Abstract
Growth temperature was found to control the expression of toxins A and B in Clostridium difficile VPI 10463, with a maximum at 37°C and low levels at 22 and 42°C in both peptone yeast (PY) and defined media. The up-regulation of toxin A and B mRNA and protein levels upon temperature upshift from 22 to 37°C followed the same kinetics, showing that temperature control occurred at the level of transcription. Experiments with Clostridium perfringens using gusA as a reporter gene demonstrated that both toxin gene promoters were temperature controlled and that their high activity at 37°C was dependent on the alternative sigma factor TcdD. Furthermore, tcdD was found to be autoinduced at 37°C. Glucose down-regulated all these responses in the C. perfringens constructs, similar to its impact on toxin production in C. difficile PY broth cultures. C. difficile proteins induced at 37°C and thus coregulated with the toxins by temperature were demonstrated by two-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis and identified as enzymes involved in butyric acid production and as electron carriers in oxidation-reduction reactions. The regulation of toxin production in C. difficile by temperature is a novel finding apparently reflecting an adaptation of the expression of its virulence to mammalian hosts.
Clostridium difficile is a gram-positive, spore-forming anaerobic bacterium that is associated with diarrhea and colitis in humans (26, 39). A reduction of the microbial flora in the colon, usually caused by antibiotic therapy, allows overgrowth of C. difficile, with symptoms ranging from mild self-limiting diarrhea to life-threatening pseudomembranous colitis. Clinically significant strains have a pathogenicity locus (PaLoc) integrated into the chromosome (6, 12, 39). In most toxin-producing strains, the PaLoc includes both the enterotoxin (toxin A) and the cytotoxin (toxin B) genes, as well as three additional open reading frames (ORFs), tcdD, tcdE, and tcdC (16). There is polymorphism in the toxin A and B genes among clinical isolates of C. difficile (42), and virulent strains producing only one of the toxins have been isolated (2, 8). The C. difficile toxins A and B belong to a class of proteins called large clostridial cytotoxins that share the same general structure and enzymatic function (46). Thus, both toxins are monoglucosyl transferases that modify small regulatory GTP-binding proteins of the Rho family in the eucaryotic cell (18, 19). The glucosylated target proteins are rendered inactive, resulting in a collapse of the actin cytoskeleton and death of colonic enterocytes. In addition to the direct cytotoxic effect, toxin A also affects neurons in the enteric nerve system to release substance P (7). Substance P is then thought to activate mast cells, resulting in the recruitment of neutrophils and increased intestinal secretion, contributing to mucosal inflammation (47).
The functions of the three additional PaLoc ORFs (tcdD, tcdE, and tcdC) are less well studied. One breakthrough in the understanding of toxin regulation came when tcdD was demonstrated to up-regulate toxin synthesis (35), and another occurred when it was shown in a heterologous Clostridium perfringens reporter gene system that tcdD encodes an alternative sigma factor involved in transcription of the toxin genes (28). The tcdD-dependent toxin expression was recently also confirmed in C. difficile (29), supporting the notion that C. perfringens is a good model organism for studying C. difficile toxin regulation. The functions of tcdE and tcdC remain poorly understood. TcdE has similarities to holins, cytolytic proteins encoded by certain bacteriophages (45). Expression of tcdC is highest during exponential growth (low toxin expression) and has been suggested to act as a negative regulator of the toxin genes (16).
The toxin yield in C. difficile is dependent on the nutrient levels in the growth medium, and much data indicates that toxin synthesis is turned on as a response to a shortage of sugars and certain amino acids (9, 13, 21, 22, 23, 24, 37, 48, 50). In addition, growth-limiting levels of the vitamin biotin leads to high toxin production (49). In complex media, but not in defined media, the presence of rapidly metabolizable carbon sources lowers the toxin yields (9, 23, 37). Among amino acids, cysteine is particularly potent in down-regulating toxin synthesis (23, 24). Cysteine concomitantly down-regulates other proteins, including enzymes involved in the formation of butyric acid and butanol, and these metabolic end products affect toxin yields when added to C. difficile cultures (23).
A transition from ambient temperature to the range 36 to 38°C, i.e., the body temperature of mammals, correlates with a dramatic up-regulation of the expression of virulence determinants in many pathogens (25, 33). In this paper, we show that 37°C is the optimum temperature for toxin synthesis by C. difficile VPI 10463. Furthermore, the temperature control of toxins A and B was found to occur at the level of transcription and to depend on the alternative sigma factor TcdD. The new finding that the toxins are regulated by temperature adds to the complexity of their control and suggests a host-specific adaptation of virulence expression in C. difficile.
MATERIALS AND METHODS
C. difficile strains and growth media.
C. difficile VPI 10463 and the serogroup type strains A (CCUG 37779), C (CCUG 37766), G (CCUG 37783), and H (CCUG 37784) were from the Culture Collection, University of Göteborg, Göteborg, Sweden. C. difficile strains were grown in peptone yeast (PY) broth or the defined medium SDM as described previously (23). Viable counts of C. difficile cultures were performed on blood agar.
Protein sampling, measurements of toxin, and short-chain fatty acid production.
One-milliliter samples of C. difficile cultures were collected and either stored immediately at −20°C or separated into a cellular pellet and supernatant by centrifugation at 4,000 × g for 5 min before storage at −20°C. Sonication, protein measurements, short-chain fatty acid assays by gas-liquid chromatography, and determination of toxins (A and B) by enzyme immunoassay were performed as described previously (23).
Toxin stability assay.
Cell culture supernatant containing toxin (10,000 U/ml) was obtained from a 48-h VPI 10463 culture grown at 37°C in PY broth without cysteine. VPI 10463 was also grown at 22, 37, and 42°C for 48 h in PY broth, and 2.5-ml aliquots of the cultures were sonicated twice for 60 s each time in order to stop growth and release intracellular components. The cell sonicates and the toxin preparations were mixed 1:2 and incubated anaerobically at the respective temperatures (22, 37, and 42°C), and the toxin levels in the mixtures were determined at time zero and after 24 h. Each experiment was performed in triplicate.
Western blots of toxins A and B.
Cellular proteins (1.5 μg) of C. difficile were separated by electrophoresis on sodium dodecyl sulfate (SDS)-polyacrylamide gels (7.5%). The proteins were transferred to polyvinylidene difluoride membranes (Millipore) using the Pharmacia Novablot transfer equipment and a continuous buffer system (39 mM glycine, 48 mM Tris, 0.0375% [wt/vol] SDS, 20% [vol/vol] methanol) according to the Multiphor II manual. The membranes were dried and blocked overnight with 0.5% (vol/vol) Tween 20 at 4°C. The blotted membranes were incubated for 1 h at room temperature with 0.2 μg of antibodies against toxin A (PCG-4; r-Biopharm) or toxin B (2CV; r-Biopharm)/ml in TST buffer (0.05 M Tris, 0.5 M NaCl, 0.1% Tween 20, pH 9). After three washes in TST, the membranes were incubated with horseradish peroxidase-conjugated anti-mouse antibodies (DAKOPATTS; diluted 1:15,000 in TST) for 1 h and finally washed three times in TST. A chemiluminiscent signal (ECL Plus; Amersham) was used to detect the bands. The relative amounts of toxins were measured on scanned X-ray films using Molecular Analyst software (Bio-Rad).
Cloning of toxin A and toxin B gene fragments.
Chromosomal DNA from C. difficile was purified using a DNeasy tissue kit (Qiagen). Using the primer pairs 5′-TGCTTCCAGGTATTCACTCTGA-3′ plus 5′-ACACTGCCCTAAAGCGAAAGC-3′ and 5′-TGCATTTTTGATAAACACATTGAA-3′ plus 5′-GCAGCAGCTAAATTCCACCT-3′, respectively, gene fragments for toxins A (280 bp) and B (402 bp) were PCR amplified with Ampli-taq Gold (Perkin-Elmer) as a DNA polymerase. The fragments were subsequently cloned into the vector PCR-Script Amp (Stratagene), creating the plasmids SK-A3 and SK-B3. The integrity of the cloned inserts was analyzed by DNA sequencing. The Escherichia coli strain Epicuran Coli XL1-Blue (Stratagene) was used as the recipient for the plasmids. Cells were grown at 37°C in Luria-Bertani medium supplemented with 50 μg of ampicillin/ml. SK-A3 and SK-B3 where then purified and used to generate radiolabeled specific antisense RNA probes (see below) for the assay of toxin A and toxin B mRNA levels. DNA restriction, ligation, agarose gel electrophoresis, and electroporation were carried out as described previously (30).
Synthesis of antisense RNA probes.
In vitro transcription of the cloned toxin A and B fragments was generated using the MAXI script kit (Ambion) according to the recommendations provided by the manufacturer. Briefly, 1 μg of linearized plasmid (SK-A3 or SK-B3) was incubated for 2 h at room temperature in the presence of T3 RNA polymerase and nucleotides. [α-32P]UTP (3 μM; Amersham Pharmacia Biotech) was used as the labeling nucleotide. The DNA template was degraded by incubating it for 15 min at 37°C in the presence of 2 U of DNase I. The reaction was stopped by adding 1 μl of 0.5 M EDTA. The RNA was denatured for 3 min at 80°C and separated on a 5% polyacrylamide-8 M urea gel. The full-length transcripts were gel purified, and the specific radioactivity of the eluted probes was determined using the Liquid Scintillation System LS 1801 (Beckman).
Preparation of C. difficile RNA.
C. difficile was harvested by adding 10% (vol/vol) of a mixture of 95% ethanol-5% phenol to the cultures and snap-frozen in liquid nitrogen. Samples were stored at −70°C and thawed on ice. The cells were pelleted by centrifugation for 5 min at 4,000 × g and 4°C, and RNA was extracted with a Fast Prep 120 incubator (Bio 101) using a FastRNA kit, blue (Bio 101), as recommended by the manufacturer. The RNA concentration was determined by spectrophotometry, and RNA integrity was determined by analysis of the 16S/23S rRNA by gel electrophoresis.
RPA.
RNase protection assays (RPA) were performed using the RPA III kit (Ambion) according to the manufacturer's recommendations. Briefly, aliquots of toxin A- and B-specific antisense RNA probes (5 × 104 cpm) were coprecipitated with 15 μg of C. difficile RNA and dissolved in 10 μl of hybridization buffer. The samples were heated for 3 min at 90°C and incubated at 42°C for at least 6 h and then digested with RNase T1. Protected fragments were precipitated, denatured for 3 min at 90°C, and separated on a 5% polyacrylamide-8 M urea gel. The gels were dried, and radioactivity was quantified using PhosphorImager Image QanNT software (Molecular Dynamics).
Construction of reporter gene fusions for C. perfringens.
The vector pTUM177, used for studying gene expression in C. perfringens, was constructed by introducing the E. coli gusA gene into the C. perfringens-E. coli shuttle vector pJIR750 (4). The gusA gene was engineered to contain the C. difficile toxB (tcdB) ribosome binding site upstream of the gusA start codon (28). The promoter and the first coding nucleotides of tcdA, tcdB, tcdD, and gdh (6, 9) were fused in frame with the gusA gene in pTUM177, creating the plasmids pTUM181, pTUM182, pTUM183, and pTUM481, respectively (28, 29). The construction of pTUM307, a TcdD-expressing plasmid, was described previously (28). To test the effect of temperature on toxA, toxB, and tcdD gene expression with tcdD in trans, plasmid pTUM181, pTUM182, or pTUM183 was introduced into electrocompetent cells of C. perfringens strain SM101 (52) with or without pTUM307 present. C. perfringens was grown in TY medium or TY medium supplemented with 1% glucose, and β-glucuronidase activity, representing reporter gene expression, was assayed as described previously (9).
2-D SDS-PAGE.
Protein samples of C. difficile cultures were collected at the appropriate times by centrifugation at 4,000 × g for 5 min at 4°C. The cells were washed twice in ice-cold phosphate-buffered saline and stored at −70°C to await further analysis. The cells were disrupted by sonication, and 20 μg of C. difficile proteins was separated on two-dimensional (2-D) SDS-polyacrylamide gel electrophoresis (PAGE) and silver stained as described previously (23). The gels were dried using Novex frames.
Protein expression analysis.
Analyzer version 6.1 software (BioImage) was used for protein spot detection, gel matching, quantification of spot intensities, and estimation of the isoelectric points (pIs) and molecular masses of proteins. Images were normalized by the total intensity of all matched spots for each gel. For each temperature experiment, gels from two independent cultures were used to create a reference image representing the average protein expression. Spots with a difference in intensity of fivefold or more between temperature experiments were selected. To minimize errors in spot intensity caused by overexposure due to the nonlinearity of protein staining by silver, the intensities of the selected proteins were converted to their Gaussian values. Finally, the selected spots were verified with those of the original gels by ocular inspection. Using C. difficile proteins previously identified (23) and known proteins loaded onto the gels as markers, the pIs and molecular masses of the proteins were calculated.
N-terminal amino acid sequencing and database analysis of temperature-regulated proteins.
2-D SDS-PAGE-separated C. difficile proteins were transferred to polyvinylidene difluoride membranes, stained with Coomassie brilliant blue, destained, washed, and dried before the proteins of interest were excised and subjected to amino-terminal sequencing by Edman degradation at the Protein Analysis Center, Karolinska Institute, Stockholm, Sweden. The amino acid sequence was matched and identified in the genome sequence database for C. difficile strain 630 at the Sanger Center. Further characterization of the proteins was made using ORF-finder and the BLAST search algorithm at the National Center for Biotechnology Information website.
RESULTS
Impact of growth temperature on C. difficile toxin expression.
The toxin yields from C. difficile strain VPI 10463, a high-level toxin-producing isolate belonging to serogroup G, were found to be highly dependent on the growth temperature. Toxin yields in 72-h PY broth cultures were maximal at 37°C (2,100 U/ml) but low at 22 (50 U/ml) and 42°C (< 0.2 U/ml) (Fig. 1). The final culture optical densities (OD) were 0.80, 1.1, 0.66, 0.65, and 0.43 for the tested growth temperatures (22 to 42°C) (Fig. 1), and viable counts at 42°C were about half those at 37°C after 72 h. Toxin stability experiments, in which a defined amount of C. difficile toxin was added to C. difficile cultures grown at different temperatures, demonstrated that the toxin levels changed only ±5% after 24 h of incubation (see Materials and Methods). Accordingly, the results from the growth and toxin stability experiments showed that the low toxin levels in cultures grown at 22 or 42°C could not be explained simply by poor growth or poor viability of the cells or proteolysis of the toxins at these temperatures. Toxin yields in four C. difficile reference strains, representing the serogroups A, C, H, and G, ranged from 10 to 60 U/ml at 37°C and thus were generally much lower than those in strain VPI 10463. The serogroup G reference strain showed evident temperature dependence, with the highest toxin yields at 37°C. This was observed to some extent in the serogroup H strain, whereas the serogroup A and C strains had low toxin yields (0 to 10 U/ml) regardless of temperature (data not shown).
FIG. 1.
Toxin yields determined by enzyme immunoassay in C. difficile VPI 10463 grown for 72 h in PY broth at indicated temperatures. The means and standard errors of three independent experiments are shown. The asterisk indicates that the toxin yield was below the detection limit of 0.2 U/ml.
Temperature regulation of C. difficile toxin expression occurs at the level of transcription.
Shifts of C. difficile VPI 10463 PY broth cultures from 22 to 37 or 42°C resulted in an expected increase in the growth rate (Fig. 2A), but only the 37°C culture showed a major induction of toxin as determined by enzyme immunoassay (Fig. 2B). Similar results were obtained in defined medium lacking biotin, a medium known to promote high toxin production (50), showing that the temperature regulation of toxin production in C. difficile VPI 10463 was not medium dependent (Fig. 2A and B, insets). Western blots confirmed that both toxins were under temperature control (Fig. 2C and D). The amount of toxin A, but not toxin B, was smaller at 1,300 min than at 300 min, which may indicate degradation of toxin A (Fig. 2C). However, no additional bands corresponding to degradation products appeared on the Western blots, and the reason for the smaller amount of toxin A at 1,300 min is not known. Toxin A mRNA and toxin B mRNA increased continuously upon a shift to 37°C, showing that both toxins were controlled by temperature at the level of transcription (Fig. 2E and F). For toxin A, the mRNA levels were 15- and 5-fold lower at 22 and 42°C than at 37°C at 300 min, and the corresponding differences for toxin B mRNA were 7- and 6-fold (Fig. 2E and F).
FIG. 2.
Bacterial growth (A), toxin yields determined by enzyme immunoassay (B) and Western blotting (C and D), and toxin mRNA levels determined by RPA (E and F) in C. difficile VPI 10463 after temperature upshifts from 22 to 37 or 42°C in PY broth. Data from corresponding experiments in biotin-limited defined medium (SDM) are shown in insets in panels A and B. The mRNA expression at 22 and 42°C is indicated as a single point at 300 min in panels E and F. Histograms (C and D) and mRNA curves (E and F) show the percentage of maximum band intensity for each experiment. The results are representative of two independent experiments.
Temperature regulation of toxin A and B promoters is dependent on TcdD.
As temperature affected C. difficile toxin gene expression at the mRNA level (Fig. 2E and F), we investigated the possible role of the alternative sigma factor TcdD in this regulation. Due to a lack of genetic tools in C. difficile, we used C. perfringens as the host for the different gusA fusion plasmids that we made (see Materials and Methods). pTUM181 (PtcdA-gusA), pTUM182 (PtcdB-gusA), or pTUM183 (PtcdD-gusA) was introduced into C. perfringens strain SM-101 lacking or carrying pTUM307, encoding TcdD (28). The C. perfringens strains were grown to stationary phase at various temperatures, and β-glucuronidase activity was used to monitor promoter activity. In the absence of tcdD, little or low gusA expression was observed from the toxin A and B promoters, regardless of temperature (Fig. 3A and B). The presence of tcdD in trans dramatically increased expression from both toxin promoters at 37°C but not at 22 or 42°C (Fig. 3A and B). Interestingly, tcdD was found to be positively autoregulated, but again only at 37°C (Fig. 3C). Similar to toxin expression in C. difficile (9, 24), the activity of the tcdA, tcdB, and tcdD promoters was suppressed by adding 1% glucose to the growth medium (Fig. 3A to C). Control experiments using the promoter of gdh, known to be under glucose control (20), were performed by introducing pTUM481 (Pgdh-gusA) into C. perfringens with or without the TcdD-encoding plasmid pTUM307 present. The expression of gusA from pTUM481 was affected little by temperature or by tcdD but was down-regulated by glucose (Fig. 3D), showing that the constructs in C. perfringens were stable and functional at all temperatures tested. Thus, the results presented in Fig. 3A to C were not caused by nonspecific effects of 22 and 42°C temperatures on gusA expression. Finally, control experiments using the gusA plasmid pTUM177 lacking any promoter yielded negligible β-glucuronidase activity irrespective of temperature or the presence of tcdD in trans (Fig. 3E). Western blots and immunoprecipitation of proteins from 37°C cultures of C. difficile VPI 10463 or C. perfringens strains carrying tcdD using polyclonal mouse or rabbit anti-TcdD antibodies failed to detect TcdD (data not shown), implying that TcdD is normally produced at low levels and/or is unstable.
FIG. 3.
(A to C) Expression of β-glucuronidase in C. perfringens strain SM101 carrying plasmids with gusA as the reporter gene fused to the promoters of tcdA (A), tcdB (B), and tcdD (C) with or without a plasmid carrying tcdD in trans. The cells were grown at 22, 37, or 42°C to stationary phase in TY medium with or without 1% glucose. (D and E) Control experiments with gusA fused to the promoter of the glutamate dehydrogenase gene (gdh) (D) or promoterless gusA (E) with or without tcdD in trans.
In summary, the pattern of temperature regulation of toxin gene expression in C. difficile VPI 10463 was also present in the C. perfringens model system, but only in the presence of tcdD. Furthermore, the results showed that TcdD was autoinduced specifically at 37°C.
Identification of temperature-regulated C. difficile proteins.
Previous studies showed that the patterns of protein expression in C. difficile differ significantly under growth conditions yielding high and low toxin production (see the introduction). To investigate whether this also applied to temperature, we analyzed changes of protein expression in C. difficile VPI 10463 PY broth cultures upon temperature upshifts. The average toxin yields 300 min after temperature shifts to 37 and 42°C were 530 and 58 U/ml, compared to 36 U/ml at 22°C. Twenty-eight proteins were found by 2-D SDS-PAGE to have their highest expression at 37°C, and seven of these were identified by N-terminal sequencing (Fig. 4A and C, no. 1 to 7, and Table 1). Several of these proteins matched other clostridial proteins known to be involved in reductive-oxidative metabolism leading to the formation of butyric acid (see Fig. 6).
FIG. 4.
(A and B) 2-D SDS-PAGE of proteins expressed by C. difficile VPI 10463 during growth in PY broth (A) and in defined medium (SDM) without biotin (B). The cultures were incubated at 22°C to an OD at 600 nm (OD600) of ∼0.2 to 0.3 (PY broth) or an OD420 of ∼0.1 to 0.2 (SDM), shifted to 37 or 42°C or left at 22°C, and harvested 300 (PY broth) or 600 (SDM) min after the temperature shift. The arrowheads indicate proteins with higher expression at 37 than at 22 and 42°C. (C) Enlarged sections of the 22, 37, and 42°C 2-D SDS-PAGE gels highlighting N-terminal sequenced proteins (see also proteins 1 to 9 in Table 1).
TABLE 1.
Selected proteins from C. difficilea
| No. | Relative intensityb
|
kDa | pI | N-terminal sequenced | Protein | Ide | Sif | Organism | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 22°C | 37°C | 42°C | ||||||||
| 1 | 1.6 | 11 | 6.9 | 56 | 5.7 | MKVLIIGGVA | NADH oxidase | 33 | 50 | C. perfringens |
| 2 | 0.3 | 4.2 | 3.7 | 51 | 5.5 | MEKAVENFED | Succinate-semialdehyde dehydrogenase | 66 | 82 | C. kluyveri |
| 3 | 0.4 | 2.3 | 1.2 | 42 | 5.1 | MKFYVYKAPD | Putative hydrolase | 51 | 69 | Salmonella enterica serovar Typhimurium |
| 4 | 0.3 | 3.8 | 1.4 | 34 | 6.3 | MKLAVIGSXT | 3-hydroxybutyryl-CoA dehydrogenase | 100 | 100 | C. difficile |
| 5 | 6.9 | 22 | 15 | 32 | 6.3 | MKLAVIGSXT | 3-hydroxybutyryl-CoA dehydrogenase | 100 | 100 | C. difficile |
| 6 | 0.2 | 1.1 | 0.7 | 23 | 5.1 | MHXIFINKDL | Nitrate reductase | 47 | 62 | C. perfringens |
| 7 | 10 | 18 | 13 | 19 | NDc | DKVEIPPEEN | No match in database | ND | ND | ND |
| 8 | 4.6 | 14 | 4.2 | 22 | 5.0 | MKKFVXTVXG | Rubrerythrin | 76 | 85 | C. acetobutylicum |
| 9 | 5.7 | 12 | 3.9 | 22 | 5.1 | MKKFVXTVXG | Rubrerythrin | 76 | 85 | C. acetobutylicum |
Relative amounts, physical properties, and identities of selected proteins from C. difficile VPI 10463 specifically induced concomitantly with its toxins at 37°C during growth in PY broth (Fig. 4A, spots 1 to 7) and in biotin-limited defined medium (Fig. 4B, spots 8 and 9). Genes were identified by searching for a high-score match of the N-terminal sequence in the Sanger Center C. difficile strain 630 sequence database (http://www.sanger.ac.uk/Projects/C_difficile/) using the BLAST algorithm. The genome segments found were further analyzed using ORF-finder (http://www.ncbi.nlm.nih.gov/), and the ORFs in the contigs were identified using the BLAST algorithm and the nonredundant database at the National Center for Biotechnology Information.
Relative spot intensity is the calculated Gaussian value (see Materials and Methods).
ND, not determined.
X, unidentified amino acid.
Id, percentage of identical amino acids found in the identified protein and the corresponding homologue.
Si, percentage of similar amino acids found in the identified protein and the corresponding homologue.
FIG. 6.
Operon structure of genes in C. difficile involved in butyric acid production compared to those in other clostridia (A) and the corresponding metabolic pathways (B). The arrows numbered 1 to 8 in panel B correspond to genes and enzymes in panel A.
We also measured protein expression in C. difficile strain VPI 10463 cultures after temperature uphifts in defined medium with or without biotin, supporting low and high toxin expression, respectively. Twelve proteins matched the criteria for having the highest expression at 37°C during biotin limitation, and thus high toxin production, and two of these were identified (Fig. 4B and C, no. 8 and 9, and Table 1). The N-terminal sequences of these two proteins were identical, and the proteins showed similarity to iron-sulfur-containing proteins involved in reduction-oxidation processes.
Relation between growth temperature and butyric acid-butanol metabolism.
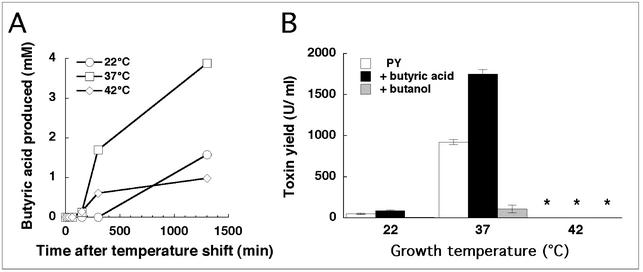
As the above-mentioned experiments indicated that butyric acid production pathways were temperature regulated like toxin production, we monitored the production of butyric acid during a temperature upshift of a C. difficile VPI 10463 culture in PY broth. The rise of butyric acid levels in the medium at 37°C (Fig. 5A) paralleled that of the toxin levels (Fig. 2B) and reached a maximum of 4 mM at 1,300 min, whereas the butyric acid levels in the 22 and 42°C cultures were three- to fourfold lower. The production of other short-chain fatty acids (acetic, propionic, isobutyric, valeric, isovaleric, caproic, and isocaproic [for details, see reference 23]) did not show any temperature dependence (data not shown).
FIG. 5.
(A) Levels of butyric acid produced in cultures of C. difficile VPI 10463 grown in PY broth after a temperature upshift from 22 to 37 or 42°C. The growth curves and toxin production during these experiments were analogous to those in Fig. 2A and B (data not shown). The values are means of two independent experiments. (B) Toxin yields, determined by enzyme immunoassay, from C. difficile VPI 10463 grown for 48 h at the indicated temperatures in PY broth or PY broth with 15 mM butyric acid or butanol added. The means and standard errors of three independent experiments are shown. The asterisks indicate that toxin levels were below the detection limit of 0.2 U/ml.
Addition of butyric acid to C. difficile cultures growing at 37 and 22°C induced toxin yields twofold (Fig. 5B). At 42°C, no toxin was detected even if butyric acid was added to the medium. The addition of butanol decreased toxin production 10-fold both at 37°C (920 to 110 U/ml) and at 22°C (51 to 5.5 U/ml) (Fig. 5B). No apparent impact on growth due to the addition of butyric acid or butanol compared to the control was observed (data not shown). Thus, although the toxin level was higher at 37°C than at 22°C, the relative change in toxin production by butyric acid or butanol was the same at both temperatures. In conclusion, butyric acid, butanol, and temperature apparently affected toxin production independently of each other, and the results also suggested that the low toxin production at 22 and 42°C was due to different mechanisms.
DISCUSSION
We have described temperature-dependent production of toxins A and B in C. difficile strain VPI 10463, controlled at the transcriptional level with maximum expression at 37°C. Among four different serotype reference strains tested, the temperature effect was most prominent in serogroup G (which includes strain VPI 10463). Interestingly, the dominant strain among clinical C. difficile isolates from hospitalized patients in the United Kingdom is related to serogroup G (43). The alternative sigma factor TcdD (28), encoded by the gene located directly upstream of tcdB on the PaLoc, was required for the temperature-dependent activity of the toxin promoters, as shown using a gusA reporter system in C. perfringens. Furthermore, tcdD was autoinduced strictly at 37°C. Adding glucose to the medium resulted in loss of the TcdD-dependent expression from the promoters of tcdA, tcdB, and tcdD and showed that the nutrient effect on toxin production observed in C. difficile is also present in C. perfringens.
Limiting the levels of cysteine leads to up-regulation of toxin production in C. difficile and also to the induction of the key enzyme in butyric acid production, 3-hydroxybutyryl coenzyme A (CoA) dehydrogenase (23). Its gene is clustered with those encoding other enzymes involved in butyric acid-butanol production on both the Clostridium acetobutylicum and the C. difficile chromosomes (Fig. 6) (36). Similar to the toxins, expression of 3-hydroxybutyryl-CoA dehydrogenase here was found to be highest at 37°C, and the largest difference (>10-fold) was found between the 22 and 37°C cultures. This temperature response was also observed for succinate-semialdehyde dehydrogenase, another enzyme involved in butyric acid formation but via a different pathway (Fig. 6). The succinate pathways in C. difficile share temperature control and down-regulation by glucose (S. Karlsson, L. G. Burman, and T. Åkerlund, unpublished data), similar to the transcriptional control of the toxin genes and tcdD. The corresponding operon structure is conserved in C. difficile, Clostridium aminobutyricum, and Clostridium kluyveri (Fig. 6) (11). An induced succinate pathway due to the absence of glucose may reflect an ongoing fermentation, and subsequent limiting levels, of certain amino acids. This may impose a metabolic stress that triggers toxin production and that can be reversed by supplementing PY broth with these amino acids or glucose (24). Many enzymatic reactions in the butyric acid production pathways are NADH dependent, and also, NADH oxidase was induced at 37°C in C. difficile. Interestingly, virulence regulation by amino acids and temperature is also found in Bordetella pertussis, where the toxin PtX is specifically induced at 37°C and is influenced by cysteine metabolism (5, 40).
High-level toxin production in C. difficile also occurs during slowed growth due to biotin limitation and can be reversed by adding the amino acid asparagine, glutamic acid, glutamine, or lysine to the growth medium (50). The effect of biotin limitation may be linked to starvation for glutamine and the subsequent block of glutamine-dependent purine biosynthesis (27). The pattern of up-regulated proteins during biotin limitation, however, was different from that in PY broth at 37°C, indicating that the metabolic stresses imposed on the bacteria to induce toxin production differed under the two conditions. Proteins previously shown to be up-regulated during biotin starvation (24) were found here to also be affected by temperature. These showed similarity to iron-sulfur binding proteins involved in reduction-oxidation reactions. However, biotin starvation leads to growth arrest at low cell densities (24), and the differential expression of various proteins observed may be related to changes in both growth phase and stress due to limiting biotin levels. Nevertheless, the up-regulation of toxin synthesis during biotin and glucose-amino acid limitation at 37 but not 22 and 42°C shows that temperature regulation is general and may act at a fundamental level.
In order for bacteria to effectively compete and survive in their ecological niche(s), they must be able to sense environmental changes and respond accordingly. Several principles of regulation of gene expression by temperature have been described in bacteria, e.g., supercoiling of DNA, secondary structure of RNA, activities of proteins, and temperature monitoring by signal transduction via two-component regulatory systems (17). Recently, fatty acids were suggested to act as temperature sensors in Bacillus and Synechocystis (1, 44). The cold shock response comprises proteins in general metabolism, as well as RNA chaperones and fatty acid desaturases, resulting in correct mRNA and membrane phospholipid structure, respectively (38). The heat shock response is triggered when cells are exposed to either cell-damaging agents or high temperature. The proteins induced are mainly involved in the maintenance of correct protein structure, e.g., chaperones, proteases, transcription factors, or ribosome binding proteins (3, 51). However, the heat shock protein GroEL has also been suggested to be virulence associated in C. difficile (14). Up-regulation of virulence determinants in response to transition from ambient temperature to that of warm-blooded animals is different from heat or cold shock and has evolved in such different bacterial genera as Bordetella, Borrelia, Escherichia, Salmonella, Shigella, Vibrio, and Yersinia (25, 33).
The alternative sigma factor TcdD of the C. difficile PaLoc has similarities to the regulatory proteins BotR in Clostridium botulinum (31), TetR in Clostridium tetani (32) and UviA in C. perfringens (10), all suggested to be sigma factors (28). TcdD also shows homology to extracellular function (ECF) sigma factors (28). ECF sigma factors are controlled by their cognate anti-sigma factors, which often are membrane proteins capable of sequestering the ECF sigma factor. At a given stimulus, the anti-sigma factor releases the ECF sigma factor to the cytosol, enabling transcription of the respective target genes (15, 41). ECF sigma factors are thought to act as general stress mediators responding to a variety of signals, such as envelope or metabolic stress, and several ECF sigma factors are cotranscribed with their specific anti-sigma factors (34, 41). The apparent positive autoregulation of tcdD suggests that a negative factor is involved to modulate TcdD activity. Two candidates are TcdE and TcdC, encoded by the PaLoc of C. difficile, and TcdC has also been suggested to negatively regulate toxin expression (16). However, expression of tcdD was blocked both by altered temperature (22 and 42°C) and by glucose in C. perfringens. C. perfringens lacks homologues to tcdE and tcdC, suggesting that these genes are not required for modulating TcdD activity. However, we cannot exclude the possibility that tcdE and/or tcdC has a regulatory role in C. difficile. In view of the various growth conditions affecting toxin production in C. difficile, TcdD activity may be modulated by a pleiotropic regulator and/or by other sigma factors competing with TcdD for RNA polymerase binding.
In summary, this is the first report of up-regulation of virulence by host temperature in clostridia. The expression of C. difficile toxins A and B and the toxin-specific sigma factor TcdD was temperature dependent, with a maximum at 37°C. The results further support the notion that toxin regulation is linked to the induction of metabolic pathways involved in butyric acid production in PY broth. The findings presented here encourage further studies regarding the regulation of virulence in C. difficile with respect to TcdD, growth temperature, and metabolism.
Acknowledgments
This work was supported by grants from the Vårdal Foundation, Stockholm, Sweden, and by a Ph.D. student grant provided by the Microbiology and Tumor biology Center at the Karolinska Institute. Protein data were obtained at the Protein Analysis Center, Karolinska Institute. The sequence data were produced by the Clostridium difficile Sequencing Group at the Sanger Center and can be obtained at http://www.sanger.ac.uk/Projects/C_difficile/.
We are grateful for the excellent technical support provided by Anna-Karin Persson. We thank Nagraj Mani for kindly providing all pTUM plasmids.
Editor: J. T. Barbieri
REFERENCES
- 1.Aguilar, P. S., A. M. Hernandez-Arriaga, L. E. Cybulski, A. C. Erazo, and D. de Mendoza. 2001. Molecular basis of thermosensing: two-component signal transduction thermometer in Bacillus subtilis. EMBO J. 20:1681-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Barrak, A., J. Embil, B. Dyck, K. Olekson, M. Alfa, and A. Kabani. 1999. An outbreak of toxin A negative, toxin B positive Clostridium difficile-associated diarrhea in a Canadian tertiary-care hospital. Can. Commun. Dis. Rep. 25:65-69. [PubMed] [Google Scholar]
- 3.Arsène, F., T. Tomoyasu, and B. Bukau. 2000. The heat shock response of Escherichia coli. Int. J. Food Microbiol. 55:3-9. [DOI] [PubMed] [Google Scholar]
- 4.Bannam, T. L., and J. I. Rood. 1993. Clostridium perfringens-Escherichia coli shuttle vectors that carry single antibiotic resistance determinants. Plasmid 29:233-235. [DOI] [PubMed] [Google Scholar]
- 5.Bogdan, J. A., J. Nazario-Larrieu, J. Sarwar, P. Alexander, and M. S. Blake. 2001. Bordetella pertussis autoregulates pertussis toxin production through the metabolism of cysteine. Infect. Immun. 89:6823-6830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braun, V., T. Hundsberger, P. Leukel, M. Sauerborn, and C. von Eichel-Streiber. 1996. Definition of the single integration site of the pathogenicity locus in Clostridium difficile. Gene 181:29-38. [DOI] [PubMed] [Google Scholar]
- 7.Castagliuolo, I., A. C. Keates, B. Qiu, C. P. Kelly, S. Nikulasson, S. E. Leeman, and C. Pothoulakis. 1997. Increased substance P responses in dorsal root ganglia and intestinal macrophages during Clostridium difficile toxin A-induced enteritis in rats. Proc. Natl. Acad. Sci. USA 94:4788-4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choen, S. H., Y. J. Tang, B. Hansen, and J. Silva, Jr. 1998. Isolation of a toxin B-deficient mutant strain of Clostridium difficile in a case of recurrent C. difficle associated diarrhea. Clin. Infect. Dis. 26:410-412. [DOI] [PubMed] [Google Scholar]
- 9.Dupuy, B., and A. L. Sonenshein. 1998. Regulated transcription of Clostridium difficile toxin genes. Mol. Microbiol. 27:107-120. [DOI] [PubMed] [Google Scholar]
- 10.Garnier, T., and S. T. Cole. 1988. Studies of UV-inducible promoters from Clostridium perfringens in vivo and in vitro. Mol. Microbiol. 2:607-614. [DOI] [PubMed] [Google Scholar]
- 11.Gerhardt, A., I. Çinkaya, D. Linder, G. Huisman, and W. Buckel. 2000. Fermentation of 4-aminobutyrate by Clostridium aminobutyricum: cloning of two genes involved in the formation and dehydration of 4-hydroxybutyryl-CoA. Arch. Microbiol. 174:189-199. [DOI] [PubMed] [Google Scholar]
- 12.Hammond, G. A., and J. L. Johnson. 1995. The toxigenic element of Clostridium difficile strain 10463. Microb. Pathog. 19:203-213. [DOI] [PubMed] [Google Scholar]
- 13.Haslam, S. C., J. M. Ketley, T. J. Mitchel, J. Stephen, D. W. Burdon, and D. C. A. Candy. 1986. Growth of Clostridium difficile and production of toxins A and B in complex and defined media. J. Med. Microbiol. 21:293-297. [DOI] [PubMed] [Google Scholar]
- 14.Hennequin, C., F. Porcheray, A. Waligora-Dupriet, A. Collignon, M. Barc, P. Bourlioux, and T. Karjalainen. 2001. GroEL (Hsp60) of Clostridium difficile is involved in cell adherence. Microbiology 147:87-96. [DOI] [PubMed] [Google Scholar]
- 15.Hughes, K. T., and K. Mathee. 1998. The anti-sigma factors. Annu. Rev. Microbiol. 52:231-286. [DOI] [PubMed] [Google Scholar]
- 16.Hundsberger, T., V. Braun, M. Weidmann, P. Leukel, M. Sauerborn, and C. von Eichel-Streiber. 1997. Transcription analysis of the genes tcdA-E of the pathogenicity locus of Clostridium difficile. Eur. J. Biochem. 244:735-742. [DOI] [PubMed] [Google Scholar]
- 17.Hurme, R., and M. Rhen. 1998. Temperature sensing in bacterial gene regulation—what it all boils down to. Mol. Microbiol. 30:1-6. [DOI] [PubMed] [Google Scholar]
- 18.Just, I., J. Selzer, M. Wilm, C. von Eichel-Streiber, M. Mann, and K. Aktories. 1995. Glucosylation of Rho proteins by Clostridium difficile toxin B. Nature 375:500-503. [DOI] [PubMed] [Google Scholar]
- 19.Just, I., M. Wilm, J. Selzer, G. Rex, C. von Eichel-Streiber, M. Mann, and K. Aktories. 1995. The enterotoxin from Clostridium difficile (ToxA) monoglucosylates the Rho proteins. J. Biol. Chem. 270:13932-13936. [DOI] [PubMed] [Google Scholar]
- 20.Kane, J. F., J. Wakim, and R. S. Fischer. 1981. Regulation of glutamate dehydrogenase in Bacillus subtilis. J. Bacteriol. 148:1002-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karasawa, T., S. Ikoma, K. Yamakawa, and S. Nakamura. 1995. A defined medium for Clostridium difficile. Microbiology 141:371-375. [DOI] [PubMed] [Google Scholar]
- 22.Karasawa, T., T. Maegawa, T. Nojiri, K. Yamakawa, and S. Nakamura. 1997. Effect of arginine on toxin production by Clostridium difficile in defined medium. Microbiol. Immunol. 41:581-585. [DOI] [PubMed] [Google Scholar]
- 23.Karlsson, S., A. Lindberg, E. Norin, L. G. Burman, and T. Åkerlund. 2000. Toxins, butyric acid, and other short-chain fatty acids are coordinately expressed and down-regulated by cysteine in Clostridium difficile. Infect. Immun. 68:5881-5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karlsson, S., L. G. Burman, and T. Åkerlund. 1999. Suppression of toxin production in Clostridium difficile VPI 10463 by amino acids. Microbiology 145:1683-1693. [DOI] [PubMed] [Google Scholar]
- 25.Konkel, M. E., and K. Tilly. 2000. Temperature-regulated expression of bacterial virulence genes. Microbes Infect. 2:157-166. [DOI] [PubMed] [Google Scholar]
- 26.Lyerly, D. M., H. C. Krivan, and T. D. Wilkins. 1988. Clostridium difficile: its disease and toxins. Clin. Microbiol. Rev. 1:1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maegawa, T., T. Karasawa, T. Otha, X. Wang, H. Kato, H. Hayashi, and S. Nakamura. 2002. Linkage between toxin production and purine biosynthesis in Clostridium difficile. J. Med. Microbiol. 51:34-41. [DOI] [PubMed] [Google Scholar]
- 28.Mani, N., and B. Dupuy. 2001. Regulation of toxin synthesis in Clostridium difficile by an alternative RNA polymerase sigma factor. Proc. Natl. Acad. Sci. 98:5844-5849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mani, N., D. Lyras, L. Barroso, P. Howarth, T. Wilkins, J. I. Rood, A. L. Sonenshine, and B. Dupuy. 2002. Environmental response and autoregulation of Clostridium difficile TxeR, a sigma factor for toxin gene expression. J. Bacteriol. 184:5971-5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maniatis, T., J. Fritsch, and J. Sambrook. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 31.Marvaud, J. C., M. Gilbert, K. Inoue, Y. Fujinaga, K. Oguma, and M. R. Popoff. 1998. BotR/A is a positive regulator of botulinum neurotoxin and associated non-toxin protein genes in Clostridium botulinum A. Mol. Microbiol. 29:1009-1018. [DOI] [PubMed] [Google Scholar]
- 32.Marvaud, J. C., U. Eisel, T. Binz, H. Nieman, and M. R. Popoff. 1998. TetR is a positive regulator of the tetanus toxin gene in Clostridium tetani and is homologous to BotR. Infect. Immun. 66:5698-5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mekalanos, J. J. 1992. Environmental signals controlling expression of virulence determinants in bacteria. J. Bacteriol. 174:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Missiakas, D., and S. Raina. 1998. The extra-cytoplasmic function sigma factors: role and regulation. Mol. Microbiol. 28:1059-1066. [DOI] [PubMed] [Google Scholar]
- 35.Moncrief, J. S., L. A. Barosso, and T. D. Wilkins. 1997. Positive regulation of Clostridium difficile toxins. Infect. Immun. 65:1105-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mullany, P., P. M. Clayton, M. J. Pallen, R. Slone, A. Al-Saleh, and S. Tabaqchali. 1994. Genes encoding homologues of three consecutive enzymes in the butyrate/butanol-producing pathway of Clostridium acetobutylicum are clustered on the Clostridium difficile chromosome. FEMS Microbiol. Lett. 124:61-69. [DOI] [PubMed] [Google Scholar]
- 37.Osgood, D. P., N. P. Wood, and J. F. Sperry. 1993. Nutritional aspects of cytotoxin production by Clostridium difficile. Appl. Environ. Microbiol. 59:3985-3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phadtare, S., J. Alsina, and M. Inouye. 1999. Cold-shock responses and cold-shock proteins. Curr. Opin. Microbiol. 2:175-180. [DOI] [PubMed] [Google Scholar]
- 39.Poxton, I. R., J. McCoubrey, and G. Blair. 2001. The pathogenicity of Clostridium difficile. Clin. Microbiol. Infect. 7:421-427. [DOI] [PubMed] [Google Scholar]
- 40.Prugnola, A., B. Aricò, R. Manetti, R. Rappuoli, and V. Scarlato. 1995. Response of the bvg regulon of Bordetella pertussis to different temperatures and short-term temperature shifts. Microbiology. 141:2529-2543. [DOI] [PubMed] [Google Scholar]
- 41.Ravio, T. L., and T. J. Silhavy. 2001. Periplasmic stress and ECF sigma factors. Annu. Rev. Microbiol. 55:591-624. [DOI] [PubMed] [Google Scholar]
- 42.Rupkin, M., V. Braun, F. Soehn, M. Janc, M. Hofstetter, R. Laufenberg-Feldmann, and C. von Eichel-Streiber. 1997. Characterization of polymorphism in the toxin A and B genes in Clostridium difficile. FEMS. Microbiol. Lett. 148:197-202. [DOI] [PubMed] [Google Scholar]
- 43.Stubbs, S. L., J. S. Brazier, G. L. O'Neill, and B. I. Duerden. 1999. PCR targeted to the 16S-23S rRNA gene intergenic spacer region of Clostridium difficile and construction of a library consisting of 116 different PCR ribotypes. J. Clin. Microbiol. 37:461-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suzuki, I., D. A. Los, Y. Kanesaki, K. Mikami, and N. Murata. 2000. The pathway for perception and transduction of low-temperature signals in Synechocystis. EMBO J. 19:1327-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tan, K. S., B. Y. Wee, and K. P. Song. 2001. Evidence for holine function of tcdE gene in the pathogenicity of Clostridium difficile. J. Med. Microbiol. 50:613-619. [DOI] [PubMed] [Google Scholar]
- 46.von Eichel-Streiber, C., P. Boquet, M. Sauerborn, and M. Thelestam. 1996. Large clostridial cytotoxins—a family of glucosyltransferase modifying small GTP-binding proteins. Trends Microbiol. 4:375-382. [DOI] [PubMed] [Google Scholar]
- 47.Wershil, B. K., I. Castagliuolo, and C. Pothoulakis. 1998. Direct evidence of mast cell involvement in Clostridium difficile toxin A-induced enteritis in mice. Gastroenterology 114:956-964. [DOI] [PubMed] [Google Scholar]
- 48.Yamakawa, K., S. Kamiya, X. Q. Meng, T. Karasawa, and S. Nakamura. 1994. Toxin production by Clostridium difficile in a defined medium with limited amino acids. J. Med. Microbiol. 41:319-323. [DOI] [PubMed] [Google Scholar]
- 49.Yamakawa, K., T. Karasawa, T. Ikoma, and S. Nakamura. 1996. Enhancement of Clostridium difficile toxin production in biotin-limited conditions. J. Med. Microbiol. 44:111-114. [DOI] [PubMed] [Google Scholar]
- 50.Yamakawa, K., T. Karasawa, T. Ohta, H. Hayashi, and S. Nakamura. 1998. Inhibition of enhanced toxin production by Clostridium difficile in biotin-limited conditions. J. Med. Microbiol. 47:767-771. [DOI] [PubMed] [Google Scholar]
- 51.Yura, T., and K. Nakahigashi. 1999. Regulation of the heat-shock response. Curr. Opin. Microbiol. 2:153-158. [DOI] [PubMed] [Google Scholar]
- 52.Zhao, Y., and S. Melville. 1998. Identification and characterization of sporulation-dependent promoters upstream of the enterotoxin gene (cpe) of Clostridium perfringens. J. Bacteriol. 180:136-142. [DOI] [PMC free article] [PubMed] [Google Scholar]