Abstract
There has been emerging evidence that immunocompetent hosts can harbor Pneumocystis in their lungs. The purpose of this study was to determine the kinetics of Pneumocystis carinii f. sp. muris infection in adult immunocompetent mice and the host immune response to the organisms. To accomplish this, we exposed adult immunocompetent mice to SCID mice infected with P. carinii f. sp. muris by cohousing. We found that P. carinii f. sp. muris was detectable in the lungs of cohoused immunocompetent mice by PCR by 3 weeks after the beginning of cohousing. At about 4 weeks of cohousing, P. carinii f. sp. muris was readily detectable in the lungs of mice by microscopic techniques. Also at this time, P. carinii f. sp. muris-specific immunoglobulin G was found in the sera of the mice, and CD62low CD4- and CD8-positve T cells accumulated in the lungs. Shortly after this immune response, the P. carinii f. sp. muris organisms were cleared from the lungs. Adult mice cohoused for only 1 week also contained P. carinii f. sp. muris cysts detectable by silver staining at 5 and 6 weeks after the beginning of cohousing. We also found that the P. carinii f. sp. muris organisms grew to greater numbers in the lungs of BALB/c mice than in those of C57BL6 mice. This indicates that immunocompetent hosts develop a mild infection with P. carinii f. sp. muris which resolves in 5 to 6 weeks when there is a detectable immune response to the organism. Once an acquired immune response was initiated, the P. carinii f. sp. muris organisms were quickly eliminated without clinical signs of disease.
Pneumocystis sp. is an important pathogen in immunocompromised individuals. It has been shown that a loss of CD4-positive T cells is the predominant cause of susceptibility to these organisms (13, 22). Thus, it is believed that immunocompetent hosts are resistant to infection by Pneumocystis. This belief is further enforced by the fact that Pneumocystis rarely causes overt disease in immunocompetent individuals.
Recent studies suggest that children may frequently harbor Pneumocystis in their lungs (17, 24, 26), and animal experiments have shown that neonatal mice, pigs, and rabbits can harbor substantial numbers of Pneumocystis organisms (3, 7, 15, 23). However, after intratracheal instillation of Pneumocystis carinii f. sp. muris into immunocompetent adult mice, the organisms grow in number in the lungs for only about 1 week and are essentially cleared from the lungs in another 1 to 2 weeks (2; our unpublished data). Because the natural mode of transmission of Pneumocystis is by an aerosol route (14), we reasoned that immunocompetent adult mice might harbor P. carinii f. sp. muris for a longer period after exposure if the organisms are transmitted by the natural aerosol mode. We thought this to be possible because with a natural aerosol exposure, very few organisms would be deposited in the lungs; this should not induce an inflammatory response as does the intratracheal instillation of a suspension of a large number of P. carinii f. sp. muris in a fluid vehicle that by itself can cause inflammation when instilled intratracheally. This may be an important point, because infection of SCID mice by cohousing (aerosol exposure) does not result in an inflammatory response (27, 28). In addition, most studies of the host immune response to Pneumocystis have been done on animals after intratracheal instillation of P. carinii f. sp. muris. With this route of administration of the organism, the host's immune system would be exposed to a bolus of P. carinii f. sp. muris organisms that are not adherent to host lung epithelial cells, whereas with cohousing, a few organisms probably reach the lung epithelium undetected and then expand in number as adherent organisms. These adherent organisms could present the host immune surveillance mechanisms with a much different profile than nonadherent organisms coming down the airways after intratracheal instillation.
The purpose of this study was twofold. First, we wished to determine the ability of P. carinii f. sp. muris to grow in the lungs of immunocompetent mice for an extended period of time after the mice were exposed by being cohoused with P. carinii f. sp. muris-infected mice. Secondly, we investigated the immune response to P. carinii f. sp. muris induced in these cohoused mice. We found that P. carinii f. sp. muris grows in the lungs of immunocompetent mice for about 5 weeks after the beginning of cohousing. At about 4 weeks of cohousing, P. carinii f. sp. muris-specific immunoglobulin G (IgG) was found in the sera of the mice and CD4- and CD8-positve T cells accumulated in the lungs. Shortly after this immune response, the P. carinii f. sp. muris organisms were cleared from the lungs. This indicates that immunocompetent hosts harbored P. carinii f. sp. muris in small numbers for up to 5 weeks after the beginning of cohousing. However, in the cohoused mice, once an acquired immune response was initiated, the P. carinii f. sp. muris was quickly eliminated.
MATERIALS AND METHODS
Infection of mice.
CB.17 scid/scid (SCID), C57BL/6 (B6), CB.17 and BALB/c mice were obtained from the Trudeau Institute animal breeding facility (Saranac Lake, N.Y.). The SCID mice were maintained in microisolator cages and fed sterilized food and water. Beginning at 3 weeks of age, the P. carinii f. sp. muris-free SCID mice were cohoused with P. carinii f. sp. muris-infected SCID mice. After 6 weeks of cohousing, the mice newly infected with P. carinii f. sp. muris were removed to their own cages and were used as P. carinii f. sp. muris source mice after an additional 2 weeks. At this time, the SCID mice contained about 107 P. carinii f. sp. muris nuclei in their lungs (data not shown). For infection experiments, 2 P. carinii f. sp. muris-infected SCID mice were cohoused with 10 adult BALB/c or B6 mice (7 to 9 weeks of age) for a period of 6 weeks.
Microscopic enumeration of P. carinii f. sp. muris organisms in mouse lungs.
The numbers of P. carinii f. sp. muris organisms in the lungs of the mice were determined microscopically as described previously (13), except that besides staining with Diff-Quick, in some experiments, the P. carinii f. sp. muris organisms were also stained by silver stain or by immunohistochemical staining, as previously described (9). With all of these microscopic techniques, the limit of detection of P. carinii f. sp. muris is determined by the amount of lung homogenate placed on the slide (as determined by the dilution and volume) and the fraction of the stained slide that is read. Thus, the limit of detection for the Diff-Quick stain was 4.2 log10 nuclei, whereas the limit of detection with the immunostaining was 3.0 log10 organisms (each cyst and each trophozoite is considered one organism) and that for the silver stain was 2.7 log10 cysts. Although the silver stain was sensitive, it detected cysts only, and because the cysts comprised only about one-tenth of the total number of organisms, the immunostaining method was the most sensitive of the microscopic methods of detection used, since it was sensitive to both cysts and trophozoites.
P. carinii f. sp. muris-specific DNA amplification by PCR.
A previously described PCR technique (4, 10) was used to amplify P. carinii f. sp. muris-specific DNA in clarified boiled lung homogenate of mice with primers pAZ102-E (5′-GATGGCTGTTTCCAAGCCCA-3′) and pAZ102-H (5′-GTGTACGTTGCAAAGTACTC-3′), which are specific for a portion of the P. carinii f. sp. muris mitochondrial rRNA gene. PCR conditions were as follows. A hot start was done at 94°C for 2 min, followed by denaturing at 94°C for 90 s, annealing at 65°C for 90 s, and extension at 72°C for 120 s; a total of 35 cycles was done. In titer determination experiments, we were able to detect mouse P. carinii f. sp. muris at a concentration of approximately 10 nuclei per ml of lung homogenate.
Measurement of P. carinii f. sp. muris-specific IgG.
A previously described enzyme-linked immunosorbent assay (5, 11) was used to determine P. carinii f. sp. muris-specific IgG in mouse serum. Flat-bottom microtiter plates (Flow Laboratories, McLean, Va.) were coated with a P. carinii f. sp. muris soluble total protein preparation from the lungs of SCID mice infected with P. carinii f. sp. muris (10 μg of protein per ml). Test sera were diluted 1:100 in phosphate-buffered saline-0.05% Tween 20. Controls for this assay included a monoclonal antibody (MAb 90-3-2B5) specific for mouse P. carinii f. sp. muris-specific glycoprotein A, normal mouse serum, and mouse hyperimmune serum produced by immunizing immunocompetent mice with subcutaneous injections of P. carinii f. sp. muris.
Lung lavage and analysis of cells by fluorescence-activated cell sorting.
Lungs were subjected to lavage, as previously described (12), with five 1-ml aliquots of phosphate-buffered saline containing 0.3 mM EDTA. Total cells in the bronchoalveolar lavage fluids (BALF) were determined with a hemocytometer, and cells were spun onto a slide with a cytocentrifuge and stained with Diff-Quik for differential counts. Aliquots of the cells were then stained with Cy-Chrome-conjugated anti-CD4 (clone GK1.5), phycoerythrin-conjugated anti-CD8 (clone TIB 210), and fluorescein isothiocyanate-conjugated CD62 (clone Mel-14). Fluorescence-labeled cells were analyzed by using a FACScan sorter (Becton Dickinson, Mountain View, Calif.), and 5,000 events were routinely acquired.
RESULTS
Susceptibility of immunocompetent CB.17 mice to P. carinii f. sp. muris after exposure by cohousing.
CB.17 mice were cohoused for 6 weeks with CB.17 SCID mice infected with P. carinii f. sp. muris. Groups of these mice, and control mice not cohoused with infected SCID mice, were euthanized at 3, 5, and 6 weeks after the start of cohousing. The lungs of the mice were subjected to lavage, the numbers of P. carinii f. sp. muris nuclei and DNA in their lung homogenates were determined by Diff-Quik staining and PCR, respectively, and serum was collected. Results of P. carinii f. sp. muris nuclear counts are shown in Table 1. No P. carinii f. sp. muris nuclei were detected in any of the control mice at any time point by either microscopy or PCR. In the cohoused mice, no P. carinii f. sp. muris were detected at 3 weeks by microscopy but P. carinii f. sp. muris DNA was found by PCR (medium density bands consistently found). At 5 weeks, most of the cohoused mice had microscopically detectable numbers of P. carinii f. sp. muris nuclei in their lungs as well as P. carinii f. sp. muris DNA detectable by PCR (heavy bands). By 6 weeks after the end of cohousing, the cohoused mice did not have microscopically detectable P. carinii f. sp. muris nuclei in their lungs, but all the mice were still weakly positive (light but consistently detected band) for P. carinii f. sp. muris DNA by PCR.
TABLE 1.
Development of Pneumocystis infection in CB.17 immunocompetent mice cohoused with infected mice
| Length of exposure (wks) | No. of P. carinii f. sp. muris nuclei (log10)
|
PCR result for P. carinii f. sp. murisa
|
||
|---|---|---|---|---|
| Cohoused mice | Control mice | Cohoused mice | Control mice | |
| 3 | <4.3 ± 0.0b | <4.3 ± 0.0 | ++ | − |
| 5 | 5.1 ± 0.8c | <4.3 ± 0.0 | +++ | − |
| 6 | <4.3 ± 0.0 | <4.3 ± 0.0 | + | − |
+, light band; ++, medium band; +++, heavy band, −, no band.
Limit of detection 4.3 log10 P. carinii f. sp. muris nuclei per mouse.
Significantly different than control (P > 0.05).
Susceptibility of B6 and BALB/c mice to infection with P. carinii f. sp. muris by cohousing.
B6 and BALB/c mice were cohoused with P. carinii f. sp. muris-infected SCID mice for 6 weeks. As a control, some of each of the two strains of mice were not cohoused with infected mice. Groups of the mice were killed at 4, 5, and 6 weeks after cohousing. The numbers of P. carinii f. sp. muris cysts in the lungs of the mice as determined by microscopy of silver-stained homogenates is shown in Fig. 1. No cysts were detected in mice of either strain that were not cohoused. In the cohoused mice, P. carinii f. sp. muris cysts were detected at 4 weeks in both the B6 and BALB/c mice. At 5 weeks, cysts were easily detected in the BALB/c mice but were undetectable in the B6 mice. Cysts were not detected by silver staining at 6 weeks in either strain of mouse.
FIG. 1.
Numbers of P. carinii f. sp. muris cysts (log10) in lungs of C57BL/6 and BALB/c mice with and without (no-coho) cohousing with P. carinii f. sp. muris-infected SCID mice. Mice were cohoused with P. carinii f. sp. muris-infected SCID mice for 4, 5, or 6 weeks; the mice were then killed, and the numbers of P. carinii f. sp. muris cysts in their lungs were determined by silver staining of lung homogenates. Other mice were not cohoused as a control. The minimal level of detection of cysts was 102.5. Data points are the average number of cysts ± standard deviation of the mean at each time point (n = 5). ∗, P < 0.05 for comparison of cohoused and control mice, as calculated by one-way ANOVA.
In order to get a more accurate picture of the kinetics of P. carinii f. sp. muris growth in immunocompetent B6 and BALB/c mice, the previous experiment was repeated, except groups of the mice were euthanized at 15, 20, 26, 30, 35, and 40 days after cohousing and lung homogenates were stained for P. carinii f. sp. muris by immunocytochemistry.
Immunocytochemical staining detects both cysts and trophozoites, whereas the silver stain normally only detects cysts, which usually are only about one-tenth the number of trophozoites in these mice. Results are shown in Fig. 2. Most of the BALB/c and B6 mice had P. carinii f. sp. muris organisms detectable by immunocytochemistry at 30 days after the end of cohousing. The numbers of organisms detected peaked at 35 days and quickly declined by 41 days in both strains. As in the previous experiment, significantly greater numbers of P. carinii f. sp. muris organisms were detected in the lungs of the BALB/c mice than in those of the B6 mice even though the immunocytochemical staining detected more organisms than did the silver staining.
FIG. 2.
Numbers of P. carinii f. sp. muris organisms (log10) in lungs of C57BL/6 and BALB/c mice with and without cohousing with P. carinii f. sp. muris-infected SCID mice. Mice were cohoused with P. carinii f. sp. muris-infected SCID mice for 15, 20, 25, 30, 35, 40, or 45 days; the mice were then killed, and the numbers of P. carinii f. sp. muris organisms in their lungs were determined by immunostaining of lung homogenates. The minimal level of detection was 103.1 organisms. The numbers of P. carinii f. sp. muris organisms are the average number of organisms ± standard deviation of the mean at each time point (n = 5). ∗, P < 0.05 for comparison of results at day 15 and those at days 30 and 35, as calculated by one-way ANOVA.
Immune response to P. carinii f. sp. muris in BALB/c and B6 mice cohoused with P. carinii f. sp. muris-infected SCID mice.
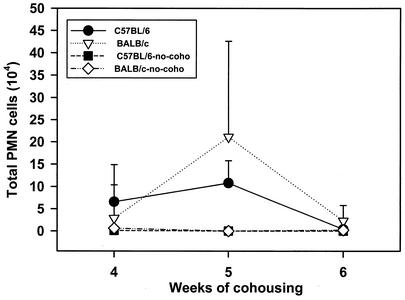
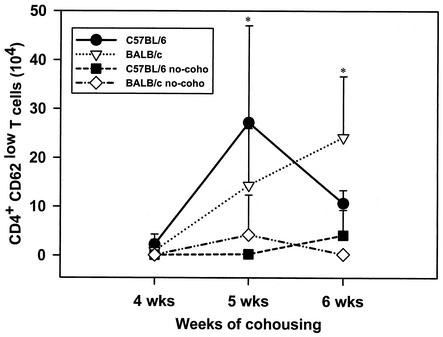
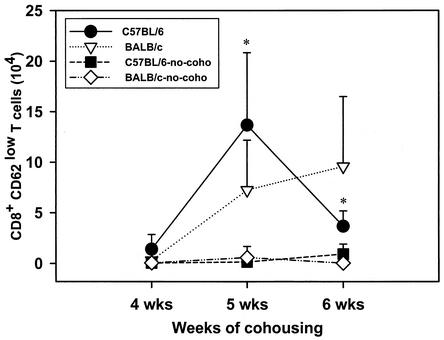
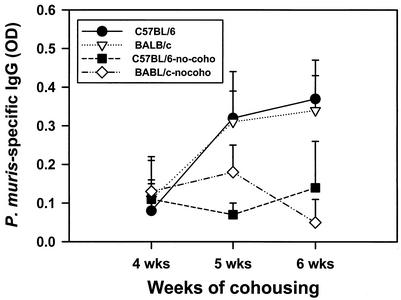
The numbers of neutrophils in BALF of the mice were determined by differential counting. Expression of CD4, CD8, and CD62 by cells in the BALF of the B6 and BALB/c mice cohoused or not cohoused with infected mice and killed at 4, 5, and 6 weeks after the end of cohousing was determined by fluorescence-activated cell sorting. Neutrophils were nearly undetectable in the BALF of either BALB/c or B6 mice (Fig. 3) not cohoused with P. carinii f. sp. muris-infected SCID mice. However, significant numbers of neutrophils were found in the BALF of both strains of mice at 4 weeks after commencement of cohousing. The numbers of neutrophils in the BALF of both strains of mice peaked at 5 weeks after the beginning of cohousing and dropped to values near those of the controls by 6 weeks. The numbers of CD4+ CD62low cells in the BALF of the mice is shown in Fig. 4, and nearly all CD4+ cells (>90%) in the BALF of mice in this experiment were typically CD62low. Few CD4+ CD62low cells were found in the BALF of mice not cohoused. However, large numbers of these cells were found in the BALF of both strains of mice at 5 weeks after the end of cohousing. At 6 weeks, the number of CD4+ CD62low cells in the BALF of the C57BL6 mice had decrease to numbers near those of the controls, but the numbers of these cells in the BALF of the BALB/c mice remained elevated. The numbers of CD8+ CD62low cells in the BALF of the mice is shown in Fig. 5; nearly all (>90%) of the CD8+ cells in BALF were CD62low. The kinetics of CD8+ CD62low cell accumulation in the lungs of the four groups of mice was similar to that of the CD4+ CD62low cells. Significant numbers of CD8+ CD62low cells were found in the BALF of the cohoused mice at 5 weeks; these cells then waned in number in the C57BL6 by 6 weeks but persisted in the BALB/c mice. The P. carinii f. sp. muris-specific IgG responses in the sera of the four groups of mice are shown in Fig. 6. Compared to the control mice not cohoused, the cohoused mice had detectable levels of P. carinii f. sp. muris-specific IgG in their sera at both 5 and 6 weeks. There were no significant differences in the amounts of specific IgG in the sera of the cohoused B6 and BALB/c mice.
FIG. 3.
Numbers of polymorphonuclear neutrophils (PMN) in lung lavage fluids of C57BL/6 and BALB/c mice after cohousing. Mice were cohoused with P. carinii f. sp. muris-infected SCID mice for 4, 5, or 6 weeks; the mice were then killed, and the numbers of PMN in their lung lavage fluids were determined. Other mice (no-coho) were not cohoused as control. Data points are the average numbers of PMN ± standard deviation of the mean at each time point (n = 5).
FIG. 4.
Numbers of CD4+ CD62low T cells in lung lavage fluids of C57BL/6 and BALB/c mice after cohousing. Mice were cohoused with P. carinii f. sp. muris-infected SCID mice for 4, 5, or 6 weeks; the mice were then killed, and the numbers of CD4+ CD62low T cells in their lung lavage fluids were determined. Other mice (no-coho) were not cohoused as a control. Data points are the average numbers of CD4+ CD62low T cells ± standard deviation of the mean at each time point (n = 5). ∗, P < 0.05 for comparison of cohoused and control mice, as calculated by one-way ANOVA.
FIG. 5.
Numbers of CD8+ CD62low T cells in lung lavage fluids of C57BL/6 and BALB/c mice after cohousing. Mice were cohoused with P. carinii f. sp. muris-infected SCID mice for 4, 5, or 6 weeks; the mice were then killed, and the numbers of CD8+ CD62low T cells in their lung lavage fluids were determined. Other mice (no-coho) were not cohoused as a control. Data points are the average numbers of CD8+ CD62low T cells ± standard deviation of the mean at each time point (n = 5). ∗, P < 0.05 for comparison of cohoused and control mice, as calculated by one-way ANOVA.
FIG. 6.
P. carinii f. sp. muris-specific IgG in lung lavage fluid of C57BL/6 and BALB/c mice after cohousing. Mice were cohoused with P. carinii f. sp. muris-infected SCID mice for 4, 5, or 6 weeks; the mice were then killed, and the levels of P. carinii f. sp. muris-specific IgG in their lung lavage fluids were determined by ELISA. Other mice (no-coho) were not cohoused as a control. Data points are the average optical densities ± standard deviation of the mean at each time point (n = 5).
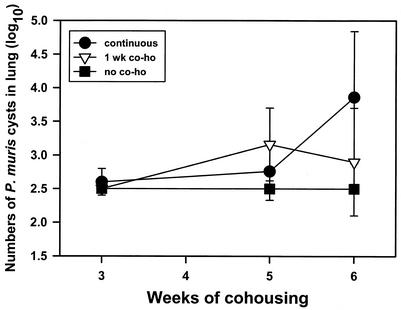
Susceptibility of CB-17 mice to infection with P. carinii f. sp. muris by continuous cohousing or a single week of cohousing.
Adult CB-17 mice were cohoused with P. carinii f. sp. muris-infected SCID mice. Half of the mice were removed from cohousing after 1 week, and the rest remained in cohousing for the duration of the experiment. Some of the mice from the two cohoused groups and some mice not cohoused were killed at 3, 5, or 6 weeks after the beginning of cohousing, and the numbers of P. carinii f. sp. muris cysts in their lungs were determined by silver staining of lung homogenates. Results are shown in Fig. 7. As seen previously, P. carinii f. sp. muris cysts were found in the lungs of continuously cohoused mice at 5 and 6 weeks after the start of cohousing. Similarly, P. carinii f. sp. muris cysts were also found in the lungs of mice cohoused for a single week when they were killed at 5 or 6 weeks after the beginning of cohousing. There was no significant difference in P. carinii f. sp. muris cyst numbers between the mice continuously cohoused and those cohoused for 1 week.
FIG. 7.
Numbers of P. carinii f. sp. muris cysts (log10) in lungs of CB-17 mice with 1 week of cohousing (1 wk co-ho) or continuous cohousing with P. carinii f. sp. muris-infected SCID mice. Mice were cohoused with P. carinii f. sp. muris-infected SCID mice for 3, 4, 5, or 6 weeks; the mice were then killed, and the numbers of P. carinii f. sp. muris cysts in their lungs were determined by silver staining of lung homogenates. Other mice (no co-ho) were not cohoused as a control. The minimal level of detection of cysts was 102.5. Data points are the average numbers of cysts ± standard deviation of the mean at each time point (n = 5).
DISCUSSION
Results presented here indicate that when immunocompetent mice are cohoused with P. carinii f. sp. muris-infected SCID mice, the P. carinii f. sp. muris organisms grow progressively in the lungs of the mice for about 5 weeks and decline to undetectable numbers (by microscopy of silver-stained or immunohistochemically stained slides) by 6 weeks. The kinetics of this infection is in contrast to what occurs in immunocompetent mice infected with P. carinii f. sp. muris by administration of organisms as a bolus directly into the lungs. In this regard, P. carinii f. sp. muris organisms grow in numbers in the lungs of immunocompetent mice for only about 1 week after intratracheal instillation of viable organisms (2) and are subsequently cleared by 3 weeks. We have found results similar to those of Beck et al. (2) in our lab (unpublished observations). The reason for this difference in persistence of infection with different means of inoculation is not known, but there are several possibilities. Others (16) have shown that by 3 h after intratracheal instillation of P. carinii f. sp. muris into immunocompetent or CD4-depleted mice, large amounts of tumor necrosis factor and numerous neutrophils are found in the BALF of the mice. This indicates that there is a significant inflammatory response in the lungs of the mice soon after P. carinii f. sp. muris is introduced by intratracheal instillation. In contrast, our current data indicate that the mice exposed to P. carinii f. sp. muris by cohousing do not mount a detectable neutrophil response in the lungs until about 4 to 5 weeks after the beginning of cohousing. In addition, we have previously shown that SCID mice infected with P. carinii f. sp. muris by cohousing do not have a detectable inflammatory response in their lungs, as indicated by a lack of both neutrophil accumulation and the expression of inflammatory cytokine mRNA in the lungs (27) until the infection progresses to the point where the lung alveoli are nearly filled with organisms (about 10 to 12 weeks of infection). This suggests that in contrast with direct intratracheal instillation, exposure to P. carinii f. sp. muris by cohousing may allow organisms to establish themselves in the lung alveoli without inducing an inflammatory response.
An inflammatory response could be a prerequisite to the induction of the acquired immune response to P. carinii f. sp. muris. In this regard, it has been shown that lung immune responses are augmented if an inflammagen is deposited in the lung together with the antigen (21). This would account for the observation that the acquired immune response to P. carinii f. sp. muris is initiated by about 10 days after intratracheal inoculation but not until about 5 weeks after exposure to P. carinii f. sp. muris by cohousing. An alternative explanation is that there is a threshold of the quantity of antigen needed to induce an immune response to the P. carinii f. sp. muris. Such a threshold could be crossed immediately with intratracheal instillation but may not be reached for some time after cohousing. Future experiments will address why, with cohousing exposure, P. carinii f. sp. muris can grow in the lungs of mice for 5 weeks before an acquired immune response is induced.
We utilized four different techniques to detect P. carinii f. sp. muris in the lungs of immunocompetent mice. As expected, PCR was the most sensitive, as it has been previously shown to detect 10 P. carinii f. sp. muris nuclei per ml of lung homogenate (4). Of the microscopic techniques we utilized, indirect fluorescent-antibody (IFA) staining detected greater numbers of P. carinii f. sp. muris organisms in the lungs of immunocompetent mice than did either the silver stain or the Diff-Quik stain. This is similar to results reported by Baughman et al. (1), who compared IFA, Diff-Quik, and silver stain sensitivities. We believe that the IFA detected more P. carinii f. sp. muris organisms than the silver stain because the IFA readily detected both cysts and trophozoites, whereas the silver stain detects only cysts. This difference is important because in our mice, there are approximately 10 times more trophozoites than cysts. Thus, quantitating both forms of P. carinii f. sp. muris by IFA gives a minimal detection number in the lungs that is 10 times lower than that obtained by using silver stain. This difference is important when trying to quantitate the few organisms present in the lungs of the immunocompetent mice.
Studies by others have shown that immunocompetent infants can harbor Pneumocystis organisms in their lungs (17, 19, 24, 26). In addition it was recently shown that healthy adults can, at least transiently, acquire Pneumocystis organisms from infected, immunodeficient patients (25). Recent work by others has shown that immunocompetent mice can transmit P. carinii f. sp. muris to immunodeficient mice (6). Thus, it is becoming apparent that Pneumocystis can infect the lungs of immunocompetent individuals. Results of the present studies establish that by 21 days after the beginning of cohousing, the lungs of all exposed immunocompetent mice become positive for P. carinii f. sp. muris DNA by PCR. By 30 days after the beginning of cohousing, the lungs of most exposed immunocompetent mice contain P. carinii f. sp. muris organisms, as determined by immunohistochemistry. By 35 days after the beginning of cohousing, activated CD4- and CD8-positive cells begin to accumulate in the lungs of the mice, and their sera contain detectable amounts of P. carinii f. sp. muris-specific IgG. By 40 to 42 days, most of the exposed mice no longer contain detectable numbers of P. carinii f. sp. muris organisms in their lungs. Thus, it takes approximately 40 days after the beginning of exposure for the P. carinii f. sp. muris to be cleared from the lungs of immunocompetent mice by the acquired immune response. The clearance of the P. carinii f. sp. muris occurs as a P. carinii f. sp. muris-specific antibody response develops. This is consistent with previous findings (8, 9, 11, 18) that antibody responses to Pneumocystis are a significant contributor to resistance to this organism. It is of interest that the cellular response occurring in the lungs of the mice as they clear the P. carinii f. sp. muris is readily detectable in the mice by analysis of the BALF but is of such a low intensity that it could easily not be noticed by histologic analysis. The minimal amount of inflammation that occurs in the immunocompetent mice as they clear the P. carinii f. sp. muris is consistent with our inability to detect overt clinical signs in these mice.
The present results indicate that P. carinii f. sp. muris persists in the lungs of immunocompetent adults for about 5 weeks before an acquired immune response clears the organisms. This could be ample time for the infected mouse to infect other mice. It is of interest that neither an environmental source nor an animal reservoir for Pneumocystis has been identified, yet the organism appears to be ubiquitous (20). It seems unlikely that in nature, the number of immunodeficient hosts is adequate to perpetuate this organism. It has already been shown that immunocompetent infant animals can acquire Pneumocystis from infected mothers (3, 7, 15, 23) and that the organisms persist for several weeks. In addition, recent work by others has shown that immunocompetent mice can transmit P. carinii f. sp. muris to immunodeficient mice (6). Therefore, our data adds to the growing evidence that immunocompetent adult and infant animals harboring Pneumocystis as a subclinical infection may be reservoirs for this organism. Studies to determine the ability of P. carinii f. sp. muris-harboring immunocompetent mice to transmit P. carinii f. sp. muris to other immunocompetent mice are currently in progress in our laboratory. In addition, whether P. carinii f. sp. muris is completely cleared from immunocompetent adult mice infected by cohousing, or whether a latent infection can persist, remains to be determined.
Acknowledgments
We acknowledge the technical assistance of Mike Tighe and Ann Harmsen.
This work was supported by NIH grants HL55002 and HL59833 as well as the Montana Agricultural Experiment Station and USDA Formula Funds.
Editor: T. R. Kozel
REFERENCES
- 1.Baughman, R. P., S. S. Strohofer, B. A. Clinton, A. D. Nickol, and P. T. Frame. 1989. The use of an indirect fluorescent antibody test for detecting Pneumocystis carinii. Arch. Pathol. Lab. Med. 113:1062-1065. [PubMed] [Google Scholar]
- 2.Beck, J. M., M. L. Warnock, J. L. Curtis, M. J. Sniezek, S. M. Arraj-Peffer, H. B. Kaltreider, and J. E. Shellito. 1991. Inflammatory responses to Pneumocystis carinii in mice selectively depleted of helper T lymphocytes. Am. J. Respir. Cell Mol. Biol. 5:186-197. [DOI] [PubMed] [Google Scholar]
- 3.Bille-Hansen, V., S. E. Jorsal, S. A. Henriksen, and O. P. Settnes. 1990. Pneumocystis carinii pneumonia in Danish piglets. Vet. Rec. 127:407-408. [PubMed] [Google Scholar]
- 4.Chen, W., F. Gigliotti, and A. G. Harmsen. 1993. Latency is not an inevitable outcome of infection with Pneumocystis carinii. Infect. Immun. 61:5406-5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, W., E. A. Havell, F. Gigliotti, and A. G. Harmsen. 1993. Interleukin-6 production in a murine model of Pneumocystis carinii pneumonia: relation to resistance and inflammatory response. Infect. Immun. 61:97-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dumoulin, A., E. Mazars, N. Seguy, D. Gargallo-Viola, S. Vargas, J. D. Cailliez, E. M. Aliouat, A. E. Wakefield, and E. Dei-Cas. 2000. Transmission of Pneumocystis carinii disease from immunocompetent contacts of infected hosts to susceptible hosts. Eur. J. Clin. Microbiol. Infect. Dis. 19:671-678. [DOI] [PubMed] [Google Scholar]
- 7.Garvy, B. A., and A. G. Harmsen. 1996. Susceptibility to Pneumocystis carinii infection: host responses of neonatal mice from immune or naive mothers and of immune or naive adults. Infect. Immun. 64:3987-3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garvy, B. A., J. A. Wiley, F. Gigliotti, and A. G. Harmsen. 1997. Protection against Pneumocystis carinii pneumonia by antibodies generated from either T helper 1 or T helper 2 responses. Infect. Immun. 65:5052-5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gigliotti, F., B. A. Garvy, and A. G. Harmsen. 1996. Antibody-mediated shift in the profile of glycoprotein A phenotypes observed in a mouse model of Pneumocystis carinii pneumonia. Infect. Immun. 64:1892-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gigliotti, F., P. J. Haidaris, C. G. Haidaris, T. W. Wright, K. R. Van Der Meid. 1993. Further evidence of host species-specific variation in antigens of Pneumocystis carinii using the polymerase chain reaction. J. Infect. Dis. 168:191-194. [DOI] [PubMed] [Google Scholar]
- 11.Harmsen, A. G., W. Chen, and F. Gigliotti. 1995. Active immunity to Pneumocystis carinii reinfection in T-cell-depleted mice. Infect. Immun. 63:2391-2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harmsen, A. G., M. J. Mason, B. A. Muggenburg, N. A. Gillett, M. A. Jarpe, and D. E. Bice. 1987. Migration of neutrophils from lung to tracheobronchial lymph node. J. Leukoc. Biol. 41:95-103. [DOI] [PubMed] [Google Scholar]
- 13.Harmsen, A. G., and M. Stankiewicz. 1990. Requirement for CD4+ cells in resistance to Pneumocystis carinii pneumonia in mice. J. Exp. Med. 172:937-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hughes, W. T. 1982. Natural mode of acquisition for de novo infection with Pneumocystis carinii. J. Infect. Dis. 145:842-848. [DOI] [PubMed] [Google Scholar]
- 15.Icenhour, C. R., S. L. Rebhollz, M. S. Collins, and M. T. Cushion. 2002. Early acquisition of Pneumocystis carinii in neonatal rats as evidence by PCR and oral swabs. Eukaryot. Cell 1:414-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kolls, J. K., J. M. Beck, S. Nelson, W. R. Summer, and J. Shellito. 1993. Alveolar macrophage release of tumor necrosis factor during murine Pneumocystis carinii pneumonia. Am. J. Respir. Cell Mol. Biol. 8:370-376. [DOI] [PubMed] [Google Scholar]
- 17.Leibovitz, E., M. Rigaud, H. Pollack, R. Lawrence, S. Chandwani, K. Krasinski, and W. Borkowsky. 1990. Pneumocystis carinii pneumonia in infants infected with the human immunodeficiency virus with more than 450 CD4 T lymphocytes per cubic millimeter. New Engl. J. Med. 323:531-533. [DOI] [PubMed] [Google Scholar]
- 18.Marcotte, H., D. Levesque, K. Delanay, A. Bourgeault, R. de la Durantaye, S. Brochu, and M. C. Lavoie. 1996. Pneumocystis carinii infection in transgenic B cell-deficient mice. J. Infect. Dis. 173:1034-1037. [DOI] [PubMed] [Google Scholar]
- 19.Morgan, D. J., S. L. Vargas, M. Reyes-Mugica, J. N. Walterspiel, W. Carver, and F. Gigliotti. 2001. Identification of Pneumocystis carinii in the lungs of infants dying of sudden infant death syndrome. Pediatr. Infect. Dis. J. 20:306-309. [DOI] [PubMed] [Google Scholar]
- 20.Peglow, S. L., A. G. Smulian, M. J. Linke, C. L. Pogue, S. Nurre, J. Crisler, J. Phair, J. W. Gold, D. Armstrong, and P. D. Walzer. 1990. Serologic responses to Pneumocystis carinii antigens in health and disease. J. Infect. Dis. 161:296-306. [DOI] [PubMed] [Google Scholar]
- 21.Peterson, L. B., R. S. Thrall, V. L. Moore, J. O. Stevens, and P. Abramoff. 1977. An animal model of hypersensitivity pneumonitis in the rabbit. Induction of cellular hypersensitivity to inhaled antigens using carrageenan and BCG. Am. Rev. Respir. Dis. 116:1007-1012. [DOI] [PubMed] [Google Scholar]
- 22.Shellito, J. E., V. V. Suzara, W. Blumenfeld, J. M. Beck, H. J. Steger, and T. H. Ermack. 1990. A new model of Pneumocystis carinii infection in mice selectively depleted of helper T lymphocytes. J. Clin. Investig. 85:1686-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamburrini, E., E. Ortona, E. Visconti, P. Mencarini, P. Margutti, M. Zolfo, S. Barca, S. E. Peters, A. E. Wakefield, and A. Siracusano. 1999. Pneumocystis carinii infection in young non-immunosuppressed rabbits. Kinetics of infection and of the primary specific immune response. Med. Microbiol. Immunol. 188:1-7. [DOI] [PubMed] [Google Scholar]
- 24.Vargas, S. L., W. T. Hughes, M. E. Santolaya, A. V. Ulloa, C. A. Ponce, C. E. Cabrera, F. Cumsille, and F. Gigliotti. 2001. Search for primary infection by Pneumocystis carinii in a cohort of normal, healthy infants. Clin. Infect. Dis. 32:855-861. [DOI] [PubMed] [Google Scholar]
- 25.Vargas, S. L., C. A. Ponce, F. Gigliotti, A. V. Ulloa, S. Prieto, M. P. Muñoz, and W. T. Hughes. 2000. Transmission of Pneumocystis carinii DNA from a patient with P. carinii pneumonia to immunocompetent contact health care workers. J. Clin. Microbiol. 38:1536-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vargas, S. L., C. A. Ponce, W. T. Hughes, A. E. Wakefield, J. C. Weitz, S. Donoso, A. V. Ulloa, P. Madrid, S. Gould, J. J. Latorre, R. Avila, S. Benveniste, M. Gallo, J. Belletti, and R. Lopez. 1999. Association of primary Pneumocystis carinii infection and sudden infant death syndrome. Clin. Infect. Dis. 29:1489-1493. [DOI] [PubMed] [Google Scholar]
- 27.Wright, T. W., C. J. Johnston, A. G. Harmsen, and J. N. Finkelstein. 1997. Analysis of cytokine mRNA profiles in the lungs of Pneumocystis carinii-infected mice. Am. J. Respir. Cell Mol. Biol. 17:491-500. [DOI] [PubMed] [Google Scholar]
- 28.Wright, T. W., C. J. Johnston, A. G. Harmsen, and J. N. Finkelstein. 1999. Chemokine gene expression during Pneumocystis carinii-driven pulmonary inflammation. Infect. Immun. 67:3452-3460. [DOI] [PMC free article] [PubMed] [Google Scholar]