Abstract
Helicobacter pylori colonizes the gastric and duodenal mucosa. The infection normally persists for life and causes peptic ulcers and gastric cancer in a subset of infected individuals. We hypothesized that the inability to clear the infection may be a consequence of H. pylori-specific regulatory T cells that actively suppress T-cell responses. Therefore, we characterized the T-cell responses to H. pylori in H. pylori-infected individuals without any subjective symptoms and in uninfected control subjects and investigated the role of regulatory CD4+ CD25high T cells during infection. The stimulation of CD4+ peripheral blood T cells with monocyte-derived dendritic cells pulsed with a membrane preparation of H. pylori resulted in proliferation and gamma interferon production in both infected and uninfected individuals. Sorted memory cells from infected individuals responded less than cells from uninfected subjects, and the unresponsiveness could be abolished by depletion of CD4+ CD25high regulatory T cells or the addition of interleukin 2. Furthermore, CD4+ CD25high T cells suppressed H. pylori-induced responses in cocultures with CD25low/− cells. Tetanus toxoid induced comparable responses in memory cells from infected and uninfected individuals in both the presence and the absence of regulatory T cells, suggesting that the suppression was H. pylori specific. In conclusion, we have shown that H. pylori-infected individuals have impaired memory CD4+ T-cell responses to H. pylori that are linked to the presence of H. pylori-specific regulatory T cells that actively suppress the responses.
Helicobacter pylori colonizes the gastric and duodenal mucosa. The bacterium causes chronic gastritis in almost all cases, and about 15% of infected individuals develop symptoms such as peptic ulcers, mucosa-associated lymphoid tissue lymphoma, or gastric adenocarcinoma (7, 11). Colonization of the mucosa leads to local infiltration of neutrophils, macrophages (11), and T and B cells specific for H. pylori antigens (8, 10, 25). In addition, circulating H. pylori-specific T cells (12, 30) and B cells (26) are also induced by the infection in most individuals. However, despite these vigorous immune responses, the host is unable to clear the bacterium from the mucosa and the infection normally persists for life.
H. pylori infection induces a T-cell response of the Th1 type (2, 34). Previous studies showed that the infiltration of gamma interferon (IFN-γ)-producing cells in the infected mucosa is accompanied by increased numbers of cells producing transforming growth factor β (TGF-β) (20), suggesting that the H. pylori-induced proinflammatory response may be counteracted by suppressive signals from regulatory cells.
Recently, the presence of regulatory T cells (Treg cells) expressing CD4 and high levels of CD25 in humans was demonstrated (1, 9, 16, 18, 29, 35, 37). CD4+ CD25+ Treg cells are nonproliferative and/or anergic in response to polyclonal stimulation but can suppress the proliferation and cytokine production of both CD4+ and CD8+ T cells via a cell-cell contact-dependent mechanism (31). Taams et al. demonstrated that the in vitro suppressive activity of Treg cells can be induced by several different types of antigens, including dietary, self, and foreign antigens (38). In animal models, in which the significance of Treg cells has been studied in more detail, these cells have been shown to be of crucial importance for maintaining self-tolerance and preventing autoimmune diseases (31). Furthermore, Treg cells have been demonstrated to be responsible for maintaining tolerance to food antigens (21, 23, 44) and to the normal intestinal flora (33). Treg cells may also play a role during infection (27). It was recently shown that Treg cells reduce H. pylori-induced gastritis in mice while allowing the bacterium to colonize the mucosa at higher densities (S. Raghavan, A. M. Svennerholm, J. Holmgren, and E. Suri-Payer, submitted for publication). Thus, Treg cells may simultaneously diminish both induction of T-cell-mediated mucosal damage and T-cell-mediated protection against bacterial colonization.
Although more detailed studies of human T-cell responses to H. pylori are essentially lacking, poor responsiveness to H. pylori antigens has been reported for T cells from gastric mucosa as well as from peripheral blood of infected individuals (4, 12, 17). We hypothesized that the poor T-cell responsiveness is caused by H. pylori-specific Treg cells, which actively suppress the responses. The aim of the present study was therefore to analyze how CD4+ memory and naive T cells as well as CD4+ CD25high Treg cells contribute to T-cell responses in H. pylori-infected and uninfected individuals. Our results show that H. pylori-specific CD4+ memory T-cell responses are reduced in infected versus uninfected individuals and that CD4+ CD25high Treg cells suppress T-cell responses to H. pylori in infected individuals.
MATERIALS AND METHODS
Volunteers and collection of specimens.
Fifteen adult Swedes who were infected with H. pylori and had no subjective symptoms (Hp+; 23 to 58 years old; 5 females) and 25 healthy, uninfected volunteers (Hp−; 24 to 40 years old; 14 females) were recruited for the study. Asymptomatic carriers were identified by screening of healthy blood donors for elevated immunoglobulin G antibody titers against H. pylori by using an in-house enzyme-linked immunosorbent assay (ELISA) (14), and their H. pylori infection status was confirmed either by culturing of H. pylori bacteria from antral biopsy specimens (nine volunteers were tested; all were found positive) or by a urea breath test (six volunteers were tested; all were found positive). Uninfected volunteers were shown to be negative for H. pylori by the in-house ELISA as well as the Pyloriset EIA-G III ELISA (Orion Diagnostica, Espoo, Finland), which has a sensitivity of 100% and a specificity of 94%. None of the volunteers had any previous history of gastrointestinal symptoms or illnesses or was on any medication during the preceding 3 weeks before recruitment for the study. Thirty to 70 ml of heparinized venous blood was collected twice, at a 1-week interval, from each individual. The study was approved by the Ethical Committee for Human Research, Göteborg University, and informed consent was obtained from each volunteer before participation.
Antigens.
A membrane preparation (MP) was prepared from H. pylori strain Hel 305. This strain is a clinical isolate from a duodenal ulcer patient; it carries an intact cag pathogenicity island and expresses the vacuolating cytotoxin (VacA s1/m1) and Lewis X. The bacteria were grown on blood agar plates under microaerobic conditions for 3 days. The MP was prepared by sonication of the bacteria followed by differential centrifugation as previously described (5). Gel electrophoresis of the MP showed that it contained more than 20 different proteins; among these, urease, the neutrophil activating protein, H. pylori adhesin A, and flagellin were identified by Western blotting with monoclonal antibodies specific for the different antigens (19, 40). The MP contained <50% (wt/wt) lipopolysaccharide (LPS), as determined by the Limulus test. LPS was purified from strain Hel 305 as previously described (43).
Cell culture conditions.
All cells were cultured in Iscove's medium supplemented with 3 μg of l-glutamine, 100 μg of gentamicin/ml, and 5% human AB-positive serum at 37°C in 5% CO2.
Generation of DCs.
From the first blood sample collected from each individual, monocytes were isolated in order to generate dendritic cells (DCs) to use as antigen-presenting cells. Peripheral blood mononuclear cells were isolated by density gradient centrifugation on Ficoll-Paque (Pharmacia, Uppsala, Sweden), followed by positive selection of CD14+ cells with magnetic beads (Miltenyi Biotec, GmbH, Bergisch Gladbach, Germany). Thereafter, 106 CD14+ cells/ml were incubated in medium supplemented with 800 U of granulocyte-macrophage colony-stimulating factor (GM-CSF) (Leucomax; Molgramostim, Schering-Plough)/ml and 500 U of interleukin 4 (IL-4) (R&D Systems Europe Ltd., Oxon, United Kingdom)/ml. After 2 days of incubation, fresh medium with IL-4 and GM-CSF was added to the cells. Two days later, the supernatants were removed, and fresh medium containing 100 U of tumor necrosis factor alpha (Pepro Tech, Inc., London, United Kingdom)/ml and antigens (5 μg of MP/ml, 2.5 μg of H. pylori LPS/ml, or 25 flocculating units of tetanus toxoid [TT] [SBL Vaccine AB, Solna, Sweden]/ml), GM-CSF, and IL-4 were added to the cells. Control cells were incubated in the absence of foreign antigens. One week after the initial cell isolation, the DCs were harvested, washed twice, and used as antigen-presenting cells. At the time of harvest, the DCs had lost the expression of CD14 but expressed high levels of HLA-DR, CD40, CD80, and CD86, and virtually all of the cells had the typical veiled morphology when inspected with a microscope. Most DC populations were devoid of any contaminating T or B cells, and the latter cells always accounted for <1% of the total cell population.
Isolation of T cells.
One week after the isolation of CD14+ cells, a new blood sample was drawn from each volunteer for the isolation of T cells. CD4+ and CD8+ T cells were isolated by positive selection with magnetic beads (Dynabeads; Dynal AS, Oslo, Norway) as previously described (22). By flow cytometric analysis, the CD4+-cell fractions were found to contain >95% CD4+ CD3+ cells (median purity, 98%) and <2% CD8+ CD3+ cells. The CD8+-cell fractions contained >90% CD8+ CD3+ cells (median purity, 95%) and <2% CD4+ CD3+ cells.
CD4+ cells from 22 volunteers (9 Hp+ and 13 Hp−) were further sorted into memory and naive cells, based on their expression of CD45RA and L-selectin (28). Purified CD4+ cells were incubated with antibody to anti-CD45RA conjugated to fluorescein isothiocyanate (anti-CD45RA-FITC) and antibody to anti-L-selectin antibodies conjugated to phycoerythrin (anti-L-selectin-PE; BD Pharmingen, San Diego, Calif.). For nine volunteers (five Hp+ and four Hp−), CD4+ cells were also labeled with anti-CD25 antibodies conjugated to allophycocyanin (anti-CD25-APC; BD Pharmingen), and a fraction of the memory cells was sorted into CD25high Treg cells and CD25low/− cells. Cell sorting was performed with a FACSVantage SE apparatus (Becton Dickinson San Jose, Calif.) operating at a sheath pressure of 22 lb/in2. On average, memory cells represented 66% (range, 36 to 85%) and naive cells represented 34% (range, 14 to 64%) of CD4+ cells. After sorting, the memory CD4+-cell fraction contained >98% CD45RA− L-selectin+/− or CD45RA+ L-selectin− cells (median purity, 99%) and the naive cell fraction contained >95% CD45RA+ L-selectin+ cells (median purity, 99%). On average, CD25high cells represented 8% (range, 4 to 15%) of the CD4+ memory cells. After sorting, the CD25high-cell fraction contained >91% CD25high memory cells (median purity, 96%) and the CD4+ CD25low/−-cell fraction contained <0.5% CD25high memory cells (median contamination, 0.3%).
Stimulation of T cells.
CD4+ or CD8+ cells (105 per well) or sorted naive or memory CD4+ cells (5 × 104 per well) were stimulated with 104 antigen-pulsed DCs in duplicate or triplicate wells of round-bottom 96-well plates. In coculture experiments, 5 × 104 CD25high or CD25low/− CD4+ memory cells were cultured together with 5 × 104 CD25low/− cells and stimulated with 104 DCs in one well. Phytohemagglutinin (PHA) (10 μg/ml; Murex Diagnostics Ltd., Temple Hill, United Kingdom) was added to control wells and, to overcome suppression, 25 U of IL-2 (BD Pharmingen)/ml was added to some wells containing memory cells. After 48 h of incubation, 100 μl of the culture medium was removed from each well and replaced with 100 μl of fresh medium. Replicate supernatants were pooled and stored at −70°C until analysis of the cytokine content. After 5 days of cultivation, cell proliferation was measured with a thymidine incorporation assay as previously described (22).
Flow cytometric analysis of proliferating cells.
The proliferation of single CD4+ memory and CD4+ CD25low/− memory cells was studied by labeling the cells with 5 μM carboxyfluorescein diacetate succinimidyl ester (CFSE) (Vybrant CFDA SE cell tracer kit; Molecular Probes Europe BV, Leiden, The Netherlands) according to the instructions of the manufacturer. After 6 days of stimulation, the cells were harvested and stained with propidium iodide and anti-CD3 antibodies conjugated to PE (BD Pharmingen) before flow cytometric analysis. The frequency of cells responding to the stimulation by proliferation in the starting population was determined by using ModFit computer software (Verity Software House, Inc.).
Detection of cytokines.
The amounts of released IFN-γ, IL-10, and TGF-β were measured by using ELISAs as previously described (22).
Statistical analysis.
The Mann-Whitney test was used to evaluate differences in proliferation and cytokine secretion for cells from Hp+ and Hp− individuals. A paired t test was used for comparisons of proliferation and IFN-γ secretion in the presence and absence of CD4+ CD25high Treg cells or exogenous IL-2.
RESULTS
CD4+ T cells from both Hp+ and Hp− individuals respond to stimulation with H. pylori antigens.
In order to investigate the contributions of CD4+ and CD8+ cells to T-cell responses to H. pylori, those T-cell subsets were isolated from peripheral blood of Hp+ and Hp− volunteers and stimulated with DCs pulsed with an MP of H. pylori or the control antigen TT.
CD4+ T cells from Hp+ and Hp− individuals responded to MP with comparable levels of proliferation and IFN-γ production (Fig. 1). The TT- and PHA-induced responses were also comparable among CD4+ T cells in the two study groups (data not shown). MP stimulation did not induce any detectable production of IL-10 or TGF-β in any cultures. CD8+ T cells responded to MP with little proliferation and IFN-γ production (1/16 and 1/4 the mean levels for CD4+ cells, respectively), and no differences were detected between Hp+ and Hp− individuals (data not shown).
FIG. 1.
Responses of CD4+ T cells after stimulation with H. pylori MP. CD4+ T cells from Hp+ and Hp− volunteers were stimulated with MP-pulsed DCs, and the resulting proliferation (A) and IFN-γ production (B) were determined. Each symbol represents the response of one individual after subtraction of the background response. Median values are indicated by horizontal bars.
Stimulation of CD4+ or CD8+ T cells with DCs pulsed with H. pylori LPS did not result in any detectable IFN-γ production. CD4+ T cells responded to LPS-treated DCs with low proliferation (1/8 the MP-induced proliferation).
Responses to H. pylori are reduced in CD4+ memory T cells from Hp+ individuals.
Since CD4+ T cells from Hp+ as well as Hp− individuals responded to H. pylori antigens with more proliferation and IFN-γ production than CD8+ T cells, we went on to study the responses of CD4+ cells in greater detail by analyzing the relative contributions of naive and memory cells. Sorted naive and memory CD4+ cells (Fig. 2A) were stimulated with MP-pulsed DCs, and the resulting proliferation and cytokine production were determined. As a control for memory cell function, cells were also stimulated with DCs pulsed with the recall antigen TT.
FIG. 2.
Responses of memory and naive CD4+ T cells after stimulation with MP or TT. (A) CD4+ cells were labeled with anti-CD45RA-FITC and anti-L-selectin-PE antibodies and sorted into memory (CD45RA− L-selectin+/− or CD45RA+ L-selectin−) and naive (CD45RA+ L-selectin+) populations. The contour plots show 1 representative experiment out of 22. The percentages of memory and naive cells are shown inside the gates. (B to E) Memory and naive cells were stimulated with DCs pulsed with MP (B and C) or TT (D and E), and the resulting proliferation and IFN-γ production were determined. Each symbol represents the response of one individual after subtraction of the background response. Median values are indicated by horizontal bars. The Mann-Whitney test was used for statistical evaluation. n.s., not significant.
First, we compared the responses in memory cells and naive cells within each study group. We found that MP stimulation induced little memory cell proliferation in Hp+ individuals, while naive cells from the same individuals proliferated vigorously in response to MP (on average, fivefold more than the memory cells) (Fig. 2B). Both memory and naive cells from Hp+ individuals produced little IFN-γ in response to MP (Fig. 2C). However, cells from Hp− individuals responded differently. Memory cells from these individuals proliferated to the same extent as naive cells in response to MP (Fig. 2B). Furthermore, memory cells from Hp− individuals produced on average 20-fold more IFN-γ than naive cells (Fig. 2C). In contrast to the MP-induced responses, TT stimulation resulted in comparable responses in cells from Hp+ and Hp− individuals (Fig. 2D and E). Thus, memory cells from both groups of subjects responded to TT with higher levels of proliferation and IFN-γ production than naive cells, demonstrating that the reduced responsiveness observed among memory cells from Hp+ individuals is limited to H. pylori-specific cells. Neither memory nor naive cells produced any detectable levels of IL-10 or TGF-β in response to MP or TT.
Next, we directly compared the responses to MP between Hp+ and Hp− individuals. Memory cells from Hp+ individuals produced on average 10-fold less IFN-γ in response to MP than memory cells from Hp− individuals. There was also a tendency toward a lower level of proliferation among memory cells from Hp+ individuals than among memory cells from Hp− individuals, although this finding was not statistically significant (P > 0.05). However, there were no differences in memory cell responses to TT between the two study groups. In contrast to the results for memory cells, we found no major differences in the responses to either MP or TT in naive cells from Hp+ and Hp− individuals.
Taken together, these results demonstrate that memory cells from Hp+ individuals have suppressed responses to MP, while their responses to TT remain normal.
IL-2 restores responses to H. pylori in CD4+ memory T cells from Hp+ individuals.
The reduced ability of memory cells from Hp+ individuals compared to those from Hp− individuals to respond to MP might have been a result of anergy among memory cells from Hp+ individuals and/or an effect of H. pylori-specific Treg cells that actively suppress the responses. In vitro, IL-2 can break T-cell anergy (3) as well as overcome the activity of suppressor cells (1). Therefore, we evaluated the effects of the addition of exogenous IL-2 on CD4+ memory T cells stimulated with MP- or TT-pulsed DCs (Fig. 3). The addition of IL-2 to memory cells from Hp+ individuals resulted in an increase in MP-induced proliferation (mean increase, 2,300 cpm); the same tendency was seen for IFN-γ production, although this finding was not statistically significant (mean increase, 12 pg/ml) (P > 0.05). In Hp− individuals, the addition of IL-2 did not result in a statistically significant increase in proliferation (mean increase, 1,500 cpm) (P > 0.05), and IFN-γ production decreased in the presence of IL-2 in most individuals. Memory T-cell responses to TT were unaffected or even reduced by the addition of IL-2.
FIG. 3.
Effects of IL-2 on MP- and TT-induced memory CD4+ T-cell responses. Memory CD4+ T cells from Hp+ (closed symbols) and Hp− (open symbols) volunteers were stimulated with DCs pulsed with MP or TT in the presence or absence of 25 U of IL-2/ml, and proliferation (A) and IFN-γ production (B) were determined. Values are shown after subtraction of background responses. Median values are indicated by horizontal bars. A paired t test was used for statistical evaluation.
CD4+ CD25high Treg cells suppress responses to H. pylori in CD4+ memory T cells from Hp+ individuals.
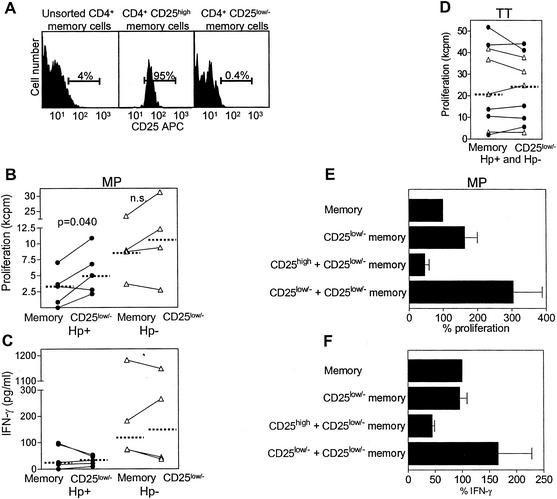
We next investigated whether CD4+ CD25high Treg cells actively suppress responses to MP in Hp+ individuals. First, CD25high cells were removed from CD4+ memory cells (Fig. 4A), and the effects on proliferation and IFN-γ production were evaluated (Fig. 4B to D). The depletion of CD25high cells led to increased proliferation in response to MP in four out of five Hp+ individuals tested (Fig. 4B), while depletion had variable effects on IFN-γ secretion in different individuals (Fig. 4C). In contrast, TT-induced memory cell proliferation was not affected by the removal of CD25high cells (Fig. 4D).
FIG. 4.
Effects of CD4+ CD25high Treg cells on MP- and TT-induced memory T-cell responses. (A) CD4+ cells were labeled with anti-CD25- allophycocyanin, anti-CD45RA- FITC, and anti-L-selectin- PE antibodies. CD45RA− L-selectin+/− or CD45RA+ L-selectin− memory cells were then sorted into CD25high and CD25low/− cells. The histograms show one representative experiment out of nine. The percentages of CD25high cells are shown inside the gates. (B to D) Memory cells and CD25low/− memory cells from Hp+ (closed symbols) and Hp− (open symbols) volunteers were stimulated with DCs pulsed with MP (B and C) or TT (D), and proliferation and IFN-γ production were determined. Values are shown after subtraction of background responses. Median values are indicated by horizontal bars. A paired t test was used for statistical evaluation. n.s., not significant. (E and F) CD25high and CD25low/− memory cells from Hp+ volunteers were cultured together (cell ratio, 1:1) and stimulated with DCs pulsed with MP, and proliferation and IFN-γ production were determined. The responses of memory cells were set to 100% in each experiment. Values shown are arithmetic means and standard errors of the means of three independent experiments.
Next, we evaluated the effects of Treg cells from Hp+ individuals in a coculture system (Fig. 4E and F). The addition of CD25high cells to CD25low/− cells (cell ratio, 1:1) reduced MP-induced proliferation and IFN-γ production in all three individuals tested (72 and 53% mean reductions, respectively). In contrast, both proliferation and IFN-γ production increased in control cultures in which CD25low/− cells were cocultured with CD25low/− cells. Thus, the observed suppression in cultures with CD25high cells cannot be explained by crowding effects in the coculture wells.
Depletion of Treg cells had less evident effects on MP-induced responses in Hp− individuals than in Hp+ individuals (Fig. 4B and C). Depletion resulted in increased proliferation in two of the Hp− individuals and had only marginal effects on proliferation in the other two individuals.
MP induces proliferation in only a small proportion of CD4+ memory T cells.
In order to exclude the possibility that the observed responses to MP in memory T cells from Hp− individuals and in naive T cells were due to polyclonal activation by mitogens present in the antigen preparation, we labeled CD4+ memory and CD4+ CD25low/− memory T cells with the fluorescent dye CFSE before stimulation and evaluated the frequency of responding cells by flow cytometry (Fig. 5). We found that after 6 days of stimulation with MP-pulsed DCs, on average 12% of CD4+ memory cells could be found in the proliferating cell population. When CD25high cells were removed from the cultures, the dividing population increased to 19%. In contrast, after mitogenic stimulation with PHA, 98% of memory or CD25low/− cells were proliferating. When the precursor frequencies of responding cells in the starting populations were calculated, we found that <1% of the total memory cell population and of the CD25low/− memory cells had initially responded to MP stimulation and that >50% of the cells had responded to PHA stimulation.
FIG. 5.
Flow cytometric analysis of proliferation induced in CD4+ memory cells by stimulation with MP and PHA. CD4+ memory and CD4+ CD25low/− memory cells were labeled with CFSE and stimulated with MP-pulsed DCs or PHA. The proliferation of CD3+ cells after 6 days of culturing was determined by flow cytometry. The histograms shown are from one experiment out of two. The percentages of proliferating cells are shown inside the gates. ag, antigen.
DISCUSSION
H. pylori infection induces massive infiltration of neutrophils, macrophages, and B and T cells into the gastric mucosa (8, 10, 11, 25). However, in spite of these immune responses, the bacteria are not cleared and a chronic infection is generally established. Furthermore, after antibiotic eradication of H. pylori, the reinfection rate is high in areas with a high prevalence of H. pylori infection (7). Clearly, H. pylori infection per se does not give rise to protective immunity. Previous studies showed that both H. pylori-infected and uninfected individuals harbor H. pylori-reactive T cells in the blood and locally in the gastric mucosa and that the reactivities of those cells to H. pylori antigens are comparable or even reduced in Hp+ subjects compared to Hp− subjects (4, 10, 12, 17, 30). In the present study, we stimulated T cells with DCs pulsed with an MP of H. pylori in order to provide optimal conditions for T-cell activation. However, even under these conditions, CD4+ and CD8+ T cells from Hp+ individuals responded to the same extent or to a lesser extent than cells from Hp− individuals.
To increase the understanding of the subtypes of CD4+ T cells that contribute to the responses in Hp+ and Hp− individuals in our system, we separated CD4+ T cells into memory and naive cells and monitored their responses to H. pylori antigens. We found that CD4+ memory T cells from Hp+ individuals show less responsiveness to H. pylori antigens than cells from Hp− individuals, whereas the antigen stimulation induces comparable responses in naive cells from the two study groups. The existence of H. pylori-reactive memory cells in uninfected individuals may be explained by the high prevalence of the H. pylori bacterium. Thus, most individuals may have encountered H. pylori antigens, giving rise to a memory T-cell response but not to sustained antibody production. Alternatively, memory cells recognizing H. pylori antigens in vitro can be the result of previous priming with cross-reactive antigens from other, related bacteria in vivo. However, our results show that persistent H. pylori infection does not increase the memory T-cell response above the baseline found in uninfected individuals. In contrast, our results suggest that infection with H. pylori leads to reduced memory cell responses to H. pylori. This unresponsiveness is limited to H. pylori-specific T cells, since the stimulation of memory and naive cells with TT resulted in comparable responses in cells from the two study groups.
Results from other systems suggest that repetitive stimulation of T cells can lead to the induction of Treg cells that actively suppress the responses of bystander cells (38). We hypothesized that due to the constant supply of H. pylori antigens over a long period of time, H. pylori-specific Treg cells that cause impaired memory T-cell responsiveness may develop. To test this hypothesis, we depleted the memory cell population of CD4+ CD25high Treg cells or, alternatively, added IL-2 to the memory cell cultures in order to overcome suppression. We found that both the depletion of Treg cells and the addition of IL-2 increased H. pylori-induced proliferation in Hp+ individuals. Furthermore, Treg cells were able to actively suppress H. pylori-induced proliferation in cocultures with CD25low/− cells. Although the depletion of Treg cells resulted in variable effects on IFN-γ production in different individuals, CD4+ CD25high cells were consistently able to suppress the IFN-γ responses of CD4+ CD25low/− cells in coculture experiments. Only terminally differentiated memory cells produce cytokines (32), and Treg cells may suppress the development of these cells in vivo. When the suppression is removed by the depletion of Treg cells in vitro, less differentiated memory cells may be able to start to proliferate but not to produce cytokines. In the coculture situation, large numbers of Treg cells are added to the few cells producing IFN-γ, a fact which may explain the observed suppression.
Several reports have described the existence of human CD4+ CD25high cells that mediate suppression after polyclonal or allogeneic stimulation (1, 9, 16, 18, 29, 35, 37). In the present study, flow cytometric proliferation analysis showed that stimulation with H. pylori membrane proteins induces less than 1% of CD4+ memory T cells to proliferate. Thus, the observed responses were not due to polyclonal activation induced by mitogens present in the antigen preparation. Furthermore, we could show that the responses to TT were not affected by the depletion of Treg cells or the addition of exogenous IL-2, supporting the notion that the suppression was specific for H. pylori antigens. Thus, our results suggest that Hp+ individuals harbor H. pylori-specific Treg cells with the ability to actively suppress responses to H. pylori. Our results also suggest that limited suppression may exist in Hp− individuals, since the depletion of CD4+ CD25high cells had less consistent effects on the H. pylori-induced responses in these individuals. Thus, we conclude that H. pylori-specific Treg cells expand in the setting of chronic H. pylori infection.
In contrast to CD4+ CD25+ memory cells, CD4+ CD25+ naive cells are not suppressive (16, 38). Accordingly, we found that naive cells from Hp+ and Hp− individuals respond to similar extents after H. pylori stimulation. The vigorous naive cell proliferation observed in most individuals is likely to be a consequence of the complex composition of the antigen preparation. Our preliminary data show that stimulation with this mixture of H. pylori antigens leads to the expansion of a broad spectrum of T-cell clones with different specificities (A. Lundgren et al., unpublished data), suggesting that proliferation is elicited by many different conventional antigens rather than by a superantigen.
Treg cells may play a role during a range of chronic infections (27). In tuberculosis, 15% of infected patients do not respond to intradermal injection with purified protein derivative (6). T cells from patients with normal responsiveness proliferate and produce IFN-γ in response to in vitro stimulation with purified protein derivative, whereas T cells from unresponsive patients fail to proliferate and to produce IFN-γ and instead produce IL-10 after stimulation. Similarly, T cells producing IL-10 circulate together with IFN-γ-producing Th1 cells in patients with chronic hepatitis C virus infection (24). However, in contrast to these reports, we could not detect any IL-10 in the culture supernatants in our study. This result may have been due to differences in the in vitro systems used or to differential induction of Treg cell phenotypes by different pathogens.
A recent report suggested that H. pylori-specific T cells in infected individuals may be deleted. This suggestion was inferred by the observed induction of FasL expression and apoptosis in different immortalized T-cell lines by sonicates from cag pathogenicity island-expressing H. pylori strains (42). Therefore, it might be argued that the CD25high Treg cells described in this report exert their function through the induction of FasL and the subsequent killing of neighboring T cells. However, in earlier studies of CD25+ Treg cells, blocking of FasL interactions did not abolish suppression and CD25+ Treg cells did not induce cell death in the suppressed responder T cells (39, 41). Furthermore, in preliminary experiments, we could not detect any FasL expression on CD25high Treg cells isolated either from peripheral blood or from stomach mucosa (Lundgren et al., unpublished).
T cells have been implicated in both protection and immunopathology during H. pylori infection. Successful vaccination strategies for mice have been associated with a shift from a Th1 to a Th2 response, whereas an increased Th1 response has been shown to aggravate H. pylori-induced gastritis (7). Recent studies indicated that Treg cells reduce the immunopathology induced by bacterial colonization (Raghavan et al., submitted). Athymic C57BL/6 nu/nu mice reconstituted with lymph node cells depleted of CD4+ CD25+ T cells and infected with H. pylori showed an earlier onset and an increased severity of gastritis but a reduced bacterial load after infection compared to mice reconstituted with whole lymph node cells. Thus, Treg cells seem to reduce the immune-mediated damage of the mucosa at the same time as they limit protection against the infection. Similar results have been reported in studies of mice with a Pneumocystis carinii lung infection, where Treg cells were also shown to dampen inflammation. An acute lethal reaction was thereby avoided, but at the expense of a protective response and bacterial clearance (15). The involvement of Treg cells in H. pylori infection is further supported by the recent finding of a higher level of infiltration of cytotoxic T lymphocyte-associated antigen 4 (CTLA-4)-expressing cells in the duodenal mucosa in patients with duodenal ulcers than in infected individuals without symptoms (36). Since duodenal ulcer patients also have a higher bacterial load in the duodenum (13), we hypothesize that an increased level of Treg cell activity in the duodenal mucosa of these patients may lead to suppression of the protective immune response, which may result in increased colonization and subsequently ulcer formation. However, the Hp+ individuals participating in the present study were all asymptomatic carriers of the infection, and in such individuals, Treg cells may maintain a balance between induced gastritis and bacterial colonization. Clearly, further studies are needed to determine whether duodenal ulcer patients have the same pattern of memory T-cell responses as asymptomatic carriers of the bacterium or even a higher level of Treg cell activity.
In conclusion, we have shown that CD4+ memory cells from Hp+ individuals have suppressed responsiveness to H. pylori antigens and that this suppression is linked to the existence of H. pylori-specific Treg cells. These cells could have important consequences both for modulation of H. pylori-induced gastritis and for protection against the infection.
Acknowledgments
This study was supported by grants from the Swedish Cancer Foundation, AstraZeneca, the Swedish Research Council, the EC council (MUCIMM), Magnus Bergvall's Foundation, and Tore Nilson's Foundation for Medical Research.
We thank Gunilla Bogren for help with recruitment of volunteers.
Editor: A. D. O'Brien
REFERENCES
- 1.Baecher-Allan, C., J. A. Brown, G. J. Freeman, and D. A. Hafler. 2001. CD4+CD25high regulatory cells in human peripheral blood. J. Immunol. 167:1245-1253. [DOI] [PubMed] [Google Scholar]
- 2.Bamford, K. B., X. Fan, S. E. Crowe, J. F. Leary, W. K. Gourley, G. K. Luthra, E. G. Brooks, D. Y. Graham, V. E. Reyes, and P. B. Ernst. 1998. Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology 114:482-492. [DOI] [PubMed] [Google Scholar]
- 3.Beverly, B., S. M. Kang, M. J. Lenardo, and R. H. Schwartz. 1992. Reversal of in vitro T cell clonal anergy by IL-2 stimulation. Int. Immunol. 4:661-671. [DOI] [PubMed] [Google Scholar]
- 4.Birkholz, S., U. Knipp, and W. Opferkuch. 1993. Stimulatory effects of Helicobacter pylori on human peripheral blood mononuclear cells of H. pylori infected patients and healthy blood donors. Zentbl. Bakteriol. 280:166-176. [DOI] [PubMed] [Google Scholar]
- 5.Bolin, I., H. Lonroth, and A. M. Svennerholm. 1995. Identification of Helicobacter pylori by immunological dot blot method based on reaction of a species-specific monoclonal antibody with a surface-exposed protein. J. Clin. Microbiol. 33:381-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boussiotis, V. A., E. Y. Tsai, E. J. Yunis, S. Thim, J. C. Delgado, C. C. Dascher, A. Berezovskaya, D. Rousset, J. M. Reynes, and A. E. Goldfeld. 2000. IL-10-producing T cells suppress immune responses in anergic tuberculosis patients. J. Clin. Investig. 105:1317-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Del Giudice, G., A. Covacci, J. L. Telford, C. Montecucco, and R. Rappuoli. 2001. The design of vaccines against Helicobacter pylori and their development. Annu. Rev. Immunol. 19:523-563. [DOI] [PubMed] [Google Scholar]
- 8.D'Elios, M. M., M. Manghetti, F. Almerigogna, A. Amedei, F. Costa, D. Burroni, C. T. Baldari, S. Romagnani, J. L. Telford, and G. Del Prete. 1997. Different cytokine profile and antigen-specificity repertoire in Helicobacter pylori-specific T cell clones from the antrum of chronic gastritis patients with or without peptic ulcer. Eur. J. Immunol. 27:1751-1755. [DOI] [PubMed]
- 9.Dieckmann, D., H. Plottner, S. Berchtold, T. Berger, and G. Schuler. 2001. Ex vivo isolation and characterization of CD4+CD25+ T cells with regulatory properties from human blood. J. Exp. Med. 193:1303-1310. [DOI] [PMC free article] [PubMed]
- 10.Di Tommaso, A., Z. Xiang, M. Bugnoli, P. Pileri, N. Figura, P. F. Bayeli, R. Rappuoli, S. Abrignani, and M. T. De Magistris. 1995. Helicobacter pylori-specific CD4+ T-cell clones from peripheral blood and gastric biopsies. Infect. Immun. 63:1102-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ernst, P. B., and B. D. Gold. 2000. The disease spectrum of Helicobacter pylori: the immunopathogenesis of gastroduodenal ulcer and gastric cancer. Annu. Rev. Microbiol. 54:615-640. [DOI] [PubMed] [Google Scholar]
- 12.Fan, X. J., A. Chua, C. N. Shahi, J. McDevitt, P. W. Keeling, and D. Kelleher. 1994. Gastric T lymphocyte responses to Helicobacter pylori in patients with H. pylori colonisation. Gut 35:1379-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamlet, A., A. C. Thoreson, O. Nilsson, A. M. Svennerholm, and L. Olbe. 1999. Duodenal Helicobacter pylori infection differs in cagA genotype between asymptomatic subjects and patients with duodenal ulcers. Gastroenterology 116:259-268. [DOI] [PubMed] [Google Scholar]
- 14.Hamlet, A. K., K. I. Erlandsson, L. Olbe, A. M. Svennerholm, V. E. Backman, and A. B. Pettersson. 1995. A simple, rapid, and highly reliable capsule-based 14C urea breath test for diagnosis of Helicobacter pylori infection. Scand. J. Gastroenterol. 30:1058-1063. [DOI] [PubMed] [Google Scholar]
- 15.Hori, S., T. L. Carvalho, and J. Demengeot. 2002. CD25+CD4+ regulatory T cells suppress CD4+ T cell-mediated pulmonary hyperinflammation driven by Pneumocystis carinii in immunodeficient mice. Eur. J. Immunol. 32:1282-1291. [DOI] [PubMed] [Google Scholar]
- 16.Jonuleit, H., E. Schmitt, M. Stassen, A. Tuettenberg, J. Knop, and A. H. Enk. 2001. Identification and functional characterization of human CD4+CD25+ T cells with regulatory properties isolated from peripheral blood. J. Exp. Med. 193:1285-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karttunen, R., G. Andersson, K. Poikonen, T. U. Kosunen, T. Karttunen, K. Juutinen, and S. Niemela. 1990. Helicobacter pylori induces lymphocyte activation in peripheral blood cultures. Clin. Exp. Immunol. 82:485-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levings, M. K., R. Sangregorio, and M. G. Roncarolo. 2001. Human CD25+CD4+ T regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J. Exp. Med. 193:1295-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindholm, C., J. Osek, and A. M. Svennerholm. 1997. Quantification of conserved antigens in Helicobacter pylori during different culture conditions. Infect. Immun. 65:5376-5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindholm, C., M. Quiding-Jarbrink, H. Lonroth, A. Hamlet, and A. M. Svennerholm. 1998. Local cytokine response in Helicobacter pylori-infected subjects. Infect. Immun. 66:5964-5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lundin, B. S. 1998. Regulation of the immune response to intestinal antigens. Ph.D. thesis. Göteborg University, Göteborg, Sweden.
- 22.Lundin, B. S., C. Johansson, and A. M. Svennerholm. 2002. Oral immunization with a Salmonella enterica serovar Typhi vaccine induces specific circulating mucosa-homing CD4+ and CD8+ T cells in humans. Infect. Immun. 70:5622-5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lundin, B. S., M. R. Karlsson, L. A. Svensson, L. A. Hanson, U. I. Dahlgren, and E. Telemo. 1999. Active suppression in orally tolerized rats coincides with in situ transforming growth factor-beta (TGF-beta) expression in the draining lymph nodes. Clin. Exp. Immunol. 116:181-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacDonald, A. J., M. Duffy, M. T. Brady, S. McKiernan, W. Hall, J. Hegarty, M. Curry, and K. H. Mills. 2002. CD4 T helper type 1 and regulatory T cells induced against the same epitopes on the core protein in hepatitis C virus-infected persons. J. Infect. Dis. 185:720-727. [DOI] [PubMed] [Google Scholar]
- 25.Mattsson, A., M. Quiding-Jarbrink, H. Lonroth, A. Hamlet, I. Ahlstedt, and A. Svennerholm. 1998. Antibody-secreting cells in the stomachs of symptomatic and asymptomatic Helicobacter pylori-infected subjects. Infect. Immun. 66:2705-2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mattsson, A., A. Tinnert, A. Hamlet, H. Lonroth, I. Bolin, and A. M. Svennerholm. 1998. Specific antibodies in sera and gastric aspirates of symptomatic and asymptomatic Helicobacter pylori-infected subjects. Clin. Diagn. Lab. Immunol. 5:288-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGuirk, P., and K. H. Mills. 2002. Pathogen-specific regulatory T cells provoke a shift in the Th1/Th2 paradigm in immunity to infectious diseases. Trends Immunol. 23:450-455. [DOI] [PubMed] [Google Scholar]
- 28.Mitra, D. K., S. C. De Rosa, A. Luke, A. Balamurugan, B. K. Khaitan, J. Tung, N. K. Mehra, A. I. Terr, A. O'Garra, L. A. Herzenberg, and M. Roederer. 1999. Differential representations of memory T cell subsets are characteristic of polarized immunity in leprosy and atopic diseases. Int. Immunol. 11:1801-1810. [DOI] [PubMed] [Google Scholar]
- 29.Ng, W. F., P. J. Duggan, F. Ponchel, G. Matarese, G. Lombardi, A. D. Edwards, J. D. Isaacs, and R. I. Lechler. 2001. Human CD4+CD25+ cells: a naturally occurring population of regulatory T cells. Blood 98:2736-2744. [DOI] [PubMed] [Google Scholar]
- 30.Quiding-Jarbrink, M., B. S. Lundin, H. Lonroth, and A. M. Svennerholm. 2001. CD4+ and CD8+ T cell responses in Helicobacter pylori-infected individuals. Clin. Exp. Immunol. 123:81-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakaguchi, S., N. Sakaguchi, J. Shimizu, S. Yamazaki, T. Sakihama, M. Itoh, Y. Kuniyasu, T. Nomura, M. Toda, and T. Takahashi. 2001. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol. Rev. 182:18-32. [DOI] [PubMed] [Google Scholar]
- 32.Sallusto, F., D. Lenig, R. Forster, M. Lipp, and A. Lanzavecchia. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401:708-712. [DOI] [PubMed] [Google Scholar]
- 33.Singh, B., S. Read, C. Asseman, V. Malmstrom, C. Mottet, L. A. Stephens, R. Stepankova, H. Tlaskalova, and F. Powrie. 2001. Control of intestinal inflammation by regulatory T cells. Immunol. Rev. 182:190-200. [DOI] [PubMed] [Google Scholar]
- 34.Sommer, F., G. Faller, P. Konturek, T. Kirchner, E. G. Hahn, J. Zeus, M. Rollinghoff, and M. Lohoff. 1998. Antrum- and corpus mucosa-infiltrating CD4+ lymphocytes in Helicobacter pylori gastritis display a Th1 phenotype. Infect. Immun. 66:5543-5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stephens, L. A., C. Mottet, D. Mason, and F. Powrie. 2001. Human CD4+CD25+ thymocytes and peripheral T cells have immune suppressive activity in vitro. Eur. J. Immunol. 31:1247-1254. [DOI] [PubMed] [Google Scholar]
- 36.Strömberg, E., A. Lundgren, A. Edebo, B. S. Lundin, A.-M. Svennerholm, and C. Lindholm. Increased frequency of activated T cells in the Helicobacter pylori-infected antrum and duodenum. FEMS Immunol. Med. Microbiol., in press. [DOI] [PubMed]
- 37.Taams, L. S., J. Smith, M. H. Rustin, M. Salmon, L. W. Poulter, and A. N. Akbar. 2001. Human anergic/suppressive CD4+CD25+ T cells: a highly differentiated and apoptosis-prone population. Eur. J. Immunol. 31:1122-1131. [DOI] [PubMed] [Google Scholar]
- 38.Taams, L. S., M. Vukmanovic-Stejic, J. Smith, P. J. Dunne, J. M. Fletcher, F. J. Plunkett, S. B. Ebeling, G. Lombardi, M. H. Rustin, J. W. J. Bijlsma, F. P. J. G. Lafeber, M. Salmon, and A. N. Akbar. 2002. Antigen-specific T cell suppression by human CD4+CD25+ regulatory T cells. Eur. J. Immunol. 32:1621-1630. [DOI] [PubMed] [Google Scholar]
- 39.Takahashi, T., Y. Kuniyasu, M. Toda, N. Sakaguchi, M. Itoh, M. Iwata, J. Shimizu, and S. Sakaguchi. 1998. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int. Immunol. 10:1969-1980. [DOI] [PubMed] [Google Scholar]
- 40.Thoreson, A. C., A. Hamlet, J. Celik, M. Bystrom, S. Nystrom, L. Olbe, and A. M. Svennerholm. 2000. Differences in surface-exposed antigen expression between Helicobacter pylori strains isolated from duodenal ulcer patients and from asymptomatic subjects. J. Clin. Microbiol. 38:3436-3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thornton, A. M., and E. M. Shevach. 1998. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med. 188:287-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang, J., E. G. Brooks, K. B. Bamford, T. L. Denning, J. Pappo, and P. B. Ernst. 2001. Negative selection of T cells by Helicobacter pylori as a model for bacterial strain selection by immune evasion. J. Immunol. 167:926-934. [DOI] [PubMed] [Google Scholar]
- 43.Westphal, O., and K. Jann. 1965. Bacterial lipopolysaccharide: extraction with phenol-water and further applications of the procedure, p. 83-92. In R. Whitler (ed.), Methods in carbohydrate chemistry. Academic Press, New York, N.Y.
- 44.Zhang, X., L. Izikson, L. Liu, and H. L. Weiner. 2001. Activation of CD25+CD4+ regulatory T cells by oral antigen administration. J. Immunol. 167:4245-4253. [DOI] [PubMed] [Google Scholar]