Abstract
Zonula occludens toxin (Zot) is produced by Vibrio cholerae and has the ability to increase mucosal permeability by reversibly affecting the structure of tight junctions. Because of this property, Zot is a promising tool for mucosal drug and antigen (Ag) delivery. Here we show that Zot acts as a mucosal adjuvant to induce long-lasting and protective immune responses upon mucosal immunization of mice. Indeed, the intranasal delivery of ovalbumin with two different recombinant forms of Zot in BALB/c mice resulted in high Ag-specific serum immunoglobulin G titers that were maintained over the course of a year. Moreover, His-Zot induced humoral and cell-mediated responses to tetanus toxoid in C57BL/6 mice and protected the mice against a systemic challenge with tetanus toxin. In addition, we found that Zot also acts as an adjuvant through the intrarectal route and that it has very low immunogenicity compared to the adjuvant Escherichia coli heat-labile enterotoxin. Finally, by using an octapeptide representing the putative binding site of Zot and of its endogenous analogue zonulin, we provide evidence that Zot may bind a mucosal receptor on nasal mucosa and may mimic an endogenous regulator of tight junctions to deliver Ags in the submucosa. In conclusion, Zot is a novel and effective mucosal adjuvant that may be useful for the development of mucosal vaccines.
Mucosal vaccines are very promising tools for the prevention of infectious diseases. Indeed, mucosal immunization stimulates systemic and local immune responses that can prevent pathogen adhesion to host mucosae, tissue invasion, and disease (19). At present, a drawback for mucosal vaccination is the paucity of effective, nontoxic adjuvants, which are necessary for the stimulation of immune responses against soluble antigens (Ags) (5, 17). For many years, cholera toxin (CT) and Escherichia coli heat-labile enterotoxin (LT) have been used in animal models as very powerful mucosal adjuvants; however, these molecules are highly toxic to humans (5). Recently, mutant CTs and LTs with decreased or absent enzymatic activities and with unmodified receptor-binding subunits have been developed (16, 27). These molecules retain their adjuvant properties and have proven useful for the stimulation of systemic and mucosal responses through different mucosal routes. However, the in vivo mechanisms of action of these molecules as well as those of CT and LT are still under investigation (16). In addition, the use of CT, LT, and mutants of these toxins has raised some concerns because these molecules bind to the ganglioside GM1, a receptor that is ubiquitous on eukaryotic cells (18), and a potential toxic effect of CT on olfactory neurons has been recently demonstrated (23). Thus, there is a need to identify new mucosal adjuvants that are efficacious and nontoxic and that have a well-defined mechanism of action.
Recently, we have described zonula occludens toxin (Zot) as a potential mucosal adjuvant for intranasal Ag delivery (15). Zot is a single polypeptide chain of 44.8 kDa encoded by the filamentous bacteriophage CTXΦ present in toxigenic strains of Vibrio cholerae (1, 25). Zot increases the permeability of the small intestine by affecting the structure of epithelial tight junctions, and this allows the passage of macromolecules through the paracellular route (9, 10). This effect of Zot has been clearly demonstrated in vivo and in vitro for rabbit, rat, and monkey intestinal epithelia and is dependent on the binding of Zot to specific receptors (11, 22). The mechanism of action of Zot on tight junctions involves a rearrangement of the epithelial cell cytoskeleton due to protein kinase C alpha-dependent F-actin polymerization (10). It is important that Zot does not cause tissue damage and that its effect on intestinal permeability is time- and dose-dependent and fully reversible (9, 10). Interestingly, a mammalian analogue of Zot has been recently described. This molecule, named zonulin, has been found on intestinal epithelial cells and acts as an endogenous regulator of tight junctions (7, 26). It has been shown that danger signals, such as bacterial loading or tissue injury in the intestine, cause zonulin to be released on the luminal side. It then binds to its receptor and modulates tight junctions (8). Interestingly, Zot and zonulin bind to the same receptor on intestinal epithelial cells and share a common binding motif at their N termini (3, 26). Thus, it appears that Zot mimics a physiological molecule in the regulation of tight junctions, and this mechanism may be responsible for its mucosal adjuvant effect. Because of its properties, Zot may be an ideal adjuvant for the development of mucosal vaccines.
A previous report has shown that intranasal delivery of Zot as an adjuvant induced Ag-specific immunoglobulin G (IgG) in serum as well as IgA in vaginal and intestinal secretions (15). Here, we investigated the efficacy of Zot for the induction of protective and long-lasting responses and asked whether it acts through mucosal routes other than the intranasal route and whether its mechanism of action involves binding to the Zot-zonulin receptor.
MATERIALS AND METHODS
Mice.
Female BALB/c and C57BL/6 mice used throughout the study were 8 to 12 weeks of age and were obtained from Charles River (Lecco, Italy).
Ags and adjuvants.
Ovalbumin (Ova) was purchased from Sigma (St. Louis, Mo.). Zot fused with the maltose-binding protein (MBP-Zot) was obtained and purified according to a previously described method (11). Briefly, the zot gene was fused in frame with the maltose-binding protein gene by using vector pMal-c2 (12). The fusion product was expressed in E. coli, and the MBP-Zot fusion protein was purified by affinity chromatography with an amylose column (MBP fusion purification system; New England Biolabs, Beverly, Mass.). We employed another recombinant form of Zot that corresponds to the whole Zot sequence and is tagged at the N terminus with a hexahistidine tail (His-Zot) (3). The biological activities of MBP-Zot and His-Zot were tested in vitro by using Ussing chambers and in vivo with the rabbit ileal loop assay (11). The octapeptide FZI/0 (GGVLVQPG) was synthesized as previously described (26).
LT, tetanus toxoid (TT), and tetanus toxin were from Chiron S.p.A., Siena, Italy.
Immunization schedules and sample collection.
Groups of five mice were intranasally immunized five times at weekly intervals (days 0, 7, 14, 21, and 28) with 5 μg of Ova or with 5 μg of TT in the absence or in the presence of mucosal adjuvants. Mice received 10 μg of MBP-Zot, 5 μg of His-Zot, or 1 μg of LT as a mucosal adjuvant. For intranasal immunization, mice were lightly anesthetized by intraperitoneal injection of ketamine and xylazine and then a final volume of 15 to 20 μl (i.e., 7.5 to 10 μl per nostril) of a solution containing Ag, with or without an adjuvant, diluted in sterile phosphate-buffered saline (PBS) was administered. In experiments in which the octapeptide FZI/0 was employed, mice were given 20 nmol of the synthetic peptide first and, after a few minutes, the vaccine. For intrarectal immunization, groups of five mice received four doses of vaccine containing 10 μg of TT with or without 10 μg of LT or 20 μg of His-Zot as an adjuvant. Mice were anesthetized as described above, and a final volume of 1,000 μl of vaccine was administered intrarectally. Serum samples were generally collected every week, 24 h before each immunization and 1 week after the last dose, and stored at −20°C until assayed.
Challenge experiments were performed by subcutaneous injection of 100 50% protective doses of tetanus toxin 74 days after the initial immunization. The appearance of any signs of paralysis and the number of deaths were monitored daily for 7 days.
Analysis of Ab isotypes.
Anti-Ova, anti-TT, and anti-Zot antibodies (Abs) were titrated in individual serum samples by using enzyme-linked immunosorbent assay (ELISA) methods (4, 14). Microplates (Microtest III; Becton Dickinson, Oxnard, Calif.) were coated with a 100-μl solution containing Ova (45 μg/ml), TT (5 μg/ml), or His-Zot (5 μg/ml) in PBS and incubated overnight at 4°C. Plates were then washed three times with PBS containing 0.05% Tween 20 (Sigma) and blocked for 2 h with 200 μl of PBS containing 1% bovine serum albumin (BSA), and serial dilutions of serum samples were added to duplicate wells. IgG titers were determined by the addition of γ-chain-specific biotin-conjugated goat anti-mouse Abs (Sigma) diluted 1:1,000 in PBS containing 0.1% BSA and 0.025% Tween 20. After incubation and washing steps, a 100-μl aliquot of horseradish peroxidase-conjugated streptavidin (Dako, Glostrup, Denmark) diluted 1:2,000 in PBS containing 0.1% BSA and 0.025% Tween 20 was added and color developed with TMB (3,3′,5,5′-tetramethylbenzidine) substrate (Kirkegaard & Perry, Gaithersburg, Md.). The color reaction was terminated after 5 to 10 min with 50 μl of 0.2 M H2SO4, and the absorbance at 450 nm was determined with an ELISA plate reader. Ab titers are expressed as the reciprocal of the sample dilution corresponding to an optical density of 0.3 U (for IgG) or 0.2 U (for IgA) above those of controls. Ab titers in each group of mice are expressed as geometric means of the individually measured titers (GMTs).
Proliferation assay and cytokine analysis.
Spleens were removed from mice that had been sacrificed by cervical dislocation. Single cell suspensions from immunized and control mice were obtained by passing organs through a 100-μm-pore-size nylon cell strainer. After lysis of erythrocytes, the splenocytes were resuspended in complete medium (RPMI 1640 containing 10% fetal bovine serum [HyClone Laboratories, Inc., Logan, Utah] and 25 mM HEPES, 2 mM l-glutamine, 100 U of penicillin/ml, 100 μg of streptomycin/ml, 1 mM sodium pyruvate, 5.5 × 10−5 M 2-mercaptoethanol, and 0.1 mM nonessential amino acids, all from GIBCO) at final concentrations of 5 × 106 cells/ml (Costar 24-well plates; Corning Inc., Corning, N.Y.) and 5 × 105 cells/well (Costar flat-bottomed 96-well plates) for cytokine analysis and the proliferation assay, respectively. The cell suspensions were stimulated with TT (1 μg/ml) for 5 to 6 days in a humidified atmosphere of 5% CO2 at 37°C. For the assessment of TT-specific T-cell proliferation, each culture condition was assessed in quadruplicate and 0.5 μCi of [3H]thymidine (Amersham Corp., Arlington Heights, Ill.) was added to each well after 5 to 6 days of culture and 12 h prior to harvest. Control wells contained unstimulated cells, and the stimulation index was calculated as follows: the number of counts per minute for experimental cultures was divided by the number of counts per minute for control cultures. Stimulatory indices above 3.0 were considered to be positive.
For the cytokine analysis, culture supernatants were harvested after 5 to 6 days of culture and stored at −70°C until assayed. Cytokine levels (gamma interferon [IFN-γ], interleukin-4 [IL-4], IL-6, IL-10, IL-12, and tumor necrosis factor alpha [TNF-α]) were measured in culture supernatants by using ELISAs as previously described (14). Briefly, 96-well Nunc-Immuno plates with MaxiSorp surfaces (Nunc) were coated with a solution containing 1 μg of monoclonal rat anti-mouse cytokine Ab (PharMingen, San Diego, Calif.)/ml (in 0.1 M Na2HPO4, pH 9.0). After overnight incubation at 4°C, plates were washed with PBS-Tween and blocked with 200 μl of PBS containing 1% BSA (Sigma) for 2 h at 37°C. After the plates were washed, serial dilutions of culture supernatants and recombinant mouse cytokines (standards) were added to the wells and the plates were incubated overnight at 4°C. The plates were then washed, appropriate biotin-conjugated rat anti-mouse cytokine Abs (PharMingen) were added to the wells, and the plates were incubated for 2 h at 37°C. Finally, after the plates were washed and horseradish peroxidase-conjugated streptavidin (Dako) was added for 2 h at 37°C, the TMB (Kirkegaard & Perry Laboratories) substrate was added to the wells, and the absorbance at 450 nm was measured after 10 to 20 min. Standard curves were generated with murine recombinant IFN-γ (rIFN-γ), rIL-4, rIL-6, rIL-10, rIL-12, and rTNF-α (PharMingen). The ELISAs were capable of detecting 20 pg of IFN-γ and IL-6/ml, 10 pg of IL-4/ml, and 80 pg of IL-10, IL-12, and TNF-α/ml.
RESULTS
Persistence of anti-Ova Abs induced by Zot in BALB/c mice.
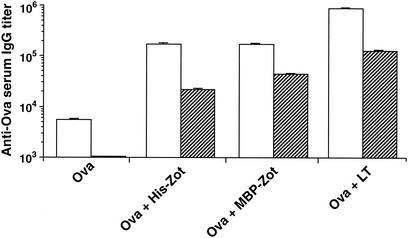
We have previously shown that Zot acts as an adjuvant for the stimulation of humoral responses against Ova in BALB/c mice following intranasal immunization (15). To determine whether the immune responses induced by Zot are maintained over a long period of time, we analyzed the memory Ab response to Ova in BALB/c mice immunized with two recombinant forms of Zot, His-Zot and MBP-Zot. We also included in the experiment a group of animals immunized with the adjuvant LT. Serum samples were taken 1 week after the fifth immunization (day 35) and 1 year after the beginning of the immunization protocol, and their anti-Ova IgG titers were compared. The results presented in Fig. 1 show that the animals that received Ova together with the adjuvants developed high titers of Ag-specific IgG at day 35 and that they maintained high serum IgG titers over the course of a year. Indeed, although a predictable decrease in the Ab levels was observed at this time, the IgG titers were still in the range of 1:20,000 or higher. The decreases in Ab titers observed in the groups that received Ova with His-Zot and MBP-Zot were 7.0- and 3.9-fold, respectively. Interestingly, although LT was slightly more potent than His-Zot and MBP-Zot at primary stimulation, the titer decrease (6.6-fold) observed after 1 year was similar to that observed for the groups immunized with the two forms of Zot. These results show that Zot is an effective mucosal adjuvant that induces long-lasting immune responses.
FIG. 1.
Persistence of serum Ab response to Ova in BALB/c mice. Mice received five weekly intranasal immunizations with Ova alone or with Ova in the presence of the indicated adjuvants and were then kept in the animal house for 11 months without receiving any further immunizations. Serum samples were collected 1 week after the last immunization (day 35, open bars) and 1 year after the beginning of the immunization protocol (striped bars). Data are expressed as Ab GMTs ± standard errors for five mice in each group.
Zot elicits Ab responses to TT in C57BL/6 mice and is poorly immunogenic.
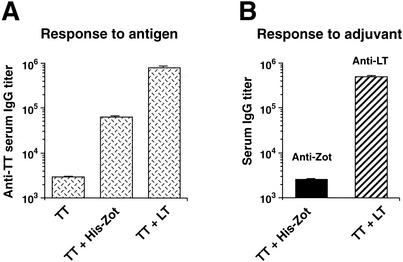
To analyze the adjuvant potency of Zot in an experimental setting where protection could be measured, we used TT as an Ag. Furthermore, the new set of experiments was performed with C57BL/6 mice to investigate the efficacy of Zot in animals with a genetic background different from that of BALB/c mice. Thus, we immunized C57BL/6 mice with TT through the intranasal route, in the presence of His-Zot or LT, and measured the serum Ab response induced. The data in Fig. 2A show that His-Zot induced high titers of anti-TT IgG Abs in serum and that LT was about 10 times more potent than Zot, a finding similar to that described for Ova with BALB/c mice (15). In addition, anti-TT IgA Abs were also found in the sera of these animals (data not shown).
FIG. 2.
Adjuvant effect and immunogenicity of His-Zot in C57BL/6 mice. Mice were intranasally immunized five times with TT alone or with TT in the presence of His-Zot or LT, and their sera were analyzed for anti-TT Ab titers (A) and for anti-His-Zot and anti-LT Ab titers (B). Data are expressed as described in the legend for Fig. 1.
Since potent mucosal adjuvants such as LT and CT are highly immunogenic, we asked whether Zot elicits a response to itself when delivered intranasally. We thus measured the adjuvant-specific serum IgG responses in the animals immunized with TT in the presence of either His-Zot or LT. The data in Fig. 2B show that His-Zot and LT differ strikingly in their immunogenicities. Indeed, after five immunizations, His-Zot induced an Ab response that was similar to that induced by the Ag alone (Fig. 2A), whereas LT elicited a much higher response to itself.
Anti-TT cell-mediated responses induced by Zot.
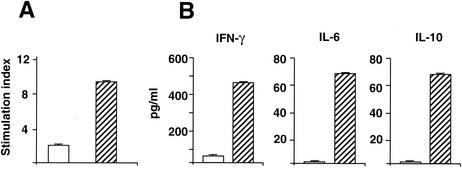
To investigate the efficacy of Zot for the induction of cell-mediated responses against the coadministered Ag and to characterize these responses, we analyzed proliferation and cytokine production in response to TT in the spleens of animals immunized with TT alone or with TT in the presence of His-Zot. Figure 3 shows that the cells from mice immunized with TT and His-Zot proliferated in response to the Ag (Fig. 3A) and secreted high amounts of IFN-γ as well as IL-6 and IL-10 (Fig. 3B), but the cells of the animals immunized with TT alone did not. In addition, we measured TT-specific IgG subclasses in the sera of these mice and found that IgG1, IgG2a, and IgG2b were all induced in the presence of His-Zot (GMTs: IgG1, 4,096; IgG2a, 256; IgG2b, 2,048). We did not observe secretion of IL-4, IL-12, or TNF-α in the splenocyte cultures of animals immunized with TT either alone or with His-Zot.
FIG. 3.
Proliferative responses (A) and cytokine secretion (B) in C57BL/6 mice after intranasal immunization with TT alone (open bars) or with TT and His-Zot (striped bars). Three mice in each group were analyzed, and the data are mean values ± standard deviations. Stimulatory indices of >3 were considered to be positive.
Zot induces protective immune responses.
To investigate whether the immune responses elicited by Zot against TT are protective, we challenged with tetanus toxin two groups of animals that had been immunized with TT alone or with TT and His-Zot. Mice were injected subcutaneously with 100 50% protective doses of tetanus toxin, and signs of paralysis and the number of deaths were monitored for 7 days. None of the seven mice challenged with tetanus toxin after immunization with TT alone survived, while all of the six mice immunized with TT and His-Zot survived and none showed signs of paralysis. The anti-TT IgG GMTs measured before challenge were 2,200 for mice immunized with TT alone and 155,872 for mice immunized with TT and His-Zot.
Zot also acts as an adjuvant through the intrarectal route.
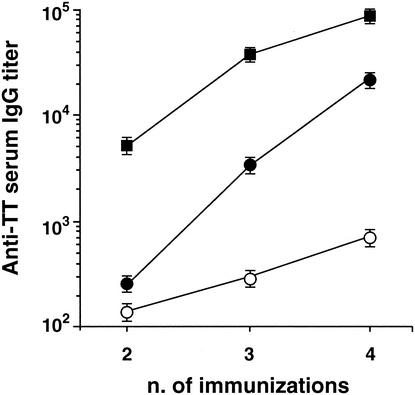
We tested whether Zot could be used through mucosal routes other than the intranasal one. We explored the possibility of using the intrarectal route, which is known to be effective and to have the potential of application for AIDS vaccines (2). C57BL/6 mice were intrarectally immunized with TT alone or with TT in the presence of His-Zot or LT, and the kinetics of anti-TT Abs were studied (Fig. 4). Although serum anti-TT IgG titers increased progressively in the three groups of animals, they remained low in the mice immunized with TT alone and greatly increased after the third dose in the animals that received His-Zot. In the mice immunized with LT, Ab titers were already high after the second dose; however, after the third and fourth doses the difference between the titers for this group and those for the group receiving His-Zot decreased progressively.
FIG. 4.
Zot acts as an adjuvant through the intrarectal route. C57BL/6 mice were immunized four times intrarectally with TT alone (unfilled circles) or with TT in the presence of His-Zot (filled circles) or LT (squares). The data are expressed as described in the legend for Fig. 1.
We also tried additional mucosal (vaginal and oral) and parenteral (subcutaneous and transcutaneous) routes for His-Zot and TT delivery; however, only subcutaneous delivery resulted in a threefold increase in IgG titers and no adjuvant effect was exerted by His-Zot through the remaining routes (data not shown).
Involvement of Zot-zonulin putative receptor in adjuvant mechanism of Zot.
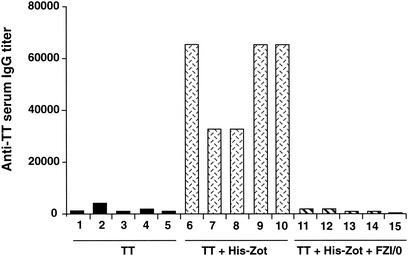
Although the exact adjuvant mechanism of Zot is not known, it is conceivable that this effect is related to the ability of Zot to open tight junctions, with the subsequent increase in permeability of the mucosal epithelial barrier (9, 11, 12). It has been reported that Zot may mimic the endogenous molecule zonulin, with which it seems to share a receptor on intestinal epithelial cells (3, 26). An octapeptide (FZI/0) homologous to the Zot-zonulin putative binding site that blocks the permeability effects of both Zot and zonulin has been synthesized (3, 26). To understand whether the putative Zot binding site is indeed involved in the adjuvant mechanism of Zot through the intranasal route, we used this peptide in vivo as a potential inhibitor of the Zot adjuvant effect. We immunized intranasally three groups of mice with (i) TT alone, (ii) TT and His-Zot, and (iii) TT with His-Zot and 20 nmol of FZI/0 and measured the serum anti-TT IgG titers. The data in Fig. 5 clearly show that the coadministration of FZI/0 with His-Zot and TT abolished the response to TT in all mice tested compared to that in the mice receiving TT and His-Zot. However, when mice received TT plus LT either in the presence or in the absence of FZI/0, they produced identical titers of TT-specific Abs (GMTs: TT and LT, 781,000; TT with LT and FZI/0, 780,000). These results indicate that FZI/0 competes with Zot for binding to its receptor on the nasal mucosa and specifically inhibits its adjuvant effect.
FIG. 5.
Inhibition of His-Zot adjuvant activity by the octapeptide FZI/0. C57BL/6 mice were intranasally immunized five times with TT alone or with TT and His-Zot in the presence or absence of the peptide FZI/0. An additional group of mice received TT and FZI/0 to test the potential toxic effect of the inhibitor; however, the octapeptide did not affect the response to the Ag alone (data not shown). Numbers identify individual mice in each group.
DISCUSSION
We have demonstrated that Zot is an effective adjuvant that induces protective and long-lasting immune responses. In addition, we have shown that Zot acts through intranasal and intrarectal routes and is efficient for the induction of humoral and cell-mediated responses. Finally, the adjuvant property of Zot has been demonstrated for different Ags and in mice with different genetic backgrounds. Two different forms of recombinant Zot, MBP-Zot and His-Zot, induced high Ab responses to the Ag, and these responses were maintained over the course of a year, a remarkable length of time relative to the murine life span. Interestingly, the adjuvant LT was slightly more efficacious than MBP-Zot and His-Zot for the initial stimulation of an immune response; however, the decrease of Ab titers observed with this molecule was comparable to that found with the two Zot proteins, indicating that LT is not superior to Zot in the maintenance of memory. Indeed, a previous study compared the efficacies of the adjuvants MBP-Zot and LT at similar intranasal doses in BALB/c mice and found that LT induced titers in serum that were 4 to 10 times higher than those induced by MBP-Zot (15). When Zot was compared with LT with intrarectal immunization, the kinetics of Ab induction were different for the two adjuvants. While LT induced a rise in Ab titers earlier than Zot, the difference in the Ab titers induced by the two molecules after the fourth dose was minimal. On the other hand, we found a striking difference in the immunogenic properties of Zot and LT. While Zot is poorly immunogenic, LT elicits a strong response to itself, and although this response does not impair subsequent immunizations (21), it nevertheless represents a useless overstimulation of the immune system. Altogether, our data indicate that the efficacy of Zot as an adjuvant is comparable to that of one of the most powerful adjuvants known.
Zot is also very efficient for the induction of protective responses. Indeed, the mice immunized with TT and His-Zot were protected from the challenge with a lethal dose of tetanus toxin. Not only did the mice survive the challenge, they also failed to show any signs of paralysis and were completely healthy.
In addition to humoral responses, we measured Ag-specific cell-mediated responses induced by His-Zot and found proliferative responses as well as cytokine production in response to TT in the group of animals that received the adjuvant. The pattern of cytokines secreted and the IgG subclasses measured in the serum of C57BL/6 mice are indicative of a mixed Th1-Th2 response, as has already been reported for BALB/c mice immunized with Ova (15). The mucosal adjuvant LT also induces mixed Th1-Th2 type responses (20), and for CT an active role in the stimulation of preferential Th2 responses has been demonstrated (13, 14). Whether Zot has an active role in the polarization of Th responses is not known and is the subject of our current studies.
Zot has been initially described as a toxin that increases intestinal permeability by modulating tight junctions, and it has been successfully used as a drug delivery system (6). Zot seems to mimic an endogenous regulator of tight junctions, zonulin, and shares with it a common receptor on intestinal epithelial cells (7). It is very likely that this activity of Zot on tight junctions is also responsible for its adjuvant effect. Our results suggest that the adjuvant effect of Zot through the intranasal route involves binding to the Zot-zonulin receptor. Indeed, the administration of the octapeptide FZI/0 containing the Zot-zonulin binding motif together with His-Zot and TT abolishes the stimulation of TT-specific immune responses, but the presence of FZI/0 does not alter the adjuvant effect of LT. Thus, our results suggest that the Zot-zonulin receptor may be expressed on nasal epithelial cells and shed light on the mechanism of action of Zot as an adjuvant. However, we cannot exclude the possibility that additional mechanisms triggered by Zot (e.g., cell recruitment or other direct effects on immune cells) may contribute to the adjuvant effect observed in vivo.
The permeabilization of epithelial barriers may be crucial for the mucosal adjuvant effect, and this notion is supported by the observation that the bacterial toxins so far described as having mucosal adjuvant activities, namely, LT, CT, Clostridium difficile toxin A, and fragilysin, all share the ability to increase intestinal permeability (16, 24; W. D. Thomas, Z. Shang, W. Lei, J. F. Torres, and T. P. Monath, Abstr. IBC 3rd Annu. Int. Conf. 1995, p. S94, 1995). The permeabilization of epithelia would allow the passage of macromolecules in the submucosa and would facilitate their encounter with cells of the immune system. In the case of CT and LT, these molecules as well as the Ag are “seen” by the immune system, whereas Zot may be sequestered by the epithelial cells as appears to happen for zonulin, and this may explain the poor immunogenicity of Zot.
The Zot adjuvant effect observed following intrarectal immunization is particularly interesting since it suggests that the receptor for Zot, although absent in the colon (12), may be present in the rectum. On the other hand, the lack of adjuvant effect of His-Zot administered through vaginal and oral routes has to be further investigated to understand whether it is due to the lack of receptors (in the case of the vaginal epithelium) or to the low stability of the protein in these mucosal environments. However, we have previously demonstrated that intranasal immunization with both MBP-Zot and His-Zot induces Ag-specific IgA Abs in vagina and intestine (15); thus, the intranasal route of immunization with Zot may be useful to induce mucosal responses at these sites.
Mucosal vaccines are very important, particularly in light of the current potential need to develop mass immunization strategies to protect against biological warfare threats. They may also increase vaccine compliance because of their noninvasiveness and offer practical advantages since their administration does not require specialized personnel and their production costs may be lower than those of injectable vaccines. Zot is a very effective adjuvant that may be useful for the development of such vaccines, and it has some advantages over other bacterial toxins with mucosal adjuvant activities. Indeed, Zot is nontoxic (9, 10) and its mechanism of action on tight junctions is well defined. Finally, the fact that Zot has homology with zonulin and uses the same receptor indicates that Zot exploits a physiological mechanism to exert its adjuvant effect.
Acknowledgments
We thank Manjusha Thakar, Antonella Riccomi, and Roberto Filippi for technical support.
This work was partially supported by the National Institutes of Health grant DK-48373 (A.F.) and by the grant 45D.5 from the AIDS Program of the Istituto Superiore di Sanità.
Editor: J. T. Barbieri
REFERENCES
- 1.Baudry, B., A. Fasano, J. Ketley, and J. B. Kaper. 1992. Cloning of a gene (zot) encoding a new toxin produced by Vibrio cholerae. Infect. Immun. 60:428-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belyakov, I. M., Z. Hel, B. Kelsall, V. A. Kuznetsov, J. D. Ahlers, J. Nacsa, D. I. Watkins, T. M. Allen, A. Sette, J. Altman, R. Woodward, P. D. Markham, J. D. Clements, G. Franchini, W. Strober, and J. A. Berzofsky. 2001. Mucosal AIDS vaccine reduces disease and viral load in gut reservoir and blood after mucosal infection of macaques. Nat. Med. 7:1320-1326. [DOI] [PubMed] [Google Scholar]
- 3.Di Pierro, M., R. Lu, S. Uzzau, W. Wang, K. Margaretten, C. Pazzani, F. Maimone, and A. Fasano. 2001. Zonula occludens toxin structure-function analysis. J. Biol. Chem. 276:19160-19165. [DOI] [PubMed] [Google Scholar]
- 4.Di Tommaso, A., G. Saletti, M. Pizza, R. Rappuoli, G. Dougan, S. Abrignani, G. Douce, and M. T. De Magistris. 1996. Induction of antigen-specific antibodies in vaginal secretions by using a nontoxic mutant of heat-labile enterotoxin as a mucosal adjuvant. Infect. Immun. 64:974-979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elson, C. O., and M. T. Dertzbaugh. 1994. Mucosal adjuvants, p. 391-401. In P. L. Ogra, J. Mestecky, M. E. Lamm, W. Strober, J. R. McGhee, and J. Bienenstock (ed.), Handbook of mucosal immunology. Academic Press, Inc., San Diego, Calif.
- 6.Fasano, A. 1998. Innovative strategies for the oral delivery of drugs and peptides. Trends Biotechnol. 16:152-157. [DOI] [PubMed] [Google Scholar]
- 7.Fasano, A. 2000. Regulation of intercellular tight junctions by Zonula occludens toxin and its eukaryotic analogue zonulin. Ann. N. Y. Acad. Sci. 915:214-222. [DOI] [PubMed] [Google Scholar]
- 8.Fasano, A. 2001. Intestinal zonulin: open sesame! Gut 49:159-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fasano, A., B. Baudry, D. W. Pumplin, S. S. Wasserman, B. D. Tall, J. M. Ketley, and J. B. Kaper. 1991. Vibrio cholerae produces a second enterotoxin, which affects intestinal tight junctions. Proc. Natl. Acad. Sci. USA 88:5242-5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fasano, A., C. Fiorentini, G. Donelli, S. Uzzau, J. B. Kaper, K. Margaretten, X. Ding, S. Guandalini, L. Comstock, and S. E. Goldblum. 1995. Zonula occludens toxin modulates tight junctions through protein kinase C-dependent actin reorganization, in vitro. J. Clin. Investig. 96:710-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fasano, A., and S. Uzzau. 1997. Modulation of intestinal tight junctions by Zonula occludens toxin permits enteral administration of insulin and other macromolecules in an animal model. J. Clin. Investig. 99:1158-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fasano, A., S. Uzzau, C. Fiore, and K. Margaretten. 1997. The enterotoxic effect of zonula occludens toxin (ZOT) on rabbit small intestine involves the paracellular pathway. Gastroenterology 112:839-846. [DOI] [PubMed] [Google Scholar]
- 13.Gagliardi, M. C., F. Sallusto, M. Marinaro, A. Langenkamp, A. Lanzavecchia, and M. T. De Magistris. 2000. Cholera toxin induces maturation of human dendritic cells and licences them for Th2 priming. Eur. J. Immunol. 30:2394-2403. [DOI] [PubMed] [Google Scholar]
- 14.Marinaro, M., P. N. Boyaka, F. D. Finkelman, H. Kiyono, R. J. Jackson, E. Jirillo, and J. R. McGhee. 1997. Oral but not parenteral interleukin (IL)-12 redirects T helper 2 (Th2)-type responses to an oral vaccine without altering mucosal IgA responses. J. Exp. Med. 185:415-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marinaro, M., A. Di Tommaso, S. Uzzau, A. Fasano, and M. T. De Magistris. 1999. Zonula occludens toxin is a powerful mucosal adjuvant for intranasally delivered antigens. Infect. Immun. 67:1287-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rappuoli, R., M. Pizza, G. Douce, and G. Dougan. 1999. A relationship between the structure and function of cholera and Escherichia coli heat-labile enterotoxins and their immunological activity at mucosal surfaces. Immunol. Today 20:493-500. [DOI] [PubMed] [Google Scholar]
- 17.Ryan, E. J., L. M. Daly, and K. H. G. Mills. 2001. Immunomodulators and delivery systems for vaccination by mucosal routes. Trends Biotechnol. 19:293-304. [DOI] [PubMed] [Google Scholar]
- 18.Spangler, B. D. 1992. Structure and function of cholera toxin and the related Escherichia coli heat-labile enterotoxin. Microbiol. Rev. 56:622-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Staats, H. F., R. J. Jackson, M. Marinaro, I. Takahashi, H. Kiyono, and J. R. McGhee. 1994. Mucosal immunity to infection with implications for vaccine development. Curr. Opin. Immunol. 6:572-583. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi, I., M. Marinaro, H. Kiyono, R. J. Jackson, I. Nakagawa, K. Fujihashi, S. Hamada, J. D. Clements, K. L. Bost, and J. R. McGhee. 1996. Mechanisms for mucosal immunogenicity and adjuvancy of Escherichia coli labile enterotoxin. J. Infect. Dis. 173:627-635. [DOI] [PubMed] [Google Scholar]
- 21.Tamura, S., H. Funato, T. Nagamine, C. Aizawa, and T. Kurata. 1989. Effectiveness of cholera toxin B subunit as an adjuvant for nasal influenza vaccination despite pre-existing immunity to CTB. Vaccine 7:503-505. [DOI] [PubMed]
- 22.Uzzau, S., R. Lu, W. Wang, C. Fiore, and A. Fasano. 2001. Purification and preliminary characterization of the zonula occludens toxin receptor from human (CaCo2) and murine (IEC6) intestinal cell lines. FEMS Microbiol. Lett. 194:1-5. [DOI] [PubMed] [Google Scholar]
- 23.van Ginkel, F. W., R. J. Jackson, Y. Yuki, and J. R. McGhee. 2000. The mucosal adjuvant cholera toxin redirects vaccine proteins into olfactory tissues. J. Immunol. 165:4778-4782. [DOI] [PubMed] [Google Scholar]
- 24.Vines, R. R., S. S. Perdue, J. S. Moncrief, D. R. Sentz, L. A. Barroso, R. L. Wright, and T. D. Wilkins. 2000. Fragilysin, the enterotoxin from Bacteroides fragilis, enhances the serum antibody response to antigen co-administered by the intranasal route. Vaccine 19:655-660. [DOI] [PubMed] [Google Scholar]
- 25.Waldor, M. K., and J. J. Mekalanos. 1996. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science 272:1910-1914. [DOI] [PubMed] [Google Scholar]
- 26.Wang, W., S. Uzzau, S. E. Goldblum, and A. Fasano. 2000. Human zonulin, a potential modulator of intestinal tight junctions. J. Cell Sci. 24:4435-4440. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto, S., H. Kiyono, M. Yamamoto, K. Imaoka, K. Fujihashi, F. W. Van Ginkel, M. Noda, Y. Takeda, and J. R. McGhee. 1997. A nontoxic mutant of cholera toxin elicits Th2-type responses for enhanced mucosal immunity. Proc. Natl. Acad. Sci. USA 94:5267-5272. [DOI] [PMC free article] [PubMed] [Google Scholar]