Abstract
Pneumolysin, a multifunctional toxin produced by all clinical isolates of Streptococcus pneumoniae, is strongly implicated in the pathogenesis of pneumococcal bronchopneumonia and septicemia. Using isogenic mutant strains, we examined the effect of deletion of the cytotoxic activity or complement-activating activity of pneumolysin on bacterial growth in lungs and blood, histological changes in infected lung tissue, and the pattern of inflammatory cell recruitment. Both of the activities of pneumolysin contributed to the pathology in the lungs, as well as the timing of the onset of bacteremia. Histological changes in the lungs were delayed after infection with either mutant compared to the changes seen after infection with the wild-type pneumococcus. The complement-activating activity of pneumolysin affected the accumulation of T cells, whereas the toxin's cytolytic activity influenced neutrophil recruitment into lung tissue.
Previous studies in our laboratory showed that the pneumococcal toxin pneumolysin has a crucial role in the pathogenesis of pneumococcal bronchopneumonia and septicemia (7, 12). Other studies showed that pneumolysin alone can reproduce the symptoms of pneumococcal disease in the lungs (8), and inactivation of the pneumolysin gene of Streptococcus pneumoniae resulted in an avirulent mutant (5). Subsequent studies showed that mutant pneumococci unable to produce pneumolysin generated much less inflammation, delayed bacteremia, limited multiplication of bacteria within the lungs (7, 12, 18), and a different pattern of host inflammatory cell infiltration into the lung tissue (12) compared with wild-type pneumococci.
Several pieces of evidence showed that pneumolysin is cytolytic to all eukaryotic cells tested, probably as a result of pore formation in target membranes, and at sublytic concentrations it alters normal functioning of immune cells (9, 17) (for example, by inhibiting human neutrophil and monocyte respiratory burst and chemotaxis [15]). In addition, low levels of the toxin can activate the complement pathway in human serum in the absence of antipneumolysin antibody (13, 16), as well as stimulate production of other host inflammatory molecules (10).
Site-directed mutagenesis showed that distinct parts of the toxin molecule are responsible for pore formation and the accompanying cytolytic and anticellular activities and for complement activation (6, 14). The site-directed mutagenesis results allowed production of pneumococcal strains that make pneumolysin which lacks one of its wild-type activities. By using these strains, it was shown previously that both anticellular (pore formation) and complement-activating activities of pneumolysin contributed to virulence during pulmonary infection following intratracheal challenge in mice (2, 19). Complement-activating activity was associated with bacterial growth in lung tissue and blood at 24 h after pulmonary infection, while the cytotoxic activity correlated with impairment of the alveolar capillary barrier and an increase in the number of bacteria during the first 6 h of infection (19). A model of pneumococcal bronchopneumonia following intranasal challenge in which complement-activating activity and anticellular activity of pneumolysin have discrete roles has also been described (2). The previous studies also suggested that a third activity contributes to virulence (2), an observation that seemed to be confirmed by recent studies (3) which showed that a fragment of pneumolysin that lacked cytolytic and complement-activating regions was able to induce gamma interferon release.
In this study we extended a previous study (2) by investigating the mechanisms by which the activities of pneumolysin individually influence in vivo events. Below we describe the histological changes and the host cellular immune response to pneumococci producing modified pneumolysin in a murine model of bronchopneumonia and septicemia. The following two isogenic mutant strains producing pneumolysins with specific amino acid substitutions that modify the toxin activity were used: strain H+/C−, carrying a point mutation (Asp385 → Asn) which results in a pneumolysin that lacks complement-activating activity but has complete anticellular activity; and strain H2−/C+, carrying a point mutation (His367 → Arg) which results in pneumolysin that has only 0.02% of the anticellular activity associated with pore formation but has 100% of the complement-activating activity (2).
MATERIALS AND METHODS
Pneumococcal strains.
Three isogenic strains of S. pneumoniae were used. The wild-type strain was serotype 2 strain D39 (= NCTC 7466 [National Collection of Type Cultures, London, United Kingdom]). The mutant strains were H+/C− and H2−/C+, which had single point mutations (Asp385 → Asn and His367 → Arg, respectively) in the chromosomal pneumolysin gene (2). The pneumococci were grown on blood agar base containing 5% (vol/vol) horse blood or in brain heart infusion broth (Oxoid, Basingstoke, United Kingdom) containing 20% (vol/vol) fetal bovine serum (Gibco, Paisley, United Kingdom) supplemented with 1 mg of erythromycin (Sigma, Poole, United Kingdom) per ml for the mutant strains.
Before use in vivo, S. pneumoniae was passaged through the peritoneal cavities of mice and then stored at −70°C (5). Pneumococci can be stored for at least 3 months at −70°C with no significant loss of viability. When needed, an aliquot was thawed at room temperature, and bacteria were harvested by centrifugation before resuspension in sterile phosphate-buffered saline (PBS) (7).
The Gram reaction, Quellung reaction, and optochin sensitivity of all strains were confirmed prior to use.
Intranasal challenge of mice.
Female MF1 outbred mice weighing 30 to 35 g were obtained from Harlan Olac, Bicester, United Kingdom. At the start of the experiment the mice had no detectable levels of antitype antibodies. Mice were each infected intranasally with 1 × 106 S. pneumoniae CFU, as described previously (12). At predetermined times following infection, groups of mice were deeply anesthetized with 5% (vol/vol) fluothane (Astra-Zeneca, Macclesfield, United Kingdom), and blood was collected by cardiac puncture. Subsequently, the mice were killed by cervical dislocation, and the lungs were removed, placed into 10 ml of sterile distilled water, weighed, and then homogenized with a Stomacher-Lab blender (Seward Medical, London, United Kingdom). Viable counts in homogenates and blood were determined by serial dilution in sterile nanopure water and plating onto blood agar plates (Oxoid) supplemented with 5% (vol/vol) horse blood.
Histology.
At the predetermined times following infection as described above, whole-lung samples were excised, embedded in Tissue-Tec OCT (Sakura), and frozen in liquid nitrogen with an isopentane heat buffer to prevent snap freezing and tissue damage. The samples were stored at −70°C. One day before sectioning, the lungs were transferred to storage at −20°C. Sections (thickness, 10 to 20 μm) were cut at −18 to −25°C with a Bright cryostat and then allowed to dry at room temperature. Once dried, the sections were stained with hematoxylin and eosin and subsequently fixed with DPX mountant (BDH) for permanent storage.
Immunohistochemistry.
Leukocyte recruitment into lung tissue was analyzed by an alkaline phosphatase anti-alkaline phosphatase staining method (11). Sections were fixed with acetone for 10 min at 4°C, air dried, and washed in PBS for 5 min. Normal rabbit serum, diluted 1:5 in PBS, was overlaid onto each section. The excess serum was tapped off, and 50-μl portions of rat anti-mouse monoclonal antibodies to T cells (pan-T-cell marker CD3), B cells (pan-B-cell marker CD19), macrophages (F/480), or neutrophils (7/4) (Serotec, Oxford, United Kingdom), all previously diluted 1:50 in PBS, were layered onto the sections. Four whole lungs collected at predetermined times were sectioned completely, and 20 sections from each lung were used for each antibody tested. After incubation for 30 min, the sections were washed for 5 min in PBS and incubated with 50 μl of rabbit anti-rat antibody (1:25; Dako, Glostrup, Denmark) for 30 min at room temperature. A 5-min wash was followed by addition of rat alkaline phosphatase anti-alkaline phosphatase monoclonal antibody diluted 1:50 in PBS. Each preparation was washed again with PBS before addition of the substrate, fast red, and levamisole to inhibit endogenous phosphatase activity. After 20 min of incubation with the substrate, the sections were washed, counterstained briefly with hematoxylin, and finally washed in tap water. The sections were mounted in aqueous mounting medium (DPX mountant). Once stained, the positively stained cells in the vicinity of inflamed bronchioles were enumerated. Sections from throughout the lung were taken, and at least 10 sections per lung were analyzed.
Statistical analysis.
Data were analyzed by analysis of variance, followed by the Bonferroni test. Statistical significance was defined as a P value of <0.05.
RESULTS
Growth of wild-type and mutant pneumococci in lung tissue and blood.
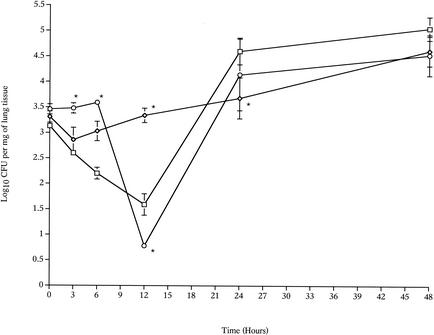
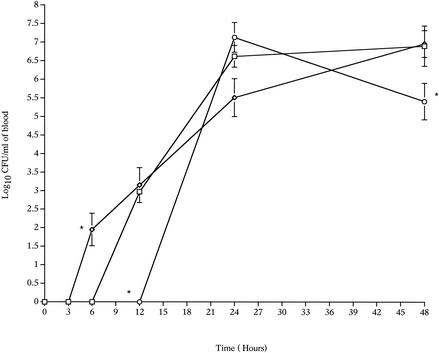
There were clear differences in the growth curves of the wild-type and mutant strains (H+/C− and H2−/C+) in the lungs (Fig. 1) and in the blood (Fig. 2). The number of H+/C− pneumococci in the lungs remained constant for 24 h postinfection, in contrast to the number of wild-type pneumococci, which declined sharply during the first 12 h postinfection (P < 0.01, compared to the zero-time value) (Fig. 1). After this, the wild-type levels increased, and by 48 h postinfection the numbers of the wild-type and H+/C− pneumococci were the same. The ability of pneumolysin to activate complement influenced the timing of bacteremia after intranasal infection. The wild-type pneumococci were detected in the blood at 12 h after infection, while the H+/C− pneumococci were detected earlier, at 6 h after infection, but after 24 and 48 h there were no differences (P > 0.05) in the numbers of the wild-type and H+/C− bacteria in the blood (Fig. 2).
FIG. 1.
Time courses for changes in the numbers of the S. pneumoniae wild-type (□), H+/C− (⋄), and H2−/C+ (○) strains in the lungs of MF1 mice infected intranasally with 106 CFU (n = 10 for each time point). The error bars indicate standard errors of the means. An asterisk indicates that the P value is <0.05 for a comparison of the H+/C− or H2−/C+ mutant with the wild-type strain.
FIG. 2.
Time courses for changes in the numbers of the S. pneumoniae wild-type (□), H+/C− (⋄), and H2−/C+ (○) strains in the blood of MF1 mice infected intranasally with 106 CFU (n = 10 for each time point). The error bars indicate standard errors of the means. An asterisk indicates that the P value is <0.05 for a comparison of the H+/C− or H2−/C+ mutant with the wild-type strain.
The growth of H2−/C+ pneumococci in lungs and blood also was assessed. In the lungs, the growth curve for H2−/C+ showed a sharp decline between 6 and 12 h postinfection (P < 0.001, compared to the zero-time value), and this was followed by notable bacterial multiplication during the following 12 h (P < 0.05 for 24-h data versus 12-h data) (Fig. 1). Compared with growth of the wild-type pneumococcus, the lack of the anticellular activity of pneumolysin influenced bacterial growth in the lungs during the very early hours after infection. Analysis of the wild-type and H2−/C+ mutant growth levels in lung tissue showed that there were significant differences at 3, 6, and 12 h postinfection (P < 0.01) but no significant differences after 12 h (P > 0.05). Elimination of the anticellular activity of pneumolysin also affected bacteremia. H2−/C+ pneumococci were not detected in the blood before 24 h postinfection (Fig. 2), and the number of H2−/C+ pneumococci in the blood after 48 h was significantly less than the number of wild-type pneumococci (P < 0.01) at the same time.
Histology.
At intervals following infection, a histological examination of lung tissue sections from mice infected with the wild-type and the mutants (H+/C− and H2−/C+) was performed. The data obtained for the wild type were identical to data obtained previously (12), and the new data are not shown.
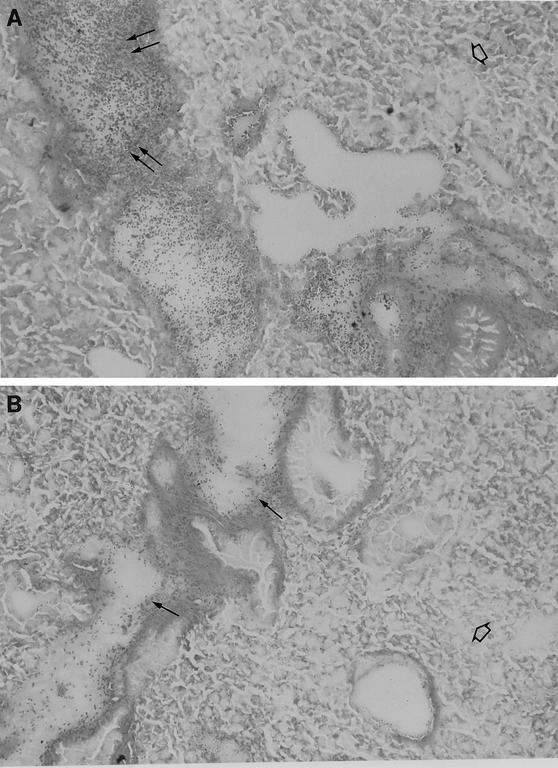
At 24 h after infection, the H+/C− pneumococcus-infected lung sections showed a heavy cellular infiltrate within infected bronchioles (Fig. 3A), and the H2−/C+ pneumococcus-infected lung sections showed slight cellular infiltration of infected bronchioles (Fig. 3B). In lungs from mice infected with either mutant, the general lung parenchyma was not involved in inflammation (Fig. 3A and B), and interstitial alveolitis (thickening of alveolar walls, along with the presence of fibrosis) was not detected at 24 h after infection (data not shown). With either mutant, the hypertrophy of bronchiole walls (enlargement of bronchial epithelial cells in the bronchiole wall) was less than that in the wild-type pneumococcus-infected tissue sections at 24 h. The main difference between H+/C− and H2−/C+ pneumococcus-infected lungs at 24 h was the difference in the extent of cellular infiltrate (Fig. 3A and B).
FIG. 3.
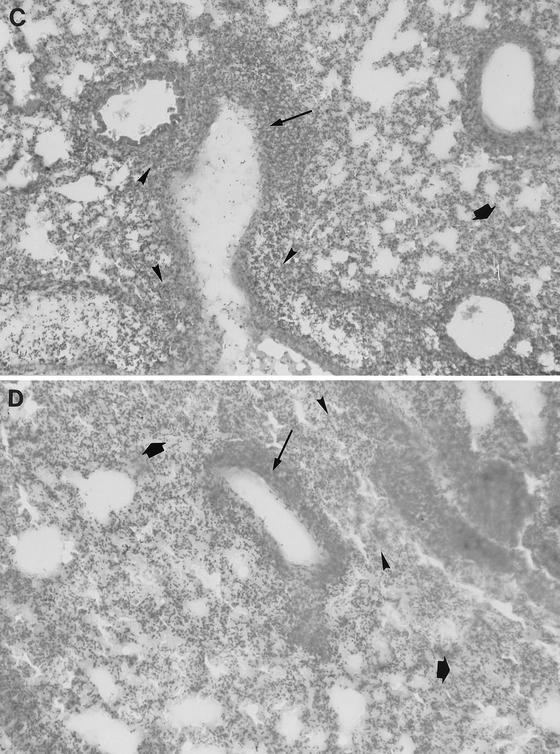
Light microscopy of lung tissue from a mouse infected with 106 CFU of S. pneumoniae H+/C− at 24 h postinfection (A), from a mouse infected with 106 CFU of S. pneumoniae H2−/C+ at 24 h postinfection (B), from a mouse infected with 106 CFU of S. pneumoniae H+/C− at 48 h postinfection (C), and from a mouse infected with 106 CFU of S. pneumoniae H2−/C+ at 48 h postinfection (D). In panel A the double arrows indicate heavy cellular infiltrate in infected bronchioles. In panel B the single arrows indicate slight cellular infiltration of infected bronchioles. In panels A and B the open arrows indicate general lung parenchyma that was not involved in inflammation. In panels C and D the thin arrows indicate hypertrophy of the inflamed bronchiole walls, the arrowheads indicate severe multifocal peribronchial infiltration of inflammatory cells, and the thick arrows indicate extensive infiltration of lung parenchyma. Magnification, ×250.
At 48 h postinfection, wild-type pneumococcus-, H+/C− pneumococcus-, and H2−/C+ pneumococcus-infected sections showed hypertrophy of the inflamed bronchiole walls, severe multifocal peribronchial infiltration of inflammatory cells, and extensive infiltration of the lung parenchyma (Fig. 3C and D). The bronchioles and lung alveoli appeared to be filled with exudate. Overall, at 48 h after infection, the majority of the lung surface in the mice infected with each strain showed consolidation of bronchiolar spaces and associated parenchyma (due to heavy infiltration of inflammatory cells and the presence of exudate) and hypertrophy of infected bronchiole cell walls.
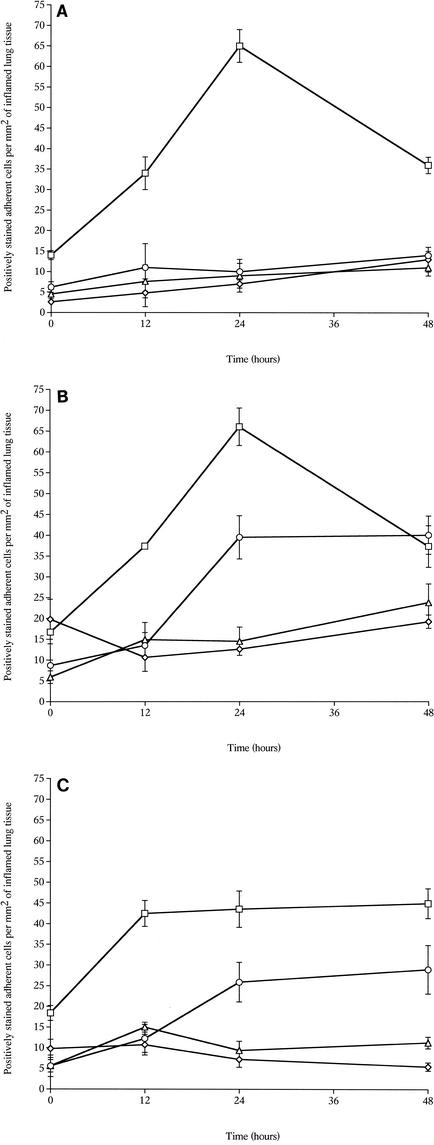
Immunohistochemical analysis of inflammatory cell infiltrates.
An immunohistochemistry analysis was performed to identify leukocytes and to analyze the change in the number of leukocytes in lung tissue over time after intranasal infection with the wild-type and mutant pneumococci.
In the inflamed areas of H+/C− pneumococcus-infected lung tissue, neutrophils showed the same pattern of recruitment that was observed in the wild-type pneumococcus-infected lung tissue. Infiltration of neutrophils was detected within inflamed bronchioles, in bronchiole walls, and in perivascular areas near inflamed bronchioles, as well as in the alveolar spaces. The number of neutrophils increased progressively over 24 h and was significantly greater (P < 0.05) at 12 and 24 h postinfection than at zero time (Fig. 4A and B). The number of neutrophils then decreased significantly (P < 0.05) by 48 h postinfection compared to the 24-h levels. Similar results were obtained for lungs infected with wild-type pneumococci (there were no significant differences between the tissues infected with the wild-type and H+/C− pneumococci at 12, 24, and 48 h).
FIG. 4.
Numbers of neutrophils (□), macrophages (⋄), T cells (○), and B cells (▵) in tissue sections from lungs of MF1 mice infected intranasally with 106 CFU of the S. pneumoniae wild-type strain (A), with 106 CFU of S. pneumoniae H+/C− (B), and with 106 CFU of S. pneumoniae H2−/C+ (C) (n = 4 for each time point) The error bars indicate standard errors of the means.
In mice infected with H2−/C+ pneumococci, the neutrophils showed a pattern of recruitment into lung tissue that was different than the pattern observed in wild-type pneumococcus-infected tissue (Fig. 4C). At 12 h postinfection the numbers of neutrophils were the same as they were in the wild-type pneumococcus-infected tissue, but after this the numbers did not change until 48 h postinfection and were significantly lower than the numbers in the mice infected with the wild-type and H+/C− pneumococci (P < 0.01 for comparisons with wild-type and H+/C− data).
In lung tissue sections from mice infected with H2−/C+ or wild-type pneumococci, the numbers of T cells were significantly greater at 24 and 48 h postinfection than at zero time (P < 0.05 for H2−/C+ and P < 0.001 for the wild type) (Fig. 4A and C). There were not significant differences in the numbers of T cells between lungs infected with the wild type and lungs infected with the H2−/C+ mutant at these times. In contrast, the numbers of T cells in lung tissue sections remained constant and did not increase during the 48 h following infection with H+/C− pneumococci (Fig. 4B).
The numbers of macrophages and B cells remained unchanged (P > 0.05) over the course of infection with either mutant pneumococcus (H+/C− or H2−/C+).
DISCUSSION
The influence of cytotoxic (hemolytic pore formation) and complement-activating properties of pneumolysin on the in vivo events in a model of bronchopneumonia was revealed by comparing events following intranasal infection with two mutant pneumococci, H+/C− and H2−/C+, and the wild-type parent strain. It was shown previously that both of these activities are required for full virulence of the pneumococcus in bronchopneumonia (2) and lobar pneumonia (19). In the studies that we describe here we extended these observations by analyzing the pulmonary growth of pneumococci in more detail and by analyzing the effects of individual activities of the toxin on the host immune response during bronchopneumonia. Our results showed that pore formation and complement activation by pneumolysin make distinct contributions to pneumococcal growth in the lungs and to bacteremia. Also, both activities are involved in the inflammation and cellular influx seen in pneumonia. Significantly, however, they contribute differently to the level of the host immune response. To our knowledge, this is the first detailed description of the pulmonary immune responses to individual pneumolysin activities.
Removal of either cytotoxic or complement-activating activity had an affect on the early growth of the pneumococci in the lungs. This may not be surprising given that these mutations significantly decrease virulence and the absence of pneumolysin has previously been shown to change the pattern of pneumococcal growth in the lungs (2, 12). However, as shown here, how the growth was affected was curious. We observed that at certain times after infection, the absence of one of the pneumolysin activities improved pneumococcal survival. Reduced cytotoxic activity was accompanied by better pneumococcal survival in the first 6 h, whereas the absence of complement activity was accompanied by significantly better pneumococcal survival at around 12 h postinfection. Therefore, for these experiments, conclusions about whether the mutations in the ply gene affect virulence depend on the definition of virulence. Judged by the outcome for the bacteria in the lungs and blood, the mutations have no effect by 48 h, but judged by the effect on the host, the absence of either activity is an important determinant of the outcome. Why does the pneumococcus retain a system that leads to damage of the host but does not apparently benefit the bacterium directly?
It has been shown previously (2) that the presence of the Asp385 → Asn and His367 → Arg mutations in the pneumococcal pneumolysin extend the survival time of mice after intranasal infection. In the study reported here we observed a correlation between survival time and timing or extent of cellular inflammation, but we did not observe a correlation between survival or inflammation and the numbers of pneumococci in the lungs. It is noteworthy that by 48 h postinfection the same numbers of mutant and wild-type pneumococci were present in the lungs. Also, there was no correlation between the timing of the onset or the extent of bacteremia and host survival time. The later appearance of H2−/C+ in the blood is consistent with the previously stated view (12) that a delay in bacteremia is associated with a delay in the time of death. However, the timing of bacteremia due to H+/C− is not consistent with this hypothesis. The early detection of H+/C− in the blood suggests that complement activation by pneumolysin plays a pivotal role in delaying bacteremia. Benton and colleagues (4) reported that point mutations in the ply gene that reduced complement activation or cytotoxicity did not individually alter the growth kinetics of pneumococci injected directly into the blood. In our study the numbers of the mutants and the wild type also increased at the same rate in the blood.
Previously, it was reported that when pneumolysin was present, pneumococci were more resistant to antimicrobial mechanisms that limit their numbers in the blood (12). The data in this paper show that the presence of either the pore formation or complement-activating activity of pneumolysin is sufficient for this resistance. In contrast to these observations, Alcantra et al. (1) suggested that an absence of complement-activating activity results in greater pneumococcal clearance after intravenous infection of rats.
It has been reported previously that an absence of pneumolysin was associated with a significant delay in and a lower severity of pulmonary inflammation (12). Also, an absence of pneumolysin resulted in significantly less intense accumulation of neutrophils and T cells at inflamed sites in the lungs compared to the responses observed with wild-type pneumococci (12). Based on the histological analysis described here, it seems that both of the pneumolysin activities that were investigated contribute to the induction of inflammation. Thus, a less severe histological result was seen with both mutant strains soon after infection. However, by 48 h the extent of inflammation was as severe as the extent of inflammation after wild-type infection. This contrasts with the situation after infection with the pneumolysin-negative mutant (12). What was also noteworthy was the observation that after infection with H+/C− pneumococci, the responding inflammatory cells were less tightly packed around the bronchioles. This implicates complement activation in mediating local localization of cells.
Pneumococcal infection is associated with a sequential influx of inflammatory cells into the lungs. We concluded that accumulation of host cells in lesions was not simply a consequence of pneumococcal numbers in the lungs but was related to the signals related to the infection, such as chemokines, cytokines, and complement factors. The data obtained in this study support this conclusion. Two other conclusions can be drawn from the data: both the cytolytic and complement-activating activities contribute to the influx of cells, and there is specificity in the consequences of each activity. Crucially, the toxin's complement-activating activity seems to be more important for the recruitment of T cells to inflammatory lesions. To our knowledge, the previous work in our laboratory was the first work to demonstrate the important involvement of T cells during pneumococcal bronchopneumonia (12). The findings presented in this paper significantly advance our understanding of the role that T cells play as part of the inflammatory host immune response to the pneumococcal toxin pneumolysin.
Conversely, the toxin's cytolytic activity is the predominant influence on neutrophil accumulation. Extrapolating from the observations of Baba et al. (3), it is likely that the H2−/C+ toxin stimulated more gamma interferon and consequently more nitric oxide than the wild-type or H+/C− toxin stimulated. Therefore, the reduced inflammation and decreased cell recruitment observed with the H2−/C+ mutant do not support the hypothesis that gamma interferon and nitric oxide are major mediators of the effects of the toxin in pneumonia. It has previously been shown that in wild-type pneumococcus-infected mice, tumor necrosis factor alpha is involved in the protective pulmonary response during pneumococcal pneumonia and that interleukin-6 levels contribute to pathogenesis of the disease rather than protection (13). Analysis at a molecular level of induction of host mediators of inflammation by pneumolysin's anticellular and complement properties is required to obtain a fuller understanding of how this toxin acts as a principal trigger of inflammation during pneumococcal pneumonia.
Acknowledgments
R.J. was the recipient of a scholarship from the University of Lebanon. The support of the Wellcome Trust also is acknowledged.
Editor: J. T. Barbieri
REFERENCES
- 1.Alcantra, R. B., L. Preheim, and M. J. Gentry. 1999. Role of pneumolysin's complement-activating activity during pneumococcal bacteremia in cirrhotic rats. Infect. Immun. 67:2862-2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander, J. E., A. M. Berry, J. C. Paton, J. B. Rubins, P. W. Andrew, and T. J. Mitchell. 1998. Amino acid changes affecting the activity of pneumolysin alter the behaviour of pneumococci in pneumonia. Microb. Pathog. 24:167-174. [DOI] [PubMed] [Google Scholar]
- 3.Baba, H., I. Kawamura, C. Kohda, T. Nomura, Y. Ito, T. Kimoto, I. Watanabe, S. Ichiyama, and M. Mitsuyama. 2002. Induction of gamma interferon and nitric oxide by truncated pneumolysin that lacks pore-forming activity. Infect. Immun. 70:107-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benton, K. A., J. C. Paton, and D. E. Briles. 1997. The hemolytic and complement activating properties of pneumolysin do not contribute individually to virulence in a pneumococcal bacteremia model. Microb. Pathog. 23:201-209. [DOI] [PubMed] [Google Scholar]
- 5.Berry, A. M., J. E. Alexander, T. J. Mitchell, P. W. Andrew, D. Hansman, and J. C. Paton. 1995. Effect of defined point mutations in the pneumolysin gene on the virulence of Streptococcus pneumoniae. Infect. Immun. 63:1969-1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boulnois, G. J., J. C. Paton, T. J. Mitchell, and P. W. Andrew. 1991. Structure and function of pneumolysin, the multifunctional thiol-activated toxin of Streptococcus pneumoniae. Mol. Microbiol. 5:2611-2616. [DOI] [PubMed] [Google Scholar]
- 7.Canvin, J. R., A. P. Marvin, M. Sivakumaran, J. C. Paton, G. J. Boulnois, P. W. Andrew, and T. J. Mitchell. 1995. The role of pneumolysin and autolysin in the pathology of pneumonia and septicaemia in mice infected with a type 2 pneumococcus. J. Infect. Dis. 172:119-123. [DOI] [PubMed] [Google Scholar]
- 8.Feldman, C., R. Reed, A. Rutman, N. C. Munro, D. K. Jeffrey, A. Brainy, P. J. Cole, and R. Wilson. 1991. The effect of Streptococcus pneumoniae on intact respiratory epithelium. Eur. Respir. J. 5:576-583. [PubMed] [Google Scholar]
- 9.Ferrante, A., B. Rowan Kelly, and J. C. Paton. 1984. Inhibition of in vitro human lymphocyte response by the pneumococcal toxin pneumolysin. Infect. Immun. 46:585-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Houldsworth, S., P. W. Andrew, and T. J. Mitchell. 1994. Pneumolysin stimulates production of tumor necrosis factor alpha and interleukin-1 beta by human mononuclear phagocytes. Infect. Immun. 62:1501-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kadioglu, A., and P. Sheldon. 1998. Steroid pulse therapy for rheumatoid arthritis: effect on lymphocytes subsets and mononuclear adhesion. Br. J. Rheumatol. 37:282-286. [DOI] [PubMed] [Google Scholar]
- 12.Kadioglu, A., N. A. Gingles, K. Grattan, A. Kerr, T. J. Mitchell, and P. W. Andrew. 2000. Host cellular immune response to pneumococcal lung infection in mice. Infect. Immun. 68:492-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kerr, A. R., J. J. Irvine, J. J. Search, N. A. Gingles, A. Kadioglu, P. W. Andrew, W. L. McPheat, C. G. Booth, and T. J. Mitchell. 2002. Role of inflammatory mediators in resistance and susceptibility to pneumococcal infection. Infect. Immun. 70:1547-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitchell, T. J., P. W. Andrew, F. K. Saunders, A. N. Smith, and G. J. Boulnois. 1991. Complement activation and antibody binding by pneumolysin via a region homologous to a human acute phase protein. Mol. Microbiol. 5:1883-1888. [DOI] [PubMed] [Google Scholar]
- 15.Paton, J. C., and A. Ferrante. 1983. Inhibition of human polymorphonuclear leukocyte respiratory burst, bactericidal activity, and migration by pneumolysin. Infect. Immun. 41:1212-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paton, J. C., B. Rowan Kelly, and A. Ferrante. 1984. Activation of human complement by the pneumococcal toxin pneumolysin. Infect. Immun. 43:1085-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paton, J. C., P. W. Andrew, G. J. Boulnois, and T. J. Mitchell. 1993. Molecular analysis of the pathogenicity of Streptococcus pneumoniae: the role of pneumococcal protein. Annu. Rev. Microbiol 47:89-115. [DOI] [PubMed] [Google Scholar]
- 18.Rubins, J. B., D. Charboneau, J. C. Paton, T. J. Mitchell, P. W. Andrew, and E. N. Janoff. 1995. Dual function of pneumolysin in the early pathogenesis of murine pneumococcal pneumonia. J. Clin. Investig. 95:142-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rubins, J. B., D. Charboneau, C. Fasching, A. M. Berry, J. C. Paton, J. E. Alexander, P. W. Andrew, T. J. Mitchell, and E. N. Janoff. 1996. Distinct role of pneumolysin's cytotoxic and complement activities in the pathogenesis of pneumococcal pneumoniae. Am. J. Respir. Crit. Care Med. 153:1339-1346. [DOI] [PubMed] [Google Scholar]