Abstract
The maintenance of a benign chronic Toxoplasma gondii infection is mainly dependent on the persistent presence of gamma interferon (IFN-γ) in the central nervous system (CNS). However, IFN-γ-activated microglia are paradoxically involved in parasitism control and in tissue damage during a broad range of CNS pathologies. In this way, nitric oxide (NO), the main toxic metabolite produced by IFN-γ-activated microglia, may cause neuronal injury during T. gondii infection. Despite the potential NO toxicity, neurodegeneration is not a common finding during chronic T. gondii infection. In this work, we describe a significant down-modulation of NO production by IFN-γ-activated microglia in the presence of conditioned medium of T. gondii-infected astrocytes (CMi). The inhibition of NO production was paralleled with recovery of neurite outgrowth when neurons were cocultured with IFN-γ-activated microglia in the presence of CMi. Moreover, the modulation of NO secretion and the neuroprotective effect were shown to be dependent on prostaglandin E2 (PGE2) production by T. gondii-infected astrocytes and autocrine secretion of interleukin-10 (IL-10) by microglia. These events were partially eliminated when infected astrocytes were treated with aspirin and cocultures were treated with anti-IL-10 neutralizing antibodies and RP-8-Br cyclic AMP (cAMP), a protein kinase A inhibitor. Further, the modulatory effects of CMi were mimicked by the presence of exogenous PGE2 and by forskolin, an adenylate cyclase activator. Altogether, these data point to a T. gondii-triggered regulatory mechanism involving PGE2 secretion by astrocytes and cAMP-dependent IL-10 secretion by microglia. This may reduce host tissue inflammation, thus avoiding neuron damage during an established Th1 protective immune response.
Despite the high prevalence of human Toxoplasma gondii infection, most infected individuals develop a benign, commonly asymptomatic infection (64). It has been shown that a persistent immunity is essential to avoid tissue damage in the central nervous system (CNS), the main site of parasite persistence during the latent infection. In immunocompromised individuals, reactivation of infection may occur and can result in life-threatening toxoplasmic encephalitis (34).
The establishment of a chronic asymptomatic T. gondii infection in the CNS requires secretion of gamma interferon (IFN-γ), the main cytokine involved in parasitism control (24, 60, 62), by both resident and nonresident CNS cells (38). In fact, host resistance appears to be dependent on the presence of IFN-γ receptors in both hematopoietic and nonhematopoietic cells (70). Further, the participation of CNS cells in the response to IFN-γ has been shown to be highly relevant (13, 30, 70). However, paradoxically the presence of Th1 cytokines in the brain parenchyma may be detrimental to the function of intense microglia activation, resulting in nitric oxide (NO) and the production of other toxic metabolites (31, 38, 58, 72). Consistently, proinflammatory mediators such as IFN-γ, tumor necrosis factor (TNF) alpha and NO were shown to have a potent neurotoxic activity both in vivo (27) and in vitro (14, 15). Although microglia activation guarantees protection from brain infections, paradoxically it may cause severe tissue damage. Among the mediators produced by activated microglia, NO is one of the most noxious to the CNS cells. In fact, neuronal injury due to microglia activation is considered to play an important role in CNS pathologies such as multiple sclerosis (27, 33), Alzheimer's disease (54), Parkinson's disease (46), and dementia complex related to AIDS (55). Surprisingly, the continuous immune response to T. gondii, which accompanies the persistence of the parasite in the CNS, does not result in neuronal degeneration or other tissue damage in immunocompetent hosts. Thus, it is reasonable to propose that immunomodulatory mechanisms might be involved in the prevention of neuronal degeneration and pathological alterations in nerve tissue during T. gondii infection.
Parasite-elicited production of prostaglandin E2 (PGE2) by human and murine monocytes during T. gondii infection has been previously reported (12, 44). To this arachidonic acid-derived product (PGE2) is attributed an immunoregulatory role, which is associated with the enhancement of intracellular levels of cyclic AMP (cAMP) in microglia cells, mediated by the activation of prostaglandin EP2 receptor (41, 42). Moreover, PGE2 appears to act selectively, suppressing Th1 cytokine production and inducing Th2 cytokines such as interleukin-10 (IL-10) (53). In the injured CNS, PGE2 may exert a neuroprotective role by means of decreasing NO production by activated microglia (4) and modulating other proinflammatory events (71). Although PGE2 production by CNS cells during T. gondii infection had not yet been shown, this prostanoid may be especially favorable in this site, modulating the immune response and contributing to the maintenance of brain cell integrity.
In vivo studies have demonstrated a remarkable increase of IL-10 expression in the brains of T. gondii-infected animals (9, 58). There is convincing evidence that Th2 cytokines exert a beneficial immunomodulatory activity in toxoplasmosis (26, 60, 68). In this context, it has been suggested that IL-10 inhibits tissue damage and contributes to evasive mechanisms that favor parasite persistence (25). Moreover, the high susceptibility of IL-10-knockout mice to T. gondii infection has been shown to be mediated by an exacerbated inflammatory process but not by a parasite overproliferation (69).
Based on this background information, the aim of the present work was to investigate cross talk between T. gondii-infected astrocytes and IFN-γ-activated microglial cells mediated by soluble factors, which might favor a neuroprotective effect. In the present study, we showed that conditioned medium of T. gondii-infected astrocytes (CMi) induced down-modulation of NO production by IFN-γ-activated microglia. Moreover, CMi eliminates the inhibition of neurite outgrowth induced by IFNγ-activated microglia. Importantly, both effects seem to be related and dependent on PGE2 production by infected astrocytes and cAMP-dependent IL-10 secretion by microglia.
MATERIALS AND METHODS
Reagents.
PGE2 was purchased from Cayman Chemical Co. Monoclonal rat anti-mouse IL-10 neutralizing antibody (clone JES052A5) was obtained from R&D systems. Aspirin (ASA), forskolin, murine recombinant IFN-γ, and sodium nitroprusside (SNP) were purchased from Sigma. RP-8-Br cAMP was purchased from Calbiochem.
Astrocyte cultures.
Murine astrocytes from BALB/c mice were cultured from the brain cortex of neonatal mice (age, less than 48 h; P0-1), following the procedure previously described (3, 66), with some modifications. Pups were decapitated, the brains were removed, the cortex was dissected, and the meninges were removed. Tissues were mechanically dissociated into a single-cell suspension with a Pasteur pipette in Dulbecco modified Eagle medium (DMEM)/F-12 (Gibco) supplemented with glucose (33 mM; Merck), glutamine (2 mM; Calbiochem), sodium bicarbonate (3 mM; Merck), penicillin-streptomycin (0.5 mg/ml), amphotericin B (Fungizone) (2.5 μg/ml), and 10% fetal bovine serum (FBS) (Gibco BRL). The dissociated cells were plated into poly-l-lysine (10 μg/ml)-coated 25-cm2 flasks. Astrocyte cultures reached confluence usually after 7 to 8 days and then were replated after dissociation with 0.25% trypsin-EDTA solution and washed in growth medium supplemented with 10% FBS. Cells were cultured in monolayers of 1 × 106 to 2 × 106 cells/25-cm2 flask. By this method, the cells were found to be >95% astrocytes as judged by positive staining for glial fibrillary acidic protein.
Microglial cell cultures.
Cells were obtained by means of a similar procedure used for astrocyte cultures, with some modifications (3, 43). To obtain microglial cell cultures, brains from five BALB/c mice were plated into 75-cm2 flasks precoated as mentioned above. The cultures were incubated at 37°C in a humidified 5% CO2, 95% air atmosphere. Cell culture medium was changed 24 h later and subsequently every five days. After 14 to 15 days, microglial cells were detached from the astrocyte monolayer by shaking the culture flasks for 30 min. The supernatants were collected and centrifuged, and the cells were reseeded on a plastic surface, in a manner similar to that described above, at a density of 5 × 104 cells/cm2. After 40 min, the medium was replaced to remove nonadherent cells, and microglial cells were allowed to grow for an additional 24 to 48 h before the experiments were started. Cells were found to be >98% microglia as judged by positive staining for isolectin b4 (lectin from Bandeiraea simplicifoliabs-I, peroxidase-labeled; Sigma). Following the same procedure, iNOS−/− (C57BL/6 background) or C57BL/6 wild-type mice were used. These iNOS−/− mice were originally obtained from the Jackson Laboratory (Bar Harbor, Maine) and were kindly provided by Jaqueline Alvarez Leite (Universidade Federal de Minas Gerais, Minas Gerais, Brazil).
Neuron cultures.
Primary dissociated cortical neurons were prepared as previously described (20) with some modifications. Briefly, timed-pregnancy mice were sacrificed at gestational day 16 to 17, and embryos were removed by Caesarian section. After cortex dissection as described above for glial cultures, cells were dissociated in DMEM/F-12 medium supplemented with 10% FBS and 2.5× 104 neurons were plated on top of microglial monolayers for coculture experiments or on 5.5-mm-diameter glass coverslips precoated with poly-l-lysine (10 μg/ml). Neuron cultures were kept for 24 h at 37°C in a humidified 5% CO2, 95% air atmosphere.
Tachyzoites of T. gondii.
Tachyzoites from a T. gondii P cystogenic strain isolated in Brazil (35) were maintained in vitro in primary astrocyte monolayers. Parasites were harvested after 3 days in culture, resuspended in DMEM/F-12 medium supplemented with 10% FBS, and used for infection of murine astrocytes.
Conditioned medium.
Astrocytes were infected with T. gondii tachyzoites for 2 h at a tachyzoite/host cell ratio of 5:1. The monolayer was then extensively washed to remove extracellular parasites and was maintained for 48 h in DMEM/F-12 medium supplemented with 10% FBS (CMi) or in the same medium supplemented with 100 μM ASA (CMiASA). Lysis of the host cells was not observed at this time point. Conditioned medium of control astrocytes (CMc) was also obtained. After harvesting, conditioned media were submitted to a 500 × g centrifugation before use.
Cytokine and drug treatment.
Microglial cells were activated with IFN-γ (500 U/ml) in fresh medium supplemented with 10% FBS or in the presence of CMc or CMi for 24 h. The assays were also performed using CMi supplemented with rat anti-mouse IL-10 neutralizing antibody (10 μg/ml) or the protein kinase A inhibitor RP-8-Br cAMP (10 mM). Two hours after the establishment of neuron-microglia cocultures, the cultures were activated for 24 h with IFN-γ (500 U/ml) or IFN-γ (500 U/ml) supplemented with CMc, CMi, CMiASA, forskolin (10 μM), PGE2 (2 ng/ml), and CMi with added rat anti-mouse IL-10 neutralizing antibody (10 μg/ml) or RP-8-Br cAMP (10 mM).
Immunocytochemistry.
Cultured cells were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 20 min and permeabilized with 0.2% Triton X-100 for 5 min at room temperature. Endogenous peroxidase activity was abolished with 3% H2O2 for 15 min followed by extensive washes with PBS. Cells were incubated with 5% bovine serum albumin (Gibco BRL) in PBS (blocking solution) for 30 min and subsequently with the specified mouse anti-human β-tubulin III antibody (1:400 dilution; Sigma), diluted in blocking solution, overnight at 4°C. After incubation, the cells were washed three times with blocking solution and then incubated with horseradish peroxidase-conjugated goat anti-mouse immunoglobulin (1:200 dilution; Gibco BRL) for 2 h at room temperature. Peroxidase activity was revealed utilizing a peroxidase substrate kit (Vector Laboratories). No reactivity was observed when the primary antibody was omitted. The preparations were dehydrated in a graded ethanol series, and coverslips were mounted in Entellan (Merck).
Measurement of NO production.
Supernatants from microglial cells and neuron-microglia cocultures were assayed for nitrite content as a reflection of NO production using Griess reagent (0.1% naphthylethylene diamine dihydrochloride and 1% sulfanilamide plus 2.5% phosphoric acid in equal volumes) as described previously (19).
Cytokine and PGE2 determinations.
Supernatants were tested for IL-10 and PGE2 using a sandwich enzyme-linked immunosorbent assay kit (Pharmingen) and an enzyme immunoassay kit (Cayman Chemical Co.), respectively, according to each manufacturer's directions.
Morphometry.
Neurons stained with β-tubulin III were photographed in a Zeiss Axioplan microscope. Photos were scanned and neurite length was analyzed using the Sigma Scan Pro Software (Jandel Scientific). Three independent experiments were performed, and at least 100 neurons were counted per sample in six or seven randomly chosen fields.
Statistical analysis.
Data were analyzed by Student's test or analysis of variance. Probability values (P) of 0.05 or less were considered significant.
RESULTS
CMi down-modulates NO production by IFN-γ-activated microglia.
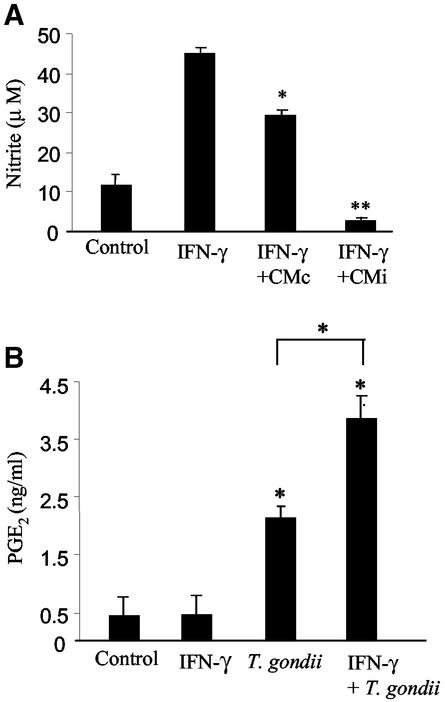
As depicted in Fig. 1A, treatment of microglial cells with IFN-γ for 24 h induced a significant increase in NO production as shown by the amount of nitrite that accumulated in the supernatant. A remarkable suppressive effect on NO production by IFN-γ-activated microglial cells was induced by CMi, whereas a slight effect was observed when activated microglia cultures were treated with CMc. These data suggest a putative role of astrocyte soluble factors in preventing NO overproduction by activated microglia during T. gondii infection.
FIG. 1.
CMi leads to an inhibition of NO production by IFN-γ-activated-microglia and may be correlated with PGE2 secretion by astrocytes. (A) Nitrite concentration was determined by the Griess reaction in the culture supernatants. Microglia cells were activated with IFN-γ (500 U/ml) for 24 h in the presence of CMi or CMc. Controls consisted of microglia cells cultured in the presence or absence of IFN-γ. (B) Supernatants of 48-h-infected astrocytes (5:1) were collected and tested for PGE2 using an enzyme immunoassay. Astrocytes were activated with IFN-γ (200 U/ml) in the presence or absence of the parasite. These data represent the means + standard deviations (error bars) of three independent experiments. Symbols: *, P < 0.05; **, P < 0.01.
Involvement of PGE2 secreted by T. gondii-infected astrocytes in the inhibition of NO production by IFN-γ-activated microglia.
Since PGE2 mediates the inhibition of NO production by microglia and other cell types (49), we first measured the levels of PGE2 in CMc and CMi. Figure 1B shows that the levels of PGE2 were fivefold (∼2 ng/ml) higher in CMi than in CMc (∼0.4 ng/ml). When infected astrocytes were treated with ASA (100 μM), an irreversible inhibitor of COX-1 and COX-2 (67a), we were unable to detect PGE2 in conditioned medium (data not shown).
Considering that in inflammatory sites both microglial cells and astrocytes are potential targets for IFN-γ (1), we tested the ability of this cytokine to regulate PGE2 production by T. gondii-infected astrocytes. As shown in Fig. 1B, while IFN-γ (200 U/ml) had no effect on PGE2 production by noninfected astrocytes, the treatment of astrocytes with IFN-γ concomitant with T. gondii infection did not eliminate the production of PGE2 induced by infection but led to a significant increase in the level of this prostanoid (>3.5 ng/ml).
Soluble factors secreted by T. gondii-infected astrocytes induce IL-10 production by microglial cells in a cAMP-dependent mechanism.
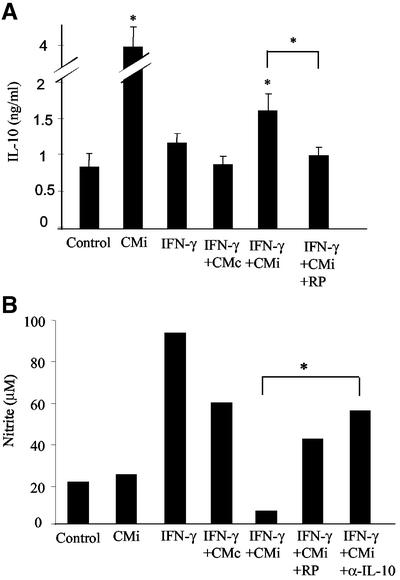
As PGE2 selectively up-regulates Th2 cytokines such as IL-10 (53) and this cytokine has been shown to modulate NO production by microglial cells (51), we investigated whether the inhibition of NO production observed here could result from the presence of IL-10 in our culture system. In fact, microglial cells showed a significant increase in IL-10 secretion after 24-h incubation with CMi (Fig. 2A). Interestingly, despite the reduction in the IL-10 level, the microglial cells continued to be able to produce IL-10 in the presence of IFN-γ and CMi, maintaining a clear statistical difference in relation to the control. In contrast, the simultaneous treatment of microglial cells with IFN-γ (500 U/ml) and supernatant of noninfected astrocytes did not alter the levels of IL-10 secreted compared to controls, whereas IFN-γ (500 U/ml) treatment had no significant effect (Fig. 2A).
FIG. 2.
Soluble factors released by T. gondii-infected astrocytes enhance IL-10 secretion by microglia, which is correlated with the NO down-modulation mechanism. Microglial cells were incubated for 24 h in the presence of CMi or treated with IFN-γ (500 U/ml) in the presence of fresh medium, CMi, or CMc. The treatment with RP-8-Br cAMP (10 mM) or with anti-IL-10 (α-IL-10) neutralizing antibodies (10 μg/ml) was performed simultaneously with IFN-γ activation and CMi treatment. (A) Supernatants from microglia cells were collected and tested for IL-10 by enzyme-linked immunosorbent assay. The data represent the means + standard deviations (error bars) of three independent experiments. (B) Supernatants were collected and tested for nitrite using the Griess reaction. The data represent one of three independent experiments. *, P < 0.05.
Recent results show that PGE2 may act via intracellular cAMP enhancement in microglial cells (42). This finding led us to investigate whether IL-10 production by microglial cells in the presence of CMi could be mediated by cAMP up-regulation. RP-8-Br cAMP, a protein kinase A inhibitor that eliminates the enhancement of intracellular cAMP levels, partially blocked the secretion of IL-10 by microglia treated with IFN-γ and CMi (Fig. 2A). These findings suggest that IL-10 secretion by microglial cells induced by CMi results from a cAMP-dependent pathway that can be triggered by PGE2. Since noninfected and 48-h-infected astrocytes did not express detectable amounts of IL-10 (data not shown), these results show that CMi induced microglial cells to secrete IL-10, which persists, although at lower levels, even in the presence of IFN-γ.
Inhibition of NO production by activated microglia induced by CMi was eliminated by RP-8 Br cAMP and anti-IL-10-neutralizing antibody.
It has been shown that PGE2 has a suppressive effect on the expression of inducible NO synthase (iNOS) (49). This effect appears to be mediated by an increase in the intracellular levels of cAMP (41). As PGE2 was detected in the supernatant of T. gondii-infected astrocytes, we investigated the effect of RP-8-Br cAMP on the production of NO by IFN-γ-activated microglia in the presence of CMi. Figure 2B shows that under this condition, NO secretion by activated microglial cells was restored, reinforcing the idea that the cAMP-dependent pathway can be activated by PGE2, leading to NO suppression induced by CMi. Indeed, in the presence of RP-8-Br cAMP the levels of nitrite, measured by the Griess reaction, in the supernatant of microglial cell cultures were similar to those obtained in the presence of IFN-γ supplemented with CMc.
It is reasonable to think that IL-10 secreted by microglia treated with CMi may act autocrinally, inhibiting NO production by IFN-γ-activated microglia. To investigate this possibility, microglial cells were activated with IFN-γ (500 U/ml) in the presence of CMi and anti-IL-10 neutralizing antibody (10 μg/ml). In this situation, NO production by microglia was also restored, reaching the levels observed in IFN-γ-activated microglia simultaneously treated with CMc (Fig. 2B). Further, CMi had no effect on the levels of NO production by nonactivated microglial cells. Altogether, these results strongly suggest that cAMP-dependent IL-10 secreted by microglia induced by conditioned medium of T. gondii-infected astrocytes has an autocrine effect, inhibiting NO secretion by IFN-γ-activated microglia.
T. gondii-infected astrocytes secrete soluble factors that prevent neuronal degeneration induced by IFN-γ-activated microglia.
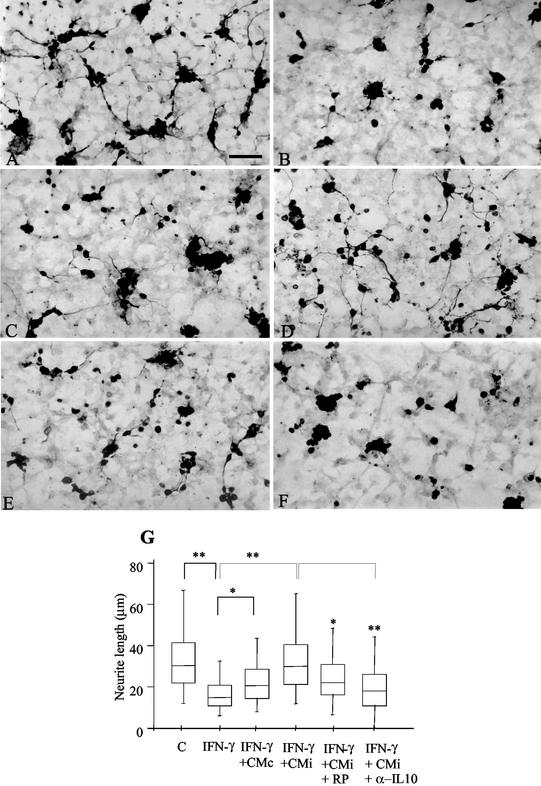
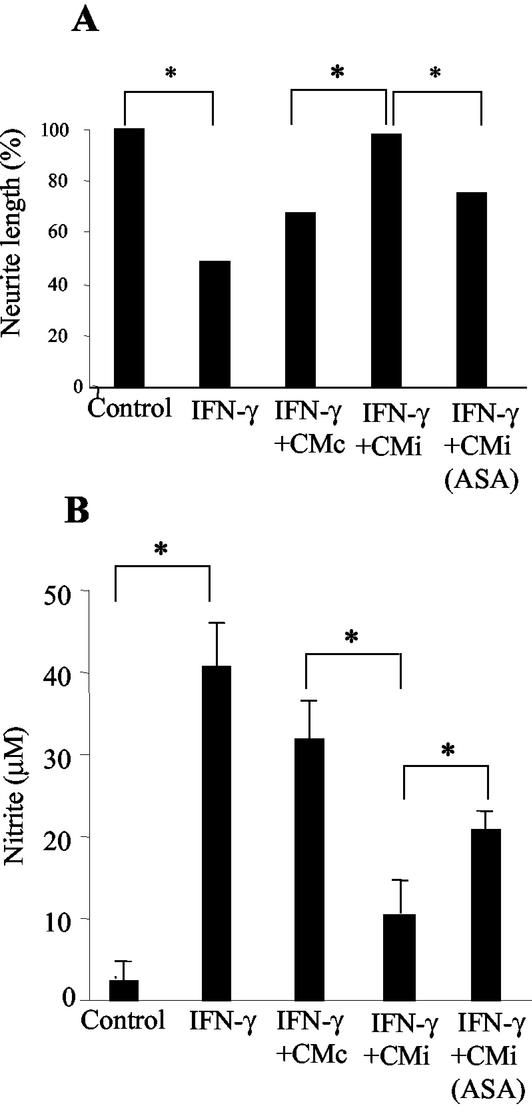
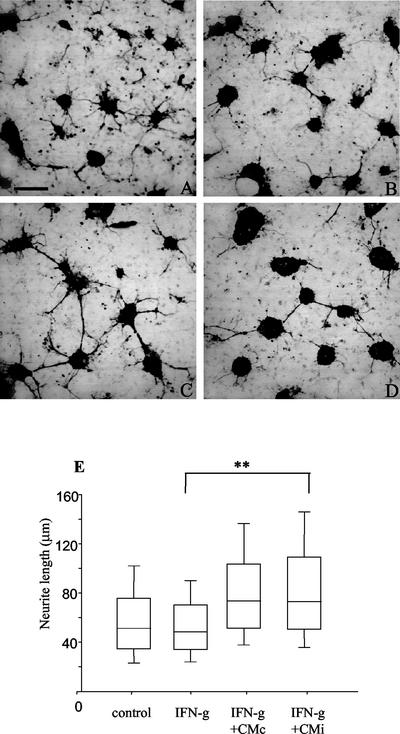
The possibility that the inhibitory effect induced by CMi on NO production by IFN-γ-activated microglia may result in a neuroprotective effect was investigated. Neurons were cocultured for 24 h on top of microglia confluent cultures, and cell viability was evaluated under different conditions, considering neurite outgrowth as a parameter to infer neuronal viability. In the presence of IFN-γ (500 U/ml), neurite outgrowth was reduced about 50% (∼16 μm) in relation to neurons cocultured with microglia in the absence of this cytokine (∼33 μm), as shown in Fig. 3 and 4A. The impairment of neurite outgrowth was accompanied by a drastic increase in NO levels in neuron-microglia coculture supernatants (Fig. 4B). Under similar experimental conditions, neurons cultured without microglia cells did not respond to IFN-γ in relationship to neurite outgrowth (data not shown). When neuron-microglia cocultures were treated with IFN-γ in the presence of CMc, a partial recovery of neurite outgrowth (∼22 μm) was observed (Fig. 3C and G and 4A), and this recovery paralleled a slight reduction (25%) in NO production (Fig. 4B). Interestingly, the addition of CMi led to a complete recovery of neurite outgrowth (Fig. 3D and G and 4A), with an average length of 32 μm, and this was accompanied by a drastic reduction (75%) in NO production (Fig. 4B), suggesting the involvement of this nitrogen-reactive radical in the impairment of neuron functions. Further, the neuroprotective effect was partially inhibited when the cocultures were exposed to CMiASA, with neurites reaching a median length of ∼25 μm, which represents a recovery of 50% in relation to the treatment with IFN-γ (Fig. 4A). In this situation, the production of NO was also partially restored (Fig. 4B), pointing to a putative role of PGE2 in the phenomenon described here. It should be noted that in the absence of IFN-γ, neurite outgrowth was equally favored by CMc or CMi (data not shown).
FIG. 3.
CMi sustains neurite outgrowth in IFN-γ-treated neuron-microglia cocultures. Morphological differences in neurons were evidenced by immunoperoxidase to β-tubulin-III, a specific marker for neurofilament. Neuron-microglial cells were cocultured as described in Materials and Methods. The cultures were treated with fresh medium (control) or IFN-γ (500 U/ml) in the absence or presence of CMc or CMi and analyzed 24 h later. Neurite length was determined as described in Materials and Methods. (A) In the control, neurite outgrowth showed normal development. Bar = 100 μm. (B) Severe impairment of neurite outgrowth was observed in cocultures treated with IFN-γ (500 U/ml). The noxious effect of IFN-γ was slightly blocked by the addition of CMc (C) and completely inhibited when CMi was added to the cultures (D). The beneficial effect of CMi was partially abolished by RP-8-Br cAMP (RP) (10 mM) (E) or anti-IL-10 (α-IL10) neutralizing antibodies (10 μg/ml) (F). (G) Statistical analysis of neurite length of three independent experiments. C, control (fresh medium). Symbols: *, P < 0.05; **, P < 0.01; error bars, range.
FIG. 4.
Neurite outgrowth is inversely proportional to NO production in neuron-microglia coculture. Neuron-microglial cells were cocultured as described in Materials and Methods. The cultures were washed with medium and treated with fresh medium (control) or IFN-γ (500 U/ml) in the absence or presence of CMc, CMi, or CMiASA and analyzed 24 h later. Neurite length (A) and nitrite concentration (B), both determined as previously described, are shown. Symbols: *, P < 0.05; error bars, standard deviations.
Neuron protective effect of CMi was abolished by cAMP antagonist and anti-IL-10 neutralizing antibodies.
Considering that several effects of PGE2 are mediated by cAMP (37), RP-8-Br cAMP was added to neuron-microglia cocultures activated with IFN-γ in the presence of CMi. In fact, the neuron protective effect of CMi was partially eliminated by RP-8-Br cAMP treatment (Fig. 3E), leading to an average neurite outgrowth of 23 μm (Fig. 3G). Interestingly, anti-IL-10 neutralizing antibody (10 μg/ml) also eliminated the favorable effect of CMi (Fig. 3F), as a median neurite length of ∼18 μm was observed (Fig. 3G). These data strongly suggest the involvement of PGE2 and IL-10 in the neuron protective effect mediated by CMi.
Effects of CMi on neuron-microglia cocultures activated with IFN-γ were mimicked by exogenous PGE2 and cAMP-elevating agent.
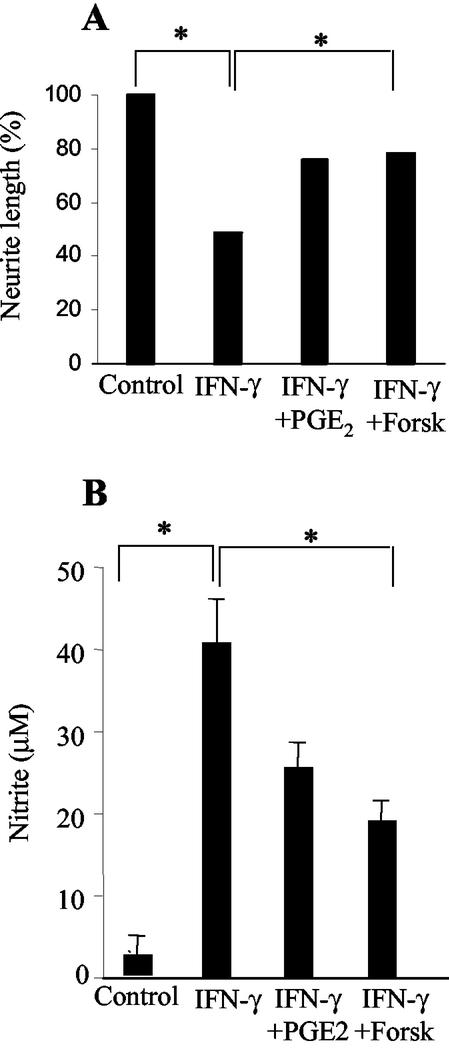
Considering that PGE2 present in CMi might indirectly contribute to the neuroprotective effect described here, neuron-microglia cocultures activated with IFN-γ were treated with exogenous PGE2 (2 ng/ml). Neurite outgrowth was partially restored (80%), reaching a median length of 29 μm (Fig. 5A). Aiming to mimic the effect of PGE2 present in CMi, neuron-microglia cocultures activated with IFN-γ were treated with an adenylate cyclase activator, forskolin (10 μM). This treatment was also able to partially restore neurite outgrowth (50%), with a median length of 26 μm (Fig. 5A). In both situations the recovery of neurite outgrowth was accompanied by a significant down-modulation of NO production (Fig. 5B).
FIG. 5.
Exogenous PGE2 and a cAMP activator, forskolin (Forsk), mimic the effect of CMi suppressing NO production and promote neurite outgrowth in neuron-microglia coculture. Neuron-microglial cells were cocultured as described in Materials and Methods. The cultures were washed with medium and treated with fresh medium (control) or IFN-γ (500 U/ml) in the absence or presence of PGE2 (2 ng/ml) or forskolin (10 μM) and analyzed 24 h later. Neurite length (A) and nitrite concentration (B), both determined as previously described, are shown. Symbols: *, P < 0.05; error bars, standard deviations.
Neurite outgrowth was equally favored by CMi and CMc in the presence of IFN-γ-activated microglia from iNOS−/− mice.
Considering that IFN-γ-activated microglia produces a broad array of toxic metabolites and inflammatory molecules (31, 38, 58, 72), the specific role of NO in the phenomenon described here should be better investigated. Microglia from mice with a targeted disruption in the iNOS gene were used. Current evidence suggests that these mice are immunologically intact, except for their inability to produce inducible NO (39). Neurons cocultured in the presence of microglia obtained from iNOS−/− mice were not injured by IFN-γ treatment, considering that there were no significant changes in neurite outgrowth between control and IFN-γ-activated cells (Fig. 6B and E). Treat the cocultures with IFN-γ in the presence of either CMc or CMi led to an enhancement of neurite outgrowth in relation to the control; however, no statistical differences between CMc and CMi were observed with regard to this neuron improvement (Fig. 6C to E). As a positive control, a coculture from wild-type C57BL/6 mice was developed in parallel. In this coculture, IFN-γ promoted a deleterious effect in neuron outgrowth, with slight recovery with CMc and dramatic recovery with CMi, as was the case for BALB/c cultures (Fig. 3). We can conclude that the results observed were directly related to the phenomenon of NO involvement proposed here.
FIG. 6.
Neurite outgrowth is equally favored by CMi and CMc in the presence of IFN-γ-activated microglia from iNOS−/− mice. Neurons were cocultured with microglia from iNOS−/− mice. The cultures were washed with medium and treated with fresh medium (control) or IFN-γ (500 U/ml) in the absence or presence of CMc or CMi and analyzed 24 h later. No statistical differences in neuron development were observed between control (A) and IFN-γ-treated (B) cultures. (A) Bar = 100 μm. In the presence of IFN-γ plus CMc (C) or CMi (D), a statistical improvement in neurite outgrowth was observed in relation to the control or IFN-γ treatment; however, no statistical difference was observed between the treatment with both conditioned media in three independent experiments (E). Symbols: *, P < 0.05; error bars, range.
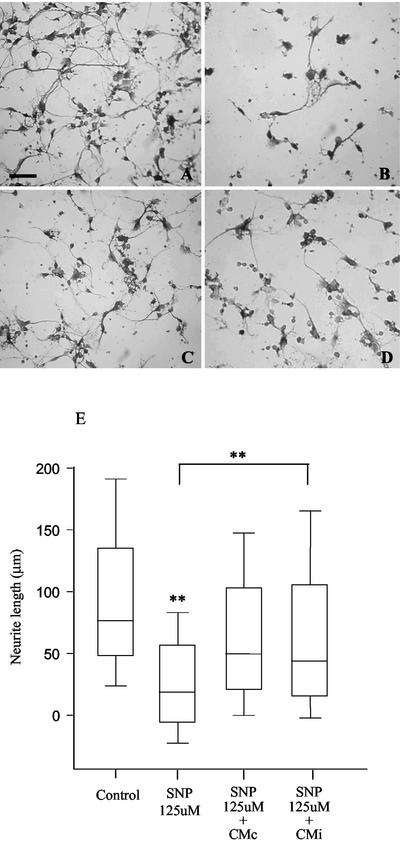
Neurons treated with the NO-exogenous donor SNP were equally favored by CMi or CMc.
In order to investigate the involvement of a direct mechanism (67), independent of the NO inhibitory effect of CMi on microglial cells, neurons were solely cultivated in the presence of the exogenous NO donor SNP, CMc, or CMi. As expected, neurite outgrowth was drastically inhibited by SNP (Fig. 7B and E), suggesting once more the involvement of NO in neurite impairment during microglia activation. Both CMc and CMi were able to partially revert the deleterious effect of SNP on neurons, but the differences were not statistically significant. This phenomenon was observed for all concentrations of SNP tested, between 31.2 and 500 μM (data not shown). These data exclude the hypothesis that the positive effect of CMi in relation to CMc on neuron-microglia coculture is due to different amounts or patterns of neurotrophins in these media.
FIG. 7.
Effects of CMi and CMc on SNP-treated neurons. Neurons were cultured as described in Materials and Methods, in the absence (A) or presence (B) of 125 μM NO-exogenous donor SNP. (A) Bar = 100 μm. Both CMc (C) and CMi (D) were able to partially revert SNP neurite impairment, but the differences were not statistically significant in three independent experiments (E). Symbols: **, P < 0.01; error bars, range.
DISCUSSION
In the present study, we provide evidence that soluble immunoregulatory mediators released by T. gondii-infected astrocytes are capable of inhibiting NO production by IFN-γ-activated microglia, which appears to favor neuron viability, sustaining neurite outgrowth even when neuron-microglia cocultures were treated with IFN-γ. The participation of astrocytes in this process was evident and may reflect the importance of the cross talk between astrocytes and microglial cells in the maintenance of CNS homeostasis during T. gondii infection. Moreover, our results point to a T. gondii-triggered mechanism that could be related to host protection, contributing to asymptomatic persistence of T. gondii in the CNS of immunocompetent hosts.
Our data show that CMc leads to a slight, but significant, reduction in NO production by IFN-γ-activated-microglia, as opposed to CMi, which provokes a robust inhibition of NO secretion. Our findings show that, instead of astrocytes and microglia synergistically acting to enhance the production of NO during T. gondii infection, a regulatory interaction supposedly beneficial to the host homeostasis occurs. It had been proposed that astrocytes play an immunoregulatory role in the CNS as a result of their ability to produce anti-inflammatory mediators (e.g., PGE2 and transforming growth factor β), restrain the activation of T cells, and inhibit the production of proinflammatory cytokines (e.g., IL-12) as well as class II major histocompatibility complex expression by microglia and macrophages. Hence, astrocytes are proposed to play an important role in limiting immune-mediated inflammation (2). In this context, our results are evidence that these immunoregulatory characteristics are intensified in infected astrocytes, which may favor, in a paracrine manner, CNS homeostasis, inhibiting the production of NO, a potentially deleterious mediator, induced by a proinflammatory cytokine, IFN-γ.
A dichotomous role for NO during acute T. gondii infection in mice is suggested. Even in models of susceptible mice, such as strain C57BL/6, in which some role in parasitism control is attributed to NO, it seems to have a detrimental or lethal side effect on the host, as demonstrated by studies with iNOS−/− mice (39).
The role of NO production as a mechanism of resistance against T. gondii infection in the brain has been actively debated (57, 60). Some authors suggest a possible role of NO in reducing replication of T. gondii by murine activated microglia (36), as observed in macrophage cells, in which NO is also considered important in triggering stage conversion (6). Recent studies, however, argue against a role for NO production as an exclusive mechanism for controlling T. gondii infection of microglial cells. According to Freund et al. (23), an unknown NO-independent mechanism leads to inhibition of parasite growth in IFN-γ-activated microglia from both BALB/c and CBA/C mice. In humans, it is also believed that the control of T. gondii replication by microglia is mediated not by NO but by a decrease in the levels of infection of activated cells (13).
Some data confined the role of NO to chronic disease control of susceptible strains (57), since iNOS−/− animals normally survive acute infection, developing brain cysts and succumbing during the chronic stage of toxoplasmosis (56). Recent data suggest that the induction of BAG-5 T. gondii antigen expression and cyst formation seems to be dependent on IFN-γ, as parasitism control is largely dependent on endogenous IFN-γ with only a partial involvement of TNF receptor p55 and iNOS (59). Since knockout mice with a susceptible genetic background, C57BL/6, were used to obtain all of these data (56, 59), the role of NO in chronic disease control by resistant BALB/c strains may not follow the same pattern. The role of NO in resistance to T. gondii in the BALB/c (toxoplasmic encephalitis [TE] resistant) mouse appears to be very different from that in the C57BL/6 (TE-susceptible) mouse. Schluter et al. (57) demonstrated that treatment of BALB/c mice with the selective iNOS inhibitor l-NG-iminoethyl-lysine did not result in reactivation of a latent infection in BALB/c mice, although it exacerbated T. gondii infection in the CNS of C57BL/6 mice. Thus, NO does not appear to play a role in maintaining a latent infection in BALB/c mice. Further experiments using IFN-γ-deficient mice on a BALB/c background demonstrated that in the absence of IFN-γ, NO is still detected in the brains of T. gondii-infected animals, although this was insufficient to determine the resistance of these animals (60). On the basis of these facts, we can suppose that the NO down-modulation described here would hardly be pivotal in the persistence of parasites in the CNS, only favoring asymptomatic infection in the resistant host. Neurite outgrowth impairment was not observed in our experiments with IFN-γ-activated microglia from iNOS−/− mice; under this condition neurons were equally favored by both CMc and CMi. These data suggest that NO inhibition plays a central role in the protection of neurons mediated by CMi in IFN-γ-activated cocultures of cells from wild-type mice. We cannot exclude the possibility that neurite outgrowth may be, in part, a secondary consequence of neuron aggregation mediated by both conditioned media. However, our results point to the NO involvement proposed here.
It has been reported that nonresident CNS cells, like monocytes and macrophages, secrete increased levels of PGE2 during T. gondii infection (44). Our results point to an enhancement of PGE2 secretion by T. gondii-infected astrocytes that is potentiated by the simultaneous treatment with IFN-γ, in contrast to what is observed in lipopolysaccharide (LPS)-activated astrocytes (32). These data suggest the existence of distinct pathways triggering PGE2 production by astrocytes in the presence of T. gondii or LPS. In fact, it appears that distinct signaling pathways are triggered in astrocytes and microglial cells by T. gondii or LPS, leading to differences in response to and production of cytokines (21).
A significant increase in the physiological levels of prostanoids in the CNS may be observed under conditions of inflammation (22). The immunomodulatory effect of PGE2 in the CNS, specifically on microglial cells, has been clearly demonstrated (41). In this context, the secretion of this prostanoid by T. gondii-infected astrocytes may be associated with the inhibition of NO production described here. This idea is supported by the demonstration that exogenous PGE2 has a suppressive effect on the expression of iNOS (49). Paradoxically the inhibition of COX-2 by nonsteroidal anti-inflammatory drugs may favor a down-modulation of iNOS expression by microglial cells (50). These data suggest the existence of different pathways mediated by PGE2 endogenously produced by microglia, and the action of this prostanoid derived from exogenous sources (50). These data may explain why even in the absence of detectable levels of PGE2, only a partial elimination of the neuroprotective effect and restoration of NO production mediated by the conditioned medium of infected astrocytes treated with ASA were observed. In this CMiASA, significant amounts of ASA were probably still available, which could directly inhibit iNOS expression and NO production by microglia, bypassing, in part, the absence of PGE2 (49, 50).
The intracellular cAMP up-regulation induced by exogenous PGE2 in microglia leads to inhibition of TNF alpha and IL-12 secretion and enhancement of IL-10 secretion (3, 4). In fact, our results show that CMi, rich in PGE2, leads to IL-10 production by microglia, which is maintained even in the presence of IFN-γ. Since RP-8-Br cAMP significantly eliminated IL-10 production by microglia triggered by CMi, this effect seems to be linked to a cAMP-dependent pathway, in accordance with observations in other models which showed PGE2-induced IL-10 secretion by microglial cells (41).
In view of the immunomodulatory effect of IL-10 observed during T. gondii infection, including inhibition of NO production (25), we can suggest that in addition to a direct action of PGE2 secreted by infected astrocytes on NO secretion by microglia, an indirect effect mediated by IL-10 produced by microglia may occur. In support of this hypothesis we observed an inverse correlation between NO and IL-10 production by microglia. In addition, both RP-8-Br cAMP and anti-IL-10 neutralizing antibody impaired NO inhibition mediated by CMi. The possibility that IL-10 present in the CMi may influence NO inhibition by activated microglia was excluded, as this cytokine was not detected in the supernatant of T. gondii-infected astrocytes, in agreement with data previously reported (21).
The secretion of IL-10 by microglial cells in the presence of infected astrocyte mediators could be part of the mechanism involved in the CNS homeostasis during T. gondii infection, reflected by asymptomatic chronic disease in the immunocompetent host. This is supported by findings showing that the presence of Th2 cytokines is correlated with the control of exacerbated inflammation induced by the parasite. In the absence of IL-10, T. gondii infection is always fatal, due to high levels of IL-12 and IFN-γ secretion by the host, followed by extensive and general tissue damage (26).
Our data clearly showed a beneficial effect of CMi on neurons, characterized by complete recovery of neurite outgrowth in IFN-γ-activated neuron-microglia cocultures. Conditioned medium of noninfected astrocytes was also able to ameliorate neurite outgrowth, suggesting a constitutive neuroprotective effect of these cells that is in agreement with the findings of other authors (29), and that may be improved by T. gondii infection. Our findings also showed that NO production is inversely related to neurite outgrowth, reinforcing the hypothesis that CMi may act to favor neuron integrity through its potential to down-regulate NO production. In the absence of microglial cells, neurons had not showed an impairment of neurite outgrowth triggered by IFN-γ treatment, showing that the noxious effect of this cytokine is indirect, probably involving NO production by microglia, as previously suggested (47). This fact could be confirmed by impairment of neurite outgrowth mediated by the NO-exogenous donor SNP, shown here.
The restoration of neurite outgrowth may in fact be explained by an indirect effect of PGE2 secreted by infected astrocytes on microglia cells that leads to an autocrine secretion and action of IL-10 on microglia. This possibility is supported by the partial or complete elimination of neuroprotection induced by CMi by treatment with RP-8-Br cAMP or anti-IL-10 neutralizing antibody. Also, when infected astrocytes were cultured in the presence of ASA, the neuroprotective ability of the conditioned medium was partially eliminated, additional support for the putative involvement of PGE2 in this process. The participation of this prostanoid was demonstrated by exogenous addition of PGE2 to IFN-γ-activated neuron-microglia cocultures, which mimicked the effect of the PGE2-containing CMi. Further, our results pointed to the activation of a cAMP-dependent pathway in microglia by soluble factors present in CMi, since forskolin, a protein kinase A activator, mimics the effect of this medium, leading to neurite outgrowth and NO inhibition. Activation of the microglia receptor of PGE2, EP2, induces protein kinase A, and its effect is mediated by adenylyl cyclase stimulation, resulting in cAMP intracellular levels (10, 42, 49). In fact, our results show that the treatment with forskolin mimics the effect of PGE2 and CMi on microglia.
One could not exclude the possibility that in our experiments T. gondii antigens, PGE2, neurotrophins, and cytokines (11, 16, 17, 45, 48, 52) also act directly on neurons, restoring neurite outgrowth. In spinal cord neurons, the protective role of PGE1 against NO toxicity was recently demonstrated. This neuroprotective effect was mediated by a stimulation of EP4 receptors present in these neurons and consequent increase in intracellular cAMP levels (40). However, this does not seem to be occurring in our model system, since in the absence of microglial cells, CMi, which is rich in PGE2 and cytokines (21), was able to partially restore neuron impairment mediated by SNP in the same fashion as CMc, without statistical difference.
The neuroprotective effect of CMi described here parallels the induction of IL-10 secretion by microglia. This finding could be explained by a direct effect of IL-10 produced by microglia on the neurons and/or by an indirect pathway involving a reduction of the oxidative stress mediated by microglia deactivation, triggered by this cytokine. Then, as previously demonstrated in other models, IL-10 may act directly by inhibiting neuron apoptosis (5) or up-regulating neuronal survival (7, 8, 28). Additionally, IL-10 may act autocrinally on the microglial cells, down-modulating NO production, which may also contribute to the recovery of neurite outgrowth. These ideas are supported by our demonstrations that in the presence of anti-IL-10 neutralizing antibodies, NO production by IFN-γ-activated microglia is restored and neurite outgrowth is impaired in neuron-microglia cocultures. Although additional studies are required, our results clearly point to a beneficial role for IL-10 during T. gondii infection. In vivo studies have demonstrated a remarkable increase in IL-10 expression in the brains of T. gondii-infected animals (9, 58). In fact, there is convincing evidence that Th2 cytokines exert an immunomodulatory beneficial activity in toxoplasmosis (26, 60, 68). In this context, it has been suggested that IL-10 eliminates tissue damage and may contribute to evasive mechanisms that favor parasite persistence (25).
The interplay between neurons, glial cells, and parasite-induced factors that may lead to CNS pathology or recovery during T. gondii infection is complex and incompletely understood. Altogether, our data are evidence supporting the notion that a potential neuroprotective role for immunoregulatory mediators such as PGE2 and IL-10 under conditions of parasite-induced CNS inflammation should be considered. Although it is less easy to predict the future role of these mediators as therapeutic tools for CNS inflammatory and/or infectious diseases, this possibility should not be discounted.
Acknowledgments
We thank Fabio Mendes, Garcia Abreu, Julio Scharfstein, Narcisa Cunha e Silva, Rossiane Vommaro, and Sergio Seabra for help in the preparation of the manuscript and Jaqueline Alavarez Leite for kindly providing iNOS−/− mice. We thank also Eleandro Joaci de Lima, Marlene Cazuza, Antônio Bosco, and Adiel Batista do Nascimento for technical assistance.
This work was supported by CNPq, FAPERJ, PRONEX, IOC-Fiocruz, and INCa.
Editor: J. M. Mansfield
REFERENCES
- 1.Aloisi, F. 1999. The role of microglia and astrocytes in CNS immune surveillance and immunopathology. Adv. Exp. Med. Biol. 468:123-133. [DOI] [PubMed] [Google Scholar]
- 2.Aloisi, F., E. Ambrosini, S. Columba-Cabezas, R. Magliozzi, and B. Serafini. 2001. Intracerebral regulation of immune responses. Ann. Med. 33:510-515. [DOI] [PubMed] [Google Scholar]
- 3.Aloisi, F., G. Penna, J. Cerase, B. Menendez Iglesias, and L. Adorini. 1997. IL-12 production by central nervous system microglia is inhibited by astrocytes. J. Immunol. 159:1604-1612. [PubMed] [Google Scholar]
- 4.Aloisi, F., R. De Simone, S. Columba-Cabezas, and G. Levi. 1999. Opposite effects of interferon-gamma and prostaglandin E2 on tumor necrosis factor and interleukin-10 production in microglia: a regulatory loop controlling microglia pro- and anti-inflammatory activities. J. Neurosci. Res. 56:571-580. [DOI] [PubMed] [Google Scholar]
- 5.Bachis, A., A. M. Colangelo, S. Vicini, P. P. Doe, M. A. De Bernardi, G. Brooker, and I. Mocchetti. 2001. Interleukin-10 prevents glutamate-mediated cerebellar granule cell death by blocking caspase-3-like activity. J. Neurosci. 21:3104-3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bohne, W., J. Heesemann, and U. Gross. 1994. Reduced replication of Toxoplasma gondii is necessary for induction of bradyzoite-specific antigens: a possible role for nitric oxide in triggering stage conversion. Infect. Immun. 62:1761-1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolanos, J. P., P. Garcia-Nogales, V. Vega-Agapito, M. Delgado-Esteban, P. Cidad, and A. Almeida. 2001. Nitric oxide-mediated mitochondrial impairment in neural cells: a role for glucose metabolism in neuroprotection. Prog. Brain Res. 132:441-454. [DOI] [PubMed] [Google Scholar]
- 8.Brewer, K. L., J. R. Bethea, and R. P. Yezierski. 1999. Neuroprotective effects of interleukin-10 following excitotoxic spinal cord injury. Exp. Neurol. 159:484-493. [DOI] [PubMed] [Google Scholar]
- 9.Burke, J. M., C. W. Roberts, C. A. Hunter, M. Murray, and J. Alexander. 1994. Temporal differences in the expression of mRNA for IL-10 and IFN-gamma in the brains and spleens of C57BL/10 mice infected with Toxoplasma gondii. Parasite Immunol. 16:305-314. [DOI] [PubMed] [Google Scholar]
- 10.Caggiano, A. O., and R. P. Kraig. 1999. Prostaglandin E receptor subtypes in cultured rat microglia and their role in reducing lipopolysaccharide-induced interleukin-1β production. J. Neurochem. 72:565-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carlson, N. G., W. A. Wieggel, J. Chen, A. Bacchi, S. W. Rogers, and L. C. Gahring. 1999. Inflammatory cytokines IL-1 alpha, IL-1 beta, IL-6, and TNF-alpha impart neuroprotection to an excitotoxin through distinct pathways. J. Immunol. 163:3963-3968. [PubMed] [Google Scholar]
- 12.Channon, J. Y., and L. H. Kasper. 1996. Toxoplasma gondii-induced immune suppression by human peripheral blood monocytes: role of gamma interferon. Infect. Immun. 64:1181-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chao, C. C., G. Gekker, S. Hu, and P. K. Peterson. 1994. Human microglial cell defence against Toxoplasma gondii. J. Immunol. 152:1246-1252. [PubMed] [Google Scholar]
- 14.Chao, C. C., S. Hu, and P. K. Peterson. 1995. Cytokines and neurotoxicity. Crit. Rev. Neurobiol. 9:189-205. [PubMed] [Google Scholar]
- 15.Chao, C. C., S. Hu, T. W. Molitor, E. G. Shaskan, and P. K. Peterson. 1992. Activated microglia mediate neuronal cell injury via a nitric oxide mechanism. J. Immunol. 149:2736-2741. [PubMed] [Google Scholar]
- 16.Chuenkova, M. V., and M. A. Pereira. 2001. The T. cruzi trans-sialidase induces PC12 cell differentiation via MAPK/ERK pathway. Neuroreport 12:3715-3718. [DOI] [PubMed] [Google Scholar]
- 17.Chuenkova, M. V., and M. A. Pereira. 2000. A trypanosomal protein synergizes with the cytokines ciliary neurotrophic factor and leukemia inhibitory factor to prevent apoptosis of neuronal cells. Mol. Biol. Cell 11:1487-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delemarre, F. G., A. Stevenhagen, F. P. Kroon, M. Y. Van Eer, P. L. Meenhorst, and R. Van Furth. 1995. Reduced toxoplasmastatic activity of monocytes and monocyte-derived macrophages from AIDS patients is mediated via prostaglandin E2. AIDS 9:441-445. [PubMed] [Google Scholar]
- 19.Ding, A. H., C. F. Nathan, and D. J. Stuehr. 1988. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J. Immunol. 141:2407-2412. [PubMed] [Google Scholar]
- 20.DiProspero, N. A., S. Meiners, and H. M. Geller. 1997. Inflammatory cytokines interact to modulate extracellular matrix and astrocytic support of neurite outgrowth. Exp. Neurol. 148:628-639. [DOI] [PubMed] [Google Scholar]
- 21.Fischer, H. G., B. Ntzgen, G. Reichmann, and U. Hadding. 1997. Cytokine responses induced by Toxoplasma gondii in astrocytes and microglial cells. Eur. J. Immunol. 27:1539-1548. [DOI] [PubMed] [Google Scholar]
- 22.Fretland, D. J. 1992. Potential role of prostaglandins and leukotrienes in multiple sclerosis and experimental allergic encephalomyelitis. Prostaglandins Leukot. Essent. Fatty Acids 45:249-257. [DOI] [PubMed] [Google Scholar]
- 23.Freund, Y. R., N. T. Zaveri, and H. S. Javitz. 2001. In vitro investigation of host resistance to Toxoplasma gondii infection in microglia of BALB/c and CBA/Ca mice. Infect. Immun. 69:765-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gazzinelli, R. T., F. T. Hakim, S. Hieny, G. M. Shearer, and A. Sher. 1991. Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-gamma production and protective immunity induced by an attenuated Toxoplasma gondii vaccine. J. Immunol. 146:286-292. [PubMed] [Google Scholar]
- 25.Gazzinelli, R. T., I. P. Oswald, S. L. James, and A. Sher. 1992. IL-10 inhibits parasite killing and nitrogen oxide production by IFN-gamma-activated macrophages. J. Immunol. 148:1792-1796. [PubMed] [Google Scholar]
- 26.Gazzinelli, R. T., M. Wysocka, S. Hieny, T. Scharton-Kersten, A. Cheever, R. Kuhn, W. Muller, G. Trinchieri, and A. Sher. 1996. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma and TNF-alpha. J. Immunol. 157:798-805. [PubMed] [Google Scholar]
- 27.Giovannoni, G., S. J. Heales, J. M. Land, and E. J. Thompson. 1998. The potential role of nitric oxide in multiple sclerosis. Mult. Scler. 4:212-216. [DOI] [PubMed] [Google Scholar]
- 28.Grilli, M., I. Barbieri, H. Basudev, R. Brusa, C. Casati, G. Lozza, and E. Ongini. 2000. Interleukin-10 modulates neuronal threshold of vulnerability to ischaemic damage. Eur. J. Neurosci. 12:2265-2272. [DOI] [PubMed] [Google Scholar]
- 29.Hailer, N. P., F. Wirjatijasa, N. Roser, G. T. Hischebeth, H. W. Korf, and F. Dehghani. 2001. Astrocytic factors protect neuronal integrity and reduce microglial activation in an in vitro model of N-methyl-D-aspartate-induced excitotoxic injury in organotypic hippocampal slice cultures. Eur. J. Neurosci. 14:315-326. [DOI] [PubMed] [Google Scholar]
- 30.Halonen, S. K., G. A. Taylor, and L. M. Weiss. 2001. Gamma interferon-induced inhibition of Toxoplasma gondii in astrocytes is mediated by IGTP. Infect. Immun. 69:5573-5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Helliwell, B. 2001. Role of free radicals in the neurodegenerative diseases. Drugs Aging 18:685-716. [DOI] [PubMed] [Google Scholar]
- 32.Hewett, S. J. 1999. Interferon-gamma reduces cyclooxygenase-2-mediated prostaglandin E2 production from primary mouse astrocytes independent of nitric oxide formation. J. Neuroimmunol. 94:134-143. [DOI] [PubMed] [Google Scholar]
- 33.Hofman, F. M., D. R. Hinton, K. Johnson, and J. E. Merrill. 1989. Tumor necrosis factor identified in multiple sclerosis brain. J. Exp. Med. 170:607-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Israelski, D. M., and J. S. Remington. 1993. Toxoplasmosis in non-AIDS immunocompromised host. Curr. Opin. Top. Infect. Dis. 13:322-356. [PubMed] [Google Scholar]
- 35.Jamra, L. M. F., and M. P. L. Vieira. 1991. Isolamento do Toxoplasma gondii de exsudato peritoneal e órgãos de camundongos com infecção experimental. Rev. Inst. Med. Trop. São Paulo 33:435-441. [PubMed] [Google Scholar]
- 36.Jun, C. D., S. H. Kim, C. T. Soh, S. S. Kang, and H. T. Chung. 1993. Nitric oxide mediates the toxoplasmastatic activity of murine microglial cells in vitro. Immunol. Investig. 22:487-501. [DOI] [PubMed] [Google Scholar]
- 37.Kammer, G. M. 1988. The adenylate cyclase-cAMP-protein kinase A pathway and regulation of the immune response. Immunol. Today 9:222-229. [DOI] [PubMed] [Google Scholar]
- 38.Kang, H., and Y. Suzuki. 2001. Requirement of non-T cells that produce gamma interferon for prevention of reactivation of Toxoplasma gondii infection in the brain. Infect. Immun. 69:2920-2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khan, I. A., J. D. Schwartzman, T. Matsuura, and L. H. Kasper. 1997. A dichotomous role for nitric oxide during acute Toxoplasma gondii infection in mice. Proc. Natl. Acad. Sci. USA 94:13955-13960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kikuchi, S., K. Shinpo, M. Niino, S. Tsuji, K. Iwabuchi, K. Onoe, and K. Tashiro. 2002. Prostaglandin E1 protects cultured spinal neurons against the effects of nitric oxide toxicity. Neuropharmacology 42:714-723. [DOI] [PubMed] [Google Scholar]
- 41.Levi, G., L. Minghetti, and F. Aloisi. 1998. Regulation of prostanoid synthesis in microglial cells and effects of prostaglandin E2 on microglial functions. Biochimie 80:899-904. [DOI] [PubMed] [Google Scholar]
- 42.Li, G., J. A. Harton, X. Zhu, and J. P. Ting. 2001. Downregulation of CIITA function by protein kinase A (PKA)-mediated phosphorylation: mechanism of prostaglandin E, cyclic AMP, and PKA inhibition of class II major histocompatibility complex expression in monocytic lines. Mol. Cell. Biol. 14:4626-4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lima, F. S. L., A. Gervais, C. Colin, M. Izembart, V. Moura-Neto, and M. Mallat. 2001. Regulation of microglial development: a novel role for thyroid hormone. J. Neurosci. 21:2028-2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luder, C. G., T. Lang, B. Beuerle, and U. Gross. 1998. Down-regulation of MHC class II molecules and inability to up-regulate class I molecules in murine macrophages after infection with Toxoplasma gondii. Clin. Exp. Immunol. 112:308-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mattson, M. P., S. W. Barger, K. Furukawa, A. J. Bruce, T. Wyss-Coray, R. J. Mark, and L. Mucke. 1997. Cellular signaling roles of TGF beta, TNF alpha and beta APP in brain injury responses and Alzheimer's disease. Brain Res. Rev. 23:47-61. [DOI] [PubMed] [Google Scholar]
- 46.McGeer, P. L., S. Itagaki, H. Akiyama, and E. G. McGeer. 1988. Rate of cell death in parkinsonism indicates active neuropathological process. Ann. Neurol. 24:574-576. [DOI] [PubMed] [Google Scholar]
- 47.McMillian, M., L. Y. Kong, S. M. Sawin, B. Wilson, K. Das, P. Hudson, J. S. Hong, and G. Bing. 1995. Selective killing of cholinergic neurons by microglial activation in basal forebrain mixed neuronal/glial cultures. Biochem. Biophys. Res. Commun. 215:572-577. [DOI] [PubMed] [Google Scholar]
- 48.Merrill, J. E. 1992. Tumor necrosis factor alpha, interleukin 1 and related cytokines in brain development: normal and pathological. Dev. Neurosci. 14:1-10. [DOI] [PubMed] [Google Scholar]
- 49.Minghetti, L., A. Nicolini, E. Polazzi, C. Creminon, J. Maclouf, and G. Levi. 1997. Prostaglandin E2 downregulates inducible nitric oxide synthase expression in microglia by increasing cAMP levels. Adv. Exp. Med. Biol. 433:181-184. [DOI] [PubMed] [Google Scholar]
- 50.Minghetti, L., A. Nicolini, E. Polazzi, C. Creminon, J. Maclouf, and G. Levi. 1997. Inducible nitric oxide synthase expression in activated rat microglia cultures is downregulated by exogenous prostaglandin E2 and by cyclooxygenase inhibitors. Glia 19:152-160. [PubMed] [Google Scholar]
- 51.Minghetti, L., E. Polazzi, A. Nicolini, and G. Levi. 1998. Opposite regulation of prostaglandin E2 synthesis by transforming growth factor-beta 1 and interleukin 10 in activated microglial cultures. J. Neuroimmunol. 82:31-39. [DOI] [PubMed] [Google Scholar]
- 52.Penkowa, M., T. Moos, J. Carrasco, H. Hadberg, A. Molinero, H. Bluethmann, and J. Hidalgo. 1999. Strongly compromised inflammatory response to brain injury in interleukin-6-deficient mice. Glia 25:343-357. [PubMed] [Google Scholar]
- 53.Phipps, R. P., S. H. Stein, and R. L. Roper. 1991. A new view of prostaglandin E regulation of the immune response. Immunol. Today 12:349-352. [DOI] [PubMed] [Google Scholar]
- 54.Rogers, J., J. Luber-Narod, S. D. Styren, and W. H. Civin. 1988. Expression of immune system-associated antigens by cells of the human central nervous system: relationship to the pathology of Alzheimer's disease. Neurobiol. Aging 9:339-349. [DOI] [PubMed] [Google Scholar]
- 55.Rostasy, K., L. Monti, C. Yiannoutsos, M. Kneissl, J. Bell, T. L. Kemper, J. C. Hedreen, and B. A. Navia. 1999. Human immunodeficiency virus infection, inducible nitric oxide synthase expression, and microglial activation: pathogenic relationship to the acquired immunodeficiency syndrome dementia complex. Ann. Neurol. 46:207-216. [PubMed] [Google Scholar]
- 56.Scharton-Kersten, T. M., G. Yap, J. Magram, and A. Sher. 1997. Inducible nitric oxide is essential for host control of persistent but not acute infection with the intracellular pathogen Toxoplasma gondii. J. Exp. Med. 185:1261-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schluter, D., M. Deckert-Schluter, E. Lorenz, T. Meyer, M. Rollinghoff, and C. Bogdan. 1999. Inhibition of inducible nitric oxide synthase exacerbates chronic cerebral toxoplasmosis in Toxoplasma gondii-susceptible C57BL/6 mice but does not reactivate the latent disease in T. gondii-resistant BALB/c mice. J. Immunol. 162:3512-3518. [PubMed] [Google Scholar]
- 58.Schluter, D., N. Kaefer, H. Hof, O. D. Wiestler, and M. Deckert-Schluter. 1997. Expression pattern and cellular origin of cytokines in the normal and Toxoplasma gondii-infected murine brain. Am. J. Pathol. 150:1021-1035. [PMC free article] [PubMed] [Google Scholar]
- 59.Silva, N. M., W. L. Tafuri, J. I. Alvarez-Leite, J. R. Mineo, and R. T. Gazzinelli. 2002. Toxoplasma gondii: in vivo expression of BAG-5 and cyst formation is independent of TNF p55 receptor and inducible nitric oxide synthase functions. Microbes Infect. 4:261-270. [DOI] [PubMed] [Google Scholar]
- 60.Stoll, G., S. Jander, and M. Schroeter. 2000. Cytokines in CNS disorders: neurotoxicity versus neuroprotection. J. Neural Transm. 59:81-89. [DOI] [PubMed] [Google Scholar]
- 61.Suzuki, Y. 1999. Genes, cells and cytokines in resistance against development of toxoplasmic encephalitis. Immunobiology 201:255-271. [DOI] [PubMed] [Google Scholar]
- 62.Suzuki, Y., M. A. Orellana, R. D. Schreiber, and J. S. Remington. 1988. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science 240:516-518. [DOI] [PubMed] [Google Scholar]
- 63.Suzuki, Y., H. Kang, S. Parmley, S. Lim, and D. Park. 2000. Induction of tumor necrosis factor-alpha and inducible nitric oxide synthase fails to prevent toxoplasmic encephalitis in the absence of interferon-gamma in genetically resistant BALB/c mice. Microbes Infect. 2:455-462. [DOI] [PubMed] [Google Scholar]
- 64.Tenter, A. M., A. R. Heckeroth, and L. M. Weiss. 2000. Toxoplasma gondii: from animals to humans. Int. J. Parasitol. 30:1217-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Théry, C., A. Dobbertin, and M. Mallat. 1994. Downregulation of in vitro neurotoxicity of brain macrophages by prostaglandin E2 and a β-adrenergic agonist. Glia 11:383-386. [DOI] [PubMed] [Google Scholar]
- 66.Trentin, A. G., M. Alvarez-Silva, and V. Moura-Neto. 2001. Thyroid hormone induces cerebellar astrocytes and C6 glioma cells to secrete mitogenic growth factors. Am. J. Physiol. Endocrinol. Metab. 281:1088-1094. [DOI] [PubMed] [Google Scholar]
- 67.Tsacopoulos, M., and P. J. Magistretti. 1996. Metabolic coupling between glia and neurons. J. Neurosci. 16:877-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67a.Vane, J. R. 2002. Back to an aspirin a day? Science 296:474-475. [DOI] [PubMed]
- 68.Villegas, E. N., U. Wille, L. Craig, P. S. Linsley, D. M. Rennick, R. Peach, and C. A. Hunter. 2000. Blockade of costimulation prevents infection-induced immunopathology in interleukin-10-deficient mice. Infect. Immun. 68:2837-2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wille, U., E. N. Villegas, B. Striepen, D. S. Roos, and C. A. Hunter. 2001. Interleukin-10 does not contribute to the pathogenesis of a virulent strain of Toxoplasma gondii. Parasite Immunol. 23:291-296. [DOI] [PubMed] [Google Scholar]
- 70.Yap, G. S., and A. Sher. 1999. Effector cells of both nonhemopoietic and hemopoietic origin are required for interferon (IFN)-gamma- and tumour necrosis factor (TNF)-alpha-dependent host resistance to the intracellular pathogen Toxoplasma gondii. J. Exp. Med. 189:1083-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang, J., and S. Rivest. 2001. Anti-inflammatory effects of prostaglandin E2 in the central nervous system in response to brain injury and circulating lipopolysaccharide. J. Neurochem. 76:855-864. [DOI] [PubMed] [Google Scholar]
- 72.Zhang, S. C., B. D. Goetz, J. L. Carre, and I. D. Duncan. 2001. Reactive microglia in dysmyelination and demyelination. Glia 34:101-109. [DOI] [PubMed] [Google Scholar]