Abstract
The Mycobacterium tuberculosis protein ESAT-6 has unusual immune stimulating activities, has been implicated in the recall of long-lived immunity, and induces protection against tuberculosis in mice. For many diseases caused by bacterial or viral pathogens, a strong cell-mediated immune (i.e., type 1) response is often required for recovery or protection. Therefore, it is important to design immunization regimens that induce agent-specific type 1 immunity. We have shown in previous studies that ESAT-6 could enhance antigen-specific type 1 immune responses in BALB/c mice against a second antigen when presented as a purified fusion protein. It was also of interest to determine if ESAT-6 could enhance the type 1 response against a second antigen beyond that afforded by DNA vaccination through CpG motifs. This was tested by using gene fusions of ESAT-6 and the Mycoplasma hyopneumoniae surface antigen P71. Modified P71 gene sequences were cloned with or without ESAT-6 sequences into a DNA vaccine vector and were used to immunize mice. Splenic lymphocytes from vaccinated mice were tested for gamma interferon (IFN-γ) and interleukin-10 (IL-10) secretion. Serum antibodies were examined for P71 antigen-specific isotype responses. When stimulated in vitro with purified P71 antigen, splenocytes from the ESAT-6:P71 vaccinates secreted higher levels of IFN-γ and lower levels of IL-10 compared to those of vaccinates receiving the P71 construct alone. Furthermore, the immunoglobulin G2a serum antibody levels were significantly higher in the ESAT-6:P71 vaccinates compared to those of the vaccinates receiving P71 alone. In conclusion, ESAT-6 was shown to enhance antigen-specific type 1 immune responses in BALB/c mice when used in DNA vaccines.
The central hypothesis of this study focuses on the unique immunological qualities of the mycobacterial protein ESAT-6. This protein has been shown to stimulate long-lived cellular immunity to Mycobacterium tuberculosis in human patients (8) and in other animal species (1, 7). In the mouse model of tuberculosis infection, the recall of long-lived immunity has been attributed to mycobacterial proteins Ag85B and ESAT-6. This recall of immunity was found to be very efficient and could control infectious challenge within the first week. The effector T cells were shown to be CD4+ and displayed a massive release of the type 1 cytokine gamma interferon (IFN-γ) (1). It was also shown in cattle experiments that the first significant T-cell response to experimental infection with Mycobacterium bovis occurred 3 weeks after the onset of infection. It was characterized by a pronounced IFN-γ response from peripheral blood mononuclear cells directed to antigens in culture filtrate of which the major antigen was ESAT-6 (7).
These properties, a rapid release of IFN-γ and induction of CD4+ cells, were the foundation of the idea that fusion of ESAT-6 with a second antigen could affect the immune response against that antigen. Previous studies in this laboratory have shown that ESAT-6 fusion proteins did result in the induction of an enhanced type 1 immune response against an antigen that induced a type 2 response in the absence of ESAT-6 (6). To further explore the immunological potential of ESAT-6, DNA vaccine vectors were constructed that contained ESAT-6 sequences in combination with the P71 gene sequences. Because P71 is a protein from Mycoplasma hyopneumoniae, it is a good test antigen as mice are immunologically naïve with respect to this antigen. To test the ability of ESAT-6 to enhance a type 1 immune response, DNA constructs were used to vaccinate BALB/c mice and the subsequent immune response was analyzed.
Plasmid constructions.
To test the effect of ESAT-6 on immune responses following DNA vaccination, the M. hyopneumoniae membrane protein P71 gene sequence was cloned into the DNA vaccine vector VR1020 (Vical, Inc.) with or without ESAT-6 gene sequences. To allow for full-length expression of the P71 sequence in the mammalian host, the mycoplasmal tryptophan coding codon TGA was altered to TGG by site-directed mutagenesis. This prevented premature truncation of the growing protein during translation in the animal host. Initially, the P71 sequence (GenBank accession number AF015665) was cloned by PCR into pTrcHis B (Invitrogen, Inc., Carlsbad, Calif.), forming pISM407 (Fig. 1 and Table 1). Site-directed mutagenesis was then performed by overlap extension PCR that converted TGA codons to TGG codons (2). Complementary primers were designed with the TGA codon replaced by TGG within the primer sequences to generate 3 fragments, A to C (Table 1). The three PCR-generated overlapping fragments having the required mutations were then joined by several rounds of overlap extension and PCR (2). The final PCR involved primers FM71.S3 and RM71.S3 with BamHI restriction sites for cloning purposes. All PCRs were performed with Pfu DNA polymerase following standard protocols. The final plasmid containing modified P71 sequences was designated pISM409. Plasmids pISM403, pISM409, and pISM410 used for purification of ESAT-6, P71, and the ESAT-6:P71 fusion protein (EsP71), respectively, have already been described (6).
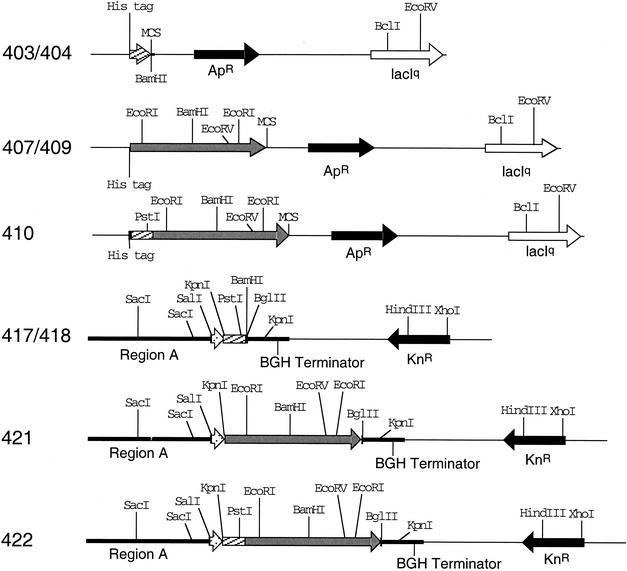
FIG. 1.
Plasmid restriction maps. Shown are the restriction site maps of plasmids constructed in this study. The plasmid numbers are shown on the left. Relevant restriction sites are also shown. The black arrows indicate antibiotic resistance markers, white arrows indicate lacIq sequences, white stipled arrows indicate tissue plasminogen activator sequences, and gray arrows indicate P71 sequences. The hatched bars indicate ESAT-6 sequences.
TABLE 1.
Primers and plasmids
| Primer | Sequence (5′-3′)a | Purpose or plasmid constructionb |
|---|---|---|
| F2Es | GAGCAGATCTATGACAGAGCAGCAGTGGAATTTC | pISM417 |
| R1Es | GGCAGATCTCTATGCGAACATCCCAGTG | pISM417 |
| R2Es | GTTGGATCCTGCGAACATCCCAGTGACG | pISM418 |
| FP71.S1 | GGGACAACAACAGAAAACTGGCTTTACC | P71 mutagenesis, fragment A |
| RP71.S1 | GGTAAAGCCAGTTTTCTGTTGTTGTCCC | P71 mutagenesis, fragment A |
| FP71.S2 | GAATTTAAAAAATGGGCAAGTATAATAAAACC | P71 mutagenesis, fragment B |
| RP71.S2 | GGTTTTATTATACTTGCCCATTTTTTAAATTC | P71 mutagenesis, fragment B |
| FP71.S3 | GATGTCTATGATAAAAGTATTTGGAATGATTCTG | P71 mutagenesis, fragment C |
| RP71.S3 | CAGAATCATTCCAAATACTTTTATCATAGACATC | P71 mutagenesis, fragment C |
| FM71.S3 | TGAAAGGATCCTGATAAAAATTTAATGGCGAA | P71 mutagenesis, pISM409 |
| RM71.S3 | ATCAGGATCCTTCCAAATACTTTTATCATAGAC | P71 mutagenesis, pISM409 |
| P71.1 | GCTAGATCTTTGCAAAAAAATTCTTTGCTTTC | pISM421 |
| P71.2 | GCTAGATCTTTAGT TTGATTTAGGCTCGGTAC | pISM421 |
Restriction sites introduced for cloning are in bold.
Primers were used in the indicated pISM plasmid constructions.
To construct the vaccine plasmids the ESAT-6 gene sequence was inserted into pVR1020 by PCR cloning with pISM403 template DNA and primers F2Es and R1Es. Necessary changes in the forward primer were incorporated to generate an in-frame protein fusion with the tissue plasminogen activator signal of VR1020 and ESAT-6. Following digestion of the fragment with BglII, it was ligated into BamHI-digested pVR1020 to create plasmid pISM417 (Fig. 1). The orientation of cloned fragments was verified by restriction mapping or by PCR, and DNA sequencing was done to verify the sequence of each plasmid constructed. Plasmid pISM417 was used as the ESAT-6 control in genetic immunization studies. To create the vector for producing EsP71, a similar construct was made lacking the stop codon and with a BamHI site downstream of ESAT-6. This was accomplished by using PCR primers F2Es and R2Es to generate plasmid pISM418. The PCR product containing the P71 structural gene was generated from pISM409 by using primers P71.1 and P71.2. The fragment was digested with BglII and cloned into the BamHI site of pVR1020 to generate plasmid pISM421 (Fig. 1). The structural gene for P71 obtained by PCR with primers P71.1 and P71.2 was also cloned into pISM418 to generate EsP71 in plasmid pISM422 (Fig. 1).
Vaccinations.
Vaccinations were performed by using plasmid preparations prepared with the Qiagen EndoFree Plasmid Giga kit. The plasmid DNA was dissolved in endotoxin-free saline by overnight suspension at 4°C. Six- to 8-week-old mycoplasma-free female BALB/c mice were immunized in groups of six with 100 μg of plasmid DNA in normal saline on day 0. Plasmids were injected intramuscularly into the thigh muscle of mice primed 2 days previously with 100 μl of 0.25% Bupivacaine. The procedure was repeated 3 weeks later. Control mice were similarly vaccinated with plasmid pVR1020 without P71 or ESAT-6 sequences. Animal care approval was obtained from the Iowa State University Committee for Animal Care in accordance with the National Institutes of Health Animal Care Guidelines.
Immunological responses.
To assess immunological responses to the DNA vaccines, IFN-γ and interleukin-10 (IL-10) levels in culture supernatants from antigen-stimulated splenocytes were measured as described previously (6). Splenocytes (5 × 106/ml) were cultured at 37°C and 5% CO2 in 48-well culture dishes with a 10-μg/ml concentration of purified recombinant P71, EsP71, or ESAT-6 (6). The negative control wells had cells alone in complete RPMI medium while the positive control wells were stimulated with 1 μg of concanavalin A/ml. The supernatant was collected after 72 h and was stored at −70°C. The antigen-specific IFN-γ and IL-10 levels in culture supernatants were measured by a capture enzyme-linked immunosorbent assay (ELISA) (6). Briefly, Immulon 2 96-well plates were coated with capture antibody (rat monoclonal antibody XMG 1.2 for IFN-γ and rat monoclonal antibody JES5-2A5 for IL-10 [PharMingen]) at 2 μg/ml in coating buffer overnight at 4°C, blocked with 1% gelatin for 1 h at 37°C, and then incubated with 100 μl of culture supernatant in triplicate wells diluted either 1:2 or 1:4 in basal RPMI medium for 1 h at 37°C. For IFN-γ estimation the plates were then incubated with 100 μl of a 1:2,000 dilution of rabbit anti-mouse IFN-γ antibodies followed by alkaline phosphatase-conjugated donkey anti-rabbit immunoglobulin G (IgG). For IL-10 estimation, plates were incubated with biotinylated rat anti-mouse IL-10 (SXC-1; PharMingen) at 1:2,000 dilution. The concentrations of IFN-γ and IL-10 in the culture supernatants were estimated from standard curves generated by using mouse recombinant IFN-γ or recombinant IL-10 (PharMingen). Statistical evaluations were performed by one-way analysis of variance or Mann Whitney test by using InStat 2.0 (GraphPad Software, San Diego, Calif.).
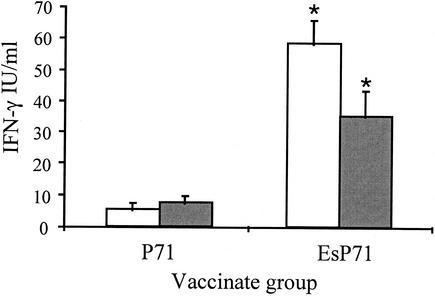
Because the focus of this work was to evaluate the effect of ESAT-6 on the resulting immune response to P71, lymphocytes were stimulated in vitro with P71 alone. Splenocytes from mice immunized with the DNA vectors containing the P71 sequence (pISM421 and pISM422) showed a significant difference (P < 0.01) in IFN-γ levels between groups following stimulation with purified P71 protein (Fig. 2). The mice vaccinated with EsP71 (pISM422) demonstrated significantly higher IFN-γ responses on days 32 and 42 compared to the responses of pISM421 (P71 alone) vaccinates (P < 0.05). The difference between the P71 and EsP71 vaccinates was about 10-fold on day 32, and it decreased to about 4- to 5-fold by day 42. When stimulated in vitro with P71, there was no detectable IFN-γ in splenocyte cultures from mice immunized with VR1020 vector only or the levels were not significantly different than background responses following vaccination with pISM417 (ESAT-6 alone) (data not shown). There was also no detectable IFN-γ in splenocyte cultures derived from mice immunized with pISM421 (P71) when stimulated with either ESAT-6 or EsP71 proteins (data not shown).
FIG. 2.
IFN-γ levels in splenocyte supernatants from P71 and EsP71 vaccinates following stimulation with P71. Mouse splenocytes from P71 and EsP71 vaccinates were seeded at a concentration of 5 × 106 cells per well and were stimulated in vitro with 10 μg of P71 antigen/ml for 72 h. The IFN-γ levels were determined in the supernatant fractions by ELISA. Data represent means plus standard errors of two experiments with triplicate wells in each experiment. White bar, day 32; gray bar, day 42. An asterisk indicates that the value was significantly different (P < 0.05) from the values for other vaccinate groups for the same time point. Splenic lymphocytes from control VR1020-vaccinated mice did not respond to the P71 antigen (data not shown).
The level of IFN-γ released following antigen stimulation is a leading indicator of the type of response (i.e., type 1 or type 2) generated against that antigen. IFN-γ is a product of Th1 cells, and two of its functions are to downregulate Th2 cells and upregulate type 1 immune responses. In these studies, the levels of IFN-γ released by splenocytes following in vitro stimulation with P71 was greatest in the mice immunized with the EsP71 construct (Fig. 2). This suggests that ESAT-6 can further enhance a type 1 response above that afforded by the DNA vaccination regimen alone.
To regulate T-cell differentiation, however, an antagonist is required. IL-10, a product of Th2 cells, serves this function by downregulating Th1 cells. If a type 1 response is induced, then IL-10 will be downregulated. Figure 3 shows the amount of IL-10 produced by stimulating splenocytes from immunized mice. The splenocyte group from pISM421-immunized mice (P71 alone) was the only group to secrete detectable IL-10 when stimulated with recombinant P71 protein (Fig. 3) (P < 0.01). The IL-10 levels were 800 and 900 pg per ml on days 32 and 42, respectively. No other group secreted detectable levels of IL-10 when stimulated with P71 (data not shown). Thus, the splenocytes recovered from EsP71 DNA vaccinates failed to produce IL-10 following stimulation with P71 (Fig. 3).
FIG. 3.
IL-10 levels in splenocyte supernatants from P71 and EsP71 vaccinates following stimulation with P71. Mouse splenocyte cultures were stimulated in vitro as described in the legend to Fig. 2, and the IL-10 levels were determined by ELISA. Data is given in international units determined from a standard curve. Data represents means plus standard errors for two experiments with triplicate wells for each experiment. White bars, day 32; gray bars, day 42. An asterisk indicates that the value was significantly different (P < 0.01) from that of the other vaccinate group for the same time point. Splenic lymphocytes from control VR1020-vaccinated mice did not respond to the P71 antigen (data not shown).
Accompanying the aforementioned cytokine profiles in type 1 immune responses is a shift in antibody isotype, reflecting the class switch mechanism influenced by the type 1 environment. Type 1 responses lead to increased levels of IgG2a antibodies in mice (9) compared to those of type 2 responses, which generate largely IgG1 antibodies following the IgM-to-IgG class switch. An ELISA was employed to measure P71-specific IgG1 and IgG2a antibody responses following vaccination. Immulon 2HB microtiter plates coated with 0.2 μg of purified P71 per well were reacted with serum samples at a dilution of 1:100 in phosphate-buffered saline and were developed with isotype-specific alkaline phosphatase-conjugated goat anti-mouse IgG1 or IgG2a (1:1,000). The results were read at 405 nm and are presented in Fig. 4 as the average optical density values of duplicate wells.
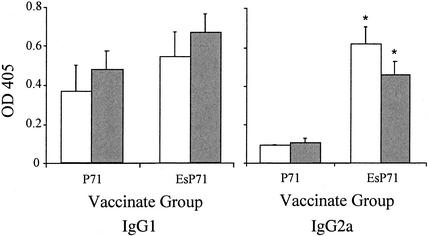
FIG. 4.
Levels of P71-specific IgG1 and IgG2a antibodies in serum from P71 and EsP71 vaccinates. Data are given as optical density at 405 nm (OD 405) and represent the means plus standard errors for two experiments that used three mice per group. White bars, day 32; gray bars, day 42. An asterisk indicates that the value was significantly different (P < 0.01) from that of the other vaccinate group for the same time point. Sera from control VR1020-vaccinated mice failed to respond to the P71 antigen (data not shown).
As is common with DNA vaccines (4), not all of the mice seroconverted (as defined by two standard deviation units above the control level) (data not shown). For IgG1, four out of the six mice seroconverted on day 32 in the pISM421 (P71)-vaccinated groups, and all six mice seroconverted by day 42. All of the mice seroconverted in the pISM422 (EsP71)-vaccinated group at both time points. The levels of IgG1 showed an increase for both P71 as well as EsP71 vaccinates from day 32 to day 42 (Fig. 4). The EsP71 vaccinates showed somewhat higher levels of IgG1 at both time points compared to levels for mice vaccinated with the P71 construct, but the differences were not significant. For the IgG2a isotype, two of the P71 vaccinates seroconverted on day 32 while five out of six seroconverted by day 42. All EsP71 vaccinates seroconverted by both days, showing that ESAT-6 also hastened the developing antibody response at day 32. In contrast to IgG1 levels, the IgG2a levels were significantly higher on both days 32 and 42 for EsP71 vaccinates than those for P71 vaccinates (P < 0.05) (Fig. 4).
DNA vaccination leads to production and presentation of the recombinant protein by the transfected cells as would normally occur with viral infections. This results in major histocompatibility complex class I presentation of antigenic epitopes and induction of type 1 immune responses. The amplitude of that response, however, may not be sufficient to provide adequate protection against an infectious agent. Since previous studies have shown an enhancement of type 1 responses when ESAT-6 was used in combination with P71 in a protein vaccine format (6), these studies showed that enhanced IFN-γ production also occurred when a DNA construct containing ESAT-6 fusions was used.
BALB/c mice are considered type 2 responders (5), and it is noteworthy that a strong type 1 immune response could be elicited in this genetic background (i.e., those receiving the EsP71 construct). Previous studies by this group with recombinant proteins have shown that the P71 protein in the absence of adjuvant induces a type 2 response in BALB/c mice (6). This did not change noticeably when P71 was presented in a DNA vaccine format based upon IgG1 and IgG2a antibody responses (Fig. 4). The presence of ESAT-6 within the vaccine construct, however, shifted the immune response significantly. In summary, the EsP71 vaccinates gave conclusive evidence of an enhanced type 1 response by the parameters measured, increased antigen-specific IFN-γ and IgG2a antibody levels and lower IL-10 levels.
Although type 1 immune responses were enhanced, the identity of the mechanism involved was not clear. In these experiments we were not able to differentiate between an effect mediated through the ESAT-6 protein product directly or through the DNA. CpG motifs within the plasmid vector have been shown to have potent stimulatory effects on the mammalian immune responses (3). A search for such motifs within the ESAT-6 gene sequence by using MacVector software (Oxford Molecular Group) revealed two such motifs at nucleotides 175 to 180 and 265 to 270. This could explain the ability of ESAT-6 DNA sequences to act as a DNA vaccine adjuvant by its mere presence within the plasmid. Given our previous studies showing enhanced type 1 responses with purified proteins, however, it is unlikely that CpG motifs could explain our results entirely. It is also unlikely that a single mechanism is involved. The presence of the tissue plasminogen activator signal sequence within pVR1020 directing the secretion of EsP71 might also explain some of the effects of ESAT-6 on the immune response. Thus, ESAT-6 may be able to bring about its adjuvant effect by a multipronged approach when used in DNA vaccinations.
Acknowledgments
This work was supported in part by the Iowa Livestock Health Advisory Council.
Editor: J. D. Clements
REFERENCES
- 1.Andersen, P., A. B. Andersen, A. L. Sorensen, and S. Nagai. 1995. Recall of long-lived immunity to Mycobacterium tuberculosis infection in mice. J. Immunol. 154:3359-3372. [PubMed] [Google Scholar]
- 2.Ge, L., and P. Rudolph. 1997. Simultaneous introduction of multiple mutations using overlap extension PCR. BioTechniques 22:28-30. [DOI] [PubMed] [Google Scholar]
- 3.Krieg, A. M., A. K. Yi, S. Matson, T. J. Waldschmidt, G. A. Bishop, R. Teasdale, G. A. Koretzky, and D. M. Klinman. 1995. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature 374:546-549. [DOI] [PubMed] [Google Scholar]
- 4.Leitner, W. W., H. Ying, and N. P. Restifo. 1999. DNA and RNA-based vaccines: principles, progress and prospects. Vaccine 18:765-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Locksley, R. M., F. P. Heinzel, M. D. Sadick, B. J. Holaday, and K. D. Gardner, Jr. 1987. Murine cutaneous leishmaniasis: susceptibility correlates with differential expansion of helper T-cell subsets. Ann. Inst. Pasteur Immunol. 138:744-749. [DOI] [PubMed] [Google Scholar]
- 6.Menon, S. A., M. J. Wannemuehler, G. G. Mahairas, and F. C. Minion. 2002. Mycobacterial ESAT-6 protein enhances mouse IFN-γ responses to Mycoplasma hyopneumoniae P71 protein. J. Interferon Cytokine Res. 22:807-813. [DOI] [PubMed] [Google Scholar]
- 7.Pollock, J. M., and P. Andersen. 1997. Prominent recognition of the ESAT-6 protein in the first phase of interferon with Mycobacterium bovis in cattle. Infect. Immun. 65:2587-2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ravn, P., A. Demissie, T. Eguale, H. Wondwosson, D. Lein, H. A. Amoudy, A. S. Mustafa, A. K. Jensen, A. Holm, I. Rosenkrands, F. Oftung, J. Olobo, F. von Reyn, and P. Andersen. 1999. Human T cell responses to the ESAT-6 antigen from Mycobacterium tuberculosis. J. Infect. Dis. 179:637-645. [DOI] [PubMed] [Google Scholar]
- 9.Stevens, T. L., A. Bossie, V. M. Sanders, R. Fernandez-Botran, R. L. Coffman, T. R. Mosmann, and E. S. Vitetta. 1988. Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature 334:255-258. [DOI] [PubMed] [Google Scholar]