Abstract
The type III secretion system (TTSS) of enteropathogenic Escherichia coli (EPEC) has been associated with the ability of these bacteria to induce secretion of proinflammatory cytokines, including interleukin-8 (IL-8), in cultured epithelial cells. However, the identity of the effector molecule directly involved in this event is unknown. In this study, we determined that the native flagellar filament and its flagellin monomer are activators of IL-8 release in T84 epithelial cells. Supernatants of wild-type EPEC strain E2348/69 and its isogenic mutants deficient in TTSS (escN) and in production of intimin (eae), grown in Luria-Bertani broth, elicited similar amounts of IL-8 secretion by T84 cells. In contrast, supernatants of EPEC fliC mutants and of B171, a nonflagellated EPEC strain, were defective in inducing IL-8 release, a phenotype that was largely restored by complementation of the fliC gene in the mutant lacking flagella. Purified flagella from E. coli K-12, EPEC serotypes H6 and H34, and enterohemorrhagic E. coli serotype H7 all induced IL-8 release in T84 cells. Induction of IL-8 by purified flagella or His-tagged FliC from EPEC strain E2348/69 was dose dependent and was blocked by a polyclonal anti-H6 antibody. Finally, the mitogen-activated protein kinases (Erk1 and -2 and Jnk) were phosphorylated in flagellin-treated T84 cells, and inhibition of the p38 and Erk pathways significantly decreased the IL-8 response induced by EPEC flagellin. Our data clearly indicate that FliC of EPEC is sufficient to induce IL-8 release in T84 cells and that activation of the Erk and p38 pathways is required for IL-8 induction.
Enteropathogenic Escherichia coli (EPEC) is the predominant bacterial cause of infant diarrhea worldwide and represents a major endemic health threat to children below the age of 6 months living in developing countries (38). EPEC causes a pathophysiology known as attaching and effacing lesion (AE), which is characterized by effacement of the intestinal epithelial cell microvilli and rearrangement of the cytoskeleton to form pedestal-like structures that cup the bacteria individually (43). In EPEC strain E2348/69, all genes necessary for the AE phenotype are encoded within a 35.6-kb pathogenicity island termed the locus of enterocyte effacement (LEE) (40). The LEE encodes an adhesin, intimin (34), its translocated intimin receptor (Tir) (37), and components of a type III secretion system (TTSS), which is responsible for secretion of EspA, EspB, EspD, EspF, EspG, Map, and Tir. Tir has been shown to bind to Nck (6, 30), talin, α-actin, and vinculin (7, 23), resulting in cytoskeletal rearrangements. Mitochondrion-associated protein targets to mitochondria, where it has membrane potential-disrupting activity (36); EspF disrupts the host intestinal barrier (41) and induces apoptosis in epithelial cells (8); and the role of EspG is still unclear (21). In addition to the LEE, the EPEC virulence plasmid (EAF) encodes the type IV bundle-forming pilus (BFP), which is involved in EPEC adherence (28) and cytotoxicity (1).
EPEC induces an inflammatory response in the gut epithelium in vivo (42), presumably by triggering production of cytokines and chemokines, including interleukin-8 (IL-8), which recruits polymorphonuclear leukocytes to the infection site (46). Although the EPEC TTSS has been implicated in triggering IL-8 production in epithelial cells (11, 15), no effector protein has yet been identified. Among the four LEE-encoded EPEC effector molecules that have been identified (36), it is not known which TTSS effectors may be involved in IL-8 stimulation.
Mitogen-activated protein kinases (MAPK) are central in many host responses including the regulation of cytokine response (24). MAPK, a group of three serine/threonine kinases with isoforms ranging from 40 to 62 kDa, form a group of three pathways, including extracellular signal-regulated protein kinases 1 and 2 (Erk1 and Erk2) and two stress-activated protein kinases designated p38 and c-Jun N-terminal kinase (Jnk). Recent reports have shown that MAPK are involved in the host cell responses to infection by Listeria monocytogenes (51), Salmonella enterica serovar Typhimurium (32), enterohemorrhagic E. coli (EHEC) (12), and EPEC (11, 15). Phosphorylation of MAPK subsequently leads to activation of transcription factors such as NF-κB and AP-1, which result in production of IL-8 among other proteins (13, 14).
Flagella are filamentous appendages required for bacterial chemotaxis and motility; they are composed of a structural repeating protein called flagellin (47). In addition to motility, flagella have been shown to play important roles in adhesion and biofilm formation in several bacterial species. The flagella from Clostridium difficile, for example, bind specifically to mouse mucus and may contribute to colonization (52). Recently, it was reported that the flagella of EPEC mediate adhesion to epithelial cells in vitro (29). The flagellins from Pseudomonas aeruginosa (17), enteroaggregative E. coli (EAEC) (50), and Salmonella serovar Typhimurium (26) have been shown to stimulate epithelial cells to produce nitric oxide (19), human beta-defensin-2 (44), and IL-8. Moreover, reports have shown that the flagellin of Salmonella serovar Typhimurium also stimulates the chemokine CCL20, which in turn triggers a specific migration of immature dendritic cells to the epithelium (48). Thus, besides their role in motility and adhesion, flagella appear to trigger native immune responses. Hence, these structures may have a significant role in the host response to bacterial infection in vivo.
In this study we demonstrate that flagellin of EPEC stimulates IL-8 production in a dose-dependent manner in T84 epithelial cells and also results in activation of the kinases Erk-1 and -2 and Jnk1 and -2. These results suggest that flagellin may play an important role in the gut inflammatory response during EPEC infection.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Bacterial strains and plasmids used in this study are shown in Table 1. Bacteria were grown overnight at 37°C with shaking at 200 rpm in 5 ml of Luria-Bertani (LB) broth or Dulbecco's modified Eagle medium (DMEM) without fetal calf serum.
TABLE 1.
E. coli strains and plasmids
| Strain or plasmid | Relevant properties | Source or reference |
|---|---|---|
| Strains | ||
| E2348/69 wt | O127:H6 clinical isolate | 39 |
| CVD206 | eae mutant of E2348/69 | 18 |
| CVD452 | escN mutant of E2348/69 | 33 |
| AGT01 | fliC mutant of E2348/69 | 29 |
| AGT02 | fliC (pfliC); AGT01 complemented with cloned fliC from E2348/69 | 29 |
| AGT03 | motB mutant of E2348/69 | 29 |
| E10 wt | O119:H6 clinical isolate | 29 |
| AGT04 | fliC mutant of E10 | 29 |
| B171 | O111:NM; clinical isolate; nonflagellated | 29 |
| EAEC O42 wt | Clinical isolate | 50 |
| EAEC O42 fliC | fliC mutant of EAEC O42 | 50 |
| ORN172 | K-12, with type I pilus genes deleted | 29 |
| E28 | O86:H34; clinic isolate | 29 |
| EHEC 86-24 O157:H7 | H7 | 29 |
| DH5α | K-12 cloning host strain | Stratagene |
| Plasmids | ||
| pQE30 | His tag expression vector | Qiagen |
| pXZ13 | pQE30 containing fliC from E2348/69 | This study |
| pREP4 | Repressor plasmid containing lacI | Qiagen |
Eukaryotic cell lines and culture conditions.
T84 human colonic carcinoma epithelial cells and HeLa cells were obtained from the American Type Culture Collection. T84 cells were grown in DMEM-F-12 medium (Gibco, Grand Island, N.Y.) supplemented with 10% heat-inactivated fetal calf serum, 100 U of penicillin/ml, and 100 μg of streptomycin/ml and were incubated at 37°C with 5% CO2, while HeLa cells were cultured in DMEM containing 10% fetal calf serum. Cells (106) were seeded onto 24-well plates and grown until 90 to 95% confluency. Prior to treatment, cells were washed twice with DMEM-F-12 medium or DMEM without serum or antibiotics and were maintained in serum-free medium for at least 2 h. Polarized T84 cells in six-well plates were prepared as described previously (26). Model epithelia were used 6 to 14 days after plating verification that T84 cells had achieved a transepithelial electrical resistance of at least 1,000 Ω/cm2.
Infection.
Prior to infection, bacteria were washed twice, resuspended, and adjusted to approximately 2 × 109 CFU/ml in sterile phosphate-buffered saline (pH 7.4) (PBS). The suspensions were added to T84 cells at a multiplicity of infection of 100. To synchronize infection, the 24-well plate was centrifuged for 10 min at 2,000 rpm by using an Allegra 6R centrifuge (Beckman). After incubation for 2.5 h at 37°C with 5% CO2, the cells were washed twice with DMEM-F-12 medium. One milliliter of DMEM-F-12 serum free medium containing 100 μg of gentamicin/ml was added to each well, and cells were incubated for 18 h at 37°C with 5% CO2. Bacterial culture supernatants were collected by centrifugation and filter sterilized by using 0.2-μm-pore-size syringe filters. One hundred microliters of the supernatant was added to each well and incubated for 18 h at 37°C with 5% CO2.
Inhibitors.
The MEK-1 inhibitor PD98059 and the p38 inhibitor SB203580 (10) (Calbiochem, San Diego, Calif.) were stored in dimethyl sulfoxide at −20°C. Prior to addition of flagella, the cells were incubated for 90 min with PD98059 (50 μM) or SD203580 (10 μM), alone or together. The inhibitors were maintained with the cells throughout the infection period.
ELISA.
Ninety-six well Maxisorp immunoplates (Nunc, Rochester, N.Y.) were coated with 100 μl of carbonate-bicarbonate buffer (pH 9.6) containing a mouse anti-human IL-8 monoclonal antibody at a concentration of 1 μg per well and were incubated overnight at 4°C. An enzyme-linked immunosorbent assay (ELISA) was performed as described in the manufacturer's instructions. Briefly, the coated plates were washed four times with PBS containing 0.1% Tween 20 (PBST) and then blocked in PBST containing 5% skim milk for 1 h at room temperature. After the plate was washed four times with PBST, 100 μl of the samples and a series of twofold dilutions of the standard were added to a 96-well plate and incubated for 2 h at room temperature. One hundred microliters of a diluted (1:2,000) biotinylated mouse anti-human IL-8 monoclonal antibody was added to each well after washing, and this was then incubated for 1 h at room temperature. After a wash, 100 μl of a 1:2,000-diluted avidin-horseradish peroxidase conjugate was added to each well. The reaction was developed with tetramethylbenzidine (TMB) substrate reagent and was stopped with 2 N H2SO4. Optical densities at 405 nm (OD405) were measured with a Labsystems Multiskan Plus reader (Fisher Scientific). Recombinant human IL-8 was used as a standard. The biotinylated mouse anti-human IL-8 monoclonal antibody, the avidin-horseradish peroxidase conjugate, and the TMB substrate reagent set were all obtained from BD PharMingen (San Diego, Calif.).
Purification of flagella.
Flagella were purified from E2348/69 (O127:H6), EDL933 (O157:H7), and E. coli K-12 ORN172 by using a CsCl2 gradient as previously described (29). Briefly, E. coli strains were cultivated on Luria agar plates overnight at 37°C and resuspended in water. The suspension was sheared briefly in a blender, and the soluble flagella were separated from bacteria and cellular debris by centrifugation at 8,000 × g for 15 min. The flagella present in the supernatant were concentrated and separated from other proteins by centrifugation at 18,000 rpm for 3 h. The pellet containing flagella was resuspended in a suitable volume of water. Flagellar crude extracts were placed in a 1.5% CsCl Sarkosyl gradient and were centrifuged at 38,000 × g for 24 h by using an LE-80 ultracentrifuge (Beckman). The band containing flagella was collected, and salt was removed by dialysis against sterile distilled water.
Cloning and expression of the EPEC flagellin.
Recombinant flagellin of EPEC strain E2348/69 was prepared as follows. The gene fragment corresponding to the flagellin gene of E2348/69 was generated by PCR amplification with the sense primer 5′ CGCGGATCCGCGCAGTCTGCGCTGTCGAGTTC and the antisense primer 5′ CGGGGTACCCCGTTATACCTGGTTGGCTTTTGCCA. Underlined nucleotides represent adaptor sequences added to the ends of primers to maintain the proper reading frame and facilitate cloning (BamHI recognition sites on sense primers and KpnI sites on antisense primers). The template DNA for PCR was a suspension of E2348/69 colonies. PCR-generated flagellin DNA was digested with BamHI plus KpnI, gel purified, and subcloned into the BamHI/KpnI sites of the pQE-30 UA vector (Qiagen, Valencia, Calif.) to produce plasmid pXZ13 so that the six-His tag is at the N terminus of FliC. The presence of the cloned fliC gene in pXZ13 was confirmed by PCR and sequencing. Plasmid pXZ13 was then transformed into E. coli DH5α(pRP4) (Qiagen Inc.), which constitutively expresses the lac repressor protein encoded by the lacI gene to prevent FliC overexpression. E. coli DH5α(pRP4, pXZ13) was harvested by centrifugation 5 h after induction of mid-log-phase cultures with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG). Cell pellets were resuspended in lysis buffer containing 50 mM NaH2PO4, 300 mM NaCl, and 10 mM imidazole (pH 8.0) and were lysed by a French pressure cell (SLM Instruments Inc.). His-tagged proteins were mixed with Ni-nitrilotriacetic acid (Qiagen Inc.), and the mixture was loaded onto a polypropylene column. The column was washed with a lysis buffer containing 20 mM imidazole, and His-tagged proteins were eluted in a similar buffer with 250 mM imidazole. Eluates were collected and dialyzed overnight at 4°C against dialysis buffer (50 mM NaH2PO4-300 mM NaCl [pH. 8.0]) by using 12,000- to 14,000-Da-cutoff Spectra/Por molecular porous membrane tubing (Spectrum Laboratories, Inc., Rancho Dominguez, Calif.).
Secretion experiments.
Secretion experiments were performed as described by Crawford and Kaper (9). Briefly, EPEC strains were grown overnight in LB medium with shaking, diluted to an OD600 of 0.05 in LB medium or DMEM, and grown as static cultures at 37°C in 5% CO2 to an OD600 of 0.2 to 0.3. Cells were pelleted by centrifugation, and the supernatant was filtered through a 0.22-μm-pore-size filter (Pall Corp., Ann Arbor, Mich.). Phenylmethylsulfonyl fluoride, aprotinin, and EDTA (pH 8.0) were added to final concentrations of 50 μg per ml, 0.5 μg per ml, and 5 mM, respectively. Trichloroacetic acid was added to a final concentration of 10%, and samples were placed on ice for at least 1 h. Protein pellets were collected in an ultracentrifuge by using an SW28 swinging bucket rotor at 28,000 rpm for 1 h. Pellets were washed with cold 95% ethanol and spun again at 28,000 rpm for 30 min. Pellets were resuspended in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer and subjected to SDS-PAGE.
SDS-PAGE and Western blotting.
To determine the production of flagellin, either total proteins from lysates of 5 × 108 CFU of EPEC or 2 μg of purified flagella or recombinant FliC was loaded per lane on a Laemmli SDS-12% PAGE gel. The proteins were transferred to a nitrocellulose membrane in standard transfer buffer (25 mM Tris [pH 8.3], 192 mM glycine, 20% methanol) at 30 V for 20 min by using a Bio-Rad semitransfer cell. After the transfer was completed, the membranes were blocked for 1 h with 2% skim milk and PBST at room temperature (RT). The membrane was probed with a rabbit anti-H6 polyclonal antibody (1:30,000) for 1 h at RT after washing and was then incubated for 2 h with a 1:2,000 dilution of horseradish perioxidase-conjugated anti-rabbit immunoglobulin G (IgG). Again the membrane was thoroughly washed as before, and it was developed by addition of the color substrate solution for horseradish peroxidase. To detect phosphorylated MAPK, the procedure of Toshchakov et al. (55) was followed. Polyclonal antibodies that recognize phosphorylated forms of Erk1 and Erk2 were from Promega (Madison, Wis.), and polyclonal antibodies directed against phosphorylated forms of the MAPK Jnk and p38 were from Cell Signaling Technology (San Francisco, Calif.).
Protein assay and statistical analysis.
Protein concentrations were determined by the bicinchoninic acid assay (Pierce, Rockford, Ill.). Data are presented as means ± standard deviations from at least two separate experiments of duplicates except where results of blots are shown, in which case the figure gives results of a representative experiment. Comparisons between two values were analyzed by Student's t test. Differences were considered significant at P values of <0.05.
RESULTS
Supernatants from a wild-type EPEC strain and its isogenic escN and eae mutants stimulate IL-8 production.
Recently it was reported that the TTSS of EPEC strain E2348/69 is responsible for inducing IL-8 secretion in T84 (45) and HeLa (15) cells. To determine whether the stimulation of IL-8 in T84 cells is dependent on the TTSS and on intimate contact of the bacteria with the cultured epithelial cells, we compared the supernatants from wild-type E2348/69 with those from its isogenic escN (TTSS) and eae (intimin) mutants grown in LB broth for IL-8 induction. Interestingly, similar amounts of IL-8 were detected in the supernatants of T84 cells treated with supernatants of E2348/69 and the escN and eae mutants (Fig. 1A). This result indicated that, at least in T84 colonic cells, induction of IL-8 release by E2348/69 is independent of intimate adherence, the TTSS, or an effector molecule secreted through this pathway. The supernatants of E2348/69 and isogenic mutant strains were tested for cytotoxicity toward T84 cells. No effect on the viability of T84 cells was observed (data not shown), a result that is consistent with previous reports stating that EPEC-induced cytotoxicity on epithelial cells is cell contact dependent (8, 54).
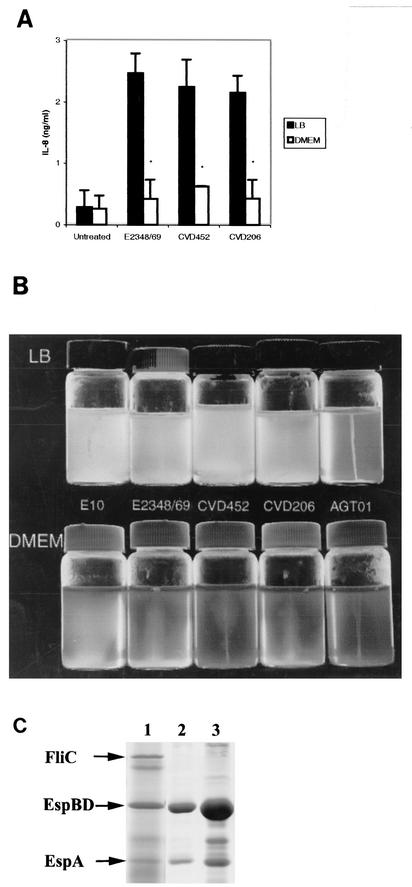
FIG. 1.
(A) Supernatants of EPEC strains stimulate IL-8 production in T84 cells. T84 cells grown in a 24-well plate were treated with 100 μl of filter-sterilized supernatants of EPEC strains grown in LB medium or DMEM for 18 h. IL-8 concentrations in supernatants of T84 cells were determined by ELISA as described in Materials and Methods. T84 cells treated with supernatants of wild-type E2348/69, CVD452 (escN mutant), or CVD206 (eae mutant) grown in LB medium secreted significantly greater amounts of IL-8 than T84 cells treated with supernatants of the same EPEC strains grown in DMEM (∗, P < 0.01). Error bars, standard deviations. (B) EPEC strains E10, E2348/69, CVD452 (escN mutant), and CVD206 (eae mutant) grown in DMEM, but not in LB medium, are deficient in motility. EPEC strains grown in LB medium were inoculated into LB or DMEM semisolid motility agar and incubated for 18 h at 37°C. Lack of motility is indicated by growth that is restricted to the line of inoculation, whereas motility is indicated by growth throughout the medium. (C) Flagellin secretion by E2348/69 strains grown in LB medium or DMEM. The wild-type strain E2348/69 was grown either to late-log phase in LB medium (lane 1) or to early-log phase (lane 2) or late-log phase (lane 3) in DMEM. Secreted proteins were collected, precipitated, and subjected to SDS-12% PAGE followed by Coomassie blue staining as described in Materials and Methods.
It has recently been reported that wild-type E2348/69 was deficient in flagellum production and nonmotile when grown in DMEM, but not when grown in LB motility agar (29). We extended this observation to several other strains, as shown in Fig. 1B. As expected, the E2348/69 flagellar mutant, AGT01, was not motile in either Luria or DMEM motility agar. All other strains tested were motile in Luria motility agar but much less motile or nonmotile in DMEM motility agar. Since flagellins from Salmonella serovar Typhimurium (19, 31), EAEC (50), and P. aeruginosa (22) have been shown to induce IL-8 secretion in epithelial cells and flagellin is secreted into supernatants, we determined whether supernatants from EPEC strains grown in DMEM were deficient in IL-8 induction in T84 cells. Interestingly, after T84 cells were treated with supernatants of E2348/69 and its isogenic mutants grown in DMEM, almost no IL-8 induction was detected (Fig. 1A). These results suggested that flagella may be involved in the IL-8 response.
To determine whether E2348/69 is deficient in flagellar secretion when grown in DMEM, secreted proteins from E2348/69 grown in LB medium or DMEM were subjected to SDS-PAGE. As shown in Fig. 1C, supernatants from wild-type E2348/69 grown to early- or late-log phase were deficient in flagellar secretion when grown in DMEM, although much greater amounts of EspB, EspD, and EspA were secreted than were seen with E2348/69 grown in LB broth. These results parallel the higher levels of IL-8 induction with LB-grown E2348/69 than with DMEM-grown E2348/69. It should be noted that EPEC strains were deficient in other factors such as BFP when grown in DMEM (29); hence, it is possible that a secreted factor(s) other than flagella may contribute to IL-8 induction in T84 cells.
FliC mutants are deficient in IL-8 induction in T84 cells.
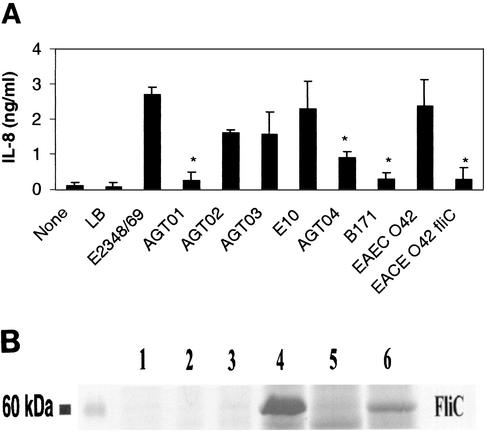
To investigate the role of EPEC flagella in IL-8 induction in T84 cells, we compared the abilities of the supernatants of EPEC strains E2348/69 and E10 and EAEC strain O42 to induce IL-8 production in T84 cells with those of their isogenic fliC mutants. Supernatants from wild-type EPEC strains E2348/69 and E10 and from EAEC strain O42 stimulated as much as 2.5 ng of IL-8/ml 18 h after treatment (Fig. 2A), whereas supernatants from their isogenic fliC mutant strains induced significantly smaller amounts of IL-8 than their wild-type parent strains (P < 0.05). These results indicated that FliC may be crucial for stimulation of IL-8 secretion in T84 cells by E2348/69, E10, and O42, a result that is consistent with the report that flagellin triggers an inflammatory response with EAEC (50). Complementation of the fliC mutation in an E2348/69 fliC mutant (AGT01) by addition of the cloned fliC gene (strain AGT02) restored the ability to stimulate IL-8, although the levels were slightly lower than those seen with its wild-type strain. When an E2348/69 motB mutant (AGT03), which is nonmotile but still produces “paralyzed” flagella, was tested, induction of IL-8 was also obtained. The motB mutant produces fewer flagella than the wild type (32) and stimulates a lower level of IL-8, equivalent to levels seen with the fliC mutant complemented strain (AGT02). Interestingly, a nonflagellated EPEC strain, B171 (O111:NM), that contains intact a type III secretion apparatus induced only low levels of IL-8 production, similar to those seen with AGT01.
FIG. 2.
(A) Supernatants of EPEC fliC mutants (AGT01 and AGT04) are deficient in stimulating IL-8 production in T84 cells. T84 cells grown in a 24-well plate were treated with 100 μl of filter-sterilized supernatants of EPEC strains grown in LB medium for 18 h. IL-8 concentrations in the supernatant were determined by ELISA. T84 cells treated with supernatants of an E2348/69 fliC mutant (AGT01), an E10 fliC mutant (AGT04), and an EAEC strain O42 fliC mutant produced significantly smaller amounts of IL-8 than those treated with supernatants from their wild-type strains (∗, P < 0.01). Complementation of the E2348/69 fliC mutation (AGT02) partially restored the ability to stimulate IL-8 in T84 cells. Error bars, standard deviations. (B) Flagellin secretion by EPEC strains grown in LB medium or DMEM. Wild-type E2348/69 (lanes 1 and 4), an E2348/69 fliC mutant (AGT01) (lanes 2 and 5), and an E2348/69 motB mutant (AGT03) (lanes 3 and 6) were grown in either DMEM (lanes 1, 2, and 3) or LB broth (lanes 4, 5, and 6). Supernatants were collected and precipitated as described in Materials and Methods. Equal amounts of supernatants were subjected to SDS-12% PAGE and visualized by Coomassie blue staining.
As shown in Fig. 2B, the EPEC motB mutant strain (AGT03) grown in LB broth secreted significantly smaller amounts of FliC (lane 6) than wild-type E2348/69 (lane 4). AGT01 (fliC mutant) was used as a negative control and did not secrete FliC, as expected (Fig. 2B, lanes 2 and 5). Consistent with previous results, wild-type E2348/69 (Fig. 2B, lane 1), AGT01 (lane 2), and AGT03 (lane 3) secreted FliC in minimal amounts when grown in DMEM, and the results were confirmed by Western blotting (data not shown). These data suggest that FliC, or a secreted protein(s) affected by fliC, is important for IL-8 induction in T84 cells.
Purified E. coli flagella induce IL-8 secretion in T84 cells.
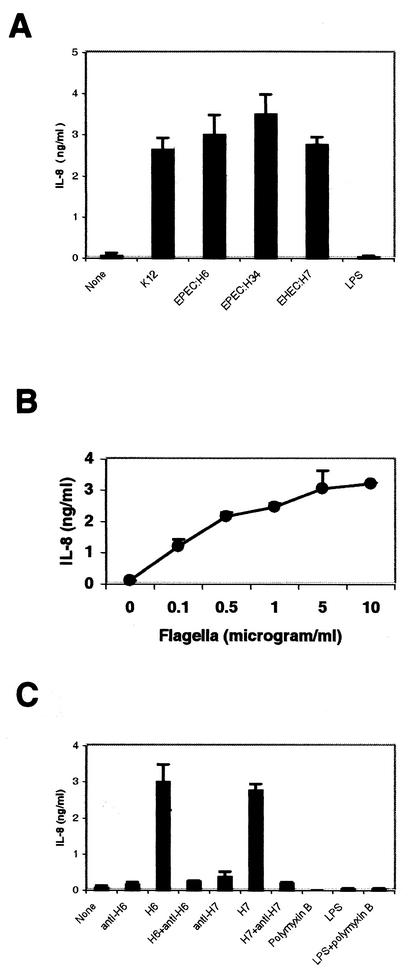
The results described above were obtained with H6 flagella. To determine whether heterologous flagella per se are able to induce IL-8 secretion in T84 cells, we purified flagella from E. coli K-12, EPEC strain E2348/69, EPEC strain E28 (O86:H34), and EHEC strain 86-24 (O157:H7). The purity of the purified flagella was assessed by SDS-PAGE and Coomasie staining and by electron microscopy as described by Girón et al. (29) (data not shown). T84 cells were incubated for 18 h with the purified flagella (1 μg/ml) obtained from the various E. coli strains, and IL-8 release was determined by ELISA (Fig. 3A). All of the flagellum types tested induced IL-8 to similar levels, suggesting that this property is inherent to both pathogenic and nonpathogenic E. coli strains and that these flagella possess a common domain responsible for IL-8 induction. The failure of LPS to stimulate IL-8 production in T84 cells is probably attributable to the lack of TLR4 and the CD14 coreceptor for LPS on epithelial cells (16). This result is consistent with reports by other researchers indicating that epithelial cells do not respond to LPS (15). The induction of IL-8 by flagella purified from EPEC strain E2348/69 was dose dependent (Fig. 3B).
FIG. 3.
(A) Purified flagella induce IL-8 production in T84 cells. T84 cells were treated with purified flagella (1 μg/ml) from EPEC, EHEC, or E. coli K-12 strains or with 100 ng of E. coli LPS/ml. IL-8 concentrations were measured by ELISA. (B) Purified H6 stimulates IL-8 production in T84 cells in a dose-dependent manner. T84 cells grown in a 24-well plate were treated with various concentrations of purified flagella from EPEC E2348/69. IL-8 production was measured by ELISA. (C) Purified flagella (1 μg/ml) from E2348/69 or an EHEC O157: H7 strain and E. coli LPS (100 ng/ml) were mixed with their respective rabbit polyclonal antibodies or with polymyxin B in DMEM-F-12 medium and were incubated for 1 h at 37°C. Polyclonal antibodies, LPS, purified flagella, and their mixtures were then applied to T84 cells grown in a 24-well plate and were incubated for 18 h. IL-8 concentrations were measured by ELISA.
To further confirm that flagella induce IL-8 production in T84 cells, purified flagella from E2348/69 and EHEC strain 86-24 were incubated at 37°C for 1 h with rabbit polyclonal antibodies against H6 and H7, respectively, and then were added to T84 cells for 18 h. As shown in Fig. 3C, anti-H6 and anti-H7 sera completely blocked IL-8 secretion in T84 cells induced by H6 and H7. Neither polyclonal anti-H6, anti-H7, LPS, normal rabbit serum, nor polymyxin B induced IL-8 induction alone. These results demonstrated that flagella from EPEC strain E2348/69 and EHEC strain 86-24 per se are sufficient to stimulate IL-8 production in T84 cells.
Purified His-tagged FliC protein induces IL-8 release.
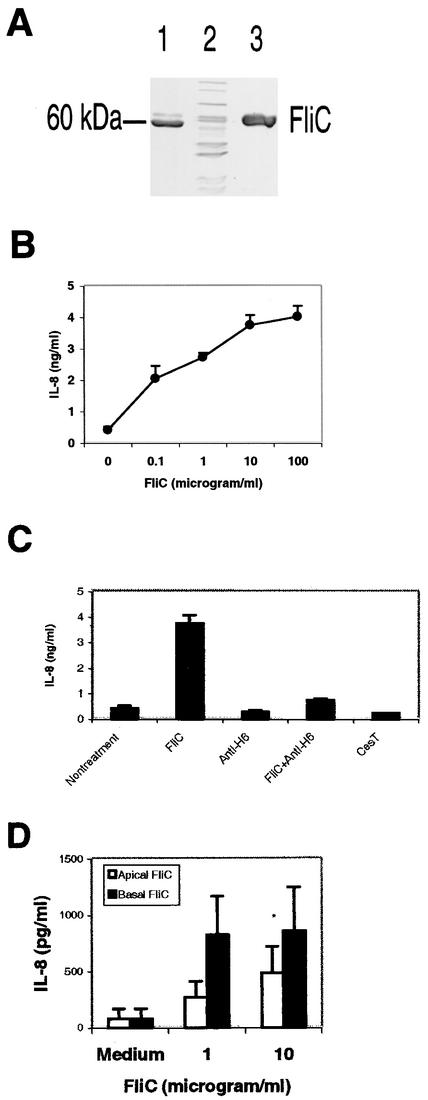
To determine whether the native structure of the flagellum filament was required for IL-8 induction, we cloned the fliC gene from E2348/69 into an expression vector containing an N-terminal six-His tag. As shown in Fig. 4A, FliC was observed in the eluate from the nickel column (lane 3) but was not present in the flowthrough (lane 2). Both purified flagella (Fig. 4A, lane 1) and recombinant FliC of E2348/69 showed a molecular size of about 60 kDa. The expressed FliC also reacted serologically with an anti-H6 antibody (data not shown). Recombinant FliC induced IL-8 release in T84 cells in a dose-dependent manner (Fig. 4B), and the ability to induce IL-8 was abolished by addition of polyclonal anti-H6 (Fig. 4C). The expressed control protein CesT, a chaperone for Tir (20), did not stimulate IL-8. These results demonstrated that the flagellin monomer FliC alone is sufficient to induce IL-8 in T84 cells.
FIG. 4.
(A) Expression and purification of FliC from EPEC E2348/69. DH5α(pRP4, pXZ13) expressing the cloned fliC gene was harvested 5 h after addition of IPTG to mid-log-phase cultures. Lysates were purified by nickel affinity chromatography and visualized on SDS-12% PAGE gels by Coomasie blue staining. Lane 1, purified flagella from EPEC strain E2348/69 (2 μg); lane 2, flowthrough from nickel column (12 μg); lane 3, eluate from column (12 μg). (B) His-tagged FliC stimulated IL-8 production in T84 cells in a dose-dependent manner. T84 cells grown in a 24-well plate were treated with various concentrations of purified recombinant His-tagged FliC from EPEC strain E2348/69. IL-8 production was measured by ELISA. (C) IL-8 production by T84 cells stimulated by purified His-tagged FliC was blocked by polyclonal rabbit anti-H6. The antibody (1:10) was mixed with FliC (final concentration, 1 μg/ml) and incubated for 1 h at 37°C. FliC (1 μg/ml), the antibody, recombinant CesT, and the mixture were added to T84 cells grown in a 24-well plate. IL-8 concentrations were measured by ELISA after 18 h of treatment. (D) Polarized T84 cells were stimulated with His-tagged FliC added to either the apical or the basolateral side. Supernatants from the basolateral chambers were removed after 18 h, and IL-8 concentrations were measured by ELISA. Asterisks indicate that a value is significantly different from the value for flagellum-treated cells (P < 0.01).
It was reported that flagellin from Salmonella serovar Typhimurium activates TLR5 on T84 cells expressed basolaterally (25). We were interested in determining whether polarized T84 cells could respond to purified His-tagged FliC presented to the basolateral side. FliC was added to the apical or the basolateral chamber of T84 cells grown as polarized monolayers. As shown in Fig. 4D, FliC added to either the apical or the basolateral side of T84 cells induced basolateral IL-8 secretion, though the level of IL-8 was higher when T84 cells were stimulated basolaterally.
MAPK are activated and required for IL-8 secretion.
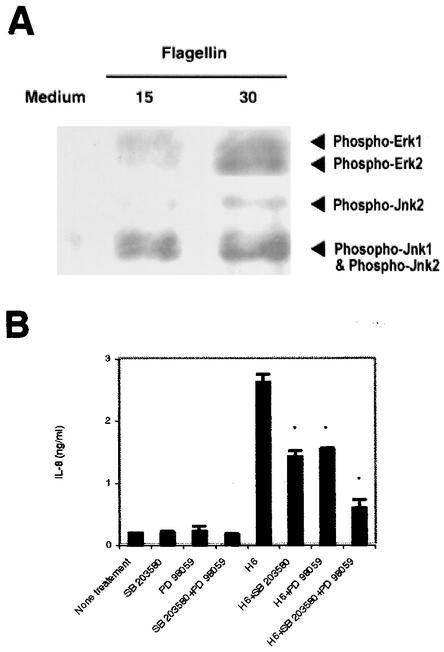
It was recently reported that a wild-type EPEC strain, but not the intimin deletion mutant (lacking eae) or the TTSS mutant strain CVD452 (lacking escN), induced activation of the Erk1 and -2, p38, and Jnk cascade in T84 cells (11). Therefore, we sought to determine whether MAPK are activated in T84 cells after flagellin treatment. As shown in Fig. 5A, kinases Erk1 and -2 and Jnk1 and -2 were phosphorylated in T84 cells as early as 15 min after stimulation. We next determined whether MAPK are involved in the IL-8 production induced by E2348/69 flagellin in T84 cells. For this purpose, T84 cells were preincubated for 90 min with PD98059 (50 μM) and SD203580 (10 μM), alone or together, prior to treatment with flagellin. These inhibitors were present during the experimental time period. Neither the combination of two inhibitors nor a single inhibitor alone affected the viability of T84 cells (data not shown). As shown in Fig. 5B, PD98059 and SB203580 reduced IL-8 production by about 50%. Similar results were obtained with purified flagella (data not shown). It is noteworthy that the concomitant presence of the two inhibitors decreased flagellum-induced IL-8 synthesis by almost 80%, suggesting that the p38 and Erk1 and -2 MAPK pathways are both required for IL-8 secretion induced by EPEC flagellin.
FIG. 5.
(A) Induction of MAPK phosphorylation by EPEC flagellin. T84 cells were treated with flagellin (1 μg/ml) from E2348/69 for 15 or 30 min, and total-cell lysates were prepared for immunoblot analysis as described in Materials and Methods. (B) Inhibition of MAPK pathway prevents IL-8 secretion in T84 cells induced by His-tagged flagellin from E2348/69. IL-8 contents in the supernatants of T84 cells after 18 h of treatment with purified H6 were estimated by ELISA. Where indicated, the inhibitor SB203580 (10 μM) or PD98059 (50 μM) or both were added 90 min prior to flagellin stimulation. Asterisks indicate that a value is significantly different from the value for flagellum-treated cells (P < 0.01).
Comparison of amino acid sequences of flagellins from other bacterial flagella.
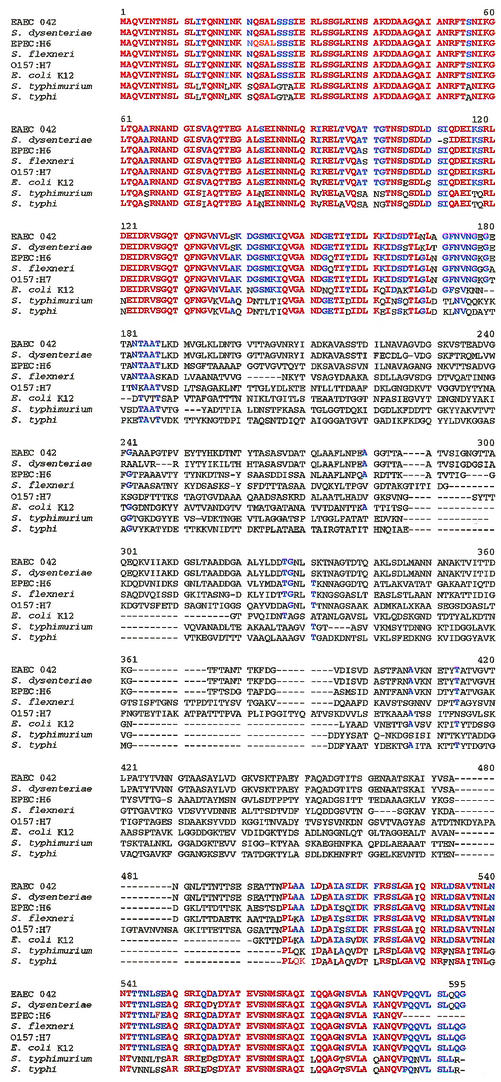
Flagellins from Salmonella serovar Typhimurium (19) and from EAEC (50) have been shown to induce inflammatory responses in gut epithelial cells. In the present study, we demonstrated that flagella and flagellin from EPEC strain E2348/69, and flagella from EHEC strain 86-24 and E. coli K-12 stimulated IL-8 secretion in T84 cells. These results suggested that flagellin from Salmonella serovar Typhimurium, EPEC, EHEC, and E. coli K-12 may share a common domain that is responsible for stimulating IL-8. Hence, we aligned the amino acid sequences of flagellins from Salmonella serovar Typhimurium (GenBank accession no. 16763390), EPEC strain E2348/69 (AF128956), an EHEC O157:H7 strain (15829254), EAEC strain O42 (AF194946), E. coli K-12 (16127994), Salmonella enterica serovar Typhi (16502975), Shigella dysenteriae (D26166), and Shigella flexneri (D16819). As shown in Fig. 6, the first 170 and last 90 amino acids are nearly identical in the eight proteins, a result consistent with the hypothesis that flagellins from these strains may recognize the same receptor on host cells.
FIG. 6.
Comparison of amino acid sequences of flagellins from EPEC strain E2348/69, an EHEC O157: H7 strain, EAEC strain O42, E. coli K-12, Salmonella serovar Typhimurium, Salmonella serovar Typhi, S. flexneri, and S. dysenteriae. Red represents conserved homology among all strains examined; blue letters indicate homologous amino acids in the majority of strains.
DISCUSSION
In this paper, we demonstrated that flagellin of EPEC is sufficient to induce IL-8 secretion in T84 cells. This conclusion is based on a number of observations. First, induction of IL-8 in T84 cells is independent of cell contact and does not require intimin or the TTSS, since the supernatants of wild-type E2348/69 and its isogenic eae and escN mutants grown in LB broth induced similar amounts of IL-8 in T84 cells. Second, supernatants of EPEC strains grown in DMEM, a condition that represses flagellum synthesis, failed to induce IL-8 in T84 cells, since EPEC strains grown in DMEM were deficient in flagellum production and secretion, and hence in motility. Third, the abilities of supernatants from an E2348/69 fliC mutant (AGT01) and an E10 fliC mutant (AGT04) to induce IL-8 were significantly lower than those of their respective wild-type strains, and the deficiency in AGT01 was restored in the complemented strain AGT02. Fourth, the supernatant from an LB-grown E2348/69 motB mutant, which is nonmotile but still produces flagella to a lesser extent than its wild-type parent, induced levels of IL-8 lower than those induced by the wild type but similar to those induced by AGT02. Finally, purified flagella and His-tagged FliC from E2348/69 induced IL-8 secretion in T84 cells in a dose-dependent manner, and the induction was completely blocked by an anti-H6 antibody.
Our results, however, are inconsistent with reports by others who showed that the TTSS was responsible for IL-8 induction in epithelial cells (11, 15). The inconsistency is probably due to differences in the infection protocols and culture conditions used. It is well established that DMEM enhances the TTSS of EPEC (33), and in the previous studies, EPEC strains were usually grown to log phase to activate the TTSS before infection (15). However, as reported recently and confirmed in this study, EPEC strains grown in DMEM produced significantly fewer flagella than those grown in LB medium, and they were not motile when grown in DMEM motility agar (29) (Fig. 1B). Furthermore, we showed that wild-type E2348/69 was also deficient in flagellum secretion when it was grown to early- or late-log phase in DMEM (Fig. 1C). Therefore, it is possible that the TTSS played a major role in the induction of IL-8 in T84 cells when EPEC strains were grown in LB medium, while flagella appeared crucial to stimulation of IL-8 secretion in T84 cells, and that both the TTSS and flagellin may play important roles in the inflammatory response of epithelial cells, as was shown in Salmonella serovar Typhimurium infection (26, 32).
The role of eae, encoding the adhesin intimin, in IL-8 induction in epithelial cells is controversial. Sakovic et al. reported that strain CVD206 (lacking eae) activated NF-κB, which in turn initiated IL-8 transcription in T84 cells (45). However, Czerucka et al. recently showed that CVD206 failed to activate p38 and Jnk or to stimulate IL-8 secretion in T84 cells (11). The reason for the discrepancy is not clear. In the present study, we showed that the supernatant from CVD206 grown in LB medium stimulated amounts of IL-8 similar to those stimulated by its wild-type parent; the result clearly demonstrates that intimate adhesion of intimin to T84 cells is not required for IL-8 induction in T84 cells. Nevertheless, it should be kept in mind that EPEC may trigger the IL-8 response in host cells by more than one signaling pathway. For example, some secreted proteins dependent on the TTSS may be crucial to trigger the signal transduction pathway in host cells; in this case, the eae gene product would be important for optimal translocation of effector proteins into host cells via the TTSS.
An inverse relationship between regulation of the TTSS and regulation of flagella may exist in EPEC strains. In the present study we showed that, on the one hand, the TTSS was activated and the flagella were down-regulated when EPEC strains were grown in DMEM, but on the other hand, the TTSS was down-regulated and the flagella were activated when these strains were grown in LB medium. The counter-regulation may be crucial in EPEC pathogenesis at different infection stages (35). In the initial phase of infection, the flagellar system is probably important for EPEC to move toward the target site and for initial adherence (29). At a later stage, motility is turned off, and the TTSS is activated once EPEC adheres to host cells. The counter-regulation system has been described for Bordetella, Salmonella, and Yersinia spp. (35). Due to the similarity of the TTSS and the flagellar secretion system, and the fact that the flagellar system is repressed under culture conditions such as DMEM, which stimulate expression of the TTSS, it is reasonable to hypothesize the existence of a flagellar repression regulator that activates the TTSS in EPEC. Though we showed that the TTSS is not required for IL-8 induction in T84 cells, we did not exclude a role for the TTSS in IL-8 induction.
Flagellin may be a common protein responsible for triggering the mucosal inflammatory response by several different enteric pathogens, which would be consistent with the highly conserved D1 and D2 domains of several enteric flagellins. For example, E. coli O157 causes acute gastroenteritis and hemorrhagic colitis that may lead to severe complications, including the hemolytic-uremic syndrome (HUS) (43). During infection by EHEC O157, inflammation of the colon accompanied by neutrophil infiltration is observed (49). The Shiga toxin (Stx) produced by EHEC has been shown to stimulate IL-8 production in epithelial cells (53). However, Stx-negative strains of EHEC still cause diarrhea (56, 57), which suggests that additional, as yet uncharacterized molecules are able to induce IL-8 release. Indeed, EHEC O157:H7 lacking stx was still able to activate IL-8 transcription in T84 cells (K. J. Kanack and J. B. Kaper, unpublished data). In the present study, we showed, to our knowledge for the first time, that flagella from E. coli O157:H7 induce IL-8 secretion in T84 cells, a fact that may be related to the inflammatory response observed in the gut after infection by O157:H7. Our finding may have significance in terms of prevention and therapeutic intervention for EHEC O157 infection. S. dysenteriae and S. flexneri are typically considered nonmotile, and they cause severe inflammation during shigellosis. Al Mamun et al. reported that all four Shigella subgroups contain cryptic flagellar operons (3, 4). Nevertheless, flagellum production was demonstrated by electron microscopy on Shigella species after growth under specific in vitro laboratory conditions (27). Thus, it would be interesting to investigate whether the flagella play a role in triggering inflammation in shigellosis in vivo.
In polarized T84 cells, we found that addition of purified His-tagged FliC from EPEC to either the apical or the basolateral epithelial compartment resulted in secretion of IL-8 on the basolateral side. The level of IL-8 induced by FliC added basolaterally was much higher than that stimulated by FliC added apically. It has recently been reported that flagellin from Salmonella serovar Typhimurium (26) or L. monocytogenes (31) can activate TLR5 and induce proinflammantory cytokine production. Since it was reported that TLR5 is distributed only on the basolateral side of T84 cells (25), our result suggested that FliC from EPEC may recognize an additional receptor(s) besides TLR5, although the mechanism is not clear and we are currently investigating it.
In the present study, we utilized pathway-specific inhibitors to demonstrate that both Erk1 and -2 and Jnk1 and -2 pathways are involved in IL-8 induction in T84 cells by EPEC flagellin, and we extended these findings by demonstrating phosphorylation of Erk and Jnk early after exposure of T84 cells to flagellin. The response of T84 cells to flagellin from Salmonella serovar Typhimurium depends on extracellular leucine-rich repeats, the intracellular Toll/IL-1R homology region of TLR-5, the adaptor protein MyD88, and activation of NF-κB (25). Although the signal transduction stimulated by flagellin seems to be conserved in diverse organisms including insects, mammals, and plants (2, 5), the receptor and its downstream signal transduction pathway triggered by EPEC flagellin remains to be determined.
In summary, we provide in this report compelling data that suggest that IL-8 stimulation by EPEC strains is independent of cell contact and the TTSS. Erk1 and -2 and Jnk1 and -2 are phosphorylated in T84 cells after flagellin stimulation, and inhibition of Erk and p38 significantly blocked IL-8 production in T84 cells. To our knowledge, this report is the first to demonstrate that flagellin of EPEC induces IL-8 production in epithelial cells. Interestingly, the induction of IL-8 in T84 cells was not restricted to enteric pathogens and was also observed with the flagella of E. coli K-12. We believe that our data provide new insights regarding the intestinal immune response triggered by EPEC infection.
Acknowledgments
We thank the entire Kaper laboratory for encouragement and support. We especially thank Vladimir Toshchakov for assistance in the Western blotting of MAPK and Neil Stokes for critical reading of the manuscript.
This work was supported by NIH grants AI-21657 and DK-58957 to J. B. Kaper and AI-18797 to S. N. Vogel. A. G. Torres was supported by research supplements for underrepresented minorities from the NIAID and NIDDK, NIH. J. A. Crawford was supported by a National Research Service award from NIH (AI-01053802).
Editor: A. D. O'Brien
REFERENCES
- 1.Abul-Milh, M., Y. Wu, B. Lau, C. A. Lingwood, and D. B. Foster. 2001. Induction of epithelial cell death including apoptosis by enteropathogenic Escherichia coli expressing bundle-forming pili. Infect. Immun. 69:7356-7364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aderem, A., and R. J. Ulevitch. 2000. Toll-like receptors in the induction of the innate immune response. Nature 406:782-787. [DOI] [PubMed] [Google Scholar]
- 3.Al Mamun, A. A., A. Tominaga, and M. Enomoto. 1997. Cloning and characterization of the region III flagellar operons of the four Shigella subgroups: genetic defects that cause loss of flagella of Shigella boydii and Shigella sonnei. J. Bacteriol. 179:4493-4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al Mamun, A. A., A. Tominaga, and M. Enomoto. 1996. Detection and characterization of the flagellar master operon in the four Shigella subgroups. J. Bacteriol. 178:3722-3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asai, T., G. Tena, J. Plotnikova, M. R. Willmann, W. L. Chiu, L. Gomez-Gomez, T. Boller, F. M. Ausubel, and J. Sheen. 2002. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415:977-983. [DOI] [PubMed] [Google Scholar]
- 6.Campellone, K. G., A. Giese, D. J. Tipper, and J. M. Leong. 2002. A tyrosine-phosphorylated 12-amino-acid sequence of enteropathogenic Escherichia coli Tir binds the host adaptor protein Nck and is required for Nck localization to actin pedestals. Mol. Microbiol. 43:1227-1241. [DOI] [PubMed] [Google Scholar]
- 7.Cantarelli, V. V., A. Takahashi, I. Yanagihara, Y. Akeda, K. Imura, T. Kodama, G. Kono, Y. Sato, and T. Honda. 2001. Talin, a host cell protein, interacts directly with the translocated intimin receptor, Tir, of enteropathogenic Escherichia coli, and is essential for pedestal formation. Cell. Microbiol. 3:745-751. [DOI] [PubMed] [Google Scholar]
- 8.Crane, J. K., B. P. McNamara, and M. S. Donnenberg. 2001. Role of EspF in host cell death induced by enteropathogenic Escherichia coli. Cell. Microbiol. 3:197-211. [DOI] [PubMed] [Google Scholar]
- 9.Crawford, J. A., and J. B. Kaper. 2002. The N-terminus of EPEC Tir mediates transport across bacterial and eukaryotic cell membranes. Mol. Microbiol. 46:855-868. [DOI] [PubMed] [Google Scholar]
- 10.Cuenda, A., J. Rouse, Y. N. Doza, R. Meier, P. Cohen, T. F. Gallagher, P. R. Young, and J. C. Lee. 1995. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 364:229-233. [DOI] [PubMed] [Google Scholar]
- 11.Czerucka, D., S. Dahan, B. Mograbi, B. Rossi, and P. Rampal. 2001. Implication of mitogen-activated protein kinases in T84 cell responses to enteropathogenic Escherichia coli infection. Infect. Immun. 69:1298-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dahan, S., V. Busuttil, V. Imbert, J. F. Peyron, P. Rampal, and D. Czerucka. 2002. Enterohemorrhagic Escherichia coli infection induces interleukin-8 production via activation of mitogen-activated protein kinases and the transcription factors NF-κB and AP-1 in T84 cells. Infect. Immun. 70:2304-2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis, R. J. 1993. The mitogen-activated protein kinase signal transduction pathway. J. Biol. Chem. 268:14553-14556. [PubMed] [Google Scholar]
- 14.Davis, R. J. 2000. Signal transduction by the JNK group of MAP kinases. Cell 103:239-252. [DOI] [PubMed] [Google Scholar]
- 15.De Grado, M., C. M. Rosenberger, A. Gauthier, B. A. Vallance, and B. B. Finlay. 2001. Enteropathogenic Escherichia coli infection induces expression of the early growth response factor by activating mitogen-activated protein kinase cascades in epithelial cells. Infect. Immun. 69:6217-6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Didierlaurent, A., J. C. Sirard, J. P. Kraehenbuhl, and M. R. Neutra. 2002. How the gut senses its content. Cell. Microbiol. 4:61-72. [DOI] [PubMed] [Google Scholar]
- 17.DiMango, E., H. J. Zar, R. Bryan, and A. Prince. 1995. Diverse Pseudomonas aeruginosa gene products stimulate respiratory epithelial cells to produce interleukin-8. J. Clin. Investig. 96:2204-2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donnenberg, M. S., and J. B. Kaper. 1991. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect. Immun. 59:4310-4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eaves-Pyles, T., K. Murthy, L. Liaudet, L. Virag, G. Ross, F. G. Soriano, C. Szabo, and A. L. Salzman. 2001. Flagellin, a novel mediator of Salmonella-induced epithelial activation and systemic inflammation: IκBα degradation, induction of nitric oxide synthase, induction of proinflammatory mediators, and cardiovascular dysfunction. J. Immunol. 166:1248-1260. [DOI] [PubMed] [Google Scholar]
- 20.Elliott, S. J., S. W. Hutcheson, M. S. Dubois, J. L. Mellies, L. A. Wainwright, M. Batchelor, G. Frankel, S. Knutton, and J. B. Kaper. 1999. Identification of CesT, a chaperone for the type III secretion of Tir in enteropathogenic Escherichia coli. Mol. Microbiol. 33:1176-1189. [DOI] [PubMed] [Google Scholar]
- 21.Elliott, S. J., E. O. Krejany, J. L. Mellies, R. M. Robins-Browne, C. Sasakawa, and J. B. Kaper. 2001. EspG, a novel type III system-secreted protein from enteropathogenic Escherichia coli with similarities to VirA of Shigella flexneri. Infect. Immun. 69:4027-4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feldman, M., R. Bryan, S. Rajan, L. Scheffler, S. Brunnert, H. Tang, and A. Prince. 1998. Role of flagella in pathogenesis of Pseudomonas aeruginosa pulmonary infection. Infect. Immun. 66:43-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freeman, N. L., D. V. Zurawski, P. Chowrashi, J. C. Ayoob, L. Huang, B. Mittal, J. M. Sanger, and J. W. Sanger. 2000. Interaction of the enteropathogenic Escherichia coli protein, translocated intimin receptor (Tir), with focal adhesion proteins. Cell. Motil. Cytoskeleton 47:307-318. [DOI] [PubMed] [Google Scholar]
- 24.Garrington, T. P., and G. L. Johnson. 1999. Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr. Opin. Cell Biol. 11:211-218. [DOI] [PubMed] [Google Scholar]
- 25.Gewirtz, A. T., T. A. Navas, S. Lyons, P. J. Godowski, and J. L. Madara. 2001. Bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J. Immunol. 167:1882-1885. [DOI] [PubMed] [Google Scholar]
- 26.Gewirtz, A. T., P. O. Simon, Jr., C. K. Schmitt, L. J. Taylor, C. H. Hagedorn, A. D. O'Brien, A. S. Neish, and J. L. Madara. 2001. Salmonella typhimurium translocates flagellin across intestinal epithelia, inducing a proinflammatory response. J. Clin. Investig. 107:99-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Girón, J. A. 1995. Expression of flagella and motility by Shigella. Mol. Microbiol. 18:63-75. [DOI] [PubMed] [Google Scholar]
- 28.Girón, J. A., A. S. Ho, and G. K. Schoolnik. 1991. An inducible bundle-forming pilus of enteropathogenic Escherichia coli. Science 254:710-713. [DOI] [PubMed] [Google Scholar]
- 29.Girón, J. A., A. G. Torres, E. Freer, and J. B. Kaper. 2002. The flagella of enteropathogenic Escherichia coli mediate adherence to epithelial cells. Mol. Microbiol. 44:361-380. [DOI] [PubMed] [Google Scholar]
- 30.Gruenheid, S., R. DeVinney, F. Bladt, D. Goosney, S. Gelkop, G. D. Gish, T. Pawson, and B. B. Finlay. 2001. Enteropathogenic E. coli Tir binds Nck to initiate actin pedestal formation in host cells. Nat. Cell Biol. 3:856-859. [DOI] [PubMed] [Google Scholar]
- 31.Hayashi, F., K. D. Smith, A. Ozinsky, T. R. Hawn, E. C. Yi, D. R. Goodlett, J. K. Eng, S. Akira, D. M. Underhill, and A. Aderem. 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410:1099-1103. [DOI] [PubMed] [Google Scholar]
- 32.Hobbie, S., L. M. Chen, R. J. Davis, and J. E. Galán. 1997. Involvement of mitogen-activated protein kinase pathways in the nuclear responses and cytokine production induced by Salmonella typhimurium in cultured intestinal epithelial cells. J. Immunol. 159:5550-5559. [PubMed] [Google Scholar]
- 33.Jarvis, K. G., J. A. Girón, A. E. Jerse, T. K. McDaniel, M. S. Donnenberg, and J. B. Kaper. 1995. Enteropathogenic Escherichia coli contains a putative type III secretion system necessary for the export of proteins involved in attaching and effacing lesion formation. Proc. Natl. Acad. Sci. USA 92:7996-8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jerse, A. E., J. Yu, B. D. Tall, and J. B. Kaper. 1990. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc. Natl. Acad. Sci. USA 87:7839-7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Josenhans, C., and S. Suerbaum. 2002. The role of motility as a virulence factor in bacteria. Int. J. Med. Microbiol. 291:605-614. [DOI] [PubMed] [Google Scholar]
- 36.Kenny, B. 2002. Mechanism of action of EPEC type III effector molecules. Int. J. Med. Microbiol. 291:469-477. [DOI] [PubMed] [Google Scholar]
- 37.Kenny, B., R. DeVinney, M. Stein, D. J. Reinscheid, E. A. Frey, and B. B. Finlay. 1997. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell 91:511-520. [DOI] [PubMed] [Google Scholar]
- 38.Levine, M. M., and R. Edelman. 1984. Enteropathogenic Escherichia coli of classic serotypes associated with infant diarrhea: epidemiology and pathogenesis. Epidemiol. Rev. 6:31-51. [DOI] [PubMed] [Google Scholar]
- 39.Levine, M. M., J. P. Nataro, H. Karch, M. M. Baldini, J. B. Kaper, R. E. Black, M. L. Clements, and A. D. O'Brien. 1985. The diarrheal response of humans to some classic serotypes of enteropathogenic Escherichia coli is dependent on a plasmid encoding an enteroadhesiveness factor. J. Infect. Dis. 152:550-559. [DOI] [PubMed] [Google Scholar]
- 40.McDaniel, T. K., K. G. Jarvis, M. S. Donnenberg, and J. B. Kaper. 1995. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc. Natl. Acad. Sci. USA 92:1664-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McNamara, B. P., A. Koutsouris, C. B. O'Connell, J. P. Nougayrede, M. S. Donnenberg, and G. Hecht. 2001. Translocated EspF protein from enteropathogenic Escherichia coli disrupts host intestinal barrier function. J. Clin. Investig. 107:621-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller, J. R., L. J. Barrett, K. Kotloff, and R. L. Guerrant. 1994. A rapid test for infectious and inflammatory enteritis. Arch. Intern. Med. 154:2660-2664. [DOI] [PubMed] [Google Scholar]
- 43.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ogushi, K., A. Wada, T. Niidome, N. Mori, K. Oishi, T. Nagatake, A. Takahashi, H. Asakura, S. Makino, H. Hojo, Y. Nakahara, M. Ohsaki, T. Hatakeyama, H. Aoyagi, H. Kurazono, J. Moss, and T. Hirayama. 2001. Salmonella enteritidis FliC (flagella filament protein) induces human beta-defensin-2 mRNA production by Caco-2 cells. J. Biol. Chem. 276:30521-30526. [DOI] [PubMed] [Google Scholar]
- 45.Savkovic, S. D., A. Koutsouris, and G. Hecht. 1997. Activation of NF-κB in intestinal epithelial cells by enteropathogenic Escherichia coli. Am. J. Physiol. 273:C1160-C1167. [DOI] [PubMed] [Google Scholar]
- 46.Savkovic, S. D., A. Koutsouris, and G. Hecht. 1996. Attachment of a noninvasive enteric pathogen, enteropathogenic Escherichia coli, to cultured human intestinal epithelial monolayers induces transmigration of neutrophils. Infect. Immun. 64:4480-4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shapiro, L. 1995. The bacterial flagellum: from genetic network to complex architecture. Cell 80:525-527. [DOI] [PubMed] [Google Scholar]
- 48.Sierro, F., B. Dubois, A. Coste, D. Kaiserlian, J. P. Kraehenbuhl, and J. C. Sirard. 2001. Flagellin stimulation of intestinal epithelial cells triggers CCL20-mediated migration of dendritic cells. Proc. Natl. Acad. Sci. USA 98:13722-13727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Slutsker, L., A. A. Ries, K. D. Greene, J. G. Wells, L. Hutwagner, and P. M. Griffin. 1997. Escherichia coli O157:H7 diarrhea in the United States: clinical and epidemiologic features. Ann. Intern. Med. 126:505-513. [DOI] [PubMed] [Google Scholar]
- 50.Steiner, T. S., J. P. Nataro, C. E. Poteet-Smith, J. A. Smith, and R. L. Guerrant. 2000. Enteroaggregative Escherichia coli expresses a novel flagellin that causes IL-8 release from intestinal epithelial cells. J. Clin. Investig. 105:1769-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tang, P., C. L. Sutherland, M. R. Gold, and B. B. Finlay. 1998. Listeria monocytogenes invasion of epithelial cells requires the MEK-1/ERK-2 mitogen-activated protein kinase pathway. Infect. Immun. 66:1106-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tasteyre, A., M. C. Barc, A. Collignon, H. Boureau, and T. Karjalainen. 2001. Role of FliC and FliD flagellar proteins of Clostridium difficile in adherence and gut colonization. Infect. Immun. 69:7937-7940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thorpe, C. M., B. P. Hurley, L. L. Lincicome, M. S. Jacewicz, G. T. Keusch, and D. W. Acheson. 1999. Shiga toxins stimulate secretion of interleukin-8 from intestinal epithelial cells. Infect. Immun. 67:5985-5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tobe, T., and C. Sasakawa. 2002. Species-specific cell adhesion of enteropathogenic Escherichia coli is mediated by type IV bundle-forming pili. Cell. Microbiol. 4:29-42. [DOI] [PubMed] [Google Scholar]
- 55.Toshchakov, V., B. W. Jones, P. Y. Perera, K. Thomas, M. J. Cody, S. Zhang, B. R. Williams, J. Major, T. A. Hamilton, M. J. Fenton, and S. N. Vogel. 2002. TLR4, but not TLR2, mediates IFN-β-induced STAT1α/β-dependent gene expression in macrophages. Nat. Immunol. 3:392-398. [DOI] [PubMed] [Google Scholar]
- 56.Tzipori, S., H. Karch, K. I. Wachsmuth, R. M. Robins-Browne, A. D. O'Brien, H. Lior, M. L. Cohen, J. Smithers, and M. M. Levine. 1987. Role of a 60-megadalton plasmid and Shiga-like toxins in the pathogenesis of infection caused by enterohemorrhagic Escherichia coli O157:H7 in gnotobiotic piglets. Infect. Immun. 55:3117-3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Waddell, T. E., C. A. Lingwood, and C. L. Gyles. 1996. Interaction of verotoxin 2e with pig intestine. Infect. Immun. 64:1714-1719. [DOI] [PMC free article] [PubMed] [Google Scholar]