Abstract
We constructed two recombinant Mycobacterium bovis BCG (rBCG) strains expressing ESAT-6 of Mycobacterium tuberculosis, named rBCG-1 and rBCG-2. rBCG-1 contained the ESAT-6 gene linked to BCG hsp60 and expressed a fusion protein, while rBCG-2, with a secretory sequence, could secret ESAT-6 into the culture medium. There was no evidence for increased virulence of the two rBCG strains when we made a comparison between them and BCG with regard to organ bacterial loads, lung histology, and survival time. rBCG-1 induced significantly higher specific antibody titers and stronger cellular immune response than BCG, whereas rBCG-2 had immunogenicity similar to that of the parental BCG strain. Both rBCG-1 and rBCG-2 conferred marked protection against M. tuberculosis infection, yet in terms of protective efficacy, they showed no significant improvements upon conventional BCG vaccine.
Tuberculosis (TB) is one of the leading infectious diseases in adults, causing over 8 million new cases and 2 million deaths annually. It is estimated that one-third of the world's population is infected with Mycobacterium tuberculosis. The human immunodeficiency virus pandemic and the emergency of multidrug-resistant strains of the causative bacilli have led to an elevated incidence of TB. The World Health Organization has recently declared the current situation to be a global emergency and has made it a priority to develop more effective vaccines against TB (11).
Mycobacterium bovis bacillus Calmette-Guérin (BCG), a live, attenuated strain of M. bovis, is currently the only available vaccine for prevention of TB. BCG has exhibited protection against severe and fatal TB in children. However, BCG has shown itself to be of varying efficacy (ranging from 0 to 80%) in protecting adults from pulmonary TB, depending on the population tested (8, 12, 30). Thus, an improved vaccine is urgently needed to replace BCG and to prevent TB effectively. Several new types of TB vaccine preparations, including subunit vaccines, live attenuated vaccines, recombinant BCG (rBCG), and DNA vaccines, are currently investigated in experiments.
ESAT-6 (1), a secretory protein from M. tuberculosis, is a dominant target for cell-mediated immunity in the early phase of TB in TB patients as well as in various animal models, causing T-cell proliferation and gamma interferon (IFN-γ) production (5, 16, 23). ESAT-6 has been considered to be a protective antigen that can be used for future vaccine development, while BCG, which lacks 15 to 16 regions of the M. tuberculosis genome, is not able to express ESAT-6 as a result of deletion of esat-6 gene (3, 15).
In the present study, we have constructed two rBCG strains expressing ESAT-6 and evaluated the virulence and the humoral, cell-mediated, and protective immune responses of them. There was no evidence for increased virulence of the two rBCG strains when we compared them with the BCG strain, and one rBCG strain that expresses ESAT-6 fused to HSP60 could elicit stronger specific immune response. This is encouraging for the future vaccine development; however, despite the enhanced in vitro immunological responses, the recombinant vaccines expressing ESAT-6 afford no greater protection against M. tuberculosis infection.
MATERIALS AND METHODS
Animals.
Pathogen-free BALB/c female mice were obtained from Sichuan Antibiotics Institute. The mice were 6 to 8 weeks old at the time of vaccination.
Bacterial strains and cultures.
Escherichia coli DH5α was grown in Luria-Bertani medium and used for cloning. M. bovis BCG (obtained from Chengdu Biological Products Institute), rBCG, and M. tuberculosis strain H37Rv were cultivated in Sauton medium (0.25 g of MgSO4 · 7H2O, 0.25 g of K2HPO4, 1 g of citric acid, 4 g of sodium glutamate, 30 ml of glycerol, 5 mg of ZnSO4, and 25 mg of ferrum-ammonium citrate in 500 ml) or on Sauton agar (1% starch and 1.5% agar in Sauton medium).
Construction of rBCG.
Genomic DNA from M. tuberculosis or M. bovis was isolated by protease K digestion and phenol-chloroform extraction. The oligonucleotide primers for the esat-6 gene were 5′ ATATGGCCAAGGGATCCACAGAGCAGCAGTGGAA 3′ (primer 1) (underlined sequences indicate BalI and BamHI sites) and 5′ CGGAATTCCTATGCGAACATCCCAG 3′ (primer 2) (underlined sequence indicates an EcoRI site). The primers for the α-antigen signal sequence of BCG were 5′ CATGGCCACAGACGTGAGCCGAAAGA 3′ (primer 3) (underlined sequence indicates a BalI site) and 5′ ATATAAGGATCCCGCGCCCGCGGTTGCCG 3′ (primer 4) (underlined sequence indicates a BamHI site). esat-6 and α-antigen signal sequences were amplified from genomic DNA of M. tuberculosis and M. bovis BCG, respectively, after an initial denaturation at 94°C for 3 min, by 30 cycles at 94°C for 45 s, 60°C for 60 s, and 72°C for 60 s. We constructed two recombinant shuttle vectors. Plasmid pME-1 was constructed by placing the BalI-EcoRI-digested esat-6 PCR product into the similarly digested parental plasmid pMV261. Plasmid pSME was obtained by first inserting the BamHI-EcoRI-digested esat-6 into pMV261 to construct plasmid pME-2 and then cloning α-antigen signal sequence into the BalI-BamHI site of pME-2. All DNA manipulations followed previously described procedures (25). The recombinant shuttle plasmids pME-1 and pSME were identified by DNA sequencing and then transformed into BCG by electroporation as described previously (28). The transformed BCG cells were plated on Sauton agar supplemented with 20 μg of kanamycin per ml. After 3 to 4 weeks growth at 37°C, individual colonies were picked and grown in Sauton medium containing 20 μg/ml of kanamycin. To identify the rBCG strains, their genomic DNA were isolated and spotted onto a nylon membrane for dot blotting, using esat-6 PCR product as a probe.
Tricine-SDS-PAGE and immunoblotting.
The rBCG strains were grown in Sauton medium for 2 weeks, and protein expression was induced by heating at 45°C for 30 min. The bacterial cells were centrifuged at 3,220 × g for 20 min. The supernatant was filtered through a 0.22-μm-pore-size membrane, concentrated by vacuum-lyophilization, and then dialyzed in distilled water for 72 h. The dialyzed culture filtrate was again vacuum lyophilized to powder and dissolved in phosphate-buffered saline (PBS) (pH 7.4). Sonicated cell lysates and culture filtrate proteins were analyzed by Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis (Tricine-SDS-PAGE) (26), using a 12.87% polyacrylamide separation gel and 4% stacking gel. After a semidry transfer with 25 mM Tris-HCl buffer (pH 8.5) containing 200 mM glycine and 20% methanol onto nitrocellulose, immunoblotting was performed with a 1:25 dilution of the monoclonal antibody (MAb) HYB76-8 (generously donated by Ida Rosenkrands, Statens Seruminstitut, Copenhagen, Denmark) (this MAb was isolated from purified protein derivative-immunized mice, with which ESAT-6 was biochemically purified, characterized and the encoding gene was cloned in 1995) (1) and horseradish peroxidase-labeled goat anti-mouse immunoglobulin G (IgG) antibodies (Zhongshan Biotech, Ltd., Beijing, China). The color was developed with diaminobenzidine as substrate.
Evaluation of virulence of rBCG.
BALB/c mice were infected subcutaneously with approximately 106 CFU of BCG, rBCG or M. tuberculosis H37Rv suspended in normal saline in a volume of 0.1 ml. Groups of mice (three mice per group) were sacrificed after 2, 4, 8, 16, and 26 weeks. The spleen, liver, and right lung of each animal were removed aseptically and then homogenized in 5 ml of Tween 80-PBS. Numbers of bacteria in these organs were determined by plating the diluted homogenates on Sauton agar. Organs from the rBCG-inoculated mice were grown on plates supplemented with kanamycin (15 μg/ml). Colonies were counted after 3 to 4 weeks of incubation at 37°C. Data are expressed as mean log10 values per experimental group. The left lung was fixed in 10% phosphate-buffered formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin.
Immunization of mice.
BALB/c mice were immunized subcutaneously on the back with 100 μl of normal saline, 106 CFU of BCG or rBCG. They were sacrificed (three mice per group) after 4, 8, 12, and 16 weeks to prepare serum and splenocytes.
Evaluation of antigen-specific antibody levels by ELISA.
Enzyme-linked immunosorbent assay (ELISA) plates were coated with 0.1 ml of culture filtrate proteins of M. tuberculosis H37Rv (5 μg/ml) overnight at 4°C. Nonspecific binding sites were blocked by 1% bovine serum albumin-PBS. Individual serum samples from three mice per group were analyzed in twofold dilutions. Horseradish peroxidase-conjugated goat anti-mouse IgG (Zhongshan Biotech, Ltd., Beijing, China) were diluted 1/1,000. Antibody titers are expressed as reciprocal end point titers.
Lymphocyte cultures.
Lymphocytes from spleens were obtained as described previously (2). The cells were washed and resuspended in complete RPMI 1640 medium supplemented with 10% fetal calf serum, 1% l-glutamine, 50 μM 2-mercaptoethanol, and 1% penicillin-streptomycin. Cells were added to 96-well plates (7×105 cells per well) in triplicate for culture with culture filtrate proteins (25 μg/ml) of M. tuberculosis H37Rv. Supernatants were harvested from cultures after 72 h of incubation for the investigation of IFN-γ.
IFN-γ ELISA.
IFN-γ production by spleen cells was measured by sandwich ELISA using paired MAbs (Jingmei Biotech, Ltd., Shenzhen, China) according to the manufacturer's instructions.
Percentages of splenocytes subsets by flow cytometry.
Splenocytes from mice 8 and 12 weeks postimmunization were prepared and cultured as above. Cells of three mice per group were pooled and centrifuged, and this was followed by incubation with rabbit anti-mouse CD4 and CD8 MAbs. After the cells were washed with PBS, goat anti-rabbit IgG-fluorescein isothiocyanate was added for incubation. The proportions of CD4+ and CD8+ cells were determined by flow cytometry.
Challenge infection.
Eight weeks after immunization, twelve mice in each group were challenged intraperitoneally by 105 CFU of virulent M. tuberculosis H37Rv. Three mice per group were sacrificed 4 and 8 weeks postchallenge. The spleen and right lung were removed aseptically and cultured for CFU of M. tuberculosis. Sauton agar contained thiophene carboxylic acid hydrazide (2 μg/ml) to selectively inhibit the growth of the residual BCG bacteria in the test organs. The left lung was fixed in 10% phosphate-buffered formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin. The challenge experiments were performed twice.
Statistical analysis.
Student's t test was used to determine the statistical significance of differences between groups. A value of P < 0.05 was considered to be significant.
RESULTS
Expression of ESAT-6 from rBCG.
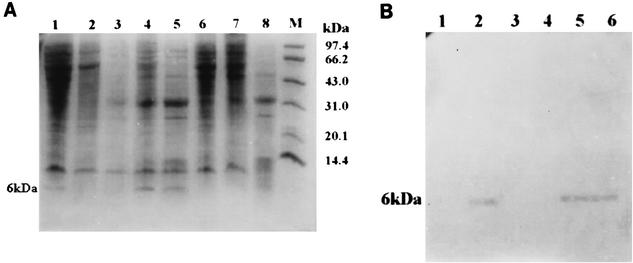
BCG cells were transformed with recombinant shuttle plasmids pME-1 and pSME, respectively, and we obtained two rBCG strains named rBCG-1 and rBCG-2. Tricine-SDS-PAGE was used to analyze the low-molecular-mass proteins that rBCG strains expressed after heat induction. Staining of Tricine-SDS-PAGE gels with Coomassie brilliant blue revealed that the expressed 6-kDa ESAT-6 was present in the cell lysates of rBCG-1 and culture filtrate of rBCG-2, suggesting that the two BCG recombinants could express ESAT-6 fusionally or secretively, respectively (Fig. 1A). This was further confirmed by immunoblotting with the MAb HYB76-8 (Fig. 1B).
FIG. 1.
Tricine-SDS-PAGE (A) and immunoblotting (B) of the expressed product of rBCG strains. (A) Cell lysates of rBCG-1 after heat induction (lane 1), cell lysates of BCG (lane 2), culture filtrate proteins of BCG (lane 3), culture filtrate proteins of rBCG-2 after heat induction (lanes 4 and 5), cell lysates of rBCG-2 after heat induction (lane 6), culture filtrate proteins of rBCG-1 after heat induction (lane 7), culture filtrate proteins of M. tuberculosis H37Rv (lane 8), and protein molecular mass markers (lane M). (B) Culture filtrate proteins of BCG (lane 1), culture filtrate proteins of rBCG-2 after heat induction (lane 2), cell lysates of rBCG-2 after heat induction (lane 3), culture filtrate proteins of rBCG-1 after heat induction (lane 4), cell lysates of rBCG-1 after heat induction (lane 5), and culture filtrate proteins of M. tuberculosis H37Rv (lane 6).
Virulence of rBCG strains.
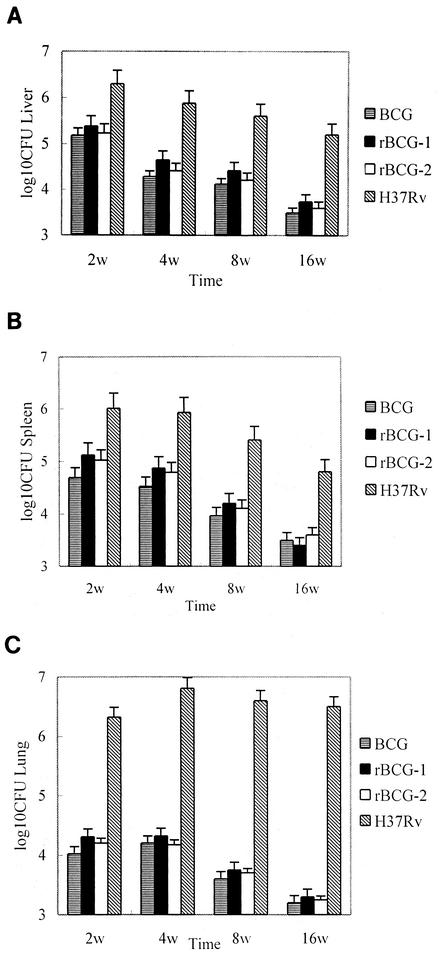
Mice were injected with 106 CFU of BCG, rBCG-1, or rBCG-2 or M. tuberculosis H37Rv subcutaneously. There were no gross pathological appearances in the lung, spleen and liver from BCG or rBCG-infected mice 2, 4, 8, 16, and 26 weeks after administration, except for a minor enlargement, while lungs of M. tuberculosis-infected mice had more severe pathological manifestations, including tubercles. Similar to that of BCG group, organ bacterial loads decreased gradually in mice infected with rBCG-1 and rBCG-2. CFU counts were noted to be higher in rBCG groups than in BCG group, but the difference is not statistically significant. Although M. tuberculosis H37Rv was progressively inactivated in spleen and liver, the CFU counts in lungs remained at high levels even 16 weeks postinoculation (Fig. 2). Lung histopathological examination only found restricted cellular infiltration in BCG, rBC G-1 and rBCG-2 groups 4 weeks after infection; there was no obvious abnormality in these groups at 26 weeks. In M. tuberculosis group, however, granulomas dominated by epithelioid macrophages and lymphocytes could be seen 8 weeks after injection. All mice in this group died before the conclusion of the 28-week observation period. In contrast, only one mouse died in BCG, rBCG-1 and rBCG-2 groups each.
FIG. 2.
Organ bacterial loads of the infected mice. BALB/c mice received injections with 106 CFU of mycobacterial strains subcutaneously. The spleens, livers, and right lungs of three mice per group were removed and homogenized in Tween 80-PBS. Numbers of bacteria in the liver (A), spleen (B), and lung (C) were determined by plating the diluted homogenates on Sauton agar. Data represent the mean and standard error (error bar) of three mice per group.
Antibody responses induced by rBCG.
Compared with control group, mice vaccinated with the three different mycobacterial strains could induce higher levels of antibody against culture filtrate proteins of M. tuberculosis H37Rv. The IgG antibody titers peaked at 8 weeks. Titers as high as 1:40960 were obtained with rBCG-1 immunization, which is significantly higher than that induced by immunization with BCG or rBCG-2 (P < 0.05) (Table 1).
TABLE 1.
Titers of specific serum antibody of immunized mice
| Group | Titera at wk:
|
|||
|---|---|---|---|---|
| 4 | 8 | 12 | 16 | |
| Control | 1:20 | 1:40 | 1:40 | 1:20 |
| BCG | 1:1,280 | 1:20,480 | 1:6,400 | 1:2,560 |
| rBCG-1 | 1:2,560 | 1:40,960 | 1:12,800 | 1:5,120 |
| rBCG-2 | 1:1,280 | 1:20,480 | 1:6,400 | 1:2,560 |
Data represent the mean values of three mice per group.
IFN-γ production of splenocytes.
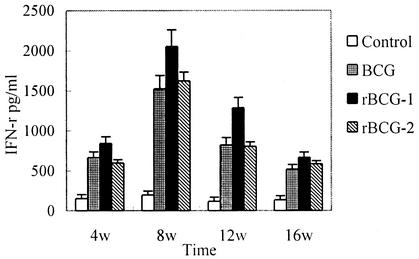
The results of IFN-γ ELISA showed that splenocytes from rBCG-1-immunized mice could produce significantly higher IFN-γ levels than those from BCG group (P < 0.05) in weeks 8 and 12, while there were no significant differences between mice vaccinated with rBCG-2 and BCG (Fig. 3).
FIG. 3.
IFN-γ production by splenocytes of immunized mice. BALB/c mice were immunized subcutaneously with 100 μl of normal saline or 106 CFU of BCG, rBCG-1, or rBCG-2. Three mice per group were sacrificed after 4, 8, 12, and 16 weeks. Splenocytes were cultured with culture filtrate proteins (25 μg/ml) of M. tuberculosis H37Rv. Supernatants were harvested after 72 h of incubation for IFN-γ assay. Data represent the mean and standard error (error bar) of three mice per group.
Percentages of splenocyte subsets.
The proportions of splenocyte subsets in immunized mice were determined by flow cytometry 8 and 12 weeks after vaccination. Results are presented as means ± standard errors. Compared with control group (23.4% ± 4.3% and 22.2% ± 3.1%, respectively), the percentages of CD8+ subset increased substantially in BCG (33.5% ± 2.1% and 30.1% ± 2.6%), rBCG-1 (38.6% ± 3.2% and 34.3% ± 1.9%) and rBCG-2 groups (35.2% ± 3.4% and 32.8% ± 3.5%). CD4/CD8 ratio decreased simultaneously. At week 8, the ratios were 1.81 ± 1.2, 0.90 ± 1.1, 0.81 ± 1.6, and 0.91 ± 1.7 in the control, BCG, rBCG-1 and rBCG-2 group, respectively. Vaccination with rBCG-1 induced significantly greater percentages of CD8+ cells than that with BCG (P < 0.05), whereas there were no significant differences between rBCG-2 and BCG groups.
Protective efficacy against M. tuberculosis challenge.
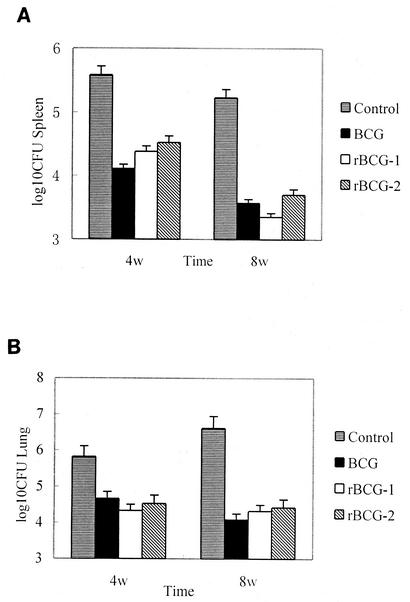
BCG and rBCG-immunized mice were challenged with M. tuberculosis H37Rv intraperitoneally. Lungs and spleens were removed 4 and 8 weeks after challenge. On visual inspection of the lung surfaces, we found that mice immunized with BCG, rBCG-1 and rBCG-2 had fewer and smaller tubercles than control group injected with normal saline only. Infection with M. tuberculosis caused an enlargement of the lungs, which was more pronounced in control group. As expected, control mice had the highest bacterial loads in both lung and spleen. Immunization with BCG, rBCG-1, or rBCG-2 could remarkably reduce CFU counts in organs, but there were no significant differences among these three groups (Fig. 4). The extent of cellular infiltration in the lungs of rBCG-1 and rBCG-2 groups was less expansive than that of control group, also the granulomas were fewer and smaller. However, it was difficult to differentiate these histopathological findings from those of BCG group.
FIG. 4.
Organ bacterial loads of the immunized mice after M. tuberculosis challenge. Immunized BALB/c mice were challenged with 105 CFU of M. tuberculosis H37Rv. The right lungs and spleens of three mice per group were removed and homogenized in Tween 80-PBS. Numbers of bacteria in the spleen (A) and lung (B) were determined by plating the diluted homogenates on Sauton agar. Data represent the mean and standard error (error bar) of three mice per group.
DISCUSSION
The present report describes the first rBCG vaccines expressing a protective antigen which BCG lacks. The two rBCG strains showed no obvious virulence in a mouse model, and more importantly, one of the recombinants induced stronger humoral response and IFN-γproduction, although their protective efficacies were not significantly higher than that of the parental BCG.
The low mass secreted ESAT-6 protein has been demonstrated to be a key molecule recognized by memory effector T lymphocytes in the long-lived immunity to TB (1). It is strongly recognized in M. tuberculosis-infected mice (7), guinea pigs (16), and in the early stage of infection with virulent M. bovis in cattle (23), and it stimulates the early release of IFN-γ. Moreover, ESAT-6 stimulates CD4+ T cells in human peripheral blood mononuclear cells from TB patients and induces IFN-γ production (24, 29). Recently, new vaccines comprising ESAT-6, including subunit vaccines, DNA vaccines, and recombinant attenuated Salmonella enterica serovar Typhimurium, have proven of use for providing some degree of protection (6, 17, 18, 19, 21). They are capable of inducing a substantial IFN-γ response in the spleen and stimulating a significant reduction in the level of M. tuberculosis infection in the lungs of mice. In addition, ESAT-6 is highly expressed in all virulent strains of M. tuberculosis and M. bovis; however, the gene is lacking in all the strains of BCG tested (15, 20). It is assumed that ESAT-6 is a protective antigen missing from virulent M. bovis during the long course of in vitro cultivation which led to the generation of attenuated BCG strain. Our research is aimed to investigate whether reintroduction of ESAT-6 gene into BCG could improve the efficacy of the vaccine.
Since a conventional lead sequence is absent from the gene encoding ESAT-6 (27), in this study we cloned the signal sequence of BCG α-antigen into the E. coli-BCG shuttle plasmid pMV261, and placed it in the 5′ end of esat-6 gene to facilitate the secretion of ESAT-6. The transformed BCG strain rBCG-2 could express ESAT-6 secretively, which is confirmed by Tricine-SDS-PAGE and immunoblotting. The other rBCG strain rBCG-1, with no signal sequence, expressed ESAT-6 fused to the first several amino acids of BCG HSP60.
Though no evidence has yet shown that ESAT-6 is definitely a virulence factor, ESAT-6 is thought to be relevant to virulence for its presence in virulent M. bovis and M. tuberculosis. In order to prevent the possibility that reversion to a virulent strain may occur with the reintroduction of esat-6 gene, we examined the manifestations of virulence of rBCG strains in mice. The CFU counts and lung histopathology suggested that like BCG, rBCG-1 and rBCG-2 were not able to multiply in organs, nor did they cause progressive pathology in the lungs. The virulence of the BCG recombinants was probably not elevated with the expression of ESAT-6; however, this needs to be substantiated by further researches in immunodeficient mice model.
Recent researches have found that ESAT-6 is one part of a complex including CFP-10. esat-6 and the neighboring lhp gene (encoding CFP-10) form an operon structure and the two genes are transcribed together in M. tuberculosis (4). Thirteen genes related to the lhp/esat-6 operon which constitute a novel gene family were identified via computer searches in the M. tuberculosis genome. This might explain the apparent lack of increased virulence in rBCG.
Since IFN-γ is a critical cytokine associated with the induction of an antimycobacterial protective response (10, 13, 22), we initially focused on this cytokine response in the vaccinated animals. rBCG-1 immunization could elicit higher IFN-γ levels as well as specific antibody titers in mice. Percentages of CD8+ subset were also increased, which may contribute to better protection because of the important role of CD8T cells in the clearance of mycobacterial infection (14). To our surprise, although the immunogenicity of rBCG-1 was greater than that of BCG, rBCG-2 expressing ESAT-6 secretively was immunogenically similar to BCG, and neither recombinant strain conferred greater protective efficacy against M. tuberculosis infection. The results highlight the discrepancy between immunological responses to antigens in vitro and protective immunity in vivo. The reasons are to be explored.
The difference in immunogenicity between the two recombinants is probably related to the quantity and stability of the expressed ESAT-6. ESAT-6 fused with HSP60 tends to be less readily degraded by hydrolytic enzymes and remains stable in phagosomes. More ESAT-6 are therefore processed and presented, leading to stronger immune responses. Obviously, a comparative quantitative analysis of ESAT-6 expression in these two recombinant strains is necessary to explain why the immunogenicity of rBCG-1 was greater than that of rBCG-2.
Genetic comparison of M. tuberculosis H37Rv and BCG revealed that 15 to 16 regions (encompassing 129 open reading frames) are deleted in the genome of BCG (3). Many immunodominant antigens including ESAT-6 and the probable transcriptional regulators predicted in sequencing of M. tuberculosis H37Rv genome (9) are within these deletion regions. The failure of rBCG strains supplemented with ESAT-6 to give more effective protection against M. tuberculosis suggests that the imperfect efficacy of BCG as a vaccine may at least not be attributable to the absence of the ESAT-6 gene alone. We hypothesize that the lack of regulating proteins encoded by missing genes might have a great influence on the normal expression of the downstream genes. It is more likely that the deletions of a cluster(s) of genes, not just one or two genes are involved in the unsatisfactory protection of BCG. Further investigation should be encouraged.
Some scholars have previously proposed that genetic engineering BCG is a candidate vaccine against TB. Despite the unsatisfactory protective efficacy yielded in our research, the increased immunogenicity compared with BCG and the potential advantages over other types of new vaccines make rBCG attractive in TB vaccine development. It contains many more antigens, many of which are protective; it is able to multiply in the host macrophages, releasing various antigens consistently; it is a strong adjuvant and live vector itself; it needs no repeated vaccination; and it also shares several important advantages of conventional BCG (e.g., it is safe to use, stable to store, available for immunization at birth, and cost-effective).
Several approaches may be considered for further enhancement of the immunogenicity and protective efficacy of rBCG vaccine: (i) using shuttle vectors with high-efficiency promoters to increase expression of antigens; (ii) adding more kinds of protective antigens, such as Ag85A, Ag85B, CFP10, and MPT64 (18, 31); (iii) coexpressing genes of cytokines, such as IL-2, IFN-γ, and IL-12; and (iv) utilizing other methods of administration, such as intranasal vaccination.
Acknowledgments
We thank Ida Rosenkrands for donating MAb HYB76-8. We are also grateful to Chengdu Biological Products Institute for BCG strains.
Editor: J. D. Clements
REFERENCES
- 1.Andersen, P., A. B. Andersen, A. L. Sorensen, and S. Nagai. 1995. Recall of long-lived immunity to Mycobacterium tuberculosis infection in mice. J. Immunol. 154:3359-3372. [PubMed] [Google Scholar]
- 2.Andersen, P., D. Askgaard, L. Ljungqvist, M. W. Bentzon, and I. Heron. 1991. T cell proliferative response to antigens secreted by Mycobacterium tuberculosis. Infect. Immun. 59:1558-1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Behr, M. A., M. A. Wilson, W. P. Gill, H. Salamon, G. K. Schoolnik, S. Rane, and P. M. Small. 1999. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science 284:1520-1523. [DOI] [PubMed] [Google Scholar]
- 4.Berthet, F. X., P. B. Rasmussen, I. Rosenkrands, P. Andersen, and B. Gicquel. 1998. A Mycobacterium tuberculosis operon encoding ESAT-6 and a novel low-molecular-mass culture filtrate protein (CFP-10). Microbiology 144:3195-3203. [DOI] [PubMed] [Google Scholar]
- 5.Boesen, H., B. N. Jensen, T. Wilcke, and P. Andersen. 1995. Human T-cell responses to secreted antigen fractions of Mycobacterium tuberculosis. Infect. Immun. 63:1491-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brandt, L., M. Elhay, I. Rosenkrands, E. B. Lindblad, and P. Andersen. 2000. ESAT-6 subunit vaccination against Mycobacterium tuberculosis. Infect. Immun. 68:791-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandt, L., T. Oettinger, A. Holm, A. B. Andersen, and P. Andersen. 1996. Key epitopes on the ESAT-6 antigen recognized in mice during the recall of protective immunity to Mycobacterium tuberculosis. J. Immunol. 157:3527-3533. [PubMed] [Google Scholar]
- 8.Colditz, G. A., T. F. Brewer, C. S. Berkey, M. E. Wilson, E. Burdick, H. V. Fineberg, and F. Mosteller. 1994. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA 271:698-702. [PubMed] [Google Scholar]
- 9.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. 3rd Barry, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, B. G. Barrell, et al. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 10.Cooper, A. M., D. K. Dalton, T. A. Stewart, J. P. Griffin, D. G. Russell, and I. M. Orme. 1993. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J. Exp. Med. 178:2243-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dolin, P. J., M. C. Raviglione, and A. Kochi. Global tuberculosis incidence and mortality during 1990-2000. Bull. W. H. O. 72:213-220. [PMC free article] [PubMed] [Google Scholar]
- 12.Fine, P. E. 1995. Variation in protection by BCG: implications of and for heterologous immunity. Lancet 346:1339-1345. [DOI] [PubMed] [Google Scholar]
- 13.Flynn, J. L., J. Chan, K. J. Triebold, D. K. Dalton, T. A. Stewart, and B. R. Bloom. 1993. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flynn, J. L., M. M. Goldstein, K. J. Triebold, B. Koller, and B. R. Bloom. 1992. Major histocompatibility complex class I restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc. Natl. Acad. Sci. USA 89:12013-12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harboe, M., T. Oettinger, H. G. Wiker, I. Rosenkrands, and P. Andersen. 1996. Evidence for occurrence of the ESAT-6 protein in Mycobacterium tuberculosis and virulent Mycobacterium bovis and for its absence in Mycobacterium bovis BCG. Infect. Immun. 64:16-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haslov, K., A. Andersen, S. Nagai, A. Gottschau, T. Sorensen, and P. Andersen. 1995. Guinea pig cellular immune responses to proteins secreted by Mycobacterium tuberculosis. Infect. Immun. 63:804-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamath, A. T., C. G. Feng, and M. Macdonald. 1999. Differential protective efficacy of DNA vaccines expressing secreted proteins of Mycobacterium tuberculosis. Infect. Immun. 67:1702-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li, Z., A. Howard, C. Kelley, G. Delogu, F. Collins, and S. Morris. 1999. Immunogenicity of DNA vaccines expressing tuberculosis proteins fused to tissue plasminogen activator signal sequences. Infect. Immun. 67:4780-4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindblad, E. B., M. J. Elhay, R. Silva, R. Appelberg, and P. Andersen. 1997. Adjuvant modulation of immune responses to tuberculosis subunit vaccines. Infect. Immun. 65:623-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahairas, G. G., P. J. Sabo, M. J. Hickey, D. C. Singh, and C. K. Stover. 1996. Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M. bovis. J. Bacteriol. 178:1274-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mollenkopf, H. J., D. Groine-Triebkorn, P. Andersen, J. Hess, and S. H. Kaufmann. 2001. Protective efficacy against tuberculosis of ESAT-6 secreted by a live Salmonella typhimurium vaccine carrier strain and expressed by naked DNA. Vaccine 19:4028-4035. [DOI] [PubMed] [Google Scholar]
- 22.Newport, M. J., C. M. Huxley, S. Huston, C. M. Hawrylowicz, B. A. Oostra, R. Williamson, and M. Levin. 1996. A mutation in the interferon-gamma-receptor gene and susceptibility to mycobacterial infection. N. Engl. J. Med. 335:1941-1949. [DOI] [PubMed] [Google Scholar]
- 23.Pollock, J. M., and P. Andersen. 1997. Predominant recognition of the ESAT-6 protein in the first phase of interferon with Mycobacterium bovis in cattle. Infect. Immun. 65:2587-2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ravn, P., A. Demissie, T. Eguale, H. Wondwosson, D. Lein, H. A. Amoudy, A. S. Mustafa, A. K. Jensen, A. Holm, I. Rosenkrands, F. Oftung, J. Olobo, F. von Reyn, and P. Andersen. 1999. Human T cell responses to the ESAT-6 antigen from Mycobacterium tuberculosis. J. Infect. Dis. 179:637-645. [DOI] [PubMed] [Google Scholar]
- 25.Sambrook, J., E. F. Fritisch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 26.Schagger, H., and G. von Jagow. 1987. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166:368-379. [DOI] [PubMed] [Google Scholar]
- 27.Sorensen, A. L., S. Nagai, G. Houen, P. Andersen, and A. B. Andersen. 1995. Purification and characterization of a low-molecular-mass T-cell antigen secreted by Mycobacterium tuberculosis. Infect. Immun. 63:1710-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stover, C. K., G. P. Bansal, M. S. Hanson, J. E. Burlein, S. R. Palaszynski, J. F. Young, S. Koenig, D. B. Young, A. Sadziene, and A. G. Barbour. 1993. Protective immunity elicited by recombinant Bacille Calmette-Guerin (BCG) expressing outer surface protein A (OspA) lipoprotein: a candidate Lyme disease vaccine. J. Exp. Med. 178:197-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ulrichs, T., M. E. Munk, H. Mollenkopf, S. Behr-Perst, R. Colangeli, M. L. Gennaro, and S. H. Kaufmann. 1998. Differential T cell responses to Mycobacterium tuberculosis ESAT6 in tuberculosis patients and healthy donors. Eur. J. Immunol. 28:3949-3958. [DOI] [PubMed] [Google Scholar]
- 30.World Health Organization. 1979. Trial of BCG vaccines in south India for tuberculosis prevention: first report—Tuberculosis Prevention Trial. Bull. W. H. O. 57:819-827. [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu, X., N. Venkataprasad, H. S. Thangaraj, M. Hill, M. Singh, J. Ivanyi, and H. M. Vordermeier. 1997. Functions and specificity of T cells following nucleic acid vaccination of mice against Mycobacterium tuberculosis infection. J. Immunol. 158:5921-5926. [PubMed] [Google Scholar]