL-2), IL-4, and gamma interferon (IFN-γ); Th1 cells secrete IFN-γ, IL-2, and tumor necrosis factor β; and Th2 cells produce IL-4, IL-5, IL-6, IL-10, and IL-13. Infection of mice with Leishmania major is widely used as a model to study the differential development and function of CD4+-Th-cell subsets in vivo. The ability of different inbred strains of mice to heal L. major infection is correlated with the induction of Th1 responses, and nonhealing, progressive disease is correlated with the expansion of IL-4-producing Th2 cells (2, 21). The recent identification of T1/ST2, an orphan receptor with homology to the IL-1 receptor, as a stable and selective marker on Th2 cells (12, 33) facilitates further analysis of this subset.
Naive Th cells do not express T1/ST2 on their surface; its expression is induced after contact with antigens on differentiated Th2 effector cells. T1/ST2 has been shown to be important for Th2 effector functions: treatment of L. major-infected mice with a polyclonal anti-T1/ST2 antiserum reduced lesion development and induced a reduction in the production of Th2 cytokines (33), and collagen-induced arthritis was exacerbated after in vivo injection of the anti-T1/ST2 antiserum (33). In addition, in different models of eosinophilic lung inflammatory diseases, treatment of mice with a monoclonal antibody (MAb) against the T1/ST2 molecule, MAb 3E10, induced a reduction in the production of IL-5 and eosinophil recruitment, indicating that the T1/ST2 molecule plays a role in Th2-mediated functions (1, 12, 31). The reduction in Th2-mediated functions after injection of anti-T1/ST2 antibodies led to the conclusion that T1/ST2 is involved in Th2-mediated functions in vivo (1, 12, 31, 33); however, studies with T1/ST2 gene knockout mice showed that the expression of the T1/ST2 molecule is not required for the differentiation and function of Th2 cells in Nippostrongylus brasiliensis infections (6, 22) and in eosinophilic lung inflammation (6). Another study, also done with T1/ST2 gene knockout mice, confirmed that T1/ST2 is not necessary for the in vitro differentiation of naive CD4+ T cells into Th2 effector cells; however, it defined a key role for T1/ST2 in the early events involved in the generation of Th2 responses in vivo (30).
In the present study, we investigated the role of CD4+ T1/ST2+ T cells in experimental leishmaniasis and prevented the interaction of T1/ST2 with its ligand in two different ways: we injected BALB/c mice with a MAb against the T1/ST2 molecule, MAb DJ8 (14), or with a T1-Fc fusion protein (14) that interfered with the binding of the T1/ST2 molecule to its ligand. Our results show that the treatment of L. major-infected BALB/c mice with an anti-T1/ST2 MAb or the T1-Fc fusion protein did not alter lesion development and parasite growth; however, the treatment induced significantly stronger type 1 responses. Surprisingly, interfering with T1/ST2 signaling did not modify the antigen-specific production of Th2 cytokines or the frequency of CD4+ T1/ST2+ Th2 cells. In addition to CD4+ T1/ST2+ Th2 cells, we could also detect a population of CD4+ T1/ST2− Th2 cells that were likely to be responsible for the nonhealing phenotype of L. major-infected BALB/c mice treated with the anti-T1/ST2 MAb or the T1-Fc fusion protein. In summary, our results show that signaling through T1/ST2 is not necessary for the differentiation of CD4+ T cells into Th2 effector cells and suggest that Th1 responses are regulated by CD4+ T1/ST2+ T cells through a mechanism independent of the Th2 cytokines IL-4 and IL-10.
MATRIALS AND METHODS
Mice.
BALB/c mice were purchased from Charles River, Nargate, United Kingdom, and 6- to 12-week-old females were used in all experiments. The animal colonies were screened regularly for mouse pathogens and consistently tested negative. Animal experiments were performed in accordance with institutional and Home Office guidelines.
Infections and treatment with anti-T1/ST2 MAb or T1-Fc fusion protein.
L. major LV39 (MRHO/SU/59/P-strain) parasites were isolated from skin lesions of infected mice and maintained as described previously (9). Infections with 2 × 106 stationary-phase L. major LV39 promastigotes in a final volume of 50 μl were performed by subcutaneous injection into one footpad. Lesion development was monitored weekly by measuring the increase in footpad thickness with a dial caliper (Kröplin Schnelltaster, Schlüchtern, Germany). Mice were injected intraperitoneally (i.p.) twice a week, starting on the day of infection and continuing throughout the course of infection, with 80 μg of anti-T1/ST2 MAb (DJ8) (14), 80 or 400 μg of T1-Fc fusion protein (14), or 80 μg of rat immunoglobulin G (IgG) (Sigma) as a control, and the experiments were terminated 4 days after the last injection. Endotoxin activities in both test preparations were tested and found to be negative (≤1 pg/ml in a Limulus amoebocyte test performed in the laboratory of C. Galanos).
LDA.
The number of living L. major parasites in infected tissues was determined by using the parasite limiting dilution assay (LDA) described previously (9, 29). Serial 10-fold dilutions of footpad homogenate were distributed in 96-well plates (Costar). After 10 to 14 days, the assay results were read by scoring the numbers of positive wells (presence of motile promastigotes) and negative wells (absence of motile promastigotes). Minimal estimates of the frequencies of viable L. major parasites in infected tissues were calculated from the single-hit Poisson model equation by the statistical method of χ2 minimization as previously described (27).
Lymphocyte cultures.
Popliteal lymph node cells (5 × 106/ml) were stimulated in the presence of 4 × 106 live L. major promastigotes (rendered replication incompetent by UV irradiation)/ml at 37°C in 5% CO2 in air in 24-well Costar plates containing a final volume of 1 ml of complete medium (Dulbecco modified Eagle medium [Gibco]) supplemented with 5% heat-inactivated fetal bovine serum (Gibco), 216 μg of l-glutamine (Gibco)/ml, 5 × 10−5 M 2-mercaptoethanol (Sigma), 100 U of penicillin (Gibco)/ml, and 100 μg of streptomycin (Gibco)/ml. Cells were cultured for 3 days, and culture supernatants were collected and frozen prior to cytokine determinations. To determine the expression of T1/ST2 on CD4+ T cells after in vitro restimulation by flow cytometry, lymphoid cells were restimulated as described above. After 6 days, the cells were harvested and a Ficoll gradient (Sigma) was used to eliminate debris.
Treatment with anti-T1/ST2 MAb and complement.
Lymphoid cells were cultured as described above. After 6 days, a Ficoll gradient was used, and 5 × 106 cells were incubated with 400 μg of anti-T1/ST2 MAb in a final volume of 1 ml at 4°C. After 45 min, the cells were washed and resuspended in a 1/10 dilution of Low-Tox rabbit complement (Cederlane). A control cell suspension was treated with complement only. The effect of the treatment on the viability of T1/ST2+ T cells was determined by fluorescence-activated cell sorting (FACS).
IL-12 responsiveness.
CD4+ T cells were purified from the lymphoid organs of BALB/c mice that had been infected for 5 weeks by magnetic bead separation with anti-CD4 microbeads (Miltenyi Biotec). Single cell suspensions were incubated with anti-CD4 MAb conjugated to microbeads (10 μl of L3T4 microbeads) ([clone GK1.5] per 107 cells) and passed through a separation column according to the protocol provided by the manufacturer. The purity of the CD4+ T cells was determined by flow cytometry to be >98%. CD4+ T cells were restimulated in the presence of L. major-infected antigen-presenting cells (APCs) prepared as follows. Single cell suspensions from the spleens of naive BALB/c mice (5 × 106/ml) were irradiated with 2,500 rads, washed, and infected with 5 × 106 live L. major promastigotes (rendered replication incompetent by UV irradiation) in 24-well Costar plates containing a final volume of 1 ml of complete medium. Purified CD4+ T cells were added to the cultures at a ratio of 1:5 in a final volume of 0.1 ml of complete medium (106), and recombinant mouse IL-12 (Genetics Institute; lot number MRB02894-1; 10 U/ml) was added to the cultures. Cells were cultured for 3 days, and culture supernatants were collected and frozen prior to gamma interferon (IFN-γ) determination.
Macrophage cultures.
Bone marrow macrophages (BMM) from BALB/c mice were derived as described previously (17). After 10 days of culturing, BMM (5 × 105/ml) were stimulated with 10 ng of lipopolysaccharide (LPS; Salmonella abortus equi; kind gift from M. Freudenberg, Max-Planck Institut für Immunbiologie, Freiburg, Germany) in the presence or in the absence of 500 μg of T1-Fc fusion protein. After 24 h, supernatants were harvested and tested for the presence of IL-6 by an enzyme-linked immunosorbent assay (ELISA).
Cytokine measurements.
IFN-γ was measured by an ELISA with rat anti-mouse MAb R4-6A2 (24) as a coating antibody and biotinylated rat anti-mouse MAb AN-18-17.24 (20) as described previously (23). IL-4, IL-6 (Endogen), and IL-10 (PharMingen, San Diego, Calif.) were measured by ELISAs with the protocols and the reagents provided by the suppliers. The detection limits were 1 U/ml for IFN-γ, 7 pg/ml for IL-4, 15 pg/ml for IL-6, and 40 pg/ml for IL-10.
Flow cytometric analysis.
For the ex vivo detection of T1/ST2 on CD4+ T cells from infected footpads and after in vitro restimulation of lymphoid cells, a Ficoll gradient was used for single cell suspensions to eliminate debris. Cells were preincubated with 1 μg of rat anti-mouse MAb CD32/CD16 (FcγII/III receptor; PharMingen) in a final volume of 50 μl of staining buffer (phosphate-buffered saline [Sigma]) supplemented with 3% heat-inactivated fetal bovine serum and 0.1% NaN3 (Sigma) for 5 min to reduce nonspecific binding. Anti-T1/ST2-fluorescein isothiocyanate (FITC) (clone DJ8; Morwell Diagnostic; 0.8 μg per 106 cells) and anti-CD4- R-phycoerythrin (PE) (clone GK1.5; PharMingen; 0.5 μg per 106 cells) were added directly to the cells in a final volume of 10 μl and incubated for 20 min.
For the detection of intracellular cytokine staining, lymphoid cells were harvested after various times, and a Ficoll gradient was used to isolate viable cells. A total of 1.5 × 106 cells were stimulated with 50 ng of phorbol 12-myristate 13-acetate (Sigma) and 500 ng of ionomycin (Calbiochem) or, as a control, in the presence of complete medium alone for 4 h; 10 μg of brefeldin A (Sigma) was included during the last 2 h. This incubation was performed at 37°C in 5% CO2 in air with complete medium. The cells were washed in staining buffer and resuspended in 50 μl of staining buffer. Before surface labeling, the cells (106) were preincubated with 1 μg of rat anti-mouse MAb to CD32/CD16 (FcγII/III receptor) for 5 min to reduce nonspecific binding. Anti-T1/ST2 (clone DJ8; 0.8 μg per 106 cells) was added directly to the cells in a final volume of 10 μl and incubated for 20 min. Cells were washed, resuspended in 500 μl of phosphate-buffered saline, and fixed with 500 μl of 4% formaldehyde (Sigma) for 20 min. This step and all of the subsequent ones were carried out at room temperature. After washing, the cells were permeabilized with a solution of staining buffer containing 5% saponin (Sigma) (permeabilizing buffer) for 10 min and washed in permeabilizing buffer. Anti-IL-4- PE (clone BVD4-1D11; PharMingen) and anti-IL-10-FITC (clone JES5-16E3; PharMingen) or appropriately labeled rat IgG1 (PharMingen) as an isotype control was added at a concentration of 0.07 μg per 106 cells. After 20 min, the cells were washed in permeabilizing buffer once, washed, and resuspended in 200 μl of staining buffer.
To determine the binding of the T1-Fc fusion protein to BMM, cells were incubated with 10 μg of T1-Fc fusion protein for 20 min as described above, and binding was revealed with 20 μl of anti-human IgG-PE (PharMingen).
The expression of intracellular cytokines and cell surface markers was detected by using an EPICS XL instrument (Beckman Coulter), and data were analyzed by using Beckman Coulter Expo32 software.
Statistical analysis.
The data were analyzed with GraphPad (San Diego, Calif.) PRISM version 2.0 by using the Mann-Whitney U test, and differences were considered statistically significant at a P value of <0.05.
RESULTS
Interfering with T1/ST2 signaling does not influence lesion development in L. major-infected BALB/c mice.
To characterize the contribution of CD4+ T1/ST2+ T cells to the outcome of nonhealing L. major infections, BALB/c mice were injected with anti-T1/ST2 MAb DJ8 throughout the course of infection. As shown in Fig. 1A, the anti-T1/ST2 MAb-treated group developed nonhealing lesions similar to those of the control infected group and the group treated with rat IgG. The experiment had to be terminated after 5 weeks due to the severity of the ulceration. The effect of the administration of anti-T1/ST2 MAb on parasite growth in the lesions of individual mice was also determined, and similar values were obtained in all groups: an average of 76 × 106 live L. major parasites were recovered from the lesions of the control group (rat IgG group; 82 × 106 live L. major parasites), and an average of 63 × 106 live L. major parasites were recovered from the lesions of the group treated with anti-T1/ST2 MAb (P = 0.24) (Fig. 1A). Similar results were obtained with another approach: T1-Fc fusion protein (80 or 400 μg), which competes with the membrane-bound T1/ST2 molecule for binding to its ligand, was injected i.p. twice a week throughout the course of infection. Kropf et al. showed previously that the footpads contain the highest level of CD4+ T1/ST2+ T cells compared to the lymphoid organs (8). Therefore, we treated BALB/c mice throughout the course of infection with the T1-Fc fusion protein (400 μg) directly in the lesions. As shown in Fig. 1B, the parasite loads were similar in the footpads of the control group and the group treated with the T1-Fc fusion protein.
FIG. 1.
Lesion development and parasite load. (A) Lesion development and parasite load in L. major-infected BALB/c mice treated with anti-T1/ST2 MAb. Groups of L. major-infected BALB/c mice (n = 4) were treated twice a week throughout the course of infection with 80 μg of anti-T1/ST2 MAb or 80 μg of rat IgG injected i.p., and one group was used as a control. Lesion development was monitored by measuring the increase in footpad thickness. At 5 weeks postinfection, the numbers of viable parasites in the lesions of individual mice were determined by an LDA. (B) Parasite load in L. major-infected BALB/c mice treated with T1-Fc fusion protein. Groups of L. major-infected BALB/c mice (n = 4) were treated twice a week throughout the course of infection with 400 μg of T1-Fc fusion protein injected subcutaneously in the footpads, and one group was used as a control. At 5 weeks postinfection, the numbers of viable parasites in the lesions of individual mice were determined by an LDA. Each symbol represents one mouse, and the horizontal lines represent the averages for four mice per group. Data show the results of one representative experiment out of three independent experiments. (C) Biological activity of T1-Fc fusion protein. BMM (5 × 105/ml) were stimulated with 10 ng of LPS in the presence or in the absence of 500 μg of T1-Fc fusion protein. After 24 h, supernatants were harvested and tested for the presence of IL-6 by an ELISA. Error bars in panels A and C indicate standard errors of the means.
To assess the biological activity of the T1-Fc fusion protein, its ability to decrease IL-6 production by LPS-stimulated BMM was determined as described previously (25). Our results showed that the T1-Fc fusion protein used in the experiments presented here reduced the production of IL-6 by 75% (Fig. 1C), demonstrating its biological activity. In addition, the capacity of the T1-Fc fusion protein to bind to BMM was determined by FACS analysis, and 1.9% of BMM were bound to the T1-Fc fusion protein (data not shown).
The anti-T1/ST2 MAb binds to the T1/ST2 molecule but does not deplete CD4+ T1/ST2+ T cells.
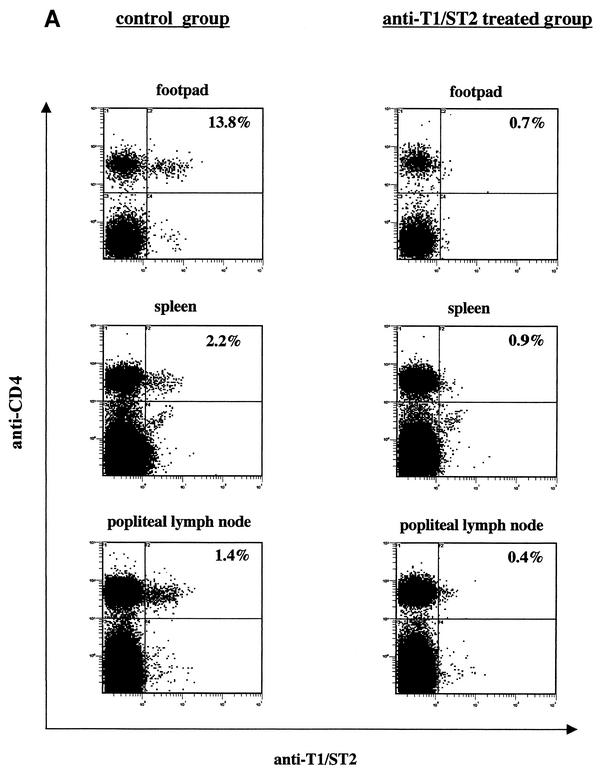
To determine whether the injection of anti-T1/ST2 MAb DJ8 resulted in the depletion of CD4+ T1/ST2+ T cells in vivo, the expression of T1/ST2 on CD4+ T cells was determined ex vivo 5 weeks postinfection. As shown in Fig. 2A, the T1/ST2 molecule was not detectable in mice treated with the anti-T1/ST2 MAb. However, when the cells were restimulated in vitro with L. major parasites, similar frequencies of CD4+ T1/ST2+ T cells were detectable in lymphoid cells from the control group and the anti-T1/ST2 MAb-treated group (Fig. 2B). Kropf et al. previously showed that T1/ST2 expression cannot be induced in vitro, as CD4+ T cells from naive mice could not be induced to express T1/ST2 after in vitro restimulation with L. major antigen (8). In addition, the expression of T1/ST2 on antigen-specific CD4+ T cells was shown to be stable, as the addition of IL-4 and anti-IFN-γ did not alter the frequency of CD4+ T1/ST2+ T cells (8), nor did it alter the frequency of CD4+ T1/ST2+ T cells expressing Th2 cytokines (11). These results strongly suggested that T1/ST2 was not reinduced after in vitro restimulation. Therefore, we hypothesized that CD4+ T1/ST2+ T cells were not depleted in vivo but that the anti-T1/ST2 MAb was binding to the T1/ST2 molecule, thereby masking the binding site for the anti-T1/ST2- FITC MAb, and that after in vitro culturing at 37°C, the anti-T1/ST2 MAb was shed from the T1/ST2 marker expressed by CD4+ T cells.
FIG. 2.
Anti-T1/ST2 MAb binds to but does not lyse CD4+ T1/ST2+ T cells in vivo. One group of L. major-infected BALB/c mice (n = 4) was treated twice a week throughout the course of infection with 80 μg of anti-T1/ST2 MAb, and one group was used as a control. At 5 weeks postinfection, the expression of T1/ST2 on CD4+ T cells was determined directly ex vivo in the footpads, spleens, and draining lymph nodes (A) or after in vitro restimulation of spleen and lymph node cells with L. major parasites (B). Data show the results for one representative mouse per group, and similar results were obtained in three independent experiments.
To test this hypothesis, we first determined whether the anti-T1/ST2 MAb was bound to the T1/ST2 molecule by using anti-rat IgG1. Similar percentages of CD4+ T1/ST2+ T cells were detected whether we used directly labeled anti-T1/ST2 (10.1%) or unlabeled anti-T1/ST2 followed by anti-rat IgG1 (11.4%); the value obtained with anti-rat IgG1 alone was 2.5%. Thus, these results showed that the anti-T1/ST2 MAb was bound to the T1/ST2 molecule and could be detected with anti-rat IgG1. In the next step, we assessed the ability of the anti-T1/ST2 MAb to lyse T1/ST2+ cells by complement-mediated lysis. In the group of lymphoid cells treated with the anti-T1/ST2 MAb and complement, T1/ST2 expression was just above the detection limit (1.5%), whereas in the control group, 13.7% of CD4+ T cells expressed T1/ST2. To determine whether the anti-T1/ST2 MAb was still bound to the T1/ST2 molecule, we used anti-rat IgG1; indeed, 12.7% of CD4+ T cells in the complement-treated group were positive for the presence of the anti-T1/ST2 MAb, as detected with anti-rat IgG1, whereas only 1.1% of CD4+ T cells in the control group were positive. After 3 days in cultures, 14.2% of CD4+ T cells in the group treated with the anti-T1/ST2 MAb and complement expressed T1/ST2, demonstrating that the anti-T1/ST2 MAb bound to the T1/ST2 molecule but did not induce cell lysis; in addition, these results showed that the anti-T1/ST2 MAb was shed after a few days in cultures. The results presented above and in Fig. 2 showed that anti-T1/ST2 MAb DJ8 bound to the T1/ST2 molecule in vivo (Fig. 2A) and was shed from the surface after in vitro restimulation (Fig. 2B). Thus, CD4+ T1/ST2+ T cells were not depleted in vivo.
Interfering with T1/ST2 signaling does not alter the levels of Th2 cytokine production.
To analyze the effect of treatment of L. major-infected BALB/c mice with the anti-T1/ST2 MAb on the levels of Th2 cytokines, the production of IL-4, IL-6, and IL-10 by draining lymph node cells was measured by an ELISA. As shown in Fig. 3, the levels of secretion of antigen-specific IL-4 (P = 0.24), IL-10 (P = 0.45), and IL-6 (P = 0.24) were similar in the anti-T1/ST2 MAb-treated group and the control group. Treatment of BALB/c mice with 80 or 400 μg of T1-Fc fusion protein, which interfered with the binding of the T1/ST2 molecule to its ligand, also did not decrease the levels of antigen-specific Th2 cytokines (data not shown). These results showed that injecting anti-T1/ST2 MAb DJ8 or T1-Fc fusion protein into L. major-infected BALB/c mice did not influence the production of Th2 cytokines.
FIG. 3.
Treatment of L. major-infected BALB/c mice with anti-T1/ST2 MAb does not alter the production of Th2 cytokines. One group of L. major-infected BALB/c mice (n = 4) was treated twice a week throughout the course of infection with 80 μg of anti-T1/ST2 MAb, and one group was used as a control. At 5 weeks postinfection, lymph node cells were restimulated with L. major parasites. After 3 days, supernatants were harvested and tested for Th2 cytokine contents by an ELISA. Each symbol represents one mouse, and the horizontal lines represent the averages for four mice per group. Data show the results of one representative experiment out of three independent experiments.
Interfering with T1/ST2 signaling results in increased IFN-γ production.
To determine whether the treatment of L. major-infected BALB/c mice with the anti-T1/ST2 MAb had an effect on the Th1 response, the levels of IFN-γ produced by lymphoid cells were measured by an ELISA. A significant (P = 0.03) increase (3.5-fold) in the production of IFN-γ was observed in supernatants of cultures of lymph node cells from the group treated with the anti-T1/ST2 MAb (average, 86.4 U/ml); the level in the control group was 24.4 U/ml (Fig. 4A) (the level in the rat IgG-treated group was 21 U/ml). A similar tendency was observed when the mice were treated with two different concentrations of T1-Fc fusion protein. IFN-γ was detected at 101 and 164 U/ml in supernatants of cultures of lymphoid cells isolated from groups of mice treated with 80 and 400 μg of T1-Fc fusion protein, respectively, and 20 U of IFN-γ/ml was detected in supernatants of cultures of lymphoid cells isolated from the L. major-infected control group; thus, the values represent 5.1- and 8.2-fold increases (Fig. 4B). The results presented in Fig. 4 showed that injecting the anti-T1/ST2 MAb or T1-Fc fusion protein during the course of L. major infection induced a type 1 response significantly stronger than that seen in control BALB/c mice.
FIG. 4.
Treatment of L. major-infected BALB/c with anti-T1/ST2 MAb or T1-Fc fusion protein induces a strong type 1 response. (A) One group of L. major-infected BALB/c mice (n = 4) was treated twice a week throughout the course of infection with 80 μg of anti-T1/ST2 MAb, and one group was used as a control. At 5 weeks postinfection, lymph node cells were restimulated with L. major parasites. After 3 days, supernatants were harvested and tested for IFN-γ content by an ELISA. Each symbol represents one mouse, and the horizontal lines represent the averages for four mice per group. Data show the results of one representative experiment out of three independent experiments. (B) An experiment similar to that shown in panel A was performed with 80 or 400 μg of T1-Fc fusion protein. The error bar shows the standard error of the mean.
Interfering with T1/ST2 signaling increases the capacity of CD4+ T cells to respond to IL-12.
Kropf et al. showed previously that CD4+ T cells from BALB/c mice immunomanipulated to control infection with L. major maintained their capacity to respond to IL-12 by increased production of IFN-γ (10). In contrast, CD4+ T cells from nonhealing BALB/c mice had a strongly reduced ability to produce IFN-γ in response to IL-12. Since IFN-γ production by total lymph node cells was higher in L. major-infected BALB/c mice treated with the anti-T1/ST2 MAb or T1-Fc fusion protein, we tested whether CD4+ T cells from the treated groups could produce higher levels of IFN-γ in response to IL-12 than CD4+ T cells from the control groups. As shown in Fig. 5A, the production of IFN-γ in response to recombinant mouse IL-12 was clearly stronger in the group of mice treated with the anti-T1/ST2 MAb: 57 U of IFN-γ/ml was detected in this group, and 16 U/ml was detected in the control group. A similar tendency was observed when the mice were treated with two different concentrations of T1-Fc fusion protein: the capacity of CD4+ T cells to produce IFN-γ in response to IL-12 was clearly higher in the treated mice (114 U/ml in the group of mice treated with 80 μg of T1-Fc fusion protein and 224 U/ml in the group of mice treated with 400 μg of T1-Fc fusion protein) (Fig. 5B) than in the control mice (7 U/ml) (Fig. 5B). These results showed that the capacity of CD4+ T cells to respond to IL-12 was stronger in BALB/c mice treated with the anti-T1/ST2 MAb or T1-Fc fusion protein than in control mice, suggesting that the interaction of CD4+ T1/ST2+ T cells with cells bearing the ligand contributes to the downregulation of IL-12 responsiveness.
FIG. 5.
CD4+ T cells from mice treated with anti-T1/ST2 MAb or T1-Fc fusion protein have an enhanced capacity to produce IFN-γ in response to IL-12. (A) One group of L. major-infected BALB/c mice (n = 4) was treated twice a week throughout the course of infection with 80 μg of anti-T1/ST2 MAb, and one group was used as a control. At 5 weeks postinfection, CD4+ T cells were purified from the lymphoid organs and restimulated in the presence of L. major-infected APCs and IL-12 (10 U/ml); the levels of IFN-γ produced in response to IL-12 were determined by an ELISA. As controls, CD4+ T cells were stimulated with APCs and antigen in the absence of IL-12. The levels of IFN-γ detected were 4.9 U/ml for the control group and 7.4 U/ml for the group treated with anti-T1/ST2 MAb, and no IFN-γ was detectable in the absence of IL-12 and antigen (data not shown). Data show the results of one representative experiment out of three independent experiments. (B) An experiment similar to that shown in panel A was performed with 80 or 400 μg of T1-Fc fusion protein. As controls, CD4+ T cells were stimulated with APCs and antigen in the absence of IL-12. The levels of IFN-γ detected were 9 U/ml for the group treated with 80 μg of T1-Fc fusion protein and 16 U/ml for the group treated with 400 μg of T1-Fc fusion protein, no IFN-γ was detectable in the control group, and no IFN-γ was detectable in the absence of IL-12 and antigen (data not shown). Error bars in both panels indicate standard errors of the means.
Interfering with T1/ST2 signaling does not influence the phenotypes of IL-4- and IL-10-producing CD4+ T cells.
Despite the increased type 1 response, our results showed that the levels of type 2 cytokines were not altered in the groups of mice treated with the anti-T1/ST2 MAb (Fig. 3) or T1-Fc fusion protein. To determine which cell type(s) produced Th2 cytokines, intracellular cytokine staining was performed. Since the anti-T1/ST2 MAb was shown to bind to CD4+ T1/ST2+ T cells, thereby masking the binding site for the labeled anti-T1/ST2 MAb, it would not have been possible to identify these cells; therefore, CD4+ T cells isolated from groups of mice treated with T1-Fc fusion protein were analyzed. The expression of IL-4 and IL-10 in CD4+ T1/ST2+ T cells and CD4+ T1/ST2− T cells was determined. CD4+ T1/ST2+ T cells isolated from the control group displayed a typical Th2 phenotype, as they expressed IL-4 and IL-10 (Fig. 6A and E). Interestingly, a population of CD4+ T cells negative for the expression of T1/ST2 also expressed IL-4 and IL-10 (Fig. 6B and F). The expression of IL-4 or IL-10 in the two CD4+-T-cell subsets, as well as the expression of T1/ST2, was shown to be stable, as the frequencies of CD4+ T1/ST2+ T cells and CD4+ T1/ST2− T cells expressing one or the other cytokine were not altered by the addition of IL-4 and anti-IFN-γ (data not shown). Both subsets of Th2 cells did not express IFN-γ, indicating that the type 2 cytokine-expressing cells were not Th0 cells (data not shown). We next compared the frequencies of Th2 cytokine-expressing CD4+ T cells in the group of mice treated with T1-Fc fusion protein and the control group. Similar frequencies of IL-4- and IL-10-expressing CD4+ T1/ST2+ T cells and CD4+ T1/ST2− T cells were detected (Fig. 6). The addition of T1-Fc fusion protein to the cultures did not alter the expression of T1/ST2 or the frequencies of IL-4- and IL-10-expressing CD4+ T1/ST2+ T cells and CD4+ T1/ST2− T cells (data not shown).
FIG. 6.
Interfering with T1/ST2 signaling does not influence the frequencies of IL-4- and IL-10-producing CD4+ T cells. One group of L. major-infected BALB/c mice (n = 4) was treated twice a week throughout the course of infection with 80 μg of T1-Fc fusion protein, and one group was used as a control. At 5 weeks postinfection, lymph node cells were restimulated with L. major parasites. After 6 days, viable cells were purified with a Ficoll gradient and stimulated with phorbol 12-myristate 13-acetate (50 ng) and ionomycin (500 ng) for 4 h; brefeldin A (10 μg) was included during the last 2 h. Cells were labeled with anti-CD4, anti-T1/ST2, anti-IL-4, or anti-IL-10. The expression of IL-4 and IL-10 in CD4+ T1/ST2+ T cells (A, C, E, and G) and CD4+ T1/ST2− T cells (B, D, F, and H) was determined by flow cytometry. An isotype control (IgG1-PE) was used at the same concentration as the antibodies, and 1% of the cells stained positive. Similar results were obtained in two independent experiments.
The results presented in Fig. 6 showed that interfering with T1/ST2 signaling did not influence the frequencies of CD4+ T1/ST2+ T cells or CD4+ T1/ST2− T cells expressing IL-4 or IL-10, indicating that signaling through the T1/ST2 molecule was not necessary for the differentiation of naive CD4+ T cells into antigen-specific CD4+ Th2 cells. In addition, our results showed that two distinct subsets of Th2 cells, which could be distinguished by the expression of T1/ST2, differentiated in vivo in response to L. major infection.
DISCUSSION
The results presented here show that interfering with the binding of T1/ST2 to its ligand by using anti-T1/ST2 MAb DJ8 or a T1-Fc fusion protein does not change the development of nonhealing disease in L. major-infected BALB/c mice. These mice developed progressive lesions that ulcerated in a manner similar to that of lesions in control infected mice (Fig. 1), and the production of antigen-specific Th2 cytokines (Fig. 3) as well as the frequencies of CD4+ T1/ST2+ T cells and CD4+ T1/ST2− T cells expressing IL-4 and IL-10 (Fig. 6) were not altered. However, the production of antigen-specific IFN-γ (Fig. 4) and the capacity of CD4+ T cells to respond to IL-12 were increased in the groups of mice treated with the anti-T1/ST2 MAb or T1-Fc fusion protein (Fig. 4B and 5A).
Three different antibodies that recognize the T1/ST2 molecule have been described to date: a polyclonal anti-T1/ST2 antiserum (33) and two MAbs, MAb 3E10 (1, 12, 31) and MAb DJ8 (14). Injection of a polyclonal antiserum enabled L. major-infected BALB/c mice to control the development of the lesions and to switch from to a Th2 to a Th1 phenotype. It is likely that the discrepancies between the results of the earlier study (33) and the results reported here are due to the different nature of the antibodies used. Indeed, the polyclonal antiserum was shown to lyse Th2 clones expressing T1/ST2 (33), whereas anti-T1/ST2 MAb DJ8 (used here) binds to the T1/ST2 molecule in vivo but does not activate complement to lyse T1/ST2+ cells (Fig. 2; see also Results). In addition, since it has been shown that the depletion of CD4+ T cells enables BALB/c mice to control L. major infection (28), the possibility that the decreased number of CD4+ T cells present after treatment with the polyclonal antiserum contributes to the healing phenotype cannot be excluded.
Anti-T1/ST2 MAb 3E10 has been used in different models of lung inflammatory diseases (1, 12, 31). It was shown that treatment of mice with anti-T1/ST2 MAb 3E10 consistently induced reductions in the level of IL-5 produced by the cells in the bronchoalveolar lavage fluid and the recruitment of eosinophils, but at the levels of the Th1 response and other Th2 cytokines, such as IL-4, the results diverged (1, 12, 31). In addition, like anti-T1/ST2 MAb DJ8 used in the present study, MAb 3E10 (1, 12, 31) is a nondepleting MAb (C. M. Lloyd, Leukocyte Biology Section, Faculty of Medicine, Imperial College of Science, Technology and Medicine, London, United Kingdom, personal communication); therefore, it is not possible to conclude that the changes in cytokine production observed in the groups of mice treated with anti-T1/ST2 MAb 3E10 were due to the depletion of CD4+ T1/ST2+ T cells (1, 31). The different results obtained with anti-T1/ST2 MAbs (1, 12, 31) in models of lung inflammatory diseases and in the model of L. major infection presented here are likely to be explained by differences in the pathogens (L. major parasites versus respiratory syncytial virus [31]) and techniques (adoptive transfer followed by exposure to ovalbumin [1, 12] versus infection) used.
The nature of the cells targeted by anti-T1/ST2 MAb DJ8 also may explain the unaltered levels of Th2 cytokines detected in L. major-infected mice. Indeed, in models of Th2-mediated lung inflammation, treatment of mice with anti-T1/ST2 MAb 3E10 was characterized by a lower level of eosinophilia and a lower level of production of IL-5 (1, 12, 31). These results suggest that CD4+ T1/ST2+ T cells may be involved in the recruitment of eosinophils and the production of IL-5. However, since eosinophils and IL-5 are not associated with pathology in L. major infection, interfering with cells involved in their regulation may not have a major effect. Further characterization of the biological activities of CD4+ T1/ST2+ T cells is under way in our laboratory.
There are conflicting data on the role of T1/ST2 in vivo and its importance for Th2-cell differentiation and Th2-mediated effector functions: studies with T1/ST2−/− mice showed that the T1/ST2 molecule does not play a unique role in the development of functional Th2 cells (6, 22). Our findings support and extend these studies, as they show that even after interference with T1/ST2 signaling, naive T cells differentiate into CD4+ T1/ST2+ Th2 effector cells in mice. Moreover, the identification of two subpopulations of antigen-specific Th2 cells in the lymphoid organs of L. major-infected mice suggests that after contact with antigen, CD4+ T cells can differentiate into at least two distinct populations of antigen-specific CD4+ Th2 cells (CD4+ T1/ST2+ and CD4+ T1/ST2− T cells). Thus, even in the absence of T1/ST2 or in the absence of an interaction of the T1/ST2 molecule with its ligand, CD4+ T1/ST2− Th2 cells can still develop and account for the detrimental Th2 response observed (Fig. 3). It should be noted that the Th2 responses detected in the studies discussed above (1, 6, 12, 22, 33) measured only type 2 cytokines at the level of total lymphoid cells. The results reported here are the first to analyze the cytokine profile at the level of antigen-specific CD4+ T1/ST2+ and CD4+ T1/ST2− T cells differentiated in vivo in response to L. major infection and demonstrate the presence of distinct subpopulations of Th2 cells (Fig. 6) distinguished by the expression of the T1/ST2 molecule.
The production of IFN-γ was significantly increased in the lymphoid organs of L. major-infected mice treated with the anti-T1/ST2 MAb or T1-Fc fusion protein. These results show that interfering with the T1/ST2 molecule and its ligand has a clear effect on the type 1 response, suggesting that CD4+ T1/ST2+ T cells play an important role in the regulation of the Th1 response. The regulation of IL-12 responsiveness could take place at the level of T1/ST2-expressing Th2 cells as well as at the level of ligand-bearing cells, and the underlying mechanism is likely to be IL-4 and IL-10 independent, as both CD4+ T1/ST2+ Th2 cells and CD4+ T1/ST2− Th2 cells express these cytokines. Interestingly, the binding of soluble T1/ST2 to the T1/ST2 ligand on macrophages resulted in the downregulation of the Toll-like receptor 4 message (25), and signaling through TLR-4 can affect IL-12 expression by specific remodeling of nucleosomes in the IL-12 p40 promoter region, an effect that is also observed following the activation of macrophages by LPS or heat-killed Listeria (32). IL-12 is crucial for the induction of Th1 responses as well as for their maintenance during infection (4, 5, 13, 26), and various investigators have shown that the reversal of nonhealing L. major infections is accompanied by the maintenance of IL-12 responsiveness (3, 7, 10). Therefore, it was unexpected that L. major-infected BALB/c mice treated with the anti-T1/ST2 MAb displayed an enhanced capacity to produce IFN-γ in response to IL-12 but could not control parasite replication. These results are in agreement with those of a recent study reporting that the constitutive expression of the IL-12 receptor β2 chain is not sufficient to induce the switch from a detrimental Th2 response to a protective Th1 response (18). Taken together, previous results (10) and those reported here indicate that the maintenance of IL-12 responsiveness is necessary but not sufficient for the healing of L. major infection.
In summary, our results show that preventing the interaction of T1/ST2 with its ligand has no effect on the development of nonhealing disease in L. major-infected BALB/c mice and does not downregulate the production of Th2 cytokines. However, it increases IFN-γ production and restores IL-12 responsiveness. Importantly, we have identified a subpopulation of Th2 cells capable of interfering with the capacity of CD4+ T cells to produce IFN-γ and to respond to IL-12.
Acknowledgments
We thank C. Galanos, M. Freudenberg, and N. Goos for the generous gift of LPS and the determination of endotoxin activity and I. Askonas, C. Bangham, and K. Senn for helpful discussions and critical reading of the manuscript.
This work was supported by a grant from The Wellcome Trust (grant 063289 to I.M.).
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Coyle, A. J., C. Lloyd, T. Nguyen, C. Erikson, L. Wang, P. Ottonson, P. Persson, T. Delaney, S. Lehar, S. Lin, L. Poisson, C. Meisel, T. Kamradt, T. Bjerke, D. Levinson, and J.-C. Gutierrez-Ramos. 1999. Crucial role of IL-1 receptor family member T1/ST2 in T helper cell type 2-mediated lung mucosal immune response. J. Exp. Med. 190:895-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Etges, B., and I. Müller. 1998. Progressive disease or protective immunity to Leishmania major infection: the result of a network of stimulatory and inhibitory interactions. J. Mol. Med. 76:372-390. [DOI] [PubMed] [Google Scholar]
- 3.Himmelrich, H., P. Launois, I. Maillard, T. Biedermann, F. Tacchini-Cottier, R. M. Locksley, M. Röcken, and J. A. Louis. 2000. In BALB/c mice, IL-4 production during the initial phase of infection with L. major is necessary and sufficient to instruct Th2 cell development resulting in progressive disease. J. Immunol. 164:4819-4825. [DOI] [PubMed] [Google Scholar]
- 4.Hondowicz, B., A. Y. Park, M. M. Elloso, and P. Scott. 2000. Maintenance of IL-12-responsive CD4+ T cells during a Th2 response in L. major-infected mice. Eur. J. Immunol. 30:2007-2014. [DOI] [PubMed] [Google Scholar]
- 5.Hondowicz, B. D., T. M. Scharton-Kersten, D. Jones, and P. Scott. 1997. Leishmania major-infected C3H mice treated with anti-IL-12 mAb develop but do not maintain a Th2 response. J. Immunol. 159:5024-5031. [PubMed] [Google Scholar]
- 6.Hoshino, K., S.-I. Kashiwamura, K. Kuribayashi, T. Kodama, T. Tsujimura, K. Nakanishi, T. Matsuyama, K. Takeda, and S. Akira. 1999. The absence of IL-1 receptor-related T1ST2 does not affect helper cell type 2 development and its effector function. J. Exp. Med. 190:1541-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones, D., M. M. Elloso, L. Showe, D. Williams, G. Trinchieri, and P. Scott. 1998. Differential regulation of the interleukin-12 receptor during the innate immune response to Leishmania major. Infect. Immun. 66:3818-3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kropf, P., Q. Bickle, S. H. Herath, R. Klemenz, and I. Müller. 2002. Organ-specific distribution of CD4+T1/ST2+ Th2 cells in Leishmania major infection. Eur. J. Immunol. 32:2450-2459. [DOI] [PubMed] [Google Scholar]
- 9.Kropf, P., K. Brunson, R. J. Etges, and I. Müller. 1997. Methods in microbiology: the leishmaniasis model, p. 419-458. In S. H. E. Kaufman and D. Kabelitz (ed.), Immunology of infections. Academic Press Ltd., London, England.
- 10.Kropf, P., R. Etges, L. Schopf, C. Chang, J. Sypek, and I. Müller. 1997. Characterisation of T cell-mediated responses in nonhealing and healing Leishmania major infections in the absence of endogenous IL-4. J. Immunol. 159:3434-3443. [PubMed] [Google Scholar]
- 11.Kropf, P., S. Herath, R. Tewari, N. Syed, R. Klemenz, and I. Müller. 2002. Identification of two distinct subpopulations of Leishmania major-specific T helper 2 cells. Infect. Immun. 70:5512-5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Löhning, M., A. Stroehmann, A. J. Coyle, J. L. Grogan, S. Lin, J.-C. Guiterrez-Ramos, D. Levinson, A. Radbruch, and T. Kamradt. 1998. T1/ST2 is preferentially expressed on murine Th2 cells, independent of IL-4, IL-5 and IL-10, and is important for Th2 effector function. Proc. Natl. Acad. Sci. USA 95:6930-6935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mattner, F., J. Magram, J. Ferrante, P. Launois, K. Di Padova, R. Behin, M. K. Gately, J. A. Louis, and G. Alber. 1996. Genetically resistant mice lacking interleukin-12 are susceptible to infection with Leishmania major and mount a polarized Th2 response. Eur. J. Immunol. 26:1553-1559. [DOI] [PubMed] [Google Scholar]
- 14.Moritz, D. R., J. Gheyselinck, and R. Klemenz. 1998. Expression analysis of the soluble and membrane-associated forms of the interleukin-1 receptor related T1 protein in primary mast cells and fibroblasts. Hybridoma 17:107-116. [DOI] [PubMed] [Google Scholar]
- 15.Mosmann, T. R., H. Cherwinski, M. W. Bond, M. A. Giedlin, and R. L. Coffman. 1986. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 136:2348-2357. [PubMed] [Google Scholar]
- 16.Mosmann, T. R., J. H. Schumacher, N. F. Street, R. Budd, A. O'Garra, T. A. T. Fong, M. V. Mond, K. W. M. Moore, A. Sher, and D. F. Fiorentino. 1991. Diversity of cytokine synthesis and function of mouse CD4+ T cells. Immunol. Rev. 123:209-229. [DOI] [PubMed] [Google Scholar]
- 17.Muller, I., M. Freudenberg, P. Kropf, A. F. Kiderlen, and C. Galanos. 1997. Leishmania major infection in C57BL/10 mice differing at the Lps locus: a new non-healing phenotype. Med. Microbiol. Immunol. 186:75-81. [DOI] [PubMed] [Google Scholar]
- 18.Nishikomori, R., S. Gurunathan, K. Nishikomori, and W. Strober. 2001. BALB/c mice bearing a transgenic IL-12 receptor β2 gene exhibit a nonhealing phenotype to L. major infection despite intact IL-12 signaling. J. Immunol. 166:6776-6783. [DOI] [PubMed] [Google Scholar]
- 19.O'Garra, A. 1998. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity 8:275-283. [DOI] [PubMed] [Google Scholar]
- 20.Prat, M., P. M. Gribaudo, P. M. Comoglio, G. Cavallo, and S. Landolfo. 1984. Monoclonal antibodies against murine γ interferon. Proc. Natl. Acad. Sci. USA 81:4115.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reiner, S. L., and R. M. Locksley. 1995. The regulation of immunity to Leishmania major. Annu. Rev. Immunol. 13:151-177. [DOI] [PubMed] [Google Scholar]
- 22.Senn, K. A., K. D. McCoy, K. J. Maloy, G. Stark, E. Fröhli, T. Rülicke, and R. Klemenz. 2000. T1-deficient and T1-Fc-trangenic mice develop a normal protective Th2-type immune response following infection with Nippostrongylus brasiliensis. Eur. J. Immunol. 30:1929-1938. [DOI] [PubMed] [Google Scholar]
- 23.Slade, S. S., and J. Langhorne. 1989. Production of Interferon-γ during infection of mice with Plasmodium chabaudi chabaudi. Immunobiology 179:353.. [DOI] [PubMed] [Google Scholar]
- 24.Spitalny, G. L., and E. A. Havell. 1984. Monoclonal antibody to murine gamma interferon inhibits lymphokin-induced anti-viral and macrophage tumoricidal activities. J. Exp. Med. 159:1560.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sweet, M. J., B. P. Leung, D. Kang, M. Sogaard, K. Schulz, V. Trajkovic, C. C. Campbell, D. Xu, and F. Y. Liew. 2001. A novel Pathway regulating LPS-induced shock by ST2/T1 via inhibition of Toll-like receptor 4 expression. J. Immunol. 166:6633-6639. [DOI] [PubMed] [Google Scholar]
- 26.Sypek, J. P., C. L. Chung, S. E. Mayor, J. M. Subramanyam, S. J. Goldman, D. S. Sieburth, F. Wolf, and R. G. Schaub. 1993. Resolution of cutaneous leishmaniasis: interleukin 12 initiates a protective T helper type 1 immune response. J. Exp. Med. 177:1797-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taswell, C. 1987. Limiting dilution assays for the separation, characterization, and quantification of biologically active particles and their clonal progeny, p. 109-145. In T. G. Pretlow and T. P. Pretlow (ed.), Cell separation: methods and selected applications, Vol. 4. Academic Press, New York, N.Y.
- 28.Titus, R. G., R. Ceredig, J. C. Cerottini, and J. A. Louis. 1985. Therapeutic effect of the anti-L3T4 monoclonal antibody GK1.5 on cutaneous leishmaniasis in genetically susceptible BALB/c mice. J. Immunol. 135:2108-2114. [PubMed] [Google Scholar]
- 29.Titus, R. G., M. Marchand, T. Boon, and J. A. Louis. 1985. A limiting dilution assay for quantifying Leishmania major in tissues of infected mice. Parasite Immunol. 7:545-555. [DOI] [PubMed] [Google Scholar]
- 30.Townsend, M. J., P. G. Fallon, D. J. Matthews, H. E. Jolin, and N. J. McKenzie. 2000. T1/ST2-deficient mice demonstrate the importance of T1/ST2 in developing primary T helper cell type 2 responses. J. Exp. Med. 191:1069-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walzl, G., S. Matthews, S. Kendall, J. C. Gutierrez-Ramos, A. J. Coyle, P. J. M. Openshaw, and T. Hussell. 2001. Inhibition of T1/ST2 during respiratory syncytial virus infection prevents Th2- but not Th1-driven immunopathology. J. Exp. Med. 193:785-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weinmann, A. S., D. M. Mitchell, S. Sanjabi, M. N. Bradley, A. Hoffmann, H. C. Liou, and S. T. Smale. 2001. Nucleosome remodeling at the IL-12 p40 promoter is a TLR-dependent, Rel-independent event. Nat. Immunol. 2:51-57. [DOI] [PubMed] [Google Scholar]
- 33.Xu, D., W. L. Chan, B. P. Leung, F.-P. Huang, R. Wheeler, D. Piedrafina, J. H. Robinson, and F. Y. Liew. 1998. Selective expression of a stable cell surface molecule on Type 2 but not Type 1 helper T cells. J. Exp. Med. 187:787-794. [DOI] [PMC free article] [PubMed] [Google Scholar]