Abstract
The objective of this study was to examine the effect of breast-feeding by immunized dams on Helicobacter colonization in newborns. Urease-based immunization regimens failed to protect nursing pups against H. felis, whereas H. felis lysate-cholera toxin resulted in protection. This observation correlated with a high recognition of cell surface-expressed bacterial antigens by milk antibodies. Protection lasted until weaning, indicating that infection is maintained at undetectable levels by passive immunity but then resumes when breast-feeding stops.
Helicobacter pylori infects half of the world population, and most individuals acquire the infection before the age of 5 years (19). Vertical transmission, especially through the mother or through older siblings is thought to be the major route of transmission of the infection (9). Infection at an early age is associated with increased risk of complications and may have adverse effects throughout childhood. Indeed, the incidence of gastric carcinoma is higher in populations where infection during infancy is common. In addition, recent evidence suggests that H. pylori infection weakens the resistance of children to diarrheal diseases such as cholera (M. L. Shahinian, D. J. Passaro, D. L. Swerdlow, E. D. Mintz, M. Rodriguez, and J. Parsonnel, Letter, Lancet 355:377-378, 2000) and impairs their growth. Thus, infants represent a major target population for preventive interventions against H. pylori, including vaccination. Yet, the role of passive immunity in preventing early acquisition of H. pylori remains largely unknown. Epidemiological studies carried out in The Gambia found a statistical association between babies breast-fed by mothers whose milk had high titers of antibodies against H. pylori and protection against early Helicobacter infection up to, but not after, the time of weaning (J. E. Thomas, S. Austin, A. Dale, P. McClean, M. Harding, W. A. Coward, and L. T. Weaver, Letter, Lancet 342:121, 1993). A couple of studies suggested that specific antibodies could be protective. Antibodies from the milk of hyperimmunized cows, when administered orally, were shown to efficiently protect humans against a large variety of pathogens including H. pylori (18). Hyperimmune bovine colostrum was also reported to be effective in the treatment of Helicobacter infection (2).
The aim of our study was to determine whether female mice immunized following protocols known to induce a good protective immunity in adults (5) could protect their babies from Helicobacter colonization.
Practically, groups of 3 to 4 female BALB/c mice (Harlan, Horst, The Netherlands) were lightly anesthetized with halothane (Halocarbon Laboratories, River Edge, N.J.) and immunized nasally four times at 1-week intervals with 30 μg of recombinant H. pylori urease (kindly provided by Acambis, Cambridge, Mass.) or 100 μg of H. felis lysate (8) combined with 5 μg of cholera toxin (CT) (Calbiochem, Lucerne, Switzerland). Other groups of mice were twice given 20-μl nasal doses of 5 × 107 live recombinant Salmonella enterica serovar Typhimurium PhoPc expressing H. pylori urease at a 2-week interval (7). For DNA immunization, mice were given intramuscular injections twice at a 2-week interval with 100 μg of pKUreA and pKUreB, two pCI-derived eukaryotic expression vectors (Promega, Wallisellen, Switzerland) encoding either the A or B subunit of H. pylori urease behind Kozak sequences, using a Gene Gun device (Bio-Rad Laboratories). Mice were then mated with males, made pregnant, and milked (12).
Immunization of adult mice with H. pylori urease or H. felis lysate triggers a specific antibody response in milk.
Specific humoral responses directed against Helicobacter antigens following immunization have been documented in blood, saliva and in intestinal secretions but not in milk. Antibody titers (Fig. 1, upper panel) were determined by end point dilutions and expressed as geometrical means of reciprocal dilutions estimated as more than two times the values observed for naive animals (7). Microtiter plates were coated with 0.5 μg of recombinant urease or 1 μg of H. felis lysate per well. Milk was serially diluted (twofold), and specific antibodies detected with biotinylated rabbit anti-mouse immunoglobulin G IgG (Amersham, Dübendorg, Germany) were used at a dilution of 1:500 and a biotinylated goat anti-mouse IgA (Sigma, Buchs, Switzerland) used at a dilution of 1:250, and this was followed by incubation with streptavidin-bound horseradish peroxidase (AP-Biotech) at a dilution of 1:5,000 (Dako, Zug, Switzerland). Immune complexes were revealed with o-phenylenediamine (Sigma) in the presence of 0.03% H2O2 as a substrate, and plates were read after 15 min of incubation.
FIG. 1.
(Upper panel) Titers of urease-specific antibodies in milk of immunized mice. Urease-specific antibody responses were measured in female BALB/c mice (n = 3 or 4) after immunization with purified H. pylori urease (AB) in the presence of CT (dark grey box), with attenuated S. enterica serovar Typhimurium PhoPc (St) expressing H. pylori urease (light grey box), after immunization with naked DNA encoding the A or the B subunits of H. pylori urease (dashed boxes) or after immunization with H. felis lysate (Hfl) and CT (black box). Titers are expressed as geometric means of reciprocal dilutions ± standard deviations (error bars). sIgAs, secretory IgAs. (Lower panel) H. felis infection in breast-fed pups. Pups breast-fed by naive dams (white boxes) or dams immunized with urease-CT (dark grey boxes), urease-Salmonella (light grey boxes), or Hfl-CT (black boxes) were infected with H. felis between day 3 and 5 of age and killed at the indicated time points. The presence of H. felis in gastric tissues was assessed by urease activity measured photometrically at an optical density at 550 nm after 3 h of incubation using a colorimetric test and by histology. abbreviations: UT, urease test; neg and pos, number of pups that were negative and positive, respectively, for Helicobacter in each experiment; ns, not significant. Error bars, standard deviations.
Nasal administration of recombinant urease and CT, recombinant Salmonella expressing urease, injected DNA constructs encoding urease, and nasal whole H. felis lysates induced local humoral responses with high titers of antigen-specific IgGs. In blood (data not shown), the IgG titers paralleled levels found in milk, although they were 1 log higher on average. Milk IgG antibody titers were lowest with DNA vaccination. Milk-specific secretory IgA antibody responses required nasal immunization with urease or H. felis lysate, no antibodies could be detected when mice were immunized with Salmonella expressing urease.
Urease-immunized mothers fail to protect against H. felis colonization.
To establish whether immunized mothers could protect their progeny against Helicobacter colonization, their pups were inoculated at day 3 with 5 × 107 H. felis organisms in 20 μl of BHI (6) and sacrificed 12 to 15 days or 16 to 22 days postinoculation (Fig. 1, lower panel). The DNA-immunized dams were not included because of their lower immune response, reflected by lower antibody titers in milk and in blood (data not shown).
The Presence of Helicobacter was assessed at sacrifice by measuring urease activity on a half-stomach sample using a colorimetric test (SC: Jatrox test; Procter and Gamble, Weiterstadt, Germany) based on quantification by photometric analysis at an optical density of 550 nm (14). The remaining half stomach was fixed in neutral buffered 10% formalin and processed routinely for histology. Five-micrometer-thick sections were stained with hematoxylin and eosin and cresyl violet. Mice were considered free of Helicobacter when both UT and histological analysis (performed by a pathologist blinded to the study) were negative. Most of the pups breast-fed by sham-immunized dams (urease-Salmonella-immunized and urease-CT-immunized ones) were found positive for H. felis. In contrast, 19 out of 24 pups breast-fed by dams immunized with whole H. felis lysates-CT presented negative urease activity and histology (P = 0.0004 versus pups breast-fed by nonimmunized mice [Fischer's exact test]). When pups were sacrificed between day 16 and 22, they were all positive for Helicobacter infection with the exception of five pups fed by naive dams.
Despite high titers of urease-specific antibodies in milk, colonization of the gastric mucosa by H. felis could not be prevented in pups breast-fed on immunized mothers. This contradicts previous experiments (8) in which oral administration of preformed immune complexes of Helicobacter and urease-specific monoclonal antibodies was shown to prevent bacterial colonization in mice (8). However, since the immune complexes were made with unpurified ascites fluid antibodies, it remains possible that protection was conferred by bactericidal factors (11).
Milk protective antibodies are directed against H. felis surface components.
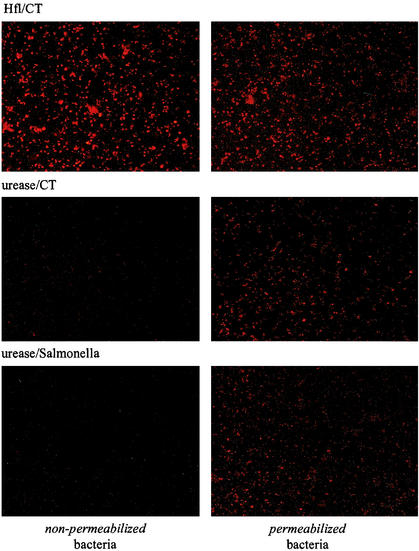
To determine whether protection was related to milk antibodies directed against H. felis, milk from naive or immunized mothers was incubated with H. felis recovered from fresh cultures. Bacterial pellets were fixed in 2% paraformaldehyde in phosphate-buffered saline, permeabilized or not with 1% Triton to allow antibodies to reach intracellular targets, incubated with milk, washed, and further incubated with phycoerythrin-labeled anti-mouse Ig antibodies. After two rinses in phosphate-buffered saline, the bacteria were spread on coverslips and examined with an Axioskop epifluorescence microscope (Zeiss, Jena, Germany).
As seen in Fig. 2 (1st row, left panel), milk from H. felis-immunized mice strongly labeled the bacteria, indicating that the milk antibodies recognized surface-exposed epitopes. Milk from urease-CT (2nd row, left panel)- or urease-Salmonella (3rd row, left panel)-immunized mice stained a few bacteria, consistent with the observation that urease can associate with the surface of a few bacteria (16). When bacteria were permeabilized with detergent prior to incubation with milk (right panels), the bacteria were labeled with milk from urease-CT- or Salmonella-urease-immunized mice. Control milk from naive lactating mice failed to stain the bacteria (data not shown).
FIG. 2.
Immunolabeling of H. felis with milk antibodies. Formaldehyde-fixed H. felis bacteria were incubated with milk antibodies from dams that had been immunized with H. felis lysate (Hfl)-CT, urease-CT, or urease-Salmonella without (left panels) or with (right panels) prior permeabilization with 1% Triton.
The lack of passive protection by urease-specific antibodies thus correlated with the lack of interaction of the antibodies with the bacterial surface. The antibodies recognized intracellular urease following membrane permeabilization, indicating that under our growth conditions very little urease leaks out of the bacteria. In contrast, milk from mice immunized with bacterial lysates protected pups and as predicted contains antibodies that react with bacterial surface exposed antigens. Therefore, we propose that, in neonatal mice, antibodies mediate protection against Helicobacter infection provided that they recognize bacterial cell surface antigens. Interestingly, in adults, protection against Helicobacter infection by active immunization with urease or H. felis lysate correlated with a strong T-cell response, and antibodies did not seem to play a key role (1, 5). In neonatal mice, protective maternal T cells may have been transferred via the placenta or milk but obviously this was not sufficient to induce protection since pups of urease-immunized dams were not protected against the infection, in contrast to the progeny of H. felis immunized dams.
In our model, absence of infection or the lowest numbers of gastric pathogens were found in the gastric mucosa of the pups breast-fed with milk containing antibodies recognizing the bacterial surface. These antibodies might facilitate the neutralization, the elimination or the destruction of Helicobacter similarly to what has been shown for other antibodies involved in the prevention of bacterial or viral infection (for reviews, see references 4, 10, and 15).
In vitro, Helicobacter-specific secretory IgA antibodies purified from human milk have been shown to inhibit the adherence of H. pylori to Kato III cells (3). However, this effect was minimal, and the role of nonimmune milk factors was not tested. Since H. felis does not adhere to murine gastric cells, it is likely that antibodies interfere with motility and/or adhesion to mucus (4). Alternatively, colostral phagocytes might facilitate the clearance of H. felis by internalizing and degrading secretory IgA-opsonized bacteria as reported for enteropathogenic Escherichia coli in infected newborns (13). Whether milk phagocytes express the recently discovered Fc α/μ receptor (17) and whether such a mechanism plays a role in the clearance of Helicobacter in mouse pups remain to be documented.
Protection mediated by immune milk disappears 16 to 22 days after birth, which corresponds to the time of weaning. This observation suggests that breast-feeding may lead to partial control of the infection, maintaining numbers of bacteria below the limits of detection. Such partial protection would nevertheless remain specifically associated with anti-H. felis immunization of the breast-feeding mothers and be transmitted via milk. It has been reported that the incidence of H. pylori infection in Gambian infants breast-fed by mothers with high H. pylori-specific IgA antibody titers was lower than that in children whose mothers had reduced specific antibody titers until the time of weaning. Based on such epidemiological studies it was proposed that maternal antibodies in populations at risk might protect newborns against Helicobacter infections (Thomas et al., letter). Our data confirm this hypothesis when mothers are immunized rather than infected.
Acknowledgments
This work was supported by the Swiss National Foundation (grants no 31-53771.98 to I.C.T. and 31-56936-99 to J.P.K.).
We thank Pierre Michetti, Bruce German, and P. Duncan for critically reading the manuscript and Jeanine Bamat for performing the immunofluorescence staining of the bacteria.
Editor: A. D. O'Brien
REFERENCES
- 1.Blanchard, T. G., S. J. Czinn, R. W. Redline, N. Sigmund, G. Harriman, and J. G. Nedrud. 1999. Antibody-independent protective mucosal immunity to gastric helicobacter infection in mice. Cell Immunol. 191:74-80. [DOI] [PubMed] [Google Scholar]
- 2.Casswall, T. H., S. A. Sarker, M. J. Albert, G. J. Fuchs, M. Bergstrom, L. Bjorck, and L. Hammarstrom. 1998. Treatment of Helicobacter pylori infection in infants in rural Bangladesh with oral immunoglobulins from hyperimmune bovine colostrum. Aliment. Pharmacol. Ther. 12:563-568. [DOI] [PubMed] [Google Scholar]
- 3.Clyne, M., J. Thomas, L. Weaver, and B. Drumm. 1997. In vitro evaluation of the role of antibodies against Helicobacter pylori in inhibiting adherence of the organism to gastric cells. Gut 40:731-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corthesy, B., and J. P. Kraehenbuhl. 1999. Antibody-mediated protection of mucosal surfaces. Curr. Top. Microbiol. Immunol. 236:93-111. [DOI] [PubMed] [Google Scholar]
- 5.Corthesy-Theulaz, I. 2000. Vaccination against Helicobacter pylori. Recent Results Cancer Res. 156:55-59. [DOI] [PubMed] [Google Scholar]
- 6.Corthesy-Theulaz, I., N. Porta, M. Glauser, E. Saraga, A. C. Vaney, R. Haas, J. P. Kraehenbuhl, A. L. Blum, and P. Michetti. 1995. Oral immunization with Helicobacter pylori urease B subunit as a treatment against Helicobacter infection in mice. Gastroenterology 109:115-121. [DOI] [PubMed] [Google Scholar]
- 7.Corthesy-Theulaz, I. E., S. Hopkins, D. Bachmann, P. F. Saldinger, N. Porta, R. Haas, Y. Zheng-Xin, T. Meyer, H. Bouzourene, A. L. Blum, and J. P. Kraehenbuhl. 1998. Mice are protected from Helicobacter pylori infection by nasal immunization with attenuated Salmonella typhimurium PhoPc expressing urease A and B subunits. Infect. Immun. 66:581-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Czinn, S. J., A. Cai, and J. G. Nedrud. 1993. Protection of germ-free mice from infection by Helicobacter felis after active oral or passive IgA immunization. Vaccine 11:637-642. [DOI] [PubMed] [Google Scholar]
- 9.Goodman, K. J., and P. Correa. 2000. Transmission of Helicobacter pylori among siblings. Lancet 355:358-362. [DOI] [PubMed] [Google Scholar]
- 10.Hodgins, D. C., S. Y. Kang, L. deArriba, V. Parreno, L. A. Ward, L. Yuan, T. To, and L. J. Saif. 1999. Effects of maternal antibodies on protection and development of antibody responses to human rotavirus in gnotobiotic pigs. J. Virol. 73:186-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Korhonen, H., E. L. Syvaoja, H. Ahola-Luttila, S. Sivela, S. Kopola, J. Husu, and T. U. Kosunen. 1995. Bactericidal effect of bovine normal and immune serum, colostrum and milk against Helicobacter pylori. J. Appl. Bacteriol. 78:655-662. [DOI] [PubMed] [Google Scholar]
- 12.Kraehenbuhl, J. P., C. Bron, and B. Sordat. 1979. Transfer of humoral secretory and cellular immunity from mother to offspring. Curr. Top. Pathol. 66:105-157. [DOI] [PubMed] [Google Scholar]
- 13.Loimaranta, V., J. Nuutila, P. Marnila, J. Tenovuo, H. Korhonen, and E. M. Lilius. 1999. Colostral proteins from cows immunised with Streptococcus mutans/S. sobrinus support the phagocytosis and killing of mutans streptococci by human leucocytes. J. Med. Microbiol. 48:917-926. [DOI] [PubMed] [Google Scholar]
- 14.Michetti, P., I. Corthesy-Theulaz, C. Davin, R. Haas, A. C. Vaney, M. Heitz, J. Bille, J. P. Kraehenbuhl, E. Saraga, and A. L. Blum. 1994. Immunization of BALB/c mice against Helicobacter felis infection with Helicobacter pylori urease. Gastroenterology 107:1002-1011. [DOI] [PubMed] [Google Scholar]
- 15.Offit, P. A., and H. F. Clark. 1985. Maternal antibody-mediated protection against gastroenteritis due to rotavirus in newborn mice is dependent on both serotype and titer of antibody. J. Infect. Dis. 152:1152-1158. [DOI] [PubMed] [Google Scholar]
- 16.Phadnis, S. H., M. H. Parlow, M. Levy, D. Ilver, C. M. Caulkins, J. B. Connors, and B. E. Dunn. 1996. Surface localization of Helicobacter pylori urease and a heat shock protein homolog requires bacterial autolysis. Infect. Immun. 64:905-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakamoto, N., K. Shibuya, Y. Shimizu, K. Yotsumoto, T. Miyabayashi, S. Sakano, T. Tsuji, E. Nakayama, H. Nakauchi, and A. Shibuya. 2001. A novel Fc receptor for IgA and IgM is expressed on both hematopoietic and non-hematopoietic tissues. Eur. J. Immunol 31:1310-1316. [DOI] [PubMed] [Google Scholar]
- 18.Tollemar, J., N. Gross, N. Dolgiras, C. Jarstrand, O. Ringden, and L. Hammarstrom. 1999. Fungal prophylaxis by reduction of fungal colonization by oral administration of bovine anti-Candida antibodies in bone marrow transplant recipients. Bone Marrow Transplant. 23:283-290. [DOI] [PubMed] [Google Scholar]
- 19.Vyas, S. P., and V. Sihorkar. 1999. Exploring novel vaccines against Helicobacter pylori: protective and therapeutic immunization. J. Clin. Pharm. Ther. 24:259-272. [DOI] [PubMed] [Google Scholar]