Abstract
Ulcerative colitis and Crohn's disease are inflammatory bowel diseases thought to involve strains of Escherichia coli. We report here that two wild-type Afa/Dr diffusely adhering E. coli (DAEC) strains, C1845 and IH11128, which harbor the fimbrial F1845 adhesin and the Dr hemagglutinin, respectively, and the E. coli laboratory strain HB101, transformed with the pSSS1 plasmid to produce Afa/Dr F1845 adhesin, all induced interleukin-8 (IL-8) production and transepithelial migration of polymorphonuclear leukocytes (PMNL) in polarized monolayers of the human intestinal cell line T84 grown on semipermeable filters. We observed that after PMNL migration, expression of decay-accelerating factor (DAF, or CD55), the brush border-associated receptor for Afa/Dr adhesins, was strongly enhanced, increasing the adhesion of Afa/Dr DAEC bacteria. When examining the mechanism by which DAF expression was enhanced, we observed that the PMNL transepithelial migration induced epithelial synthesis of tumor necrosis factor alpha and IL-1β, which in turn promoted the upregulation of DAF.
Although the etiology of inflammatory bowel diseases (IBD) has not yet been identified, animal models and clinical data obtained from patients with IBD strongly suggest that bacteria are required to sustain the inflammatory reaction (16, 25, 27). Pathogenic Escherichia coli strains present in the colon may play a crucial role in the pathogenesis of IBD (41, 67, 68, 71, 73). Several studies have demonstrated the presence of E. coli strains in patients with Crohn's disease (CD) and ulcerative colitis (14, 15, 22, 34, 45). However, the mechanism(s) by which these strains of E. coli may be involved in the pathogenicity of IBD has not been identified.
Afa/Dr diffusely adhering E. coli (DAEC) strains are a family of DAEC strains expressing the afimbrial Afa-I and Afa-III adhesins, Dr hemagglutinin, and fimbrial F1845 adhesin. Although a correlation between Afa/Dr DAEC intestinal infection and IBD has yet to be established, a recent report indicates that Afa/Dr DAEC infection of cultured human intestinal Caco-2 cells leads to increased expression of major histocompatibility complex class I-related MICA, a molecule expressed to a greater extent on the surfaces of epithelial cells in colonic biopsy specimens from patients with CD (72). It has already been demonstrated that Afa/Dr adhesins recognize a glycosylphosphatidylinositol (GPI)-anchored protein, complement-regulating decay-accelerating factor (DAF, also known as CD55), as a receptor (55), Interestingly, Afa/Dr DAEC strains recognize the short consensus repeat 3 (SCR3) domain of DAF, which plays a pivotal role in the regulatory function of DAF. To avoid excess complement activation and to protect the host, the complement system is tightly controlled by proteins present in the fluid phase and on cell membranes (43). In the normal colonic mucosa or in other human intestinal cell lines, immunofluorescence labeling shows that DAF is expressed on the luminal surface of the epithelium (74) and at the brush border of polarized intestinal cells (5). Previous studies have demonstrated that DAF is more strongly expressed both in the colonic epithelium and in stool specimens of patients with IBD than in those from patients with normal mucosae (7, 74). Proinflammatory cytokines can modulate DAF expression in various human cells (9, 52). More specifically, interleukin-4 (IL-4), IL-1β, or tumor necrosis factor alpha (TNF-α) can stimulate in vitro expression of DAF in epithelial cells (2, 3, 54, 66, 69). Production of proinflammatory interleukins also contributes to the recruitment of polymorphonuclear leukocytes (PMNL). It is interesting that expression of these cytokines is strongly increased in the intestinal mucosae of patients with IBD, particularly during the acute phase of the disease characterized by the influx of PMNL (44).
In this study, we used the human intestinal epithelial cell line T84 grown on semipermeable filters to investigate whether Afa/Dr DAEC infection is accompanied by a proinflammatory response linked to the interaction between Afa/Dr DAEC adhesins and DAF. We investigated (i) whether the Afa/Dr DAEC infection can induce PMNL transepithelial migration, (ii) whether DAF is upregulated after PMNL transmigration and so impacts on Afa/Dr DAEC infection, and (iii) whether the DAF upregulation during Afa/Dr-induced PMNL-T84 transmigration results from production of cytokines by epithelial cells.
MATERIALS AND METHODS
Reagents, antibodies, and plasmids.
The enhanced chemiluminescence (ECL) kit was obtained from Amersham International (Little Chalfont, Buckinghamshire, United Kingdom). Trizol reagent was from Life Technologies (Cergy Pontoise, France). Biotin sulfo-N-hydroxysuccinimide ester was from Pierce Biochemicals (Rockford, Ill.). The cytokine TNF-α (10 ng/ml) was from Euromedex (Mundolshein, France), and the cytokine IL-1β (10 ng/ml) was from Valbiotech (Paris, France). The N-formyl-l-methionyl-leucyl-l-phenylalanine peptide (f-MLP) was from Sigma (Paris, France). Plasmid pcDNA-DAF was from the StripEZ DNA kit (Ambion, Austin, Tex.). The anti-DAF SCR3 monoclonal antibody (MAb) 1H4 (20 μg/ml) was from D. M. Lublin (Washington University, St. Louis, Mo.). The control immunoglobulin G1 (IgG1) isotype (20 μg/ml; MOPC21) was from Sigma. The anti-DAF MAb F4-29D9 (20 μg/ml) was from Valbiotech. The polyclonal anti-DAF antibody H-319 (20 μg/ml) was from Santa Cruz Biotechnologies (Santa Cruz, Calif.). The anti-membrane cofactor protein (MCP) MAb GB24 (2 μg/ml) was produced by INSERM Unit 364 (Nice, France) (37). The anti-TNF-α MAb IPV-1 (5 μg/ml) was from Abcys (Paris, France). The anti-IL-1β MAb BIO 065 (5 μg/ml) was from Euromedex (Paris, France). Phosphatase-conjugated goat anti-TNF-α and anti-IL-1β polyclonal antibodies were from Sandoz Pharmaceutical (Rueil-Malmaison, France). The peroxidase-conjugated secondary antibodies (anti-rabbit IgG) were supplied by Dakopatts (Copenhagen, Denmark). Cycloheximide (Sigma) was used at 10 μg/ml for 1 h. Phosphatidylinositol-specific phospholipase C (PI-PLC) isolated from Bacillus cereus (Sigma) was used at 100 mU/ml for 30 min. Lipopolysaccharide (LPS) (E. coli O26:B6; Sigma) was used at 100 ng/ml for 1 h. Phorbol myristate acetate (Sigma) was used at 100 ng/ml for 1 h. EDTA (Sigma) was used at 2 · 10−3 mM for 15 min.
In order to characterize IL-8 secretion after preincubation of T84 monolayers with various strains of E. coli, the lower reservoirs were assayed in triplicate for IL-8 by enzyme-linked immunosorbent assay (ELISA). ELISA was carried out by using a polyclonal rabbit anti-IL-8 antibody and a polyclonal goat anti-IL-8 antibody (Sandoz Pharmaceutical). Phosphatase-conjugated rabbit anti-goat IgG (Sigma) was used as the reporting antibody.
The pcDNA3-DAF plasmid consists of the human DAF cDNA inserted into the EcoRI site of pcDNA3 (Invitrogen, Cergy Pontoise, France).
Bacterial strains and growth conditions.
We used the wild-type Afa/Dr DAEC strains C1845 and IH11128, harboring fimbrial F1845 adhesin (8) and Dr hemagglutinin (56), respectively, and the E. coli laboratory strain HB101 transformed with the pSSS1 plasmid to produce Afa/Dr F1845 adhesin (8), For the confocal microscopy study, we used the GFP-IH11128 strain. The E. coli laboratory strain K12-HB101 (a gift of Patrice Boquet, INSERM 452, Nice, France) was used as the negative control. Strains were grown on colonization factor agar (CFA) agar, containing 1% Casamino Acids (Difco Laboratories, Detroit, Mich.), 0.15% yeast extract, 0.005% magnesium sulfate, and 0.0005% manganese chloride in 2% agar, for 18 h at 37°C. The E. coli laboratory strain HB101 was grown at 37°C for 18 h on Luria agar.
Cell culture.
T84 cells (a human colonic carcinoma cell line; ATCC CCL 248, passages 65 to 90; American Type Culture Collection, Manassas, Va.) were grown and maintained as confluent monolayers on collagen-coated permeable supports with minor modifications (58). Monolayers were grown on 0.33-cm2 ring-supported polycarbonate filters with a pore size of 3 μm (Costar, Cambridge, Mass.) and used 6 to 14 days after plating. Confluent monolayers on permeable supports were constructed to permit basolateral-to-apical migration of PMNL (“inverted inserts”) as previously described (58). To assess currents, transepithelial potentials, and resistances, a commercial voltage clamp (Bioengineering Department, University of Iowa) was used, as previously described (36). Cells were used at a density of 3 × 106/ml for Western blotting.
HeLa cells (a human cervix carcinoma cell line; ATCC CCL-2) were maintained in Dulbecco's modified Eagle's medium (Life Technologies) supplemented with 10% fetal calf serum and 1% l-glutamine (Life Technologies). HT-29 (a human colon carcinoma cell line; ATCC HTB-38) and CHO (a hamster ovarian carcinoma cell line; ATCC CR2-9606) cells were maintained in Dulbecco's modified Eagle's medium and Ham F-12 medium (Sigma) supplemented with 10% fetal calf serum and 1% l-glutamine (Life Technologies). The mutant CHO cells (kindly provided by P. Boquet, INSERM 452, and F. G. van der Goot, Department of Biochemistry, University of Geneva, Geneva, Switzerland) are deficient in GPI proteins (1). The pcDNA3-DAF plasmid was transfected into the HT-29 and CHO cell lines by using EXGEN500 (Euromedex; Souffelweyersheim, France) according to the manufacturer's instructions. The same plasmid was transfected into HeLa cells by calcium phosphate precipitation. Cells were used at a density of 3 × 106/ml for Western blotting.
Epithelial infection by Afa/Dr DAEC strains.
T84 cells were infected by a method described previously (38, 39, 49). Briefly, inverted T84 monolayers were rinsed extensively in Hanks balanced salt solution containing Ca2+ and Mg2+ [HBSS (+); Sigma] to remove residual serum components. Ten microliters of each bacterial sample [washed twice with HBSS (+) and representing an inoculation ratio of approximately 20 bacteria per epithelial cell] was gently distributed onto the apical surfaces of T84 monolayers. In some experiments, the T84 cells were preincubated for 45 min with MAb 1H4 or MAb MOPC21. Bacterial invasion was assessed after 3 h by measuring Afa/Dr DAEC attachment to and entry into T84 cells. Cell-associated bacteria were the bacteria that had become attached to and/or internalized in the T84 monolayers and were then released by incubation with 0.1 ml of 1% Triton X-100. Internalized bacteria were those obtained after lysis of the epithelial cells with 1% Triton X-100 45 min after addition of gentamicin (50 μg/ml). Preliminary gentamicin dose-response studies defined the conditions required to achieve bactericidal effects on the strain used (data not shown). For both cell-associated and internalized bacteria, 0.9 ml of Luria broth was then added, each sample was then mixed vigorously, and the CFU were counted after plating on MacConkey agar medium. Finally, the number of attached bacteria was determined by subtracting the number of cell-internalized bacteria from the number of cell-associated bacteria (since the cell-associated bacteria included both attached and internalized bacteria).
Infection experiments were conducted after PMNL transepithelial migration. Briefly, following 3 h of PMNL migration, polarized T84 cell monolayers were washed six times in HBSS buffer to remove PMNL and were returned to fresh wells containing HBSS buffer. T84 cells were then incubated for 3 h at 37°C with 10 μl of each bacterial sample per monolayer, and cells were processed as described above. In all these experiments, the E. coli K-12 strain HB101 was used as the negative-control strain, since this strain does not adhere to epithelial cells. All experiments were performed at a temperature of 37°C.
Preparation of PMNL.
Human PMNL were isolated from whole blood by using a gelatin sedimentation technique (58). Briefly, whole blood anticoagulated with citrate-dextrose was centrifuged at 300 × g for 20 min (20°C). The plasma and buffy coat were removed, and the gelatin-cells mixture was incubated at 37°C for 30 min to remove contaminating red blood cells. Residual red blood cells were then lysed with isotonic ammonium chloride. After a wash in HBSS free from Ca2+ and Mg2+, the cells were counted and resuspended at 5 × 107 PMNL/ml. PMNL (95% pure) with 98% viability by trypan blue exclusion were used within 1 h after isolation.
PMNL transmigration assays.
The physiologically (basolateral-to-apical) directed PMNL transepithelial migration assay has been described previously (35, 36, 39, 58). PMNL transmigration experiments were performed at 37°C on 0.33-cm2 inverts. T84 monolayers were washed three times in warm HBSS, and approximately 5 × 107 CFU of E. coli in a volume of 100 μl was gently placed on the apical surface and incubated for 4 h at 37°C. Nonadherent bacteria were removed from the monolayers by thorough washing, and cells were then transferred back into the 24-well tissue culture tray containing 10 ml of HBSS in each lower reservoir (apical membrane now colonized with E. coli) and 100 μl in the upper reservoir (basolateral interface). A total of 106 PMNL were added to the inverts. Control transmigration of PMNL was initiated by adding f-MLP (10−7 M) to the lower reservoir and incubating for 15 min to allow a transepithelial chemotactic gradient to form before addition of PMNL. Transmigration of PMNL was assayed by using the azurophil granule marker myeloperoxidase, as described previously (39, 42, 58).
Electron microscopy study.
Inverted T84 monolayers were rinsed thoroughly in HBSS. Approximately 5 × 107 CFU of E. coli in a volume of 100 μl was gently placed on the apical surface and incubated for 4 h at 37°C and pH 7.4. Nonadhering bacteria were removed from the monolayers by thorough washing, and the cells were then transferred back into the 24-well tissue culture tray containing HBSS. After removal from the inserts, the T84 monolayers were fixed with 2% freshly prepared paraformaldehyde in 0.1 M sodium cacodylate, pH 7.4, for 1 h at 4°C. Monolayers were rinsed in cacodylate buffer, postfixed in 1% OsO4 for 1 h, dehydrated through graded alcohols, and embedded in epoxy resin. Oriented 1-mm sections were obtained with diamond knives, and multiple areas were thin sectioned. Ultrathin sections were examined by using a JEOL 1200 EXII electron microscope. The numbers of adherent bacteria observed per 50 epithelial cells were counted in random sections.
Cytokine production.
In order to characterize the secretion of IL-1β and TNF-α, these cytokines were assayed in triplicate in the lower reservoirs by ELISA. ELISA was carried out with a monoclonal antibody to TNF-α or IL-1β and phosphatase-conjugated goat anti-TNF-α or anti-IL-1β polyclonal antibodies (26).
Western blotting, biotinylation, and Northern blotting for DAF and MCP.
Monolayers of T84 cells were washed first in HBSS (Sigma) and then in lysis buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 2 mM EDTA, 1% NP-40, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, 25 μM leupeptin, 5 mM benzamidine, 1 μM pepstatin, 25 μM aprotinin, 50 mM sodium glycerophosphate, 20 mM sodium pyrophosphate, 0.5 mM dithiothreitol) at 4°C, at a density of 5 × 107 cells/ml. After sonication (2 pulses, each lasting 8 s), the lysates were centrifuged at 15,000 × g for 15 min at 4°C and were denatured by boiling in reducing sodium dodecyl sulfate (SDS) sample buffer. Protein lysates (50 μg per sample) were analyzed by migration in an SDS-polyacrylamide gel electrophoresis gel and subsequently were electrophoretically transferred to a nitrocellulose membrane. The nitrocellulose membrane was incubated in saturation buffer and then probed with an anti-DAF antibody after being incubated overnight at 4°C. This labeling was visualized by peroxidase-conjugated secondary antibodies (anti-rabbit IgG) (1:3,000) and by enhanced chemiluminescence by use of the ECL kit. In some experiments, the T84 cells were pretreated with cycloheximide (Sigma) before the PMNL transepithelial migration.
For surface biotinylation, T84 cells grown on 5-cm2 permeable supports were cooled to 4°C and washed with cold HBSS. Apical or basolateral surfaces were selectively biotinylated by addition of 0.5 mg of biotin sulfo-N-hydroxysuccinimide ester. Then experiments involving anti-DAF and anti-MCP antibodies were conducted as previously described (21, 49).
Total cellular RNA (10 μg) was extracted from the cells by using Trizol reagent (Life Technologies) and reverse transcribed with avian myeloblastosis virus reverse transcriptase (Roche, Meylan, France) according to the manufacturer's instructions. PCR amplification of s26 consisted of 35 1-min denaturation cycles at 95°C, annealing for 50 s at 60°C, and extension at 72°C on the RoboCycler apparatus (Stratagene, Amsterdam, The Netherlands). The primer set was 5′-GCCACGTGCAGCCTATTCGC-3′ and 5′-GCACCCGCAGGTCTAAATCG-3′ (s26). PCR products (270 bp) were analyzed on a 1.5% agarose gel stained with ethidium bromide. Some of them were purified from the gel by using the Qiaquick gel extraction kit (Qiagen), radiolabeled, and used as probes for Northern blots.
Total RNA from T84 cells was prepared with Trizol (Life Technologies) according to the manufacturer's instructions. Total RNA (10 μg) was separated on a 1% agarose gel containing 40 mM morpholinepropanesulfonic acid (MOPS), 10 mM sodium acetate, 1 mM EDTA, and 1% formaldehyde and was then transferred to nylon membranes. After total RNA was fixed under calibrated UV irradiation, the membranes were hybridized with [32P]dATP-radiolabeled riboprobes (s26 or human DAF cDNA) by using a Strip-Ez RNA kit (Ambion) in Express-Hyb buffer (Clontech, Palo Alto, Calif.). After being washed, membranes were exposed to X-ray film (Hyperfilm; Amersham Pharmacia).
Data analysis.
Resistance time courses were compared by two-factor analysis of variance. Myeloperoxidase assays were compared by Student's t test. Values were expressed as means ± standard errors of the means from n experiments.
RESULTS
Adhesion of Afa/Dr DAEC strains to the T84-apical membrane induces PMNL transepithelial migration.
Before starting the transmigration assays, we checked to make sure that none of the Afa/Dr DAEC strains affected the transepithelial resistance of T84 cells (data not shown), as previously reported for Caco-2/TC7 cells (60). As shown in Table 1, the T84 monolayers were controlled for transepithelial migrations of PMNL in the physiologic basolateral-to-apical direction by the PMNL transmigration-inducing peptide f-MLP (10−7 M). Apical colonization of T84 monolayers by the wild-type Afa/Dr DAEC strains C1845 and IH11128 resulted in marked transepithelial migrations of PMNL in the physiologic basolateral-to-apical direction, which were, respectively, 72 and 76% of that induced by f-MLP (10−7 M). Interestingly, a similar increase in PMNL transepithelial migration was observed in T84 cells infected with the recombinant HB101-pSSS1 E. coli expressing the Afa/Dr F1845 adhesin, whereas E. coli K12-HB101, used as a control, did not stimulate any detectable transepithelial migration of PMNL. This finding suggests that the induced PMNL transepithelial migration follows the adhesin-receptor interaction.
TABLE 1.
Induction of PMNL transepithelial migration (basolateral to apical) follows apical colonization of T84 intestinal cells with Afa/Dr DAEC strains
| Strain or peptidea | Transmigrated PMNL equivalents (104)b
|
|
|---|---|---|
| Infected cells | Infected cells preincubated with anti-CD55 MAb (1H4) | |
| Wild-type C1845 | 15.3 ± 2.1 | 3.5 ± 1.1** |
| E. coli HB101-pSSS1 (F1845) | 12.4 ± 1* | 1.8 ± 1.2** |
| Wild-type IH11128 | 16.1 ± 0.5* | 2.8 ± 1** |
| E. coli HB101 | 2.1 ± 1.4 | 1.8 ± 1.2 |
| f-MLP (10−7 M) | 21.3 ± 1 | 22.4 ± 2.1* |
f-MLP, PMNL transmigration-inducing peptide.
Data are means ± standard deviations from triplicate samples. *, P < 0.01 compared with HB101; **, P < 0.01 compared with infected cells without anti-CD55 MAb 1H4.
DAF (CD55) is the intestinal brush border-associated receptor for Afa/Dr DAEC adhesins (6). In order to find out whether the mechanism of Afa/Dr DAEC-induced PMNL transepithelial migration involves Afa/Dr adhesion and DAF interaction, similar transmigration assays were conducted with T84 monolayers that had been preincubated with the anti-DAF antibody 1H4. As shown in Table 1, both the wild-type Afa/Dr DAEC strains and the recombinant E. coli strain HB101-pSSS1 expressing F1845 adhesin failed to induce PMNL transmigration across T84 monolayers after preincubation with this antibody. Two control experiments showed that (i) Afa/Dr DAEC-induced PMNL transmigration was not affected by preincubation with MAb MOPC21, an isotype-matched antibody control for MAb 1H4 [(16.4 ± 3) × 104 versus (17.5 ± 2.3) × 104 PMNL cell equivalents for C1845-infected cells without and with MAb MOPC21 pretreatment, respectively], and (ii) the PMNL transepithelial migration induced by f-MLP (10−7 M) was not reduced after preincubation of T84 monolayers with MAb 1H4 (Table 1).
Because several pathogenic bacteria elicit basolateral secretion of the PMNL chemokine IL-8 by epithelial cells (38, 48, 49), we looked to see whether Afa/Dr DAEC interaction with the apical pole of T84 monolayers could induce IL-8 secretion. Infection of polarized T84 cell monolayers by wild-type Afa/Dr DAEC strains or recombinant E coli pSSS1 expressing F1845 adhesin was accompanied by a 20-fold increase in the basolateral secretion of IL-8 over that by control T84 cells. Levels of IL-8 secretion, in nanograms per milliliter, were 0.15 ± 0.11 for control cells, 3.5 ± 1 for C1845-infected cells, 2.67 ± 1.2 for IH11128-infected cells, 3.8 ± 1.5 for HB101 pSSS1-infected cells, and 0.10 ± 0.20 for E. coli K12-HB101-infected cells; P < 0.01 for each of C1845-infected, IH11128-infected, and HB101 pSSS1-infected cells versus control cells).
Taken together, these data show that Afa/Dr DAEC infection of T84 monolayers is followed by production of IL-8 and PMNL transepithelial migration, a cell response which is controlled by the interaction of Afa/Dr adhesins with the brush border-associated DAF.
PMNL-induced transepithelial migration upregulates DAF expression in T84 cells.
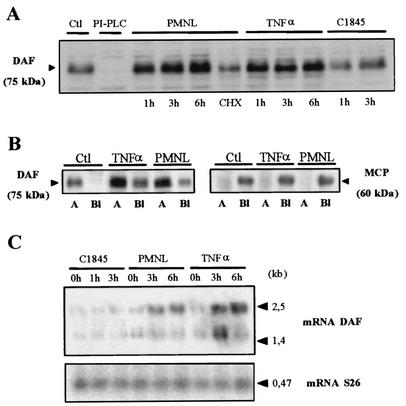
Recruitment of PMNL is a hallmark of inflammatory situations, in which expression of DAF has been shown to be increased (2, 3, 54, 66, 69). DAF upregulation was observed after PMNL transmigration in C1845-infected T84 cells (data not shown). In order to dissect the mechanism by which the DAF upregulation occurs, we conducted experiments to find out whether this phenomenon develops after Afa/Dr DAEC infection alone or whether it also requires PMNL-induced transmigration (Fig. 1). As a control, we compared the expression of DAF in T84 cells to that of the other complement-regulating protein, MCP. Western blot analysis (Fig. 1A) shows that no DAF upregulation was observed in T84 monolayers infected for 1 or 3 h with the Afa/Dr strain C1845. In contrast, DAF expression in T84 cells following f-MLP-induced PMNL transmigration was greater than that in control monolayers. This upregulation was detected after 1 h of transmigration and peaked after 6 h of transmigration. Cycloheximide pretreatment of T84 cells decreased the PMNL-induced upregulation of DAF.
FIG. 1.
DAF is upregulated in T84 cells after f-MLP-induced PMNL transepithelial migration. (A) Western blot analysis revealed that the expression of DAF (CD55) in T84 cells after f-MLP-induced PMNL transmigration was greater than that in control T84 monolayers (Ctl). This upregulation was detected after 1 h of migration and peaked after 6 h of migration. The increased expression at 6 h of PMNL transmigration was not observed if the monolayers had been preincubated with cycloheximide (CHX). TNF-α treatment induced a marked increase in DAF expression after incubation of epithelial cells for 1 to 6 h. DAF upregulation was not observed in T84 monolayers infected for 1 or 3 h with strain C1845. DAF was not observed on T84 cells exposed to PI-PLC, which breaks down the GPI anchor of DAF. (B) Biotinylation assays showed that DAF was expressed only at the apical side (lanes A), not at the basolateral side (lanes Bl); either PMNL transepithelial migration for 2 h or exposure to TNF-α (2 h) induced increased DAF expression at the apical side of the T84 cells (relative to expression by control cells). Under these conditions, DAF expression also developed at the basolateral side of the T84 cells. MCP expression on T84 cells was used as a control: basolateral expression of MCP was not modified by TNF-α or PMNL transepithelial migration, and no MCP expression appeared at the apical side of the cells after PMNL transepithelial migration or TNF-α treatment (results of one of three experiments are shown). (C) DAF mRNA levels were increased in T84 cells after PMNL transmigration (3 or 6 h) or TNF-α treatment (3 or 6 h) but not in T84 monolayers infected for 1 or 3 h with strain C1845. In all panels, micrographs are representative of three to four experiments.
As a result of polarization, the intestinal epithelial T84 cells are organized to form two distinct domains, the apical and basolateral domains, which are separated by a junctional domain. We carried out biotinylation assays to analyze the levels of DAF expression in the apical and basolateral domains. Figure 1B shows that DAF was expressed only at the apical side of the control monolayers. DAF expression was markedly modified after f-MLP-induced PMNL transmigration. In the apical domain, DAF expression was increased, and DAF was now also found to be expressed at the opposite cell membrane domain of the T84 cells, i.e., in the basolateral domain. In contrast, the basolateral expression of MCP found in T84 cells after f-MLP-induced PMNL transmigration was the same as in control cells. Moreover, in T84 cells after f-MLP-induced PMNL transmigration, no MCP was found at the opposite cell membrane domain, i.e., in the apical domain.
DAF mRNA levels were increased in T84 cells following f-MLP-induced PMNL transmigration (Fig. 1C). This upregulation was observed after 1 h (data not shown) but was greater after 3 or 6 h. DAF mRNA levels were not increased in T84 monolayers infected for 1 or 3 h with the Afa/Dr DAEC strain C1845 (Fig. 1C). Conversely, MCP mRNA levels were not modified in T84 cells following f-MLP-induced PMNL transmigration (data not shown).
These data combined reveal that the upregulation of DAF in T84 cell monolayers results from PMNL transepithelial migration, a phenomenon that is promoted by Afa/Dr DAEC infection.
DAF upregulation is linked to TNF-α and/or IL-1β production by T84 cells.
It has been reported previously that various cytokines upregulate DAF expression in intestinal epithelial cells (3, 54, 69). Consistent with these reports, we show here that TNF-α induced a time-dependent increase in DAF expression in the apical domain of T84 cells (Fig. 1A and B). Similar results were obtained after pretreatment of T84 cells with IL-1β (data not shown). As reported above for f-MLP-induced PMNL transmigration, after treatment with TNF-α, DAF expression was promoted at the opposite cell membrane domain of T84 cells, i.e., the basolateral domain. In contrast, the basolateral expression of MCP was unchanged after TNF-α treatment, and no expression of MCP was observed at the opposite cell membrane domain, i.e., the apical domain (Fig. 1B). DAF mRNA levels were increased time dependently in T84 cells following TNF-α treatment (Fig. 1C), whereas MCP mRNA levels were not (data not shown).
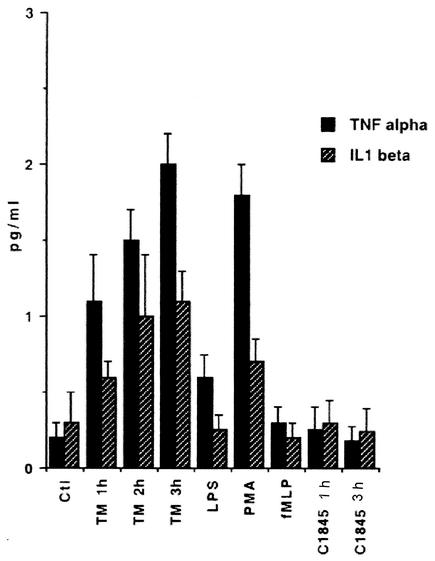
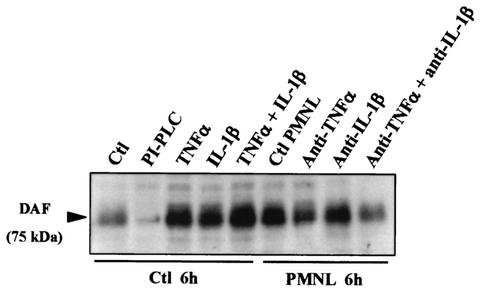
We used an ELISA to examine the production of TNF-α and IL-1β in T84 cell monolayers before and after f-MLP-induced PMNL transepithelial migration or Afa/Dr DAEC infection. As shown in Fig. 2, f-MLP-induced PMNL transepithelial migration promoted time-dependent increases in TNF-α and IL-1β production. No production of these cytokines was observed in f-MLP-treated T84 cell monolayers (Fig. 2) or in monolayers incubated in the presence of unstimulated PMNL (data not shown). Interestingly, no increase in TNF-α or IL-1β production was observed in T84 cell monolayers infected for 1 or 3 h with the Afa/Dr DAEC strain C1845 (Fig. 2). Furthermore, Western blot analysis of DAF expression in T84 cells (Fig. 3), shows that the DAF upregulation observed after f-MLP-induced PMNL transepithelial migration or after TNF-α and/or IL-1β treatment was not observed after the cells were incubated with a combination of anti-IL-1β and anti-TNF-α MAbs.
FIG. 2.
TNF-α and IL-1β production during the time-course of f-MLP-induced PMNL transepithelial migration (TM) in T84 cells. Cytokine production was measured by ELISAs. f-MLP-treated T84 cells or T84 cell monolayers infected for 1 or 3 h with strain C1845 served as negative controls. T84 cells treated with LPS or phorbol myristate acetate (PMA) served as positive controls (n = 4).
FIG. 3.
DAF upregulation observed in T84 monolayers during f-MLP-induced PMNL transmigration is linked to TNF-α and IL-1β production. DAF expression in T84 cells was analyzed by Western blotting after incubation of cells with or without MAbs against TNF-α and/or IL-1β added to the lower reservoir and subsequent PMNL transepithelial migration for 6 h. DAF upregulation observed after f-MLP-induced PMNL transepithelial migration or incubation of epithelial cells with TNF-α and/or IL-1β was decreased after treatment of T84 monolayers with either the anti-IL-1β or the anti-TNF-α MAb alone and was further decreased when the two MAbs were combined. In control (Ctl) cells, exposure to PI-PLC, which breaks down the GPI anchor of DAF, caused DAF to disappear. Micrographs are representative of three to four experiments.
Taken together, these results indicate that Afa/Dr DAEC infection of T84 cells is followed by a cascade of cell responses, including PMNL transepithelial migration, that in turn lead to production of TNF-α and IL-1β, two cytokines which upregulate the expression of DAF, the receptor for Afa/Dr adhesins.
The ability of Afa/Dr DAEC strains to adhere to T84 cell monolayers increases after PMNL transepithelial migration.
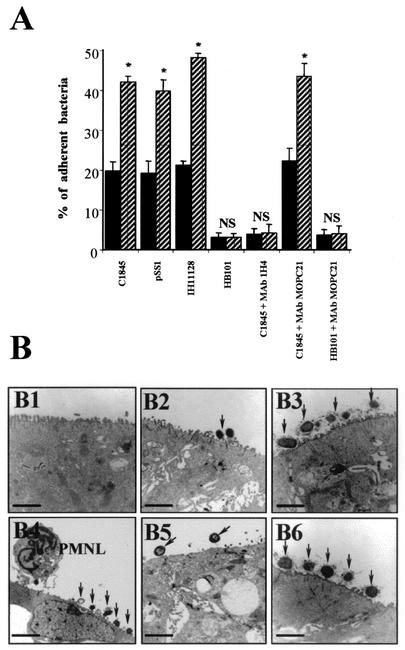
As shown in Fig. 4A, the percentage of Afa/Dr DAEC bacteria attached to epithelial cells was significantly higher after f-MLP-induced PMNL transepithelial migration than in control Afa/Dr DAEC-infected cells. Similar results were obtained for the wild-type Afa/Dr DAEC strains IH11128 and C1845 and for the recombinant strain HB101-pSSS1 expressing F1845 adhesin. Under all experimental conditions, a highly significant decrease in bacterial adhesion was observed after the cells had been apically exposed to the anti-DAF MAb 1H4. In contrast, no decrease in bacterial adhesion was observed after preincubation of T84 cells with the control IgG1 isotype MAb MOPC21. In a control experiment, preincubation of T84 cells with MAb 1H4 without and with PMNL transmigration did not modify the adhesion of the weakly adhering E. coli strain K12-HB101.
FIG. 4.
Quantification and observation by transmission electron microscopy of the attachment of Afa/Dr DAEC strains to T84 monolayers in the absence of or after PMNL transepithelial migration. (A) Cell-attached bacteria were distinguished from those that had entered the cells by treatment with gentamicin as described in Materials and Methods. T84 cell monolayers were incubated without or with wild-type Afa/Dr DAEC strain C1845 or IH11128 or with recombinant E. coli HB101-pSSS1 harboring Afa/Dr F1845 adhesin. E. coli K12-HB101 served as a negative control. The involvement of apical membrane-bound DAF in Afa/Dr adhesin attachment was investigated by using the anti-DAF MAb 1H4 and the isotype-matched control MAb MOPC21. Solid bars, bacterial attachment in control T84 monolayers after incubation with the bacteria for 2 h. Hatched bars, bacterial attachment after a 2-h incubation of T84 monolayers with the bacteria preceded by PMNL transmigration for 2 h. Data are expressed as the percentages of the inoculum that were associated with the cells. Data are means ± standard deviations from triplicate experiments. NS, not significantly different; ∗, P < 0.01. (B) Electron micrographs showing attachment of Afa/Dr DAEC bacteria to T84 monolayers in the absence of or after PMNL transepithelial migration. (B1) Control T84 monolayers; (B2) T84 monolayers incubated with IH11128 bacteria for 2 h; (B3) T84 monolayers incubated with IH11128 bacteria for 2 h followed by PMNL transepithelial migration for 2 h; (B4) T84 monolayers incubated with C1845 bacteria for 2 h followed by PMNL transepithelial migration for 2 h; (B5) C1845 bacteria adhering to T84 cells that had been exposed to MAb 1H4 before PMNL transepithelial migration for 2 h; (B6) C1845 bacteria adhering to T84 cells that had been exposed to MAb MOPC21 before PMNL transepithelial migration for 2 h. Arrows point to Afa/Dr DAEC bacteria adhering to the apical pole of the epithelial cells. Micrographs are representative of three experiments. Bars, 25 μm for panels B1 and B4; 10 μm for panels B2, B3, B5, and B6.
Afa/Dr DAEC bacteria adhering to epithelial T84 cells with and without PMNL transmigration were examined in electron microscopy experiments (Fig. 4B). Control monolayers incubated for 2 h with IH11128 bacteria showed bacteria adhering to the brush border present on the apical side of polarized T84 cells (Fig. 4B2). Consistent with the results reported above, the numbers of Afa/Dr DAEC strain IH11128 (Dr hemagglutinin) and C1845 (F1845 adhesin) bacteria attached to the brush border of the cells were dramatically increased after 2 h of PMNL transmigration (Fig. 4B3 and 4B4, respectively). A few Afa/Dr bacteria were observed adhering to the membranes of the PMNL cells still present on the apical side of the T84 monolayers (Fig. 4B4). Moreover, despite the expression of DAF at the basal domain of monolayers after PMNL transmigration, no Afa/Dr DAEC bacteria were attached to the basolateral side of the epithelial cells (data not shown). The increase in bacterial adhesion was markedly reduced when epithelial cells were apically preincubated with MAb 1H4 (Fig. 4B5). In contrast, preincubation of T84 monolayers with the control IgG1 isotype MAb MOPC21 had no effect on the increase in Afa/Dr DAEC C1845 adhesion induced by PMNL transepithelial migration (Fig. 4B6).
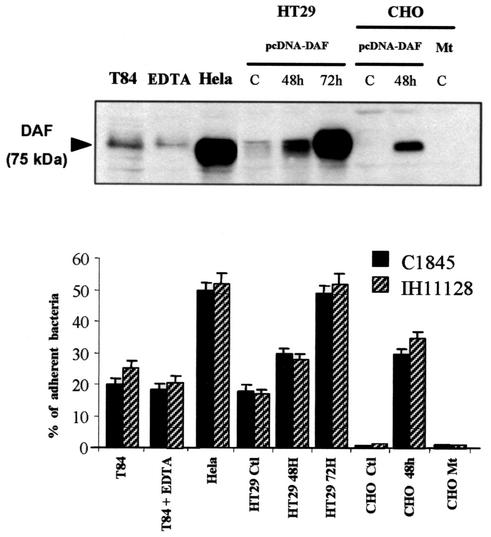
We next carried out experiments with epithelial cells to demonstrate that the level of DAF expression directly controls the level of Afa/Dr DAEC adhesion. For this purpose, we used a series of cells with a range of levels of DAF expression or which did not express DAF (Fig. 5). We used CHO and stably transfected CHO-DAF cells. In agreement with a previous report (55), no Afa/Dr DAEC binding was found in CHO cells that did not express DAF, and transfection of pcDNA-DAF onto CHO cells was shown to permit Afa/Dr DAEC binding. Adhesion disappeared in CHO cells transfected with a mutated pcDNA-DAF. We next used several epithelial cell lines constitutively expressing different levels of DAF. Levels of DAF expression were determined by Western blotting (Fig. 5, top). As shown in the bar graph in Fig. 5 (bottom), the levels of adhering Afa/Dr DAEC bacteria (strains IH11128 and C1845) associated with T84 and HeLa epithelial cells correlated with the levels of DAF expressed by these cell lines. Indeed, binding was higher in HeLa cells, in which a high level of DAF was observed, than in T84 cells. Interestingly, transfection of pcDNA-DAF into HT-29 cells, which constitutively express a low level of DAF, promoted increased DAF expression as a function of the posttransfection culture time, which fits well with the increase in Afa/Dr DAEC binding. Finally, a control experiment using EDTA to open the tight junctions was conducted. As shown in Fig. 5 (bottom), no increase in Afa/Dr DAEC binding was observed in such EDTA-treated T84 cell monolayers, indicating that increased attachment of Afa/Dr DAEC after PMNL migration was not due to basolateral access of the bacteria.
FIG. 5.
Adhesion of Afa/Dr DAEC strains to various epithelial cell lines is correlated with the level of DAF expression. (Top) Different levels of DAF expression in T84 (untreated or treated with EDTA), HeLa, HT-29, and CHO cell lines were analyzed by Western blotting. HT-29 cells, expressing DAF at a low level, and CHO cells, which did not express DAF, were first transfected with pcDNA-DAF and then subcultured at different times posttransfection for DAF expression. C, control; CHO Mt, CHO mutant without GPI expression. The immunoblot is representative of three experiments. (Bottom) Percentages of Afa/Dr DAEC strains (C1845 or IH11128 bacteria) adhering to the epithelial T84, HeLa, and HT29 cells after 6 h of incubation were strongly correlated with DAF levels. EDTA treatment of T84 monolayers before incubation with bacteria did not modify the percentage of adherent bacteria in comparison with that for control monolayers. In CHO cells, transfection of cDNA-DAF promoted Afa/Dr DAEC bacterial adhesion, a phenomenon abolished in CHO cells without GPI expression (CHO Mt). E. coli K12-HB101 was used as a control, and no binding was found in any of the cell lines used (data not shown). Data are expressed as the percentages of the inoculum that were associated with the cells. Data are means ± standard deviations from triplicate experiments.
DISCUSSION
Bacterial pathogens have developed many sophisticated ways of infecting their hosts and causing disease. In particular, bacterial pathogens subvert functional membrane-bound proteins as receptors for colonizing epithelia and exploit cell signaling pathways to cross talk with the host cells. Taken together, the results presented here indicate that wild-type Afa/Dr DAEC strains expressing adhesins of the Afa/Dr family (57) are able to induce a cascade of responses in T84 cells. These responses include IL-8 production and PMNL transepithelial migration. Production of TNF-α and IL-1β follows PMNL migration, and these two cytokines upregulate the expression of DAF, the receptor for the Afa/Dr adhesins, thus increasing the brush border adhesion of Afa/Dr DAEC bacteria. Considering that Afa/Dr DAEC adhesins are known to recognize DAF as a receptor (55), the results obtained with the recombinant E. coli strain containing the gene coding for the Afa/Dr F1845 adhesin and the anti-DAF MAb demonstrate that the cascade of cellular events following Afa/Dr DAEC infection in the presence of PMNL follows the interaction of Afa/Dr adhesins with their brush border-associated receptor, DAF. Moreover, we have provided evidence that the upregulation of DAF is the consequence of the induced PMNL migration, since Afa/Dr DAEC infection in the absence of PMNL failed to promote the production of TNF-α and IL-1β or the upregulation of DAF.
Transepithelial migration of PMNL across colonic intestinal cell monolayers has been reported for enteropathogenic E. coli (EPEC) (64), Salmonella enterica serovar Typhimurium (46), Helicobacter pylori (39), and Shigella flexneri (51). Production of IL-8, resulting from the activation of mitogen-activated protein kinase signaling, generally accompanied the infection of T84 cell monolayers by bacterial pathogens (4, 19, 20, 63, 65, 70). However, the mechanism by which bacterial pathogens elicit PMNL transepithelial migration appears to be different for each pathogen. For example, cadaverine treatment specifically inhibited S. flexneri induction of PMNL transepithelial migration but had no effect on the ability of Salmonella or EPEC to induce PMNL migration (47). Neutralization of IL-8 failed to inhibit Salmonella-elicited PMNL transmigration, suggesting that following Salmonella infection, IL-8 acts in concert with a transcellular chemotactic factor(s) which directs PMNL migration across the intestinal epithelium (46). It has been established that the primary role for basolateral secretion of IL-8 by the intestinal epithelium is recruitment of PMNL through the matrix to the subepithelial space rather than direction of the final movement of PMNL across the epithelium (48). Indeed, an array of chemokines secreted in a polarized manner directs PMNL movement. Notably, IL-8, secreted basolaterally, directs PMNL through the lamina propria, whereas pathogen-elicited epithelial chemoattractant (PEEC), secreted apically, directs PMNL migration across the epithelial monolayer to the intestinal lumen (50). Moreover, it has been demonstrated that distinct signaling pathways mediate induction of IL-8 secretion and induction of PEEC secretion by Salmonella serovar Typhimurium (28). It remains to be determined whether or not the PMNL migration observed after Afa/Dr DAEC infection in T84 cell monolayers requires PEEC, as in Salmonella infection. Interestingly, it has been reported that in human uroepithelial cell layers in which addition of recombinant IL-8 to unstimulated cell layers was sufficient to induce PMNL migration, an anti-IL-8 antibody and antibodies against IL-8 receptor A (IL-8RA) reduced E. coli-induced neutrophil migration, indicating a central role for this chemokine in the E. coli-induced migration process (30).
We found that following PMNL transepithelial migration, GPI-anchored DAF expression also develops at the basolateral domain of polarized intestinal T84 cells. Under physiological conditions, DAF is strikingly expressed at the brush border in polarized intestinal cells (5, 6). The newly developed basolateral expression of DAF in T84 cells observed after PMNL transepithelial migration could be of interest in terms of the proinflammatory situation. Indeed, DAF has recently been identified as a cellular ligand of CD97, a member of the epidermal growth factor-TM7 family of class II seven-span transmembrane receptors (24). CD97 receptors have been found on leukocytes, macrophages, numerous hematopoietic and nonhematopoietic cells, and gastrointestinal tract cancer cells (23, 32, 40). Moreover, upregulated expression of CD97 has been found at the site of inflammatory reaction in epithelial tumors (24). The DAF abnormally expressed in the basolateral domain of T84 cells could serve as a receptor for activated PMNL expressing the CD97 molecule (23). Further experiments would be required to investigate the possible role of DAF-CD97 interaction in Afa/Dr DAEC-induced PMNL transepithelial migration.
Production of cytokines could be a generalized mechanism used by bacterial pathogens to initiate diarrhea or could reflect a stereotyped innate host response to bacterial infection. For example, EPEC infection is accompanied by production of proinflammatory cytokines and promotes transmigration of PMNL (75). These cellular responses cause considerable tissue damage, which in turn contributes to inducing diarrhea. The observation that the induced PMNL transepithelial migration in T84 cells promotes production of TNF-α and IL-1β could be of interest in terms of bacterium-induced functional lesions. The effects of IL-1β on intestinal functions have been reported elsewhere; for example, IL-1β stimulates anion secretion (18), whereas IL-1α inhibits Na+ and Cl− absorption and stimulates SGLT1-dependent glucose transport (18, 33). This implies that the production of proinflammatory ILs, such as IL-1β, could contribute to physiological changes in response to the inflammatory process, for example, during Afa/Dr DAEC-induced diarrhea.
It has been reported previously that infection of cultured human intestinal Caco-2/TC7 cells by Afa/Dr DAEC strains is followed by an apical downregulation of DAF expression accompanied by recruitment of the GPI-anchored protein around the adhering bacteria (31). Here, we found that the DAF expressed at the apical membrane of polarized T84 cells was upregulated after PMNL transepithelial migration, which is promoted by Afa/Dr DAEC infection. This upregulation was found to be time dependent and to be associated with an increased level of RNA DAF in T84 cells. Finally, we found that the upregulation of DAF was linked to enhanced cytokine production. This led us to suggest that one of the consequences of the PMNL influx observed in patients with IBD may be induction of DAF overexpression in colonocytes. Host tissues are protected from damage by autologous complement activation through the activity of several cell-associated complement-regulatory proteins such as DAF, MCP, and protectin (CD59) (12, 43). One of these molecules, DAF, is a 70- to 80-kDa glycoprotein anchored to the cell membrane by a GPI linkage which protects host tissues from autologous complement activation by preventing the assembly of C3 convertases, as well as by dissociating deposited C3 convertases. DAF is expressed on the plasma membranes of all cell types, and notably on epithelial cells derived from various tissues lining the extracellular compartments. The fact that DAF is localized on the apical surfaces of polarized intestinal epithelial cells (5, 7) suggests that it may play an important role in protecting epithelial cells from activation of the autologous complement cascade at the luminal surface. DAF expression in colonic epithelial cells is increased in patients with IBD, but the significance and consequence of this enhanced expression are unknown (74). During IBD, several proinflammatory cytokines may act as inducers of DAF upregulation on colonic epithelial cells. Thus, DAF production in intestinal epithelial cells can be independently upregulated by TNF-α, IL-1β, IL-6, IL-4, and/or gamma interferon (2, 3, 66).
Initiation of IBD is an extremely complex chain of events that involves host genetic elements and physiology in addition to multiple factors related to the nature of the gut microflora and/or to the emergence of “silent pathogenic E. coli ” bacteria that are repressed when the gut microflora is functioning normally. PMNL transepithelial migration is a major part of the epithelial defense in inflammatory and infectious diseases involving mucosal surfaces. Histological observation of mucosal preparations reveals the accumulation of neutrophil exudates within dilated crypts. PMNL infiltration is a common hallmark of several large-bowel diseases, such as ulcerative colitis, CD, bacterial colitis, drug-induced colitis, and ischemia injury. During inflammatory states, ILs regulate the intensity of the intestinal immune response either directly or via the production of additional effector molecules. Bacteria, such as the pathogenic E. coli strains present in the colon, may play a pivotal role in the pathogenesis of IBD (14, 15, 34). In this regard, a previous study has shown that E. coli antibody titers are higher in patients with CD than in controls (71). Moreover, immunocytochemistry assays have revealed the presence of E. coli antigens in most intestinal resection specimens from patients with CD (17). It was recently shown that some E. coli strains are abnormally predominant in early and chronic ileal lesions observed in CD (10, 11, 22, 45) and that E. coli from IBD patients shows more ability to adhere to epithelia (14, 68). However, data from studies on adherence are inconsistent (13, 34). Finally, a new E. coli strain, named LF82, isolated from the ileal mucosa of a patient with CD showed strong invasive ability in vitro (10, 11) and was able to survive and replicate within macrophages (29). Despite the fact that very few daac-positive DAEC strains have been found in intestinal resection specimens from patients with CD (22), we can speculate, based on the findings reported here, that during the acute phase of IBD, the PMNL-epithelial cell interaction may increase intestinal colonization by Afa/Dr DAEC strains, leading to structural and functional lesions of the brush border.
The results reported here provide new insights into the mechanism of Afa/Dr DAEC pathogenicity, and they confirm and extend previous observations suggesting that these bacteria are subversive pathogens (6, 59-62). The prototype of subversive bacterial pathogens is EPEC, which colonizes the intestinal epithelium by means of a type III secretion system that forms a pore in the infected cells, permitting direct translocation of bacterial proteins including the bacterium's own receptor, the translocated intimin receptor (Tir) (75). The observation that the PMNL transmigration process promotes apical upregulation of the brush border receptor for the Afa/Dr adhesins, DAF, is interesting in terms of microbial pathogenicity. Indeed, modification of the expression of a membrane-bound bacterial receptor should be considered a potential new mechanism of bacterial pathogenicity. It has previously been reported that Neisseria gonorrhoeae upregulates the opacity-associated (Opa) protein adhesin receptor, the carcinoembryonic antigen-related cellular adhesion molecule (CEACAM1, or CD66a; previously known as C-CAM1, or biliary glycoprotein [BGP]) (53). This process, which occurs through an LPS-dependent mechanism involving NF-κB-dependent signaling by Toll-like receptor 4, may be a general mechanism used by bacteria releasing LPS. Here, we report that as a result of Afa/Dr adhesin-DAF interaction, the induced transmigration of PMNL and the associated production of proinflammatory cytokines in intestinal cells promote upregulation of the Afa/Dr adhesin receptor. As far as we are aware, this process is the first example of a bacterial pathogen's ability to autoinduce the expression of its host's cellular receptor by a specific mechanism involving adhesin-receptor interaction.
Acknowledgments
We thank Mireille Mari, Bernard Ferrua, and Antoine Galmiche for excellent technical assistance.
F. Bétis and P. Brest contributed equally to this work.
Editor: V. J. DiRita
REFERENCES
- 1.Abrami, L., M. Fivaz, T. Kobayashi, T. Kinoshita, R. G. Parton, and F. G. van der Goot. 2001. Cross-talk between caveolae and glycosylphosphatidylinositol-rich domains. J. Biol. Chem. 276:30729-30736. [DOI] [PubMed] [Google Scholar]
- 2.Andoh, A., Y. Fujiyama, K. Sumiyoshi, H. Sakumoto, and T. Bamba. 1996. Interleukin 4 acts as an inducer of decay-accelerating factor gene expression in human intestinal epithelial cells. Gastroenterology 111:911-918. [DOI] [PubMed] [Google Scholar]
- 3.Andoh, A., Y. Fujiyama, K. Sumiyoshi, H. Sakumoto, H. Okabe, and T. Bamba. 1997. Tumour necrosis factor-alpha up-regulates decay-accelerating factor gene expression in human intestinal epithelial cells. Immunology 90:358-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berin, M. C., A. Darfeuille-Michaud, L. J. Egan, Y. Miyamoto, and M. F. Kagnoff. 2002. Role of EHEC O157:H7 virulence factors in the activation of intestinal epithelial cell NF-κB and MAP kinase pathways and the upregulated expression of interleukin 8. Cell. Microbiol. 4:635-648. [DOI] [PubMed] [Google Scholar]
- 5.Bernet-Camard, M. F., M. H. Coconnier, S. Hudault, and A. L. Servin. 1996. Differential expression of complement proteins and regulatory decay accelerating factor in relation to differentiation of cultured human colon adenocarcinoma cell lines. Gut 38:248-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernet-Camard, M. F., M. H. Coconnier, S. Hudault, and A. L. Servin. 1996. Pathogenicity of the diffusely adhering strain Escherichia coli C1845: F1845 adhesin-decay accelerating factor interaction, brush border microvillus injury, and actin disassembly in cultured human intestinal epithelial cells. Infect. Immun. 64:1918-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berstad, A. E., and P. Brandtzaeg. 1998. Expression of cell membrane complement regulatory glycoproteins along the normal and diseased human gastrointestinal tract. Gut 42:522-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bilge, S. S., C. R. Clausen, W. Lau, and S. L. Moseley. 1989. Molecular characterization of a fimbrial adhesin, F1845, mediating diffuse adherence of diarrhea-associated Escherichia coli to HEp-2 cells. J. Bacteriol. 171:4281-4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bjorge, L., T. S. Jensen, and R. Matre. 1996. Characterisation of the complement-regulatory proteins decay-accelerating factor (DAF, CD55) and membrane cofactor protein (MCP, CD46) on a human colonic adenocarcinoma cell line. Cancer Immunol. Immunother. 42:185-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boudeau, J., N. Barnich, and A. Darfeuille-Michaud. 2001. Type 1 pili-mediated adherence of Escherichia coli strain LF82 isolated from Crohn's disease is involved in bacterial invasion of intestinal epithelial cells. Mol. Microbiol. 39:1272-1284. [DOI] [PubMed] [Google Scholar]
- 11.Boudeau, J., A. L. Glasser, E. Masseret, B. Joly, and A. Darfeuille-Michaud. 1999. Invasive ability of an Escherichia coli strain isolated from the ileal mucosa of a patient with Crohn's disease. Infect. Immun. 67:4499-4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brodbeck, W. G., L. Kuttner-Kondo, C. Mold, and M. E. Medof. 2000. Structure/function studies of human decay-accelerating factor. Immunology 101:104-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brook, M. G., H. R. Smith, B. A. Bannister, M. McConnell, H. Chart, S. M. Scotland, A. Sawyer, M. Smith, and B. Rowe. 1994. Prospective study of verocytotoxin-producing, enteroaggregative and diffusely adherent Escherichia coli in different diarrhoeal states. Epidemiol. Infect. 112:63-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burke, D. A., and A. T. Axon. 1988. Adhesive Escherichia coli in inflammatory bowel disease and infective diarrhoea. BMJ 297:102-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burke, D. A., and A. T. Axon. 1988. Hydrophobic adhesin of E. coli in ulcerative colitis. Gut 29:41-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campieri, M., and P. Gionchetti. 2001. Bacteria as the cause of ulcerative colitis. Gut 48:132-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cartun, R. W., H. J. Van Kruiningen, C. A. Pedersen, and M. M. Berman. 1993. An immunocytochemical search for infectious agents in Crohn's disease. Mod. Pathol. 6:212-219. [PubMed] [Google Scholar]
- 18.Chang, E. B., M. W. Musch, and L. Mayer. 1990. Interleukins 1 and 3 stimulate anion secretion in chicken intestine. Gastroenterology 98:1518-1524. [DOI] [PubMed] [Google Scholar]
- 19.Czerucka, D., S. Dahan, B. Mograbi, B. Rossi, and P. Rampal. 2001. Implication of mitogen-activated protein kinases in T84 cell responses to enteropathogenic Escherichia coli infection. Infect. Immun. 69:1298-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dahan, S., V. Busuttil, V. Imbert, J. F. Peyron, P. Rampal, and D. Czerucka. 2002. Enterohemorrhagic Escherichia coli infection induces interleukin-8 production via activation of mitogen-activated protein kinases and the transcription factors NF-κB and AP-1 in T84 cells. Infect. Immun. 70:2304-2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D'Andrea, L., C. Lytle, J. B. Matthews, P. Hofman, B. Forbush III, and J. L. Madara. 1996. Na:K:2Cl cotransporter (NKCC) of intestinal epithelial cells. Surface expression in response to cAMP. J. Biol. Chem. 271:28969-28976. [DOI] [PubMed] [Google Scholar]
- 22.Darfeuille-Michaud, A., C. Neut, N. Barnich, E. Lederman, P. Di Martino, P. Desreumaux, L. Gambiez, B. Joly, A. Cortot, and J. F. Colombel. 1998. Presence of adherent Escherichia coli strains in ileal mucosa of patients with Crohn's disease. Gastroenterology 115:1405-1413. [DOI] [PubMed] [Google Scholar]
- 23.Eichler, W. 2000. CD97 isoform expression in leukocytes. J. Leukoc. Biol. 68:561-567. [PubMed] [Google Scholar]
- 24.Eichler, W., J. Hamann, and G. Aust. 1997. Expression characteristics of the human CD97 antigen. Tissue Antigens 50:429-438. [DOI] [PubMed] [Google Scholar]
- 25.Elson, C. O., R. B. Sartor, G. S. Tennyson, and R. H. Riddell. 1995. Experimental models of inflammatory bowel disease. Gastroenterology 109:1344-1367. [DOI] [PubMed] [Google Scholar]
- 26.Ferrua, B., P. Becker, L. Schaffar, A. Shaw, and M. Fehlmann. 1988. Detection of human IL-1α and IL-1β at the subpicomolar level by colorimetric sandwich enzyme immunoassay. J. Immunol. Methods 114:41-48. [DOI] [PubMed] [Google Scholar]
- 27.French, N., and S. Pettersson. 2000. Microbe-host interactions in the alimentary tract: the gateway to understanding inflammatory bowel disease. Gut 47:162-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gewirtz, A. T., A. M. Siber, J. L. Madara, and B. A. McCormick. 1999. Orchestration of neutrophil movement by intestinal epithelial cells in response to Salmonella typhimurium can be uncoupled from bacterial internalization. Infect. Immun. 67:608-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glasser, A. L., J. Boudeau, N. Barnich, M. H. Perruchot, J. F. Colombel, and A. Darfeuille-Michaud. 2001. Adherent invasive Escherichia coli strains from patients with Crohn's disease survive and replicate within macrophages without inducing host cell death. Infect. Immun. 69:5529-5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Godaly, G., A. E. Proudfoot, R. E. Offord, C. Svanborg, and W. W. Agace. 1997. Role of epithelial interleukin-8 (IL-8) and neutrophil IL-8 receptor A in Escherichia coli-induced transuroepithelial neutrophil migration. Infect. Immun. 65:3451-3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guignot, J., I. Peiffer, M. F. Bernet-Camard, D. M. Lublin, C. Carnoy, S. L. Moseley, and A. L. Servin. 2000. Recruitment of CD55 and CD66e brush border-associated glycosylphosphatidylinositol-anchored proteins by members of the Afa/Dr diffusely adhering family of Escherichia coli that infect the human polarized intestinal Caco-2/TC7 cells. Infect. Immun. 68:3554-3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamann, J., C. Stortelers, E. Kiss-Toth, B. Vogel, W. Eichler, and R. A. van Lier. 1998. Characterization of the CD55 (DAF)-binding site on the seven-span transmembrane receptor CD97. Eur. J. Immunol. 28:1701-1707. [DOI] [PubMed] [Google Scholar]
- 33.Hardin, J., K. Kroeker, B. Chung, and D. G. Gall. 2000. Effect of proinflammatory interleukins on jejunal nutrient transport. Gut 47:184-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hartley, M. G., M. J. Hudson, E. T. Swarbrick, A. E. Gent, M. D. Hellier, and R. H. Grace. 1993. Adhesive and hydrophobic properties of Escherichia coli from the rectal mucosa of patients with ulcerative colitis. Gut 34:63-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hofman, P., L. D'Andrea, D. Carnes, S. P. Colgan, and J. L. Madara. 1996. Intestinal epithelial cytoskeleton selectively constrains lumen-to-tissue migration of neutrophils. Am. J. Physiol. 271:C312-C320. [DOI] [PubMed] [Google Scholar]
- 36.Hofman, P., G. Flatau, E. Selva, M. Gauthier, G. Le Negrate, C. Fiorentini, B. Rossi, and P. Boquet. 1998. Escherichia coli cytotoxic necrotizing factor 1 effaces microvilli and decreases transmigration of polymorphonuclear leukocytes in intestinal T84 epithelial cell monolayers. Infect. Immun. 66:2494-2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hofman, P., B. L. Hsi, S. Manie, P. Fenichel, A. Thyss, and B. Rossi. 1994. High expression of the antigen recognized by the monoclonal antibody GB24 on human breast carcinomas: a preventive mechanism of malignant tumor cells against complement attack? Breast Cancer Res. Treat. 32:213-219. [DOI] [PubMed] [Google Scholar]
- 38.Hofman, P., G. Le Negrate, B. Mograbi, V. Hofman, P. Brest, A. Alliana-Schmid, G. Flatau, P. Boquet, and B. Rossi. 2000. Escherichia coli cytotoxic necrotizing factor-1 (CNF-1) increases the adherence to epithelia and the oxidative burst of human polymorphonuclear leukocytes but decreases bacteria phagocytosis. J. Leukoc. Biol. 68:522-528. [PubMed] [Google Scholar]
- 39.Hofman, V., V. Ricci, A. Galmiche, P. Brest, P. Auberger, B. Rossi, P. Boquet, and P. Hofman. 2000. Effect of Helicobacter pylori on polymorphonuclear leukocyte migration across polarized T84 epithelial cell monolayers: role of vacuolating toxin VacA and cag pathogenicity island. Infect. Immun. 68:5225-5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jaspars, L. H., W. Vos, G. Aust, R. A. Van Lier, and J. Hamann. 2001. Tissue distribution of the human CD97 EGF-TM7 receptor. Tissue Antigens 57:325-331. [DOI] [PubMed] [Google Scholar]
- 41.Langman, M. J., and R. N. Allan. 1999. Escherichia coli for ulcerative colitis. Lancet 354:2000-2001. [DOI] [PubMed] [Google Scholar]
- 42.Le'Negrate, G., E. Selva, P. Auberger, B. Rossi, and P. Hofman. 2000. Sustained polymorphonuclear leukocyte transmigration induces apoptosis in T84 intestinal epithelial cells. J. Cell Biol. 150:1479-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liszewski, M. K., T. C. Farries, D. M. Lublin, I. A. Rooney, and J. P. Atkinson. 1996. Control of the complement system. Adv. Immunol. 61:201-283. [DOI] [PubMed] [Google Scholar]
- 44.MacDermott, R. P. 1999. Chemokines in the inflammatory bowel diseases. J. Clin. Immunol. 19:266-272. [DOI] [PubMed] [Google Scholar]
- 45.Masseret, E., J. Boudeau, J. F. Colombel, C. Neut, P. Desreumaux, B. Joly, A. Cortot, and A. Darfeuille-Michaud. 2001. Genetically related Escherichia coli strains associated with Crohn's disease. Gut 48:320-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McCormick, B. A., S. P. Colgan, C. Delp-Archer, S. I. Miller, and J. L. Madara. 1993. Salmonella typhimurium attachment to human intestinal epithelial monolayers: transcellular signalling to subepithelial neutrophils. J. Cell Biol. 123:895-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCormick, B. A., M. I. Fernandez, A. M. Siber, and A. T. Maurelli. 1999. Inhibition of Shigella flexneri-induced transepithelial migration of polymorphonuclear leucocytes by cadaverine. Cell. Microbiol. 1:143-155. [DOI] [PubMed] [Google Scholar]
- 48.McCormick, B. A., P. M. Hofman, J. Kim, D. K. Carnes, S. I. Miller, and J. L. Madara. 1995. Surface attachment of Salmonella typhimurium to intestinal epithelia imprints the subepithelial matrix with gradients chemotactic for neutrophils. J. Cell Biol. 131:1599-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCormick, B. A., A. Nusrat, C. A. Parkos, L. D'Andrea, P. M. Hofman, D. Carnes, T. W. Liang, and J. L. Madara. 1997. Unmasking of intestinal epithelial lateral membrane β1 integrin consequent to transepithelial neutrophil migration in vitro facilitates inv-mediated invasion by Yersinia pseudotuberculosis. Infect. Immun. 65:1414-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McCormick, B. A., C. A. Parkos, S. P. Colgan, D. K. Carnes, and J. L. Madara. 1998. Apical secretion of a pathogen-elicited epithelial chemoattractant activity in response to surface colonization of intestinal epithelia by Salmonella typhimurium. J. Immunol. 160:455-466. [PubMed] [Google Scholar]
- 51.McCormick, B. A., A. M. Siber, and A. T. Maurelli. 1998. Requirement of the Shigella flexneri virulence plasmid in the ability to induce trafficking of neutrophils across polarized monolayers of the intestinal epithelium. Infect. Immun. 66:4237-4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moutabarrik, A., I. Nakanishi, M. Namiki, T. Hara, M. Matsumoto, M. Ishibashi, A. Okuyama, D. Zaid, and T. Seya. 1993. Cytokine-mediated regulation of the surface expression of complement regulatory proteins, CD46(MCP), CD55(DAF), and CD59 on human vascular endothelial cells. Lymphokine Cytokine Res. 12:167-172. [PubMed] [Google Scholar]
- 53.Muenzner, P., M. Naumann, T. F. Meyer, and S. D. Gray-Owen. 2001. Pathogenic Neisseria trigger expression of their carcinoembryonic antigen-related cellular adhesion molecule 1 (CEACAM1; previously CD66a) receptor on primary endothelial cells by activating the immediate early response transcription factor, nuclear factor-κB. J. Biol. Chem. 276:24331-24340. [DOI] [PubMed] [Google Scholar]
- 54.Nasu, J., M. Mizuno, T. Uesu, K. Takeuchi, T. Inaba, S. Ohya, M. Kawada, K. Shimo, H. Okada, T. Fujita, and T. Tsuji. 1998. Cytokine-stimulated release of decay-accelerating factor (DAF;CD55) from HT-29 human intestinal epithelial cells. Clin. Exp. Immunol. 113:379-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nowicki, B., A. Hart, K. E. Coyne, D. M. Lublin, and S. Nowicki. 1993. Short consensus repeat-3 domain of recombinant decay-accelerating factor is recognized by Escherichia coli recombinant Dr adhesin in a model of a cell-cell interaction. J. Exp. Med. 178:2115-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nowicki, B., J. Moulds, R. Hull, and S. Hull. 1988. A hemagglutinin of uropathogenic Escherichia coli recognizes the Dr blood group antigen. Infect. Immun. 56:1057-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nowicki, B., R. Selvarangan, and S. Nowicki. 2001. Family of Escherichia coli Dr adhesins: decay-accelerating factor receptor recognition and invasiveness. J. Infect. Dis. 183(Suppl. 1):S24-S27. [DOI] [PubMed] [Google Scholar]
- 58.Parkos, C. A., S. P. Colgan, C. Delp, M. A. Arnaout, and J. L. Madara. 1992. Neutrophil migration across a cultured epithelial monolayer elicits a biphasic resistance response representing sequential effects on transcellular and paracellular pathways. J. Cell Biol. 117:757-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peiffer, I., M.-F. Bernet-Camard, M. Rousset, and A. L. Servin. 2001. Impairments in enzyme activity and biosynthesis of brush border-associated hydrolases in human intestinal Caco-2/TC7 cells infected by members of the Afa/Dr family of diffusely adhering Escherichia coli. Cell. Microbiol. 3:341-357. [DOI] [PubMed] [Google Scholar]
- 60.Peiffer, I., A. B. Blanc-Potard, M. F. Bernet-Camard, J. Guignot, A. Barbat, and A. L. Servin. 2000. Afa/Dr diffusely adhering Escherichia coli C1845 infection promotes selective injuries in the junctional domain of polarized human intestinal Caco-2/TC7 cells. Infect. Immun. 68:3431-3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peiffer, I., J. Guignot, A. Barbat, C. Carnoy, S. L. Moseley, B. Nowicki, A. L. Servin, and M. F. Bernet-Camard. 2000. Structural and functional lesions in brush border of human polarized intestinal Caco-2/TC7 cells infected by members of the Afa/Dr diffusely adhering family of Escherichia coli. Infect. Immun. 68:5979-5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peiffer, I., A. L. Servin, and M. F. Bernet-Camard. 1998. Piracy of decay-accelerating factor (CD55) signal transduction by the diffusely adhering strain Escherichia coli C1845 promotes cytoskeletal F-actin rearrangements in cultured human intestinal INT407 cells. Infect. Immun. 66:4036-4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Savkovic, S. D., A. Koutsouris, and G. Hecht. 1997. Activation of NF-κB in intestinal epithelial cells by enteropathogenic Escherichia coli. Am. J. Physiol. 273:C1160-C1167. [DOI] [PubMed] [Google Scholar]
- 64.Savkovic, S. D., A. Koutsouris, and G. Hecht. 1996. Attachment of a noninvasive enteric pathogen, enteropathogenic Escherichia coli, to cultured human intestinal epithelial monolayers induces transmigration of neutrophils. Infect. Immun. 64:4480-4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Savkovic, S. D., A. Ramaswamy, A. Koutsouris, and G. Hecht. 2001. EPEC-activated ERK1/2 participate in inflammatory response but not tight junction barrier disruption. Am. J. Physiol. Gastrointest. Liver Physiol. 281:G890-G898. [DOI] [PubMed] [Google Scholar]
- 66.Schmitt, C. A., W. Schwaeble, B. M. Wittig, K. H. Meyer zum Buschenfelde, and W. G. Dippold. 1999. Expression and regulation by interferon-gamma of the membrane-bound complement regulators CD46 (MCP), CD55 (DAF) and CD59 in gastrointestinal tumours. Eur. J. Cancer 35:117-124. [DOI] [PubMed] [Google Scholar]
- 67.Schultsz, C., M. Moussa, R. van Ketel, G. N. Tytgat, and J. Dankert. 1997. Frequency of pathogenic and enteroadherent Escherichia coli in patients with inflammatory bowel disease and controls. J. Clin. Pathol. 50:573-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shen, W., H. Steinruck, and A. Ljungh. 1995. Expression of binding of plasminogen, thrombospondin, vitronectin, and fibrinogen, and adhesive properties by Escherichia coli strains isolated from patients with colonic diseases. Gut 36:401-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Spiller, O. B., O. Criado-Garcia, S. Rodriguez De Cordoba, and B. P. Morgan. 2000. Cytokine-mediated up-regulation of CD55 and CD59 protects human hepatoma cells from complement attack. Clin. Exp. Immunol. 121:234-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Steiner, T. S., J. P. Nataro, C. E. Poteet-Smith, J. A. Smith, and R. L. Guerrant. 2000. Enteroaggregative Escherichia coli expresses a novel flagellin that causes IL-8 release from intestinal epithelial cells. J. Clin. Investig. 105:1769-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tabaqchali, S., D. P. O'Donoghue, and K. A. Bettelheim. 1978. Escherichia coli antibodies in patients with inflammatory bowel disease. Gut 19:108-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tieng, V., C. Le Bouguenec, L. du Merle, P. Bertheau, P. Desreumaux, A. Janin, D. Charron, and A. Toubert. 2002. Binding of Escherichia coli adhesin AfaE to CD55 triggers cell-surface expression of the MHC class I-related molecule MICA. Proc. Natl. Acad. Sci. USA 99:2977-2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tiveljung, A., J. D. Soderholm, G. Olaison, J. Jonasson, and H. J. Monstein. 1999. Presence of eubacteria in biopsies from Crohn's disease inflammatory lesions as determined by 16S rRNA gene-based PCR. J. Med. Microbiol. 48:263-268. [DOI] [PubMed] [Google Scholar]
- 74.Uesu, T., M. Mizuno, H. Inoue, J. Tomoda, and T. Tsuji. 1995. Enhanced expression of decay accelerating factor and CD59/homologous restriction factor 20 on the colonic epithelium of ulcerative colitis. Lab. Investig. 72:587-591. [PubMed] [Google Scholar]
- 75.Vallance, B. A., and B. B. Finlay. 2000. Exploitation of host cells by enteropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 97:8799-8806. [DOI] [PMC free article] [PubMed] [Google Scholar]