Abstract
Oligodendrocytes develop from a subpopulation of precursor cells within the ventral ventricular zone of the spinal cord. The molecular cues that direct this spatially and temporally restricted event seem to originate in part from structures ventral to and within the spinal cord. Here, we present evidence that the family of ligands termed neuregulins are necessary for the normal generation of mouse spinal cord oligodendrocytes. Oligodendrocytes mature in spinal cord explants from wild-type mice and mice heterozygotic for a null mutation in the neuregulin gene (NRG +/−) in a temporal sequence of developmental events that replicates that observed in vivo. However, in spinal cord explants derived from mice lacking neuregulin (NRG −/−), oligodendrocytes fail to develop. Addition of recombinant neuregulin to spinal cord explants from NRG −/− mice rescues oligodendrocyte development. In wild-type spinal cord explants, inhibitors of neuregulin mimic the inhibition of oligodendrocyte development that occurs in NRG −/− explants. In embryonic mouse spinal cord, neuregulins are present in motor neurons and the ventral ventricular zone where they likely exert their influence on early oligodendrocyte precursor cells.
Neuregulins play multiple essential roles in vertebrate embryogenesis, including development of rhombomeres, neural-crest derivatives, the neuromuscular junction, and cardiac morphogenesis (1–6). The influence of neuregulins on cell types from different stem cell origins is pleiotropic. For example, neuregulins are mitogenic for breast epithelial cells (7) and induce the differentiated property of acetylcholine receptor synthesis in skeletal muscle (8). Furthermore, neuregulins can have pleiotropic effects on cells within a single lineage. In the peripheral nervous system, neuregulins promote the generation of glia, rather than neurons, from the neural crest (9) and act as survival factors for Schwann cell precursors (10) and mitogens for mature Schwann cells (11, 12). The difference in biological response most likely reflects not only the cellular context but also the repertoire and number of neuregulin receptors expressed on responding cells.
Neuregulins are a family of ligands that include heregulin, neu differentiation factor, acetylcholine receptor-inducing activity, and glial growth factor (7, 8, 13, 14). Neuregulin isoforms differ in both their extracellular and intracellular domains. However, ligand binding to receptor and all biologic activity seem to reside in the epidermal-growth-factor-like domain (7, 15–18), which is common to all isoforms, and most isoforms are synthesized as transmembrane proteins. In general, neuregulins bind to and activate the erbB family of receptor tyrosine kinases, erbB2 (HER2), erbB3 (HER3), and erbB4 (HER4) (19–21) as functional heterodimers and homodimers (22).
METHODS
Neuregulin Null Mice and Spinal Cord Explants.
To generate neuregulin gene (NRG +/+), NRG +/−, and NRG −/− embryos, litters were obtained from timed pregnancies of NRG +/− female and NRG +/− male matings 9.5 days post conception (dpc). Each embryo was given a code number, and spinal cords were excised and cut transversely into 1- to 2-mm fragments for explant culture onto polylysine- and laminin-coated cover glasses in DMEM, N2 additives, 2% fetal bovine serum, and 10 ng/ml platelet-derived growth factor-AA. The remainder of each embryo was used to isolate genomic DNA for PCR analysis.
PCR.
PCR primers were NRG-4786 (5′-GAGATGGTCATGTCCTTGTCACTAACC), the mouse genomic sequence 5′ of exon 7 with an sense orientation; NRG-4807 (5′-TGCTGCTTTCTTCGCTCTTCAGAAGC), the mouse genomic sequence 5′ of exon 7 with an antisense orientation; and neo2 (5′-CGAATTCGCCAATGACAAGACGCTGG), a neocassette with an antisense orientation. PCR was performed with a denaturation of 45 sec at 94°C, an annealing period of 30 sec at 68°C, and an elongation of 50 + n sec at 72°C for 40 cycles. Primers NRG-4786 and NRG-4807 generate a 370-bp band for the wild-type allele. Primers NRG-4786 and neo2 generate a 520-bp band for the mutant allele.
Immunohistochemistry.
For O4 staining, living explant cultures were incubated for 20 min with the O4 mAb hybridoma supernatant at a 1:3 dilution, washed with PBS three times, then fixed in fresh 4% (wt/vol) paraformaldehyde in PBS for 7 min at ambient temperature, washed three times with PBS, then incubated with Cy3-conjugated secondary antibody (Jackson ImmunoResearch), and visualized by epifluorescence. For neurofilament staining, cultures were made permeable with 0.125% Triton X-100 in PBS for 20 min before incubation with a rabbit polyclonal antibody against neurofilaments (a gift from Peter Hollenbeck, Purdue University, West Lafayette, IN) that is recognized by fluorescein isothiocyanate-conjugated goat anti-rabbit secondary antibody.
RESULTS
Specific Loss of Oligodendrocytes in Spinal Cords Lacking Neuregulin.
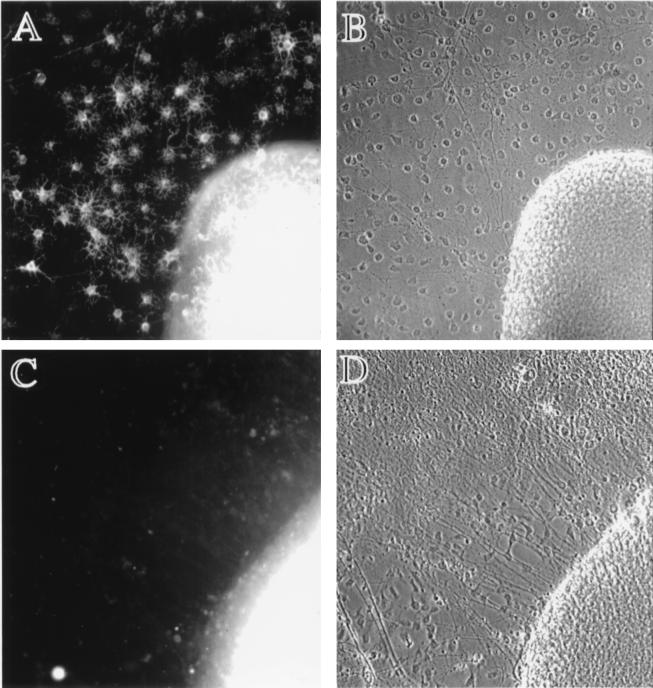
We examined the requirement for neuregulins during oligodendrocyte development by using spinal cord explant cultures from neuregulin knock-out mice (NRG −/−; ref. 1), mice heterozygotic for a null mutation in the neuregulin gene (NRG +/−), and wild-type littermates (NRG +/+). NRG −/− embryos die from a defect in cardiac morphogenesis between 10.5 and 11.5 dpc (1); therefore, spinal cord explant cultures were generated from litters ≈9.5 dpc to ensure the explants came from viable embryos. The development of oligodendrocytes in explant cultures correlates spatially and temporally with the appearance of oligodendrocytes in vivo (23–27) and is thus a reliable model for studying events necessary for oligodendrocyte development. Spinal cord explants were plated onto poly-l-lysine- and laminin-coated glass coverslips in DMEM supplemented with 1% fetal bovine serum, N2 additives, and 10 ng/ml platelet-derived growth factor-AA (23–25). Platelet-derived growth factor-AA is a known mitogen and survival factor for cells in the oligodendrocyte lineage (28–31) and was added to eliminate the possibility that neuregulin was simply influencing the synthesis of this ligand. After 7–11 days in vitro, explants were stained for oligodendrocytes with mAb O4, which identifies immature oligodendrocytes (32), and assessed for oligodendrocyte numbers before unblinding results of genotyping. Representative micrographs of explants from NRG +/− and NRG −/− embryos are shown in Fig. 1. The cellular outgrowth from NRG +/− explants contains numerous O4+ oligodendrocytes (Fig. 1A). In contrast, there are no identifiable O4+ cells within the cellular outgrowth from NRG −/− explants nor within the explant itself (Fig. 1C). The requirement of neuregulins over this developmental period seems to be specific for the oligodendrocyte lineage, because the absence of neuregulin neither affected growth of other cell types from the explant, nor did it effect the neuritic outgrowth (Fig. 1D).
Figure 1.
Oligodendrocytes fail to develop in spinal cord explants from neuregulin knock-out mice. Timed pregnancies from NRG +/− female and NRG +/− male matings were carried to ≈9.5 dpc, at which time females were killed, and embryos were removed. Each embryo was assigned a code number used to blind investigators from the genotyping. Genotyping was carried out by PCR on DNA extracted from the head of each embryo. Spinal cords were excised from embryos, cut transversely into segments, plated onto poly-l-lysine- and laminin-coated cover glasses, and cultured for 9 days in DMEM supplemented with 1% fetal bovine serum, N2 additives, and 10 ng/ml platelet-derived growth factor-AA. Cultures were stained with mAb O4 as described (32). Genotyping was unblinded after assessment of oligodendrocyte numbers and photography of explant cultures. (A and B) Micrographs of explants from NRG +/− embryos. (C and D) Micrographs of explants from NRG −/− embryos. O4 staining images are shown on the Left adjacent to the corresponding phase-contrast images on the Right. There are no identifiable O4+ cells in explants from NRG −/− embryos (the fluorescence within the explant is background not cellular staining). In contrast, explants from NRG +/− embryos have numerous O4+ oligodendrocytes. NRG −/− explants do, however, have an extensive neuritic outgrowth and growth of cells other than oligodendrocytes as seen in the phase-contrast images.
Recombinant Neuregulin Rescues Oligodendrocyte Development.
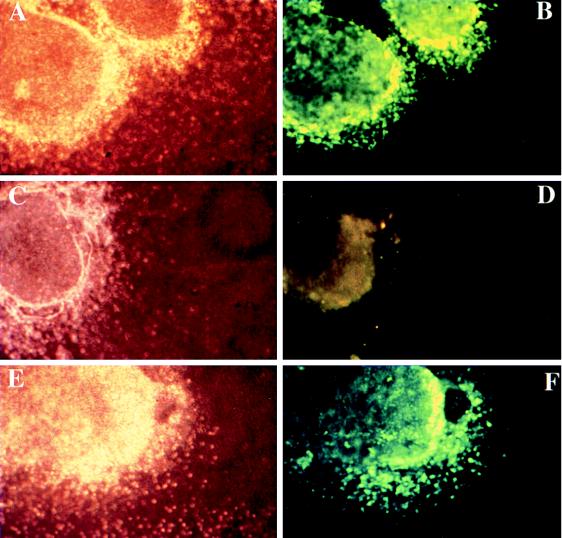
We next wanted to determine whether neuregulins were necessary before or after 9.5 dpc for oligodendrocyte development to proceed normally. To address this question, spinal cords from E9.5 embryos were used to generate parallel cultures. One culture received 1 nM recombinant neuregulin, and the other received a control buffer at the time of plating. The addition of recombinant neuregulin rescued oligodendrocyte development in explants from NRG −/− mice (Fig. 2), and thus we conclude that neuregulin is necessary for oligodendrocyte development in spinal cord after ≈9.5 dpc. Because recombinant neuregulin can rescue oligodendrocyte development when added at ≈9.5 dpc, it is unlikely that neuregulin is required for the survival, differentiation, or proliferation of a primitive, multipotent precursor cell present before this stage.
Figure 2.
Exogenous recombinant neuregulin will rescue oligodendrocyte development from NRG −/− explant cultures. Spinal cord explant cultures were generated from litters ≈9.5 dpc and genotyped as described in the legend to Fig. 1. Spinal cords initially were divided into six to eight segments. Segments that did not obviously contain both ventral and dorsal spinal cord were discarded. Explants from a single embryo received either 0.1% BSA in PBS as a control or 1 nM neuregulin (epidermal-growth-factor-like β1 domain) at the time of plating. Explant cultures were then allowed to grow for an additional 8 days, equivalent to ≈17 dpc, and processed for O4 staining as described in the legend to Fig. 1. (C–F) Micrographs of explant cultures from NRG −/− embryos. (A and B) Micrographs of explant cultures from a wild-type littermate. The NRG −/− explants (C and D) as well as the wild-type explants (A and B) received 0.1% BSA in PBS. The explants shown in E and F received 1 nM neuregulin for the entire in vitro period. O4 staining images are shown on the Right adjacent to the corresponding phase-contrast images on the Left. The addition of neuregulin to NRG −/− explants restores oligodendrocyte development, and cells are indistinguishable from those in wild-type explants.
Neuregulin Inhibitors Block Oligodendrocyte Development in Wild-Type Spinal Cords.
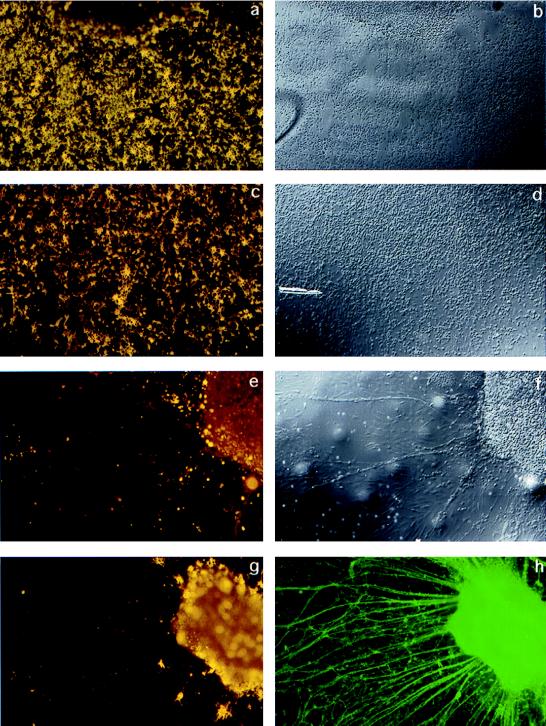
To confirm the temporal requirement for neuregulin signaling during oligodendrocyte development, an independent set of experiments was performed on wild-type spinal cord explants by using the neuregulin inhibitor IgB4, a chimeric protein composed of the extracellular (ligand-binding) domain of erbB4 and the Fc portion of human IgG1 (33). Spinal cord explant cultures were generated from E9.5 wild-type mouse embryos and cultured as described for 2.5 days, at which time cultures received either IgB4 or a control buffer (0.1% BSA in PBS). Explants were then cultured for an additional 9 days before staining for cells in the oligodendrocyte lineage with the O4 mAb. In cultures from wild-type explants that received IgB4, there were few to no oligodendrocytes, whereas explants in control cultures had numerous oligodendrocytes both within the explant and among the surrounding cells (Fig. 3). As with the neuregulin knock-out explants, functionally inhibiting neuregulin in wild-type cultures influenced neither neuritic outgrowth nor the outgrowth of other cell types (Fig. 3).
Figure 3.
Neutralizing neuregulin activity inhibits the formation of oligodendrocytes in wild-type spinal cord explant cultures. IgB4 is a chimeric protein consisting of the extracellular domain of erbB4 (ligand-binding domain) and the Fc portion of human IgG1. Spinal cord explants were generated from E9.5 wild-type mice and cultured for 2.5 days in standard growth medium then 9 days in the presence of human Fc fragment as a control (a–d) or IgB4 (e–h). At day 11, cultures were surface stained with mAb O4 and O1 combined to identify immature and mature oligodendrocytes, fixed, and, in some cases, double-labeled with antineurofilament. (a, c, e, g) Combined O4 and O1 staining. b, d, and f are Nomarski images corresponding to a, c, and e, respectively. h shows the neurofilament staining corresponding to g. In cultures treated with IgB4, there are few or no oligodendrocytes identified. In contrast, wild-type explant cultures treated with the control buffer containing human Fc fragment had abundant numbers of oligodendrocytes.
Localization of Neuregulin in Embryonic Spinal Cord.
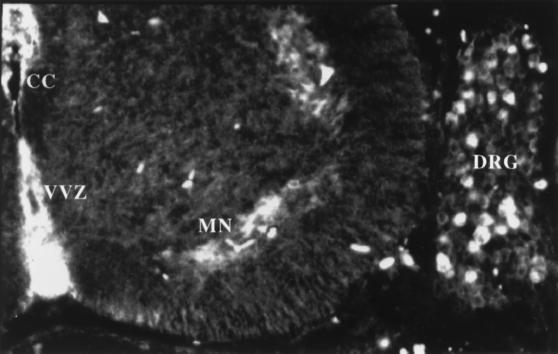
It was clear from our experiments with the neuregulin knock-out mice and from the functional inhibition experiments with IgB4 that neuregulin is necessary for oligodendrocyte development. If neuregulins are required for oligodendrocyte development in the spinal cord, then one would expect them to be expressed in or near the spinal cord during the appropriate developmental period. In the mouse, oligodendrocytes first appear between 12 and 14 dpc. Thus, we examined E14 wild-type mice for neuregulin expression by using an antibody that recognizes the cytoplasmic domain of neuregulin. In transverse sections of E14 mouse spine, neuregulin is present within motor neurons and in the ventral ventricular zone (VVZ; Fig. 4). Because the antibody used for immunohistochemistry recognizes cytoplasmic epitopes, immunoreactivity most likely represents the site of synthesis of neuregulin and excludes staining of secreted ligand adhering to neuregulin receptors. The presence of neuregulins in these structures is consistent with studies showing that ventrally derived signals are required for generation of the oligodendrocyte lineage (34). Because the oligodendrocyte lineage arises from the VVZ (23–27), neuregulins derived from cells within the VVZ may act in a paracrine or autocrine fashion.
Figure 4.
Neuregulin is present in developing mouse spinal cord within motor neurons and the VVZ. E14 mouse embryos were emersion fixed in 4% paraformaldehyde, cryoprotected, and mounted in TissueTec, and 20-μm frozen transverse sections were mounted on glass slides. Sections were stained with a rabbit polyclonal antiserum that recognizes the “a” cytoplasmic domain of neuregulin, detected by fluorescein isothiocyanate-conjugated goat anti-rabbit IgG antiserum, and visualized by epifluorescence. Neuregulin is present within motor neurons (MN), dorsal root ganglion neurons (DRG), and in the VVZ itself. CC = central canal.
DISCUSSION
The data presented in this report indicate that neuregulins are required at an early stage of development for oligodendrogliogenesis to proceed normally. The loss of functional neuregulin seems to affect the oligodendrocyte lineage specifically. The morphology of spinal cord neurons seems normal, and the neurons extend an extensive network of neurites from NRG −/− explants similar to wild-type explants. In addition, the outgrowth from the NRG −/− explants contains numerous cells, many of which are astrocytes (not shown) that appear normal by morphologic criteria. The presence of neurons and astrocytes in NRG −/− explant cultures and the ability of exogenous neuregulin added at 9.5 dpc to rescue oligodendrocyte development support the postulate that neuregulins are required specifically for development of the oligodendrocyte lineage but not for the development or survival of a primitive, multipotent precursor cell that ultimately gives rise to the oligodendrocyte and other cell lineages.
The molecular components of the signaling pathway from axon to glial cell that results in initiation of myelination and maintenance of the myelin internode are unknown. Work from our and others’ laboratories suggests that candidate ligand-receptor pairs involved in myelination are the neuregulins and their receptors. In an earlier report, we showed that neuregulins act as morphogens for mature oligodendrocytes by causing the extension of large sheet-like processes consistent with a myelinating phenotype (35, 36). Other investigators also have found an effect of neuregulins on promoting the extension and complexity of oligodendrocyte processes (37). However, in multiply passaged oligodendrocyte progenitors, neuregulins are able to act as mitogens (38). The data presented in this report show that neuregulins are necessary for the development of the oligodendrocyte lineage at early developmental stages. In this respect, the role of neuregulins in development of the oligodendrocyte lineage may be analogous to the Schwann cell lineage, in which the same ligand causes differentiation, survival, or proliferation at different stages of development (9–12).
Acknowledgments
We thank Dr. Carmen Birchmeier for providing the neuregulin knock-out mouse, Ms. Kim Dyer, Ms. Elizabeth Mole, and Mr. Christopher Severson for technical assistance, and Amgen Biologicals for the recombinant epidermal-growth-factor-like β1 domain neuregulin. This work was supported by National Institute of Neurologic Disorders and Stroke Grants NS02028 (to T.K.V.), NS18458 (to G.D.F.), and NS30800 (to R.H.M.) and the U.S. National Multiple Sclerosis Society Grant RG2912-A-1 (to T.K.V.).
ABBREVIATIONS
- dpc
days post conception
- En
number of embryonic days
- NRG
neuregulin gene
- VVZ
ventral ventricular zone
References
- 1.Meyer D, Birchmeier C. Nature (London) 1995;378:386–390. doi: 10.1038/378386a0. [DOI] [PubMed] [Google Scholar]
- 2.Gassmann M, Casagranda F, Orioli D, Simon H, Lai C, Klein R, Lemke G. Nature (London) 1995;378:390–394. doi: 10.1038/378390a0. [DOI] [PubMed] [Google Scholar]
- 3.Lee K F, Simon H, Chen H, Bates B, Hung M C, Hauser C. Nature (London) 1995;378:394–398. doi: 10.1038/378394a0. [DOI] [PubMed] [Google Scholar]
- 4.Britsch S, Li L, Kirchhoff S, Theuring F, Brinkmann V, Birchmeier C, Riethmacher D. Genes Dev. 1998;12:1825–1836. doi: 10.1101/gad.12.12.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sandrock A W J, Dryer S E, Rosen K M, Gozani S N, Kramer R, Theill L E, Fischbach G D. Science. 1997;276:599–603. doi: 10.1126/science.276.5312.599. [DOI] [PubMed] [Google Scholar]
- 6.Erickson S L, O’Shea K S, Ghaboosin N, Loverro L, Frantz G, Bauer M, Lu L H, Moore M W. Development (Cambridge, UK) 1997;124:4999–5011. doi: 10.1242/dev.124.24.4999. [DOI] [PubMed] [Google Scholar]
- 7.Holmes W E, Sliwkowski M X, Akita R W, Henzel W J, Lee J, Park J W, Yansura D, Abadi N, Raab H, Lewis G D, et al. Science. 1992;256:1205–1210. doi: 10.1126/science.256.5060.1205. [DOI] [PubMed] [Google Scholar]
- 8.Falls D L, Rosen K M, Corfas G, Lane W S, Fischbach G D. Cell. 1993;72:801–815. doi: 10.1016/0092-8674(93)90407-h. [DOI] [PubMed] [Google Scholar]
- 9.Shah N M, Marchionni M A, Isaacs I, Stroobant P, Anderson D J. Cell. 1994;77:349–360. doi: 10.1016/0092-8674(94)90150-3. [DOI] [PubMed] [Google Scholar]
- 10.Dong Z, Brennan A, Liu N, Yarden Y, Lefkowitz G, Mirsky R, Jessen K R. Neuron. 1995;15:585–596. doi: 10.1016/0896-6273(95)90147-7. [DOI] [PubMed] [Google Scholar]
- 11.Levi A D, Bunge R P, Lofgren J A, Meima L, Hefti F, Nikolics K, Sliwkowski M X. J Neurosci. 1995;15:1329–1340. doi: 10.1523/JNEUROSCI.15-02-01329.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morrissey T K, Levi A D, Nuijens A, Sliwkowski M X, Bunge R P. Proc Natl Acad Sci USA. 1995;92:1431–1435. doi: 10.1073/pnas.92.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marchionni M A, Goodearl A D, Chen M S, Bermingham M O, Kirk C, Hendricks M, Danehy F, Misumi D, Sudhalter J, Kobayashi K, et al. Nature (London) 1993;362:312–318. doi: 10.1038/362312a0. [DOI] [PubMed] [Google Scholar]
- 14.Wen D, Peles E, Cupples R, Suggs S V, Bacus S S, Luo Y, Trail G, Hu S, Silbiger S M, Levy R B, et al. Cell. 1992;69:559–572. doi: 10.1016/0092-8674(92)90456-m. [DOI] [PubMed] [Google Scholar]
- 15.Peles E, Yarden Y. BioEssays. 1993;15:815–824. doi: 10.1002/bies.950151207. [DOI] [PubMed] [Google Scholar]
- 16.Carraway K L, III, Burden S J. Curr Opin Neurobiol. 1995;5:606–612. doi: 10.1016/0959-4388(95)80065-4. [DOI] [PubMed] [Google Scholar]
- 17.Ben B N, Yarden Y. Proc Soc Exp Biol Med. 1994;206:221–227. doi: 10.3181/00379727-206-43746. [DOI] [PubMed] [Google Scholar]
- 18.Carraway K L, III, Sliwkowski M X, Akita R, Platko J V, Guy P M, Nuijens A, Diamonti A J, Vandlen R L, Cantley L C, Cerione R A. J Biol Chem. 1994;269:14303–14306. [PubMed] [Google Scholar]
- 19.Kraus M H, Issing W, Miki T, Popescu N C, Aaronson S A. Proc Natl Acad Sci USA. 1989;86:9193–9197. doi: 10.1073/pnas.86.23.9193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coussens L, Yang F T, Liao Y C, Chen E, Gray A, McGrath J, Seeburg P H, Libermann T A, Schlessinger J, Francke U, et al. Science. 1985;230:1132–1139. doi: 10.1126/science.2999974. [DOI] [PubMed] [Google Scholar]
- 21.Plowman G D, Culouscou J M, Whitney G S, Green J M, Carlton G W, Foy L, Neubauer M G, Shoyab M. Proc Natl Acad Sci USA. 1993;90:1746–1750. doi: 10.1073/pnas.90.5.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carraway K L, III, Cantley L C. Cell. 1994;78:5–8. doi: 10.1016/0092-8674(94)90564-9. [DOI] [PubMed] [Google Scholar]
- 23.Fok S J, Miller R H. J Neurosci Res. 1994;37:219–235. doi: 10.1002/jnr.490370208. [DOI] [PubMed] [Google Scholar]
- 24.Noll E, Miller R H. Development (Cambridge, UK) 1994;120:649–660. doi: 10.1242/dev.120.3.649. [DOI] [PubMed] [Google Scholar]
- 25.Noll E, Miller R H. Development (Cambridge, UK) 1993;118:563–573. doi: 10.1242/dev.118.2.563. [DOI] [PubMed] [Google Scholar]
- 26.Pringle N P, Richardson W D. Development (Cambridge, UK) 1993;117:525–533. doi: 10.1242/dev.117.2.525. [DOI] [PubMed] [Google Scholar]
- 27.Yu W P, Collarini E J, Pringle N P, Richardson W D. Neuron. 1994;12:1353–1362. doi: 10.1016/0896-6273(94)90450-2. [DOI] [PubMed] [Google Scholar]
- 28.Barres B A, Hart I K, Coles H S, Burne J F, Voyvodic J T, Richardson W D, Raff M C. Cell. 1992;70:31–46. doi: 10.1016/0092-8674(92)90531-g. [DOI] [PubMed] [Google Scholar]
- 29.McMorris F A, Dubois D M. J Neurosci Res. 1988;21:199–209. doi: 10.1002/jnr.490210212. [DOI] [PubMed] [Google Scholar]
- 30.McMorris F A, Furlanetto R W, Mozell R L, Carson M J, Raible D W. Ann NY Acad Sci. 1990;605:101–109. doi: 10.1111/j.1749-6632.1990.tb42385.x. [DOI] [PubMed] [Google Scholar]
- 31.Gard A L, Pfeiffer S E. Dev Biol. 1993;159:618–630. doi: 10.1006/dbio.1993.1269. [DOI] [PubMed] [Google Scholar]
- 32.Gard A L, Pfeiffer S E. Neuron. 1990;5:615–625. doi: 10.1016/0896-6273(90)90216-3. [DOI] [PubMed] [Google Scholar]
- 33.Pinkas-Kramarski R, Eilam R, Alroy I, Levlowitz G, Lonai P, Yarden Y. Oncogene. 1997;15:2803–2815. doi: 10.1038/sj.onc.1201466. [DOI] [PubMed] [Google Scholar]
- 34.Orentas D M, Miller R H. Dev Biol. 1996;177:43–53. doi: 10.1006/dbio.1996.0143. [DOI] [PubMed] [Google Scholar]
- 35.Vartanian T, Goodearl A, Viehover A, Fischbach G. J Cell Biol. 1997;137:211–220. doi: 10.1083/jcb.137.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vartanian T, Corfas G, Li Y, Fischbach G D, Stefansson K. Proc Natl Acad Sci USA. 1994;91:11626–11630. doi: 10.1073/pnas.91.24.11626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raabe T D, Suy S, Welcher A, DeVries G H. J Neurosci Res. 1997;50:755–768. doi: 10.1002/(SICI)1097-4547(19971201)50:5<755::AID-JNR12>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 38.Canoll P D, Musacchia J M, Hardy R, Reynolds R, Marchionni M, Salzer J L. Neuron. 1996;17:229–243. doi: 10.1016/s0896-6273(00)80155-5. [DOI] [PubMed] [Google Scholar]