Abstract
To determine the role of endogenous interleukin-10 (IL-10) in local host defense during pneumococcal meningitis, the inflammatory responses of IL-10-gene-deficient and wild-type mice after the induction of meningitis were compared. The absence of IL-10 was associated with higher cytokine and chemokine concentrations and a more pronounced infiltrate, but antibacterial defense or survival was not influenced.
Streptococcus pneumoniae is a major pathogen in bacterial meningitis in developed countries (22). Knowledge of the pathogenesis of pneumococcal meningitis is important not only because of the severity of the disease but also because resistance to penicillin and other antimicrobial agents has increased dramatically during the past decade (2, 3, 15).
Interleukin-10 (IL-10) is a pleiotropic, homodimeric cytokine produced by a large variety of cells, including monocytes/macrophages, B and T lymphocytes, and resident brain cell populations such as microglia and neurons (7, 21). IL-10 is considered a prototypic anti-inflammatory cytokine and potently inhibits the production of proinflammatory cytokines in vitro and in vivo (13, 14, 16, 18, 20).
Elevated levels of IL-10 have been found in the cerebrospinal fluid (CSF) of patients with bacterial (10, 24), aseptic (6, 8), cryptococcal (4, 6), and tuberculous (12) meningitis. Autoantibodies against IL-10 have been found in CSF from rats with experimental bacterial meningitis and are considered to play a regulatory role in the cytokine cascade (1). However, knowledge of the role of IL-10 in bacterial meningitis is highly limited. Therefore, in the present study, we induced pneumococcal meningitis in IL-10-gene-deficient (IL-10−/−) C57BL/6 mice (Jackson Laboratory, Bar Harbor, Maine) whose sex matched that of the C57BL/6 wild-type (WT) mice (Harlan Spaque Dawley, Inc., Horst, The Netherlands) used. All mice were inoculated at 3 to 4 weeks of age. Each experimental group consisted of 14 mice per time point; 22 mice per group were inoculated for survival analysis. All animal experimentation guidelines were followed in animal studies. Meningitis was induced as described previously (25). Briefly, the mice were lightly anesthetized by inhalation of isoflurane (Abott, Queensborough, Kent, United Kingdom), and 50 μl of inoculum (8 × 104 CFU of S. pneumoniae type 6A plus 180 U of hyaluronidase [Sigma, St. Louis, Mo.]) was inoculated intranasally. The control mice were inoculated with isotonic saline. At 24, 48, or 72 h after inoculation, the mice were sacrificed and blood, CSF, and brains were collected. Leukocytes in the CSF were counted immediately, and bacterial outgrowth in CSF and blood was determined by serial dilutions onto blood agar plates. The brains were removed; one-half of each brain was fixed in 10% buffered formalin for histopathologic study, while the other half was homogenized in sterile saline, plated on blood agar to determine bacterial outgrowth, and incubated with lysis buffer (300 mM NaCl, 15 mM Tris, 2 mM MgCl2, 2 mM CaCl2, 2 mM Triton X-100, pepstatin A [20 ng/ml], leupeptin [20 ng/ml], aprotinin [20 ng/ml], pH 7.4). Levels of cytokines were measured in supernatants from brain homogenates by using commercially available enzyme-linked immunospecific assays, according to the recommendations of the manufacturers (IL-1β, KC, macrophage inflammatory protein 2, and tumor necrosis factor alpha [TNF-α], from R&D Systems, Minneapolis, Minn., and IL-6 and IL-10 from Pharmingen, San Diego, Calif.). Data are expressed as means ± standard errors of the means. Statistical analysis was performed by using the Mann-Whitney U test and the Kaplan-Meier test. P values of <0.05 were considered significant.
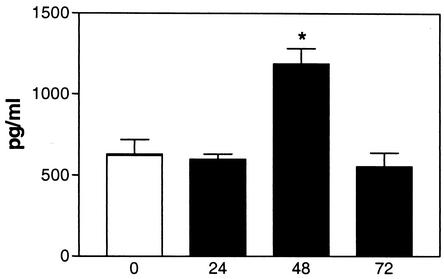
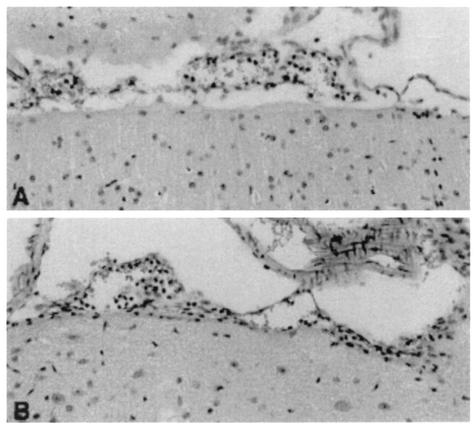
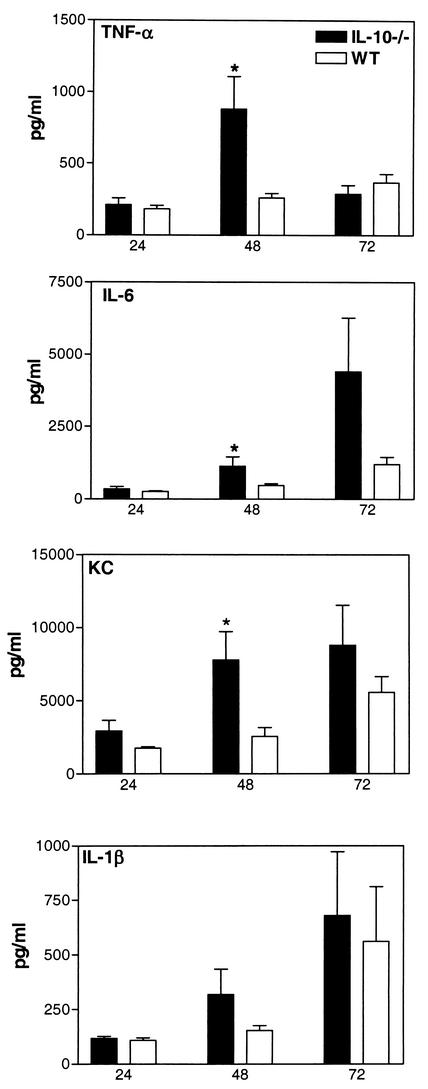
The induction of meningitis was associated with an upregulation of IL-10 in brain tissue (Fig. 1). At 24, 48, and 72 h after inoculation, higher percentages of inoculated IL-10−/− mice had developed meningitis than had the WT mice (at 24 h, 21 versus 29%; at 48 h, 71 versus 54%; at 72 h, 79 versus 64%, respectively; all differences were not significant). To determine the role of IL-10 in early host defense, we evaluated bacterial outgrowth in the CSF and blood of WT and IL-10−/− mice with meningitis. The bacterial counts determined for the CSF, brain homogenates, and blood from the IL-10−/− and WT mice were similar (Table 1). Bacterial meningitis is characterized by a pleocytosis of CSF. Although the number of leukocytes in the CSF from the IL-10−/− and WT mice did not differ significantly [at 24 h, (2.7 ± 1.5) × 105 versus (2.0 ± 1.0) × 104 cells/ml; at 48 h, (1.3 ± 0.5) × 106 versus (0.5 ± 0.4) × 106 cells/ml; at 72 h, (3.8 ± 2.0) × 106 versus (2.5 ± 1.1) × 106 cells/ml, respectively; no differences were significant], histologic examination of their respective brain tissues showed a more pronounced inflammatory infiltrate around the meninges in IL-10−/− than around those of the WT mice. At 24 h after inoculation, the WT mice showed only a slight influx of leukocytes (Fig. 2A), whereas the meningeal infiltrate, mainly consisting of neutrophils, in IL-10−/− mice was moderate (Fig. 2B). To gain further insight into the role of IL-10 in the local inflammatory response, we measured proinflammatory cytokines and chemokines in brain tissue. At 24 h after inoculation, no significant differences in cytokine and chemokine concentrations were found between IL-10−/− and WT mice; however, at 48 h after inoculation, IL-6, TNF-α, and KC levels were significantly higher in the brain tissues from the IL-10−/− mice than in those from the WT mice (Fig. 3). IL-1β was also elevated in IL-10−/− mice, but the difference did not reach statistical significance; the levels of macrophage inflammatory protein 2 in the IL-10−/− and WT mice were not different (data not shown). To determine the role of endogenous IL-10 beyond the early phase of the infection, survival was observed for 3 weeks after inoculation; no significant difference between the IL-10−/− and the WT mice was observed (hazard ratio, 0.65; 95% confidence interval, 0.28 to 1.5; P = 0.29). Because more IL-10−/− mice than WT mice developed meningitis after inoculation, we also determined the survival times of all nonsurvivors and found them to be comparable for the IL-10−/− and WT mice (3.3 ± 0.6 days [mean ± standard errors of the means] [range, 1.6 to 9.6] versus 3.8 ± 1.4 days [range, 1.9 to 13.6], respectively; no differences were significant).
FIG. 1.
IL-10 concentrations in brain tissue during meningitis. IL-10 expression levels (means ± standard errors) in brain homogenates of NaCl-injected WT mice and WT mice with meningitis at 24, 48, and 72 h after inoculation with 8 × 104 CFU of S. pneumoniae plus 180 U of hyaluronidase are shown. *, P <0.05
TABLE 1.
Bacterial outgrowth in CSF, brain homogenates, and blood of IL-10−/− and WT mice with meningitisa
| Sample | Time (h) | Bacterial count (CFU/ml)b
|
|
|---|---|---|---|
| IL-10−/− mice | WT mice | ||
| CSF | 24 | (3.7 ± 0.8) × 104 | (3.9 ± 1.9) × 104 |
| 48 | (1.0 ± 0.8) × 106 | (6.1 ± 3.9) × 104 | |
| 72 | (1.9 ± 0.9) × 105 | (3.5 ± 0.9) × 105 | |
| Brain homogenate | 24 | (6.7 ± 4.1) × 105 | (1.2 ± 0.5) × 105 |
| 48 | (4.9 ± 1.9) × 106 | (1.7 ± 0.9) × 106 | |
| 72 | (5.1 ± 4.4) × 107 | (9.2 ± 5.0) × 107 | |
| Blood | 24 | (1.9 ± 0.6) × 106 | (0.6 ± 0.2) × 106 |
| 48 | (4.7 ± 3.8) × 104 | (5.8 ± 2.7) × 104 | |
| 72 | (1.5 ± 0.9) × 106 | (2.9 ± 1.9) × 105 | |
Mice were inoculated with 8 × 104 CFU of S. pneumoniae plus 180 U of hyaluronidase.
None of the differences in values between IL-10−/− and WT mice were significant. Data are means ± standard errors of the means. P > 0.05.
FIG. 2.
Brain histology. Representative histologic sections of brain tissue from WT (A) and IL-10−/− (B) mice are shown 24 h after inoculation with 8 × 104 CFU of S. pneumoniae plus 180 U of hyaluronidase, with a more pronounced inflammatory infiltrate evident in IL-10−/− mice than in WT mice. Hematoxylin and eosin stain was used. Magnification, ×66.
FIG. 3.
Cytokine and chemokine concentrations in brains from IL-10−/− and WT mice. Concentrations (means ± standard errors) of IL-1β, IL-6, TNF-α, and KC in brain homogenates at 24, 48, and 72 h after inoculation with 8 × 104 CFU of S. pneumoniae plus 180 U of hyaluronidase are shown. *, P <0.05.
The IL-10−/− mice demonstrated an increased immune response as reflected by a more pronounced inflammatory infiltrate around the meninges and higher concentrations of proinflammatory cytokines and chemokines in brain tissue. These observations are in line with those of previous studies in which treatment with IL-10 attenuated the release of proinflammatory cytokines during experimental bacterial meningitis (9, 19). The local inflammatory response is considered to play an important role in tissue damage and death during meningitis. However, in this model, the increased inflammatory response in the IL-10−/− mice did not result in significantly impaired survival. As far as the effects of IL-10 on bacterial clearance are concerned, it has been shown that this cytokine directly inhibits neutrophil and macrophage phagocytic and bactericidal activities in vitro (11, 17). However, in two recent studies, a limited role for IL-10 in antibacterial defense in the central nervous system has been demonstrated. Control of bacteria was unchanged in IL-10−/− mice intracerebrally challenged with Listeria monocytogenes (5), and IL-10 treatment only slightly delayed bacterial clearance in an experimental rabbit model of Haemophilus influenzae meningitis (19). Interestingly, endogenous IL-10 was previously shown to impair antibacterial host defense mechanisms during pneumococcal pneumonia (23). The effect of IL-10 on in vivo phagocytosis and killing of microorganisms during meningitis may be different, in particular since the central nervous system is a compartment of the body in which the immune response is determined by the interaction of resident immunocompetent cells and recruited leukocytes.
In conclusion, these results indicate that IL-10 regulates cytokine and chemokine production but does not play an essential role in antibacterial host defense during murine pneumococcal meningitis.
Acknowledgments
We thank A. Maas and J. Daalhuisen for excellent technical support.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Bakhiet, M., M. Mustafa, J. Zhu, R. Harris, L. Lindquist, H. Link, and A. Diab. 1998. Induction of cytokines and anti-cytokine autoantibodies in cerebrospinal fluid (CSF) during experimental bacterial meningitis. Clin. Exp. Immunol. 114:398-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradley, J. S., and W. M. Scheld. 1997. The challenge of penicillin-resistant Streptococcus pneumoniae meningitis: current antibiotic therapy in the 1990s. Clin. Infect. Dis. 24(Suppl. 2):S213-S221. [DOI] [PubMed]
- 3.Breiman, R. F., J. C. Butler, F. C. Tenover, J. A. Elliott, and R. R. Facklam. 1994. Emergence of drug-resistant pneumococcal infections in the United States. JAMA 271:1831-1835. [PubMed] [Google Scholar]
- 4.Chaka, W., R. Heyderman, I. Gangaidzo, V. Robertson, P. Mason, J. Verhoef, A. Verheul, A. I. Hoepelman, et al. 1997. Cytokine profiles in cerebrospinal fluid of human immunodeficiency virus-infected patients with cryptococcal meningitis: no leukocytosis despite high interleukin-8 levels. J. Infect. Dis. 176:1633-1636. [DOI] [PubMed] [Google Scholar]
- 5.Deckert, M., S. Soltek, G. Geginat, S. Lütjen, M. Montesinos-Rongen, H. Hof, and D. Schlüter. 2001. Endogenous interleukin-10 is required for prevention of a hyperinflammatory intracerebral immune response in Listeria monocytogenes meningoencephalitis. Infect. Immun. 69:4561-4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallo, P., S. Sivieri, L. Rinaldi, X. B. Yan, F. Lolli, A. De Rossi, and B. Tavolato. 1994. Intrathecal synthesis of interleukin-10 (IL-10) in viral and inflammatory diseases of the central nervous system. J. Neurol. Sci. 126:49-53. [DOI] [PubMed] [Google Scholar]
- 7.Howard, M., and A. O'Garra. 1992. Biological properties of interleukin 10. Immunol. Today 13:198-200. [DOI] [PubMed] [Google Scholar]
- 8.Ishiguro, A., Y. Suzuki, Y. Inaba, A. Komiyama, H. P. Koeffler, and T. Shimbo. 1996. Production of interleukin-10 in the cerebrospinal fluid in aseptic meningitis of children. Pediatr. Res. 40:610-614. [DOI] [PubMed] [Google Scholar]
- 9.Koedel, U., A. Bernatowicz, K. Frei, A. Fontana, and H. W. Pfister. 1996. Systemically (but not intrathecally) administered IL-10 attenuates pathophysiologic alterations in experimental pneumococcal meningitis. J. Immunol. 157:5185-5191. [PubMed] [Google Scholar]
- 10.Kornelisse, R. F., H. F. Savelkoul, P. H. Mulder, M. H. Suur, P. J. van der Straaten, A. J. van der Heijden, R. N. Sukhai, K. Hahlen, H. J. Neijens, and R. de Groot. 1996. Interleukin-10 and soluble tumor necrosis factor receptors in cerebrospinal fluid of children with bacterial meningitis. J. Infect. Dis. 173:1498-1502. [DOI] [PubMed] [Google Scholar]
- 11.Laichalk, L. L., J. M. Danforth, and T. J. Standiford. 1996. Interleukin-10 inhibits neutrophil phagocytic and bactericidal activity. FEMS Immunol. Med. Microbiol. 15:181-187. [DOI] [PubMed] [Google Scholar]
- 12.Mastroianni, C. M., F. Paoletti, M. Lichtner, C. D'Agostino, V. Vullo, and S. Delia. 1997. Cerebrospinal fluid cytokines in patients with tuberculous meningitis. Clin. Immunol. Immunopathol. 84:171-176. [DOI] [PubMed] [Google Scholar]
- 13.Molina-Holgado, F., R. Grencis, and N. J. Rothwell. 2001. Actions of exogenous and endogenous IL-10 on glial responses to bacterial LPS/cytokines. Glia 33:97-106. [PubMed] [Google Scholar]
- 14.Moore, K. W., A. O'Garra, R. de Waal Malefyt, P. Vieira, and T. R. Mosmann. 1993. Interleukin-10. Annu. Rev. Immunol. 11:165-190. [DOI] [PubMed] [Google Scholar]
- 15.Novak, R., B. Henriques, E. Charpentier, S. Normark, and E. Tuomanen. 1999. Emergence of vancomycin tolerance in Streptococcus pneumoniae. Nature 399:590-593. [DOI] [PubMed] [Google Scholar]
- 16.Opal, S. M., J. C. Wherry, and P. Grint. 1998. Interleukin-10: potential benefits and possible risks in clinical infectious diseases. Clin. Infect. Dis. 27:1497-1507. [DOI] [PubMed] [Google Scholar]
- 17.Oswald, I. P., T. A. Wynn, A. Sher, and S. L. James. 1992. Interleukin 10 inhibits macrophage microbicidal activity by blocking the endogenous production of tumor necrosis factor alpha required as a costimulatory factor for interferon gamma-induced activation. Proc. Natl. Acad. Sci. USA 89:8676-8680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pajkrt, D., L. Camoglio, M. C. Tiel-van Buul, K. de Bruin, D. L. Cutler, M. B. Affrime, G. Rikken, T. van der Poll, J. W. ten Cate, and S. J. van Deventer. 1997. Attenuation of proinflammatory response by recombinant human IL-10 in human endotoxemia: effect of timing of recombinant human IL-10 administration. J. Immunol. 158:3971-3977. [PubMed] [Google Scholar]
- 19.Paris, M. M., S. M. Hickey, M. Trujillo, A. Ahmed, K. Olsen, and G. H. McCracken, Jr. 1997. The effect of interleukin-10 on meningeal inflammation in experimental bacterial meningitis. J. Infect. Dis. 176:1239-1246. [DOI] [PubMed] [Google Scholar]
- 20.Sawada, M., A. Suzumura, H. Hosoya, T. Marunouchi, and T. Nagatsu. 1999. Interleukin-10 inhibits both production of cytokines and expression of cytokine receptors in microglia. J. Neurochem. 72:1466-1471. [DOI] [PubMed] [Google Scholar]
- 21.Strle, K., J. H. Zhou, W. H. Shen, S. R. Broussard, R. W. Johnson, G. G. Freund, R. Dantzer, and K. W. Kelley. 2001. Interleukin-10 in the brain. Crit. Rev. Immunol. 21:427-449. [PubMed] [Google Scholar]
- 22.Townsend, G. C., and W. M. Scheld. 1998. Infections of the central nervous system. Adv. Intern. Med. 43:403-447. [PubMed] [Google Scholar]
- 23.van der Poll, T., A. Marchant, C. V. Keogh, M. Goldman, and S. F. Lowry. 1996. Interleukin-10 impairs host defense in murine pneumococcal pneumonia. J. Infect. Dis. 174:994-1000. [DOI] [PubMed] [Google Scholar]
- 24.van Furth, A. M., E. M. Seijmonsbergen, J. A. Langermans, P. H. Groeneveld, C. E. de Bel, and R. van Furth. 1995. High levels of interleukin 10 and tumor necrosis factor alpha in cerebrospinal fluid during the onset of bacterial meningitis. Clin. Infect. Dis. 21:220-222. [DOI] [PubMed] [Google Scholar]
- 25.Zwijnenburg, P. J., T. van der Poll, S. Florquin, S. J. van Deventer, J. J. Roord, and A. M. van Furth. 2001. Experimental pneumococcal meningitis in mice: a model of intranasal infection. J. Infect. Dis. 183:1143-1146. [DOI] [PubMed] [Google Scholar]