Abstract
The present studies in Nramp1−/− BALB/c and Nramp1+/+ CBA mice question the significance of this genotype as a determinant of the level of gut colonization following oral administration of naturally attenuated or highly virulent Salmonella strains. In line with previous results in BALB/c hosts, vector priming of CBA mice with Salmonella enterica serovar Stanley was found to significantly compromise the immunogenicity of a recombinant construct expressing a foreign pilus protein.
The construction of Salmonella-based multivalent vaccines, which can simultaneously confer protection against typhoid and other infectious diseases, would expedite mass immunization programs in developing countries. This strategy is threatened, however, by the possible adverse consequences of the environmental priming of potential vaccinees. Experimental studies have yielded conflicting data concerning the impact of deliberate priming with the vector strain on the immunogenicity of recombinant Salmonella vaccines. Two studies in which aroA Salmonella enterica serovar Dublin vectors were used failed to detect any reduction in responses to foreign antigen delivered orally or systemically to vector-primed hosts (2, 19). Studies in three other laboratories employed aroA S. enterica serovar Typhimurium (11, 14) or the naturally attenuated S. enterica serovar Stanley (1, 18) as vectors and concluded that prior exposure to the vector significantly compromises both serum and intestinal antibody responses to a range of foreign antigens. The factors that determine the impact of vector priming remain unclear but include the nature of the foreign antigen and the Salmonella vector (18).
To date, all studies of the significance of vector priming have been performed in BALB/c mice, which have the Nramp1−/− genotype and are highly susceptible to Salmonella infection. The Nramp1-associated difference in the capacity to control the growth of systemically administered virulent serovar Typhimurium is dramatic, with 50% lethal doses typically 1,000-fold higher in resistant Nramp1+/+ strains (10, 13). The wild-type Nramp1 protein is primarily expressed in macrophages within the spleen and liver (8, 17), where it is recruited to the membrane of Salmonella-containing phagosomes. Nramp1 functions as a divalent metal ion transporter, suggesting that its key function is to reduce cation availability within the phagosome, but a role in promoting phagosome maturation has also been proposed (3, 7).
The relevance of Nramp1 in the present context derives from Eisenstein's suggestion (5) that Nramp1+/+ mice might be more appropriate hosts for studies of Salmonella infection, which are designed to assist the development of typhoid vaccines. This was based on a consideration of the relative significance of humoral and cell-mediated responses in immunity to Salmonella. Whereas cell-mediated immunity is critical for the protection of Nramp1−/− hosts, antibodies induced by inactivated vaccines are sufficient to protect Nramp1+/+ mice and humans from (low-dose) challenge with virulent Salmonella (5).
The aims of this study were twofold. First, we sought to ascertain whether the Nramp1 genotype influences the extent to which orally administered Salmonella can colonize the gut-associated lymphoid tissue (GALT). Since the systemic spread of attenuated S. enterica serovar Typhi vaccines is deemed undesirable (16), the immunogenicity of recombinant vaccines based upon such vectors will depend on their capacity to persist within the GALT. The second objective was to determine whether vector priming compromises the immunogenicity of recombinant Salmonella vaccines in Nramp1+/+ mice. This also has clear implications for the development of human vaccines.
Salmonella infection in Nramp1−/− and Nramp1+/+ hosts.
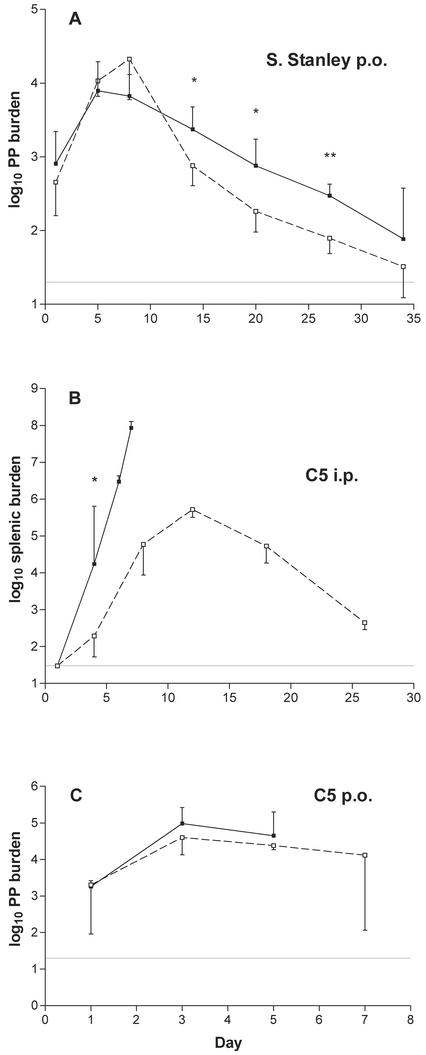
Serovar Stanley carries the same O-4,5,12 lipopolysaccharide (LPS) as serovar Typhimurium but is naturally attenuated for mice; after oral administration, it shows prolonged GALT colonization (1) but is unable to spread systemically (data not shown). Groups of BALB/c (Nramp1−/−) and CBA (Nramp1+/+) mice were orally dosed with 1.1 × 109 CFU of serovar Stanley, and the levels of GALT colonization were compared by determining Peyer's patch burdens at various intervals, as done previously (18). Similar numbers of bacteria were recovered from the two strains at each of the first three time points, with mean burdens of ca. 104 CFU on days 5 through 8 (Fig. 1A). Thereafter, significantly fewer bacteria were recovered from the Nramp1+/+ hosts. These data suggest that innate immunity in the early period is similarly effective in the two strains, with the CBA mice subsequently benefiting from more effective major histocompatibility complex-linked acquired immune responses.
FIG. 1.
Comparative growth of Salmonella strains in BALB/c and CBA mice. Animals were dosed orally (p.o. [A and C]) or intraperitoneally (i.p. [B]) with serovar Stanley (A) or serovar Typhimurium strain C5 (B and C), as described in the text. Bacterial burdens in Peyer's patches (PP [A and C]) or spleens (B) of BALB/c (▪, solid lines) and CBA (□, broken lines) mice were determined by plating homogenates on XLD medium. Graphs show log10 recoveries (geometric mean ± standard deviation; n = 4 at each time point in each experiment), with broken lines showing limits of detection. Student's t test (two-tailed) was used to assess the significance of the observed differences (*, P < 0.05; **, P < 0.01).
The similarity of the early colonization profiles was unexpected and prompted an experiment to confirm the Nramp1 status of the mice being used. Groups of CBA and BALB/c mice were injected intraperitoneally with 40 CFU of the highly virulent C5 strain of serovar Typhimurium. Spleens were removed at various time points, and bacterial loads were determined by plating homogenates on XLD medium (Oxoid). BALB/c mice were unable to control C5 growth in the spleen, and only 4 (of 13) mice were still alive on day 7; these animals had splenic burdens of ca. 108 CFU by this time. In contrast, bacterial loads in the spleens of CBA mice were significantly lower on day 4 (P < 0.05) and reached a peak burden of <106 on day 12 (Fig. 1B). Thereafter, the infection was cleared and no mouse of this strain died. This experiment confirmed the presumed difference in the Nramp1 status of our animals.
The benefit conferred by Nramp1, in terms of restricting the systemic growth of Salmonella, is less apparent with strains of lower virulence or with aroA-attenuated vectors (6, 10, 15). It was therefore of interest to ascertain whether an Nramp1-related difference in GALT colonization might be apparent if mice were orally infected with the highly virulent C5 strain. Again, there was no difference in the Peyer's patch colonization profiles seen in BALB/c or CBA mice (dose of 1.1 × 108 bacteria [Fig. 1C]). The last four BALB/c mice died as a result of infection before day 7, whereas the remaining CBA mice showed no visible signs of illness at this time, consistent with their capacity to restrict systemic growth.
Significance of vector priming: relevance of host Nramp1 status.
Groups of BALB/c and CBA mice were orally dosed with 1.1 × 109 CFU of serovar Stanley on day −70, with additional mice kept aside as controls. Ten weeks after primary infection, all animals were dosed with 1.3 × 109 CFU of serovar Stanley-K88, a recombinant expressing the Escherichia coli pilus protein K88 (1). Serum and fecal pellet samples were collected at intervals for the determination of anti-K88 and anti-LPS antibody responses by enzyme-linked immunosorbent assay (ELISA), as detailed elsewhere (18).
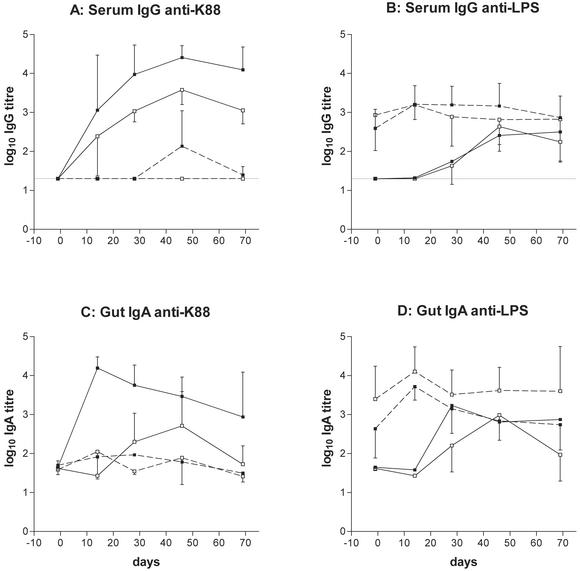
The efficacy of vector priming was illustrated by the secondary serum (Fig. 2B) and mucosal (Fig. 2D) anti-LPS responses observed in both CBA and BALB/c mice. Control and vector-primed BALB/c mice developed serum anti-K88 (Fig. 2A) and anti-LPS (Fig. 2B) responses comparable to those observed previously (1, 18). Naïve animals displayed strong, consistent immunoglobulin G (IgG) responses to K88, with comparatively weak and delayed responses to vector LPS. Vector priming completely prevented the development of the former and resulted in secondary responses to LPS. A similar pattern of responses was seen in control and vector-primed CBA mice; again, the serum IgG anti-K88 response was completely inhibited as a consequence of prior exposure to the vector strain (Fig. 2A). Of interest was the finding that the primary serum response to K88 was significantly lower in CBA than in BALB/c mice (P < 0.01 on days 28 and 69 by one-way analysis of variance with Bonferroni's posttest).
FIG. 2.
Impact of vector priming in Nramp1+/+ mice. Control and vector-primed BALB/c (▪) and CBA (□) animals were dosed with serovar Stanley-K88 on day 0. Blood and fecal pellet samples were collected at intervals, and serum IgG (A and B) and gut IgA responses (C and D) were determined by ELISA. The latter are expressed as antibody titer per milligram of IgA to correct for varying immunoglobulin content. Graphs show log10 anti-K88 (A and C) and anti-LPS (B and D) ELISA titers (geometric mean ± standard deviation; n = 5 at each time point) in control (solid lines) and vector-primed (broken lines) animals. Broken lines in panels A and B show the limit of detection of serum ELISAs at 1 in 20.
The state of hyporesponsiveness to K88 extended to the mucosal IgA responses in both mouse strains (Fig. 2C), and in BALB/c mice, the impact of vector priming was highly significant (P < 0.01 on days 14, 28, 46, and 69). However, the mucosal anti-K88 response in control CBA mice was only modest and consequently, the differences between the (corrected) antibody titers of control and vector-primed mice did not achieve statistical significance. As with the serum IgG response, the primary intestinal IgA response to K88 was significantly weaker in CBA than in BALB/c mice on days 14, 28 (P < 0.01), and 69 (P < 0.05).
IgG subclass analysis of serum anti-LPS responses.
Sera collected in the previous experiment were analyzed to ascertain the IgG1:IgG2a subclass ratios of antibodies to LPS in BALB/c and CBA mice. Samples collected 49 and 69 days after primary infection with serovar Stanley and at the same intervals after immunization (of 20-week-old controls) with serovar Stanley-K88 were tested. In all cases, the CBA sera showed a slightly higher proportion of IgG1 antibodies, and at three of the four time points, this difference was significant at the 5% level (Table 1).
TABLE 1.
IgG subclass ratios of serum anti-LPS responses in CBA and BALB/c micea
| S. enterica strain and sampling time pointb | CBA | BALB/c |
|---|---|---|
| Serovar Stanley | ||
| D49 | 1.04 ± 0.26 | 0.81 ± 0.16 |
| D69 | 1.22 ± 0.26 | ∗0.87 ± 0.10 |
| Serovar Stanley-K88 | ||
| D49 | 1.30 ± 0.38 | ∗0.87 ± 0.23 |
| D69 | 1.06 ± 0.17 | ∗0.62 ± 0.21 |
Serum IgG anti-LPS responses of CBA and BALB/c mice (from the experiment shown in Fig. 2) were dissected into IgG1 and IgG2a responses. Data show ratios of IgG1:IgG2a ELISA titers (geometric mean ± standard deviation; n = 5), and an asterisk denotes significance at the 5% level, as determined by the two-tailed t test.
D, day.
Our failure to detect any significant difference in the GALT colonization profiles of attenuated or virulent Salmonella strains in mice of different Nramp1 genotypes suggests that the complement of resident and migratory phagocytic cells within the infected gut fails to exert any Nramp1-mediated control of bacterial growth. This contrasts with the well-recognized and dramatic impact of the Nramp1 genotype on the fate of systemically administered Salmonella. Evidently, the advantage conferred by the Nramp1+/+ genotype is expressed only systemically, with even Nramp1+/+ hosts experiencing colonization of the GALT, presumably in deference to the paramount need to restrict systemic growth. Consistent with this notion, Salmonella strains capable of prolonged GALT colonization but unable to translocate to organs of the reticuloendothelial system are markedly attenuated compared with wild-type strains such as serovar Typhimurium C5. Examples include mutant strains cured of the virulence plasmid (9) and naturally attenuated strains such as serovar Stanley.
The immunogenicity of recombinant Salmonella vaccines designed for use in man will be dependent upon the capacity of the vector strain to colonize the GALT, as the systemic spread of vaccine organisms is undesirable. In this context, the lack of any detectable Nramp1-related impact on GALT colonization by Salmonella is encouraging, if the Nramp1+/+ host indeed represents a more appropriate model for vaccine development (5). The present findings will need to be confirmed in experiments with Nramp1 congenic mouse strains, however.
BALB/c and CBA mice displayed similar anti-LPS responses following oral infection with serovar Stanley or serovar Stanley-K88, consistent with the similarity of their GALT colonization profiles. However, the significantly different subclass ratios of the serum IgG responses suggest a different Th1/Th2 bias in the two strains. Moreover, antibody responses to the foreign antigen were significantly lower in the Nramp1+/+ hosts, although this could reflect the involvement of H-2 genes. The latter might determine responsiveness to a particular foreign antigen (6) and/or effect the more rapid clearance of the vaccine construct from the GALT (Fig. 1A). Further studies with Nramp1 or H-2 congenic mice would clarify this issue, but the significant difference in the anti-K88 responses seen here confirms the value of including outbred mice when assessing the immunogenicity of recombinant vaccines (6).
A recent study by Soo et al. (15) used congenic mouse strains to test the impact of Nramp1 on responses to foreign antigens delivered by recombinant Salmonella. In this instance, total antibody responses (to a fragment of tetanus toxin) were similar in Nramp1+/+ and Nramp1−/− mice although IgE and cytokine responses were suggestive of a Th1 bias in the former and a Th2 bias in the latter. Whether Salmonella infection elicits Nramp1-associated differences in the patterns of cytokine production remains controversial, however (4, 12).
A key finding of the present study was that preexisting antivector immunity compromises the immunogenicity of the serovar Stanley-K88 construct in Nramp1+/+ mice. This observation extends our previous findings in BALB/c mice (1, 18) to hosts that might better reflect the responses of human vaccine recipients (5). This study therefore supports other recent data (18) indicating that vector priming could prejudice the use of recombinant Salmonella vaccines in regions where these bacteria may be endemic. Further studies will be needed to elucidate the mechanism of hyporesponsiveness seen in vector-primed hosts before compensating strategies can be designed and evaluated.
Acknowledgments
This work was supported by the Pest Animal Control Cooperative Research Centre, Canberra, Australia.
Editor: J. D. Clements
REFERENCES
- 1.Attridge, S. R., R. Davies, and J. T. LaBrooy. 1997. Oral delivery of foreign antigens by attenuated Salmonella: consequences of prior exposure to the vector strain. Vaccine 15:155-162. [DOI] [PubMed] [Google Scholar]
- 2.Bao, J. X., and J. D. Clements. 1991. Prior immunologic experience potentiates the subsequent antibody response when Salmonella strains are used as vaccine carriers. Infect. Immun. 59:3841-3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cuellar-Mata, P., N. Jabado, J. Liu, W. Furuta, B. B. Finlay, P. Gros, and S. Grinstein. 2002. Nramp1 modifies the fusion of Salmonella typhimurium-containing vacuoles with cellular endomembranes in macrophages. J. Biol. Chem. 277:2258-2265. [DOI] [PubMed] [Google Scholar]
- 4.Eckmann, L., J. Fierer, and M. F. Kagnoff. 1996. Genetically resistant (Ityr) and susceptible (Itys) congenic mouse strains show similar cytokine responses following infection with Salmonella dublin. J. Immunol. 156:2894-2900. [PubMed] [Google Scholar]
- 5.Eisenstein, T. K., L. M. Killar, and B. M. Sultzer. 1984. Immunity to infection with Salmonella Typhimurium: mouse-strain differences in vaccine- and serum-mediated protection. J. Infect. Dis. 150:425-435. [DOI] [PubMed] [Google Scholar]
- 6.Fayolle, C., D. O'Callaghan, P. Martineau, A. Charbit, J. M. Clement, M. Hofnung, and C. Leclerc. 1994. Genetic control of antibody responses induced against an antigen delivered by recombinant attenuated Salmonella typhimurium. Infect. Immun. 62:4310-4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forbes, J. R., and P. Gros. 2001. Divalent-metal transport by NRAMP proteins at the interface of host-pathogen interactions. Trends Microbiol. 9:397-403. [DOI] [PubMed] [Google Scholar]
- 8.Govoni, G., F. Canonne-Hergaux, C. G. Pfeifer, S. L. Marcus, S. D. Mills, D. J. Hackam, S. Grinstein, D. Malo, B. B. Finlay, and P. Gros. 1999. Functional expression of Nramp1 in the murine macrophage line RAW264.7. Infect. Immun. 67:2225-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gulig, P. A., and R. Curtiss III. 1987. Plasmid-associated virulence of Salmonella typhimurium. Infect. Immun. 55:2891-2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hormaeche, C. E. 1979. Natural resistance to Salmonella typhimurium in different inbred mouse strains. Immunology 37:311-318. [PMC free article] [PubMed] [Google Scholar]
- 11.Kohler, J. J., L. B. Pathangey, S. R. Gillespie, and T. A. Brown. 2000. Effect of pre-existing immunity to Salmonella on the immune response to recombinant Salmonella enterica serovar Typhimurium expressing a Porphyromonas gingivalis hemagglutinin. Infect. Immun. 68:3116-3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lalmanach, A.-C., and F. Lantier. 1999. Host cytokine response and resistance to Salmonella infection. Microbes Infect. 1:719-726. [DOI] [PubMed] [Google Scholar]
- 13.Plant, J., and A. A. Glynn. 1974. Natural resistance to Salmonella infection, delayed hypersensitivity and Ir genes in different strains of mice. Nature 248:345-347. [DOI] [PubMed] [Google Scholar]
- 14.Roberts, M., A. Bacon, J. Li, and S. Chatfield. 1999. Prior immunity to homologous and heterologous Salmonella serotypes suppresses local and systemic anti-fragment C antibody responses and protection from tetanus toxin in mice immunized with Salmonella strains expressing fragment C. Infect. Immun. 67:3810-3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soo, S.-S., B. Villareal-Ramos, C. M. A. Khan, C. E. Hormaeche, and J. M. Blackwell. 1998. Genetic control of immune response to recombinant antigens carried by attenuated Salmonella typhimurium vaccine strain: Nramp1 influences T-helper subset responses and protection against leishmanial challenge. Infect. Immun. 66:1910-1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tacket, C. O., M. B. Sztein, S. S. Wasserman, G. Losonsky, K. L. Kotloff, T. L. Wyant, J. P. Nataro, R. Edelman, J. Perry, P. Bedford, D. Brown, S. Chatfield, G. Dougan, and M. M. Levine. 2000. Phase 2 clinical trial of attenuated Salmonella enterica serovar Typhi oral live vector vaccine CVD 908-htrA in U.S. volunteers. Infect. Immun. 68:1196-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vidal, S. M., D. Malo, K. Vogan, E. Skamene, and P. Gros. 1993. Natural resistance to infection with intracellular parasites: isolation of a candidate for Bcg. Cell 73:469-485. [DOI] [PubMed] [Google Scholar]
- 18.Vindurampulle, C. J., and S. R. Attridge. 2003. Impact of vector priming on the immunogenicity of recombinant Salmonella vaccines. Infect. Immun. 71:287-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whittle, B. L., and N. K. Verma. 1997. The immune response to a B-cell epitope delivered by Salmonella is enhanced by prior immunological experience. Vaccine 15:1737-1740. [DOI] [PubMed] [Google Scholar]