Abstract
Pneumocystis carinii remains an important and potentially fatal cause of opportunistic pneumonia. Animal studies reveal that substantial quantities of surfactant protein D (SP-D) accumulate in the airspaces during P. carinii pneumonia and are particularly abundant in aggregates of organisms. Due to the multimeric structure of SP-D, we hypothesized that SP-D mediates aggregation of the organism. From previous clinical studies it is known that aggregated organisms are conspicuous in sections of lung tissue and bronchoalveolar lavage (BAL) fluids of humans with active P. carinii pneumonia. Herein, we observe that SP-D levels increased at least fourfold in BAL fluids of patients with P. carinii pneumonia. Next, a spectrophotometric sedimentation assay was developed to assess the aggregation of P. carinii in vitro by SP-D. P. carinii organisms were first stripped with glutathione to remove bound SP-D and subsequently incubated in the presence of SP-D and 2 mM calcium. P. carinii incubated with natural SP-D (10 μg/ml) containing dodecamers and higher-order forms exhibited aggregation and enhanced sedimentation compared to that of glutathione-stripped P. carinii. Aggregation was also enhanced by the concentrated supernatant of rat BAL fluid, and this effect was abolished by the selective removal of SP-D from the lavage fluid. P. carinii aggregation was reduced by maltose, mannose, and EDTA, consistent with the role of the SP-D C-type lectin domain (CRD) in the aggregation event. Comparisons of different molecular forms of SP-D showed that dodecamers—but not trimeric subunits—mediate optimal aggregation of P. carinii. Aggregation of P. carinii by SP-D was shown to be responsible for the impaired phagocytosis of the organisms by alveolar macrophages. Thus, SP-D-mediated aggregation of P. carinii may represent one means by which the organism avoids elimination by the host.
Pneumocystis carinii pneumonia remains an important, life-threatening opportunistic infection in immunocompromised patients. It is particularly prevalent among those with AIDS, hematologic or solid malignancies, or transplanted organs and among patients receiving chronic immunosuppressive therapies, particularly corticosteroids (23, 25, 35, 39, 59). Histologically, P. carinii pneumonia is characterized by filling of the alveolar space with distinctive protein-rich frothy exudates containing prominent aggregates of organisms (32). The exact chemical nature of the proteinaceous alveolar exudates is not known, but prior studies have shown that these exudates are particularly rich in surfactant proteins A and D (SP-A and SP-D), fibronectin, and vitronectin (54). Interactions of these host components, particularly the surfactant-associated proteins, with P. carinii has been an area of intensive investigation (37, 47, 53, 58, 60).
Pulmonary surfactant is a complex mixture of lipids and proteins synthesized by alveolar type II cells and secreted into the alveolar spaces. This surfactant exerts multiple functions including reduction of alveolar surface tension and modulation of host defense and inflammatory responses (20, 29). It contains at least four associated proteins, of which two, SP-B and SP-C, are hydrophobic and two, SP-A and SP-D, are hydrophilic. SP-A and SP-D have been demonstrated to accumulate during P. carinii pneumonia, while SP-B and SP-C are suppressed during this infection (3, 42, 44). These surfactant-associated proteins have further been shown to modulate the interaction of P. carinii with host cells and to regulate host inflammatory responses to the organism (26, 30, 42, 57).
SP-D is a soluble, collagenous protein synthesized and secreted by type II pneumocytes and nonciliated bronchiolar cells (43). Structurally, it belongs to the group III, mammalian C-type lectin family that includes SP-A, mannose binding protein, and bovine conglutinin (21). SP-D is composed of 43-kDa monomers, each consisting of four major domains: a short cysteine-containing amino-terminal region, a triple helical collagenous domain, a trimeric coiled neck region, and a globular carboxy-terminal carbohydrate recognition domain (CRD) (11). These monomers are assembled into triple helical trimers, which form a single collagenous “arm” displaying the CRDs on the end. Four trimeric subunits undergo disulfide cross-linking within their amino-terminal domains to form a cruciform dodecameric structure (10). Although rat SP-D is assembled as dodecamers, human SP-D appears to consist of a complex of dodecamers and variable proportions of higher-order multimers and trimers (11). In addition, the extent of SP-D binding to P. carinii ligands appears to correlate with the number of CRDs in higher-order multimers of the molecule (55).
Recent investigations indicate that SP-D exerts various functions in innate immunity during infection of the respiratory tract. For instance, SP-D has been demonstrated to bind lipopolysaccharide present on the surface of Escherichia coli and to mediate agglutination of the organism (28). SP-D has also been reported to exert protective activities against influenza A virus (17, 19). In addition, SP-D participates in the interactions of Mycobacterium tuberculosis with alveolar macrophages (15).
It has previously been found that SP-D accumulates during P. carinii pneumonia in a rodent model and binds to the organisms through the CRD of SP-D (42). More recently, increased accumulation and expression of SP-D have been demonstrated in SCID mice with P. carinii pneumonia (3). The SP-D CRD interacts with both glycoprotein A (gpA), a major surface antigen of trophic and cyst forms of P. carinii, and β-glucan components of the cyst wall (2, 55, 56). Interestingly, the binding of SP-D to P. carinii results in enhanced attachment of organisms to rat alveolar macrophages, though macrophage uptake of P. carinii is reduced in the presence of SP-D (42). This impairment of macrophage uptake may be related to the formation of large aggregates of the organisms. The cruciform shape of dodecameric SP-D provides a structure potentially capable of aggregating the organism into such large agglomerates.
The following investigations were therefore undertaken to test the general hypothesis that SP-D induces aggregation of P. carinii. Studies were first performed to confirm for human P. carinii pneumonia the accumulation of SP-D that had previously been observed only in animal models of infection. Secondly, a spectrophotometric sedimentation assay was designed to evaluate the role of SP-D in P. carinii aggregation. Additional investigations were performed to evaluate the roles of the CRD and the structure of SP-D in mediating these effects. Finally, the effects of SP-D-induced aggregation on P. carinii uptake by macrophages were measured.
MATERIALS AND METHODS
Materials.
General reagents were obtained from Sigma Chemical Co. (St. Louis, Mo.) or Fisher Scientific Co. (Pittsburgh, Pa.) unless otherwise specified. Pneumocystis carinii f. sp. carinii was originally obtained through the American Type Culture Collection (Manassas, Va.) and was propagated in rats as reported previously (33). Ciprofloxacin was the kind gift of Barbara Painter of Miles Pharmaceuticals, Inc. (West Haven, Conn.).
Measurement of SP-D in human BAL fluid.
Prior studies with rodent models of P. carinii pneumonia indicate that SP-D accumulates during the course of infection (3, 42). To determine the extent to which SP-D accumulates in the lower respiratory tract during P. carinii infection in humans, bronchoalveolar lavage (BAL) fluids were obtained from 11 patients with P. carinii pneumonia and concurrently from 11 immunocompromised patients without P. carinii pneumonia who were undergoing lavage for clinical evaluation of pulmonary infiltration. The clinical features of these patients and the methods of flexible fiber optic bronchoscopy, lavage, and separation of the recovered BAL specimens into cellular and fluid components have been described previously (35, 36, 59). All lavage fluids were stored at −20°C until assay. SP-D concentrations in the BAL fluids were determined in duplicate by using a competitive enzyme-linked immunosorbent assay (ELISA) modified from the method of Neese et al. (40). Ninety-six-well ELISA plates were coated with human SP-D (5 μg/ml) in 100 mM NaHCO3 by overnight incubation at 37°C. Plates were then washed with Tris-HCl balanced salt solution (TBS) containing 1 mg of heat-denatured bovine serum albumin/ml, 1 mM CaCl2, and 1 mM MgCl2 and were further incubated for an additional hour with TBS containing 3 mg of heat-denatured bovine serum albumin/ml, 1 mM CaCl2, and 1 mM MgCl2 to block nonspecific protein binding sites. In separate test tubes, standard concentrations of SP-D and samples were incubated with a rabbit polyclonal antibody generated against the SP-D CRD (1 μg/ml) for 1 h at 37°C prior to plating onto the SP-D-coated wells (55). The plates were then incubated for an additional hour and washed, and a horseradish peroxidase-conjugated goat anti-rabbit antibody (dilution, 1:5,000; ICN, Costa Mesa, Calif.) was added to each well and incubated for an additional hour. After a wash, o-phenylenediamine dihydrochloride (OPD) substrate was placed in each well. After development, colorimetric reactions were stopped with 1 N H2SO4 and the absorbances were read at 450 nm. A standard curve was generated and used to derive SP-D concentrations in BAL fluids.
Preparation of P. carinii organisms.
P. carinii pneumonia was induced in rats by immunosuppression with dexamethasone (4, 34). Specific-pathogen-free rats (Harlan Sprague-Dawley, Inc., Indianapolis, Ind.) were provided with drinking water containing dexamethasone (2 mg/liter), tetracycline hydrochloride (500 mg/liter), and nystatin (200,000 U/liter). On a weekly basis, the animals also received oral ciprofloxacin (0.45 g/liter) for two consecutive days to further reduce the risk of bacterial infection (55). After 5 days, rats were intratracheally inoculated with P. carinii (500,000 organisms). Following an additional 6 weeks of immunosuppression, rats were exsanguinated and the lungs were perfused with Na Ca HEPES buffer (150 mM NaCl, 1.8 mM CaCl2, 25 mM HEPES [pH 7.4, 329 mosM]). The lungs were excised, minced in Na Ca HEPES, and homogenized in a stomacher laboratory blender for 5 min at room temperature. The remaining large tissue pieces were removed by filtration through gauze. The suspension was centrifuged (at 1,000 × g for 10 min) and treated with 0.85% NH4Cl, pH 6.8, for 5 min to lyse host cells. Following two washes with Na Ca HEPES, the material was sequentially filtered, first through polycarbonate membranes with 10-μm pores and finally through filters with 5-μm pores (Poretics Corp., Livermore, Calif.) (4, 48, 50). P. carinii isolates were quantified by counting P. carinii nuclei as described previously (33). P. carinii trophic forms represented more than 99% of the material on Diff-Quick-stained smears (24). In order to remove surface molecules from P. carinii, specified preparations were also treated with glutathione (0.5%) and EDTA (10 mM) and were washed again prior to use (22). This procedure has been previously documented to remove >99.5% of SP-A from the surfaces of P. carinii organisms (22).
Generation of concentrated BAL proteins from P. carinii-infected rats.
After 6 weeks of immunosuppression, rats were exsanguinated, and whole-lung lavage was performed with 50 ml of Hanks' balanced salt solution (HBSS) in sequential 10-ml aliquots. After centrifugation at 1,000 × g for 10 min, the lavage supernatant was concentrated 30-fold (Centriprep-3 concentrator; cutoff, 3,000 Da; Amicon, Inc., Beverly, Mass.). To remove SP-D from the BAL proteins, the concentrated solution was divided and a portion was treated with Toyopearl-amino-maltose resin (TosoHaas, Inc., Montgomeryville, Pa.) prepared as previously reported (55). One-fourth volume of packed Toyopearl-amino-maltose resin was added to the concentrated BAL proteins and adsorbed with rocking over 3 h. BAL proteins were recovered by centrifugation and stored at −70°C until use.
Spectrophotometric sedimentation assay.
To remove EDTA and glutathione prior to the assay, P. carinii suspensions were twice centrifuged at 1,000 × g for 10 min at 4°C, resuspended with TBS (50 mM Tris HCl-150 mM NaCl [pH 7.4]), and adjusted to a concentration of 7.5 × 106 P. carinii organisms per ml. The subsequent aggregation of P. carinii was quantified by using a modification of the spectrophotometric sedimentation assay of Ericson and coworkers (14, 28). The time course of macroscopic P. carinii agglutination was monitored using a spectrophotometer (model DU-74; Beckman Coulter Inc., Fullerton, Calif.) at a 700-nm wavelength (measuring optical density at 700 nm [OD700]). The baseline spectrophotometer reading was set at an OD700 of zero by using reference TBS solutions without organisms. Two milliliters of a suspension containing 15 × 106 P. carinii organisms was inserted into a 4.5-ml cuvette and equilibrated for 15 min at 37°C. Test solutions of SP-D (1 ml each) were prepared separately and added to the suspension at time zero in order to yield a final suspension concentration of 10 × 106 P. carinii organisms, 10 μg of SP-D/ml, 100 mM sugar as specified, and 2 mM calcium chloride. Some experiments also tested SP-D (10 μg/ml) in the presence of 5 mM EDTA. Equal volumes of identical buffers without SP-D served as controls. The OD700 was monitored over the subsequent 6 h at 37°C. Estimates of percent aggregation were derived from the sedimentation assay data by taking the OD700 at 5 min after addition and mixing of the test solutions as 0% aggregation, and taking an OD700 of zero as 100% aggregation.
The conditions selected for these sedimentation assays were supported by our previous kinetic assays of binding of 125I-labeled SP-D to whole P. carinii organisms in the presence of 2 mM calcium. Binding of 125I-labeled SP-D to whole P. carinii organisms was rapid, achieving equilibrium binding as early as 15 min of incubation at 37°C. P. carinii possesses multiple binding epitopes for SP-D, including the mannose-rich gpA surface complex and cell wall β-glucans (54-56). Competitive binding assays further indicated that more than 65% of 125I-labeled SP-D binding to P. carinii was competitively inhibited by a 100-fold excess of cold SP-D. Furthermore, EDTA (10 mM) inhibited 125I-labeled SP-D binding to P. carinii by >90%. Saturable binding of SP-D to P. carinii occurred at concentrations higher than 1.25 μg/ml.
SP-D preparations.
Natural rat SP-D was isolated from the 10,000 × g supernatant of BAL fluids obtained from rats with silica-induced alveolar lipoproteinosis as previously described (55). SP-D was purified by affinity chromatography on maltosyl-agarose (13, 18). The purity of SP-D preparations was verified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and silver staining. Natural rat SP-D preparations demonstrated a single 43-kDa band under reducing conditions. The majority of natural SP-D obtained from these silica-treated rats was in the dodecameric form, with 4.0% of the material representing higher-order multimers of dodecameric forms (55). To evaluate the role of multiple CRD motifs in facilitating aggregation of P. carinii gpA, two recombinant rat SP-D proteins were tested. A full-length recombinant rat SP-D (rSP-D) was generated and purified as previously described (12). The rSP-D protein comigrated with natural rat SP-D on SDS-PAGE gels under reducing and nonreducing conditions, bound efficiently to maltosyl-agarose, and coeluted predominantly with natural rat SP-D dodecamers in nondenaturing gel filtration over 4% agarose (12). In addition, mutant SP-D monomers which assemble into homotrimers (single arm) but do not form dodecamers were studied. This mutant (RrSP-Dser15,20) represents a full-length peptide containing serine substitutions for the two amino-terminal cysteines, which mediate disulfide cross-linking in the generation of dodecamers (7). Previous studies have shown that the mutant is fully active as a lectin but is defective in mediating bridging interaction between particulate ligands (7).
Uptake of P. carinii by alveolar macrophages.
To address the functional consequences of P. carinii aggregation, we measured macrophage uptake of glutathione-stripped P. carinii before and following SP-D aggregation. Uptake of P. carinii by macrophages was assayed by 51Cr labeling of the organisms (33, 34, 46). P. carinii organisms were isolated from rats as described above in the presence of EDTA (10 mM) and glutathione (0.5%) to strip SP-D from the surface. The organisms were radiolabeled by incubation for 8 h at 37°C in 2 ml of Dulbecco's modified Eagle medium containing 20% fetal calf serum and 200 μCi of sodium [51Cr]chromate (New England Nuclear; Boston, Mass.) and were then washed in HBSS with 0.5 mM EDTA to remove unincorporated label and to again disaggregate the organisms. Rat alveolar macrophages, obtained by BAL of healthy animals, were plated in tissue culture plates (105 cells/well) that had been precoated with normal immunoglobulin G (IgG) (100 μg/ml for 60 min) in order to ensure firm adherence of the macrophages (9). After 1 h, the macrophages were gently washed with HBSS to remove nonadherent cells. 51Cr-labeled P. carinii organisms were aggregated with either SP-D or mutant homotrimeric (single-arm) RrSP-Dser15,20 (10 μg/ml) for 3 h. The P. carinii organisms were gently rinsed three times in HBSS containing 2 mM calcium, added to the macrophages (200 P. carinii organisms per macrophage), and incubated for six additional hours. Additional control P. carinii organisms were cultured with the macrophages in the presence of EDTA (10 mM). To measure the combined number of bound and internalized P. carinii organisms, the macrophages were incubated at 37°C. Parallel cultures were also incubated for 6 h at 4°C to measure only the number of organisms bound to the macrophage surfaces, but not those internalized. After the incubations, nonadherent P. carinii organisms were removed by gentle washing. The macrophage monolayers containing associated P. carinii organisms were solubilized in 1 N NaOH and quantified. Phagocytosis of P. carinii was measured as the difference between the counts of organisms both bound and internalized (measured at 37°C) and the counts of organisms only bound to the macrophage surfaces (measured at 4°C).
Statistical analyses.
Data are expressed as means ± standard errors of the means (SEM). Differences between experimental and control data groups were determined by using two-tailed Student t tests for normally distributed variables. Statistical testing was performed with the Statview II statistical package (Abacus Concepts, Inc., Berkeley, Calif.). Statistical differences between groups were considered significant if P values were <0.05.
RESULTS
SP-D accumulates in the lower respiratory tract during human P. carinii pneumonia.
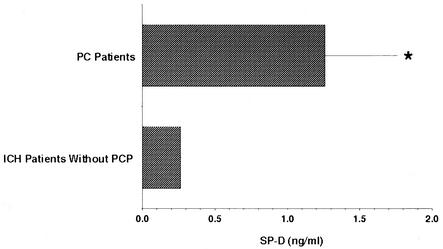
Because SP-D is known to interact with both P. carinii organisms and alveolar macrophages, we quantified this surfactant-associated protein in the lower respiratory tracts of patients with P. carinii pneumonia. BAL fluids were obtained from 11 patients with P. carinii pneumonia and concurrently from 11 immunocompromised patients without P. carinii infection, all of whom underwent bronchoscopy for clinical evaluation of diffuse pulmonary infiltration. SP-D concentrations in BAL supernatants were determined in duplicate by competitive ELISA (Fig. 1). BAL fluids from patients with P. carinii pneumonia contained 1.260 ± 0.512 μg of SP-D/ml versus 0.264 ± 0.162 μg/ml in BAL fluids from patients without P. carinii pneumonia (P = 0.0432). Thus, as observed in prior studies with rat and murine models of infection, SP-D is present in significantly increased amounts in the lower respiratory tract during P. carinii pneumonia in humans.
FIG. 1.
SP-D is present in increased quantities in BAL fluids of patients with P. carinii pneumonia. BAL fluids from 11 patients with P. carinii pneumonia (PCP) and 11 immunocompromised patients without P. carinii who were sampled concurrently were quantified by competitive ELISA. *, P = 0.0432 for comparison between these two patient groups. ICH, immunocompromised host.
SP-D promotes the aggregation of P. carinii.
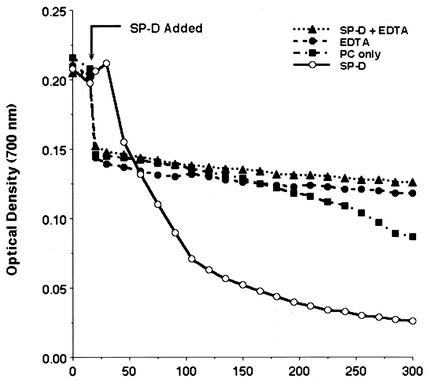
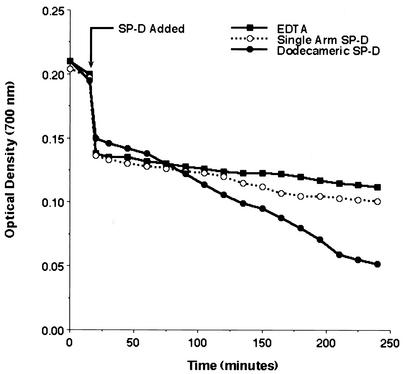
Our prior studies have shown that SP-D is associated with clusters of P. carinii in the rodent lung and in lavage fluid, suggesting that it could be responsible for aggregation of the organisms. To test this hypothesis, P. carinii organisms were freshly isolated and stripped with glutathione and EDTA, which effectively remove surfactant proteins associated with the surfaces of the organisms (22). We next quantified the aggregation of P. carinii in the presence and absence of SP-D by using a spectrophotometric sedimentation assay as previously described (28). The initial P. carinii suspension appeared turbid and displayed an OD of 0.220 ± 0.022. Over time, however, as more P. carinii organisms aggregated and settled out of suspension, the OD700 decreased. Addition of SP-D to the sedimentation assay incubation markedly enhanced aggregate formation and sedimentation (Fig. 2). There was also macroscopic aggregation and precipitation of P. carinii in the presence of SP-D. The diameters of some SP-D-induced P. carinii aggregates increased to as much as 1.5 mm over the ensuing 4 to 5 h. In contrast, P. carinii incubated with SP-D in the presence of EDTA did not show this accelerated sedimentation rate. It should be noted that P. carinii organisms stripped of surface-associated host proteins still exhibited a low rate of autoaggregation. P. carinii cultured in the presence of EDTA exhibited minimal sedimentation, similar to that of P. carinii cultured in the presence of both SP-D and EDTA. After 240 min, P. carinii incubated in the presence of SP-D exhibited 81.8% ± 1.9% aggregation while P. carinii incubated in the absence of SP-D exhibited only 28.8% ± 3.1% aggregation (P = 0.0001; n = 3 experiments). After 5 h, P. carinii incubated with SP-D demonstrated 85.0% ± 0.6% aggregation compared to only 43.2% ± 4.0% aggregation by P. carinii alone (P = 0.0046; n = 3 experiments). Taken together, these data indicate that SP-D markedly enhances the aggregation of P. carinii organisms, in a manner requiring divalent cation-mediated binding of the collectin with the organism.
FIG. 2.
SP-D promotes the aggregation of P. carinii. P. carinii organisms were freshly isolated and stripped of associated host proteins by use of glutathione and EDTA. P. carinii aggregation over time was monitored by a spectrophotometric sedimentation assay. Addition of SP-D (10 μg/ml) accelerated the aggregation of P. carinii. P. carinii aggregation was abolished in the presence of EDTA (5 mM). P. carinii incubated in the absence of SP-D and EDTA also exhibited autoaggregation, though at a significantly reduced rate compared to that in the presence of SP-D. Shown are results of an experimental run representative of three separate experiments. For statistical comparisons of aggregation conditions, see Results.
BAL proteins also enhance P. carinii aggregation.
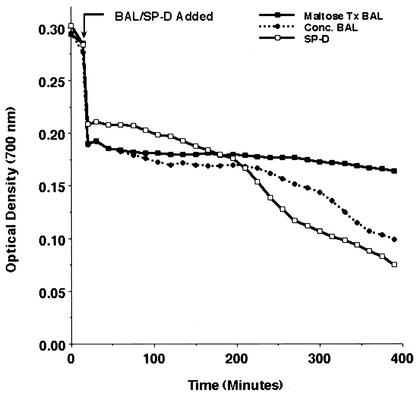
To next determine whether proteins present within BAL fluid also promoted aggregation of the organisms, we evaluated the sedimentation rate of glutathione-stripped P. carinii incubated in the presence of concentrated BAL protein (net effective concentration, 10-fold) (Fig. 3). For comparison, we evaluated identically concentrated lavage proteins that had been treated with immobilized maltose resin, which efficiently removes SP-D, but not SP-A, from the BAL protein. Though not as potent as purified SP-D (10 μg/ml), the 10-fold-concentrated BAL proteins promoted aggregation, and this effect was eliminated when the BAL proteins were treated with maltosyl resin. After 6 h, P. carinii incubated in the presence of total concentrated BAL proteins exhibited 43.2% ± 2.0% aggregation, compared to only 12.8% ± 0.9% aggregation for P. carinii exposed to concentrated BAL proteins treated with immobilized maltose to remove SP-D (P = 0.0001; n = 3 experiments). These observations indicate that BAL fluid contains proteins capable of mediating aggregation of P. carinii, and they indicate that SP-D, or a lectin with a similar specificity, could promote the aggregation of P. carinii in vivo.
FIG. 3.
Alveolar proteins recovered by BAL promote P. carinii aggregation. P. carinii organisms were freshly isolated and stripped of associated host proteins by use of glutathione and EDTA. BAL proteins were obtained by 10-fold concentration of BAL cell-free filtrates. Identical aliquots of concentrated BAL protein were exposed to maltosyl-Toyopearl resin to selectively remove SP-D. P. carinii aggregation was monitored by a spectrophotometric sedimentation assay. Concentrated BAL protein promoted the aggregation of P. carinii in a manner parallel to that of purified SP-D (10 μg/ml). The effect of the concentrated BAL proteins was reversed by treatment of the BAL with maltosyl-Toyopearl resin. Shown are results of an experimental run representative of three separate experiments. For statistical comparisons of aggregation conditions, see Results.
SP-D mediates P. carinii aggregation through its CRD.
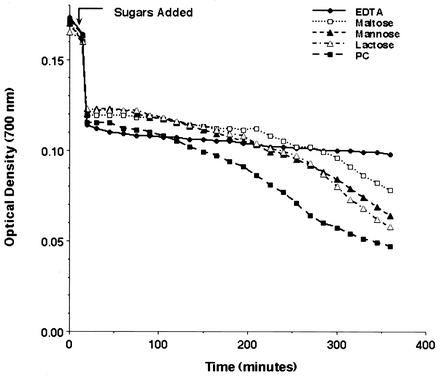
Our prior studies have indicated that SP-D binds P. carinii epitopes, including the gpA major surface glycoprotein complex and P. carinii cell wall β-glucans, through its CRD. We therefore hypothesized that SP-D similarly mediates the aggregation of P. carinii via its CRD. To test this, we compared the SP-D-induced sedimentation of P. carinii organisms in the presence of maltose, mannose, and lactose (100 mM each). Calcium chloride (2 mM) was present to facilitate CRD binding (Fig. 4). For comparison, EDTA (5 mM), which completely inhibits P. carinii aggregation, was tested in parallel. P. carinii aggregation was inhibited by these sugars in the following order of potency, from highest to lowest: maltose, mannose, and lactose (Fig. 4). Once again, divalent cations were required for P. carinii aggregation. This pattern of sugar inhibitory capacity is compatible with prior observations of SP-D binding to whole organisms and gpA (55). Taken together, these observations indicate that SP-D promotes P. carinii aggregation through interaction of its CRD with the organism surface.
FIG. 4.
SP-D induced aggregation of P. carinii is inhibited by soluble sugars. P. carinii organisms were freshly isolated (without glutathione-EDTA stripping) and aggregation was monitored in the presence of SP-D (10 μg/ml) and competitive sugars (100 mM) or EDTA (5 mM). The following conditions were evaluated: EDTA (solid circles), maltose (open squares), mannose (solid triangles), lactose (open triangles), and P. carinii in buffer alone, used as a control (solid squares). Competitive inhibition of SP-D-induced aggregation of P. carinii showed that maltose had the highest inhibitory potency, followed by mannose and lactose, in that order. Shown are results of an experimental run representative of three separate experiments.
Dodecameric forms of SP-D mediate P. carinii aggregation.
Based on our prior observations that SP-D interacts with P. carinii through its CRD, we further proposed that aggregation of the organism requires multimeric SP-D. Native SP-D is present largely as dodecameric structural forms (10, 55). These dodecamers are assembled from trimeric arms, each containing three chains composed of a triple helical collagenous domain, a neck region, and three CRDs at its carboxy terminus. The three CRDs of mannose binding lectin, a related collectin, have been shown to interact with ligands in a planar fashion (11). The amino-terminal association and cross-linking of four such trimeric arms result in the formation of the typical cruciform dodecameric structure (10). We hypothesized that single-arm trimeric SP-D would similarly bind organisms only in a planar fashion, would not effectively cross-link multiple organisms, and hence would have less effect on organism aggregation. To test this, we compared the abilities of recombinant rat SP-D dodecamers (rSP-D) and trimeric subunits of rSP-D (RrSP-Dser15,20) to aggregate P. carinii (Fig. 5). As predicted, the trimeric single-arm full-length SP-D exhibited minimal ability to promote the aggregation and sedimentation of P. carinii compared to dodecameric SP-D. After 240 min, the trimeric RrSP-Dser15,20 showed only 36.3% ± 10.1% aggregation, only slightly more than the negative control. By contrast, the rSP-D dodecamers showed 62.1% ± 8.4% aggregation (P = 0.005; n = 4 experiments). Thus, efficient aggregation of P. carinii by SP-D requires dodecameric or higher-order forms to most effectively cross-link and agglutinate adjacent organisms.
FIG. 5.
SP-D dodecamers induce greater aggregation than identical concentrations of trimeric SP-D subunits. P. carinii organisms were stripped of associated host proteins by use of glutathione and EDTA, and aggregation was monitored in the presence of recombinant dodecameric rat SP-D or mutant RrSP-Dser15,20, which assembles as trimeric single-arm structures (10 μg/ml each). EDTA (5 mM) was studied in parallel. P. carinii incubated in the dodecameric SP-D exhibited significantly greater aggregation than P. carinii incubated in equal concentrations of RrSP-Dser15,20. Shown are results of an experiment representative of four experimental runs. For statistical comparisons of aggregation conditions, see Results.
SP-D-induced aggregation of P. carinii impairs phagocytosis by alveolar macrophages.
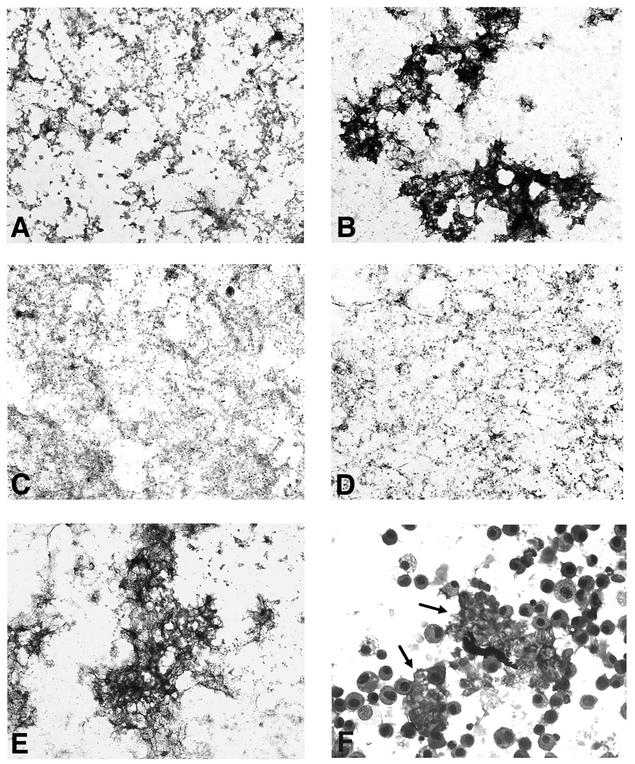
Lastly, we sought to determine the functional significance of SP-D-induced aggregation of P. carinii during interactions with alveolar macrophages, the principal cells responsible for phagocytosis of this organism (33). To first address the relative sizes of P. carinii aggregates and alveolar macrophages, microscopy was performed on glutathione stripped P. carinii following treatment with SP-D, EDTA, and inhibitory sugars (Fig. 6). Microscopic analysis revealed that SP-D-induced aggregates of P. carinii were quite large and amorphous (Fig. 6B). SP-D-mediated P. carinii aggregates ranged in size from 50 μm to 1.5 mm in the longest dimension, with most aggregates in the size range of 200 to 500 μm. While Pneumocystis organisms stripped of surface proteins with glutathione and EDTA appeared mainly as individual dispersed organisms (Fig. 6A), incubation with SP-D resulted in the development of dense aggregates of P. carinii organisms. Aggregation was effectively impaired by incubation of P. carinii with SP-D in the presence of either EDTA or maltose, but not in the presence of lactose, consistent with our earlier observations. For size comparison, we observed SP-D-aggregated P. carinii in the presence of alveolar macrophages (Fig. 6F). The SP-D-induced P. carinii aggregates were substantially larger than the typical alveolar macrophages, which range from 13 to 20 μm in diameter (27), supporting our hypothesis that SP-D-induced P. carinii aggregates are too large for effective alveolar macrophage uptake. The overwhelming majority of the P. carinii organisms were incorporated within the amorphous aggregates. The exact number of organisms in the aggregates was impossible to quantify, because aggregated organisms are tightly packed on top of each other. Nonetheless, by visual impression most (∼75%) of the P. carinii organisms treated with SP-D appeared incorporated into these large aggregates.
FIG. 6.
SP-D induces the formation of amorphous aggregates of P. carinii. (A) P. carinii organisms were isolated and stripped of host proteins by use of glutathione and EDTA and were incubated in TBS buffer for 4 h in the absence of SP-D. Organisms were recovered by centrifugation, spotted onto glass slides, and stained with modified Wright-Giemsa stain (Diff-Quik). This stains P. carinii nuclei dark blue. P. carinii handled in this manner appeared as dispersed individual organisms or small aggregates containing several organisms. (B) In contrast, incubation of P. carinii in the presence of SP-D (10 μg/ml) for 4 h resulted in formation of large amorphous aggregates containing dozens of organisms. (C) Incubation of P. carinii with SP-D in the presence of EDTA inhibited formation of aggregates. (D) Similarly, maltose (100 mM) also inhibited aggregation of P. carinii. (E) In contrast, incubation with lactose (100 mM) continued to permit SP-D-mediated aggregation of the organisms. (F) For size comparison, alveolar macrophages incubated with aggregated P. carinii are shown. The alveolar macrophages bind the periphery of the aggregated organisms but are much smaller than SP-D-aggregated P. carinii (arrows). Magnification, ×630.
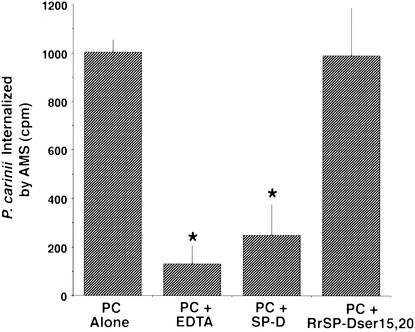
Prior studies had indicated that SP-D does facilitate binding of P. carinii to alveolar macrophages (42). However, uptake of the organisms was not enhanced by SP-D in those studies (42). To further address the effect of SP-D-induced aggregation of the organisms, we measured the phagocytosis of glutathione-stripped P. carinii by alveolar macrophages following 3 h of SP-D aggregation compared to phagocytosis of nonaggregated control P. carinii (Fig. 7). Macrophages cultured with control P. carinii in the presence of 2 mM calcium exhibited significantly greater uptake than macrophages cultured with P. carinii in the absence of calcium (with EDTA; P = 0.004). Approximately 35.1% ± 3.5% of the radiolabeled control P. carinii organisms were internalized by the macrophages over the 6-h incubation period. Furthermore, aggregation of P. carinii with SP-D (in the presence of calcium) also resulted in a dramatic reduction of organism uptake by alveolar macrophages (P = 0.0326 for comparison to control P. carinii). In contrast, treatment of P. carinii with mutant homotrimeric (single arm) RrSP-Dser15,20 in the presence of calcium, a reagent that causes minimal aggregation of P. carinii, resulted in no significant alteration in phagocytosis of the organisms by alveolar macrophages. Thus, aggregation of P. carinii induced by dodecameric SP-D (but not trimeric SP-D) was associated with impaired uptake of the organism by macrophages.
FIG. 7.
Dodecameric SP-D, but not the single-armed trimeric SP-D mutant, inhibits organism uptake by alveolar macrophages (AMS). P. carinii organisms (PC) were stripped of associated host proteins by use of glutathione and EDTA and were radiolabeled with 51Cr. Labeled organisms were aggregated for 3 h with either recombinant dodecameric rat SP-D or the mutant RrSP-Dser15,20, which assembles as trimeric single-arm structures (10 μg/ml each). Control P. carinii cultures were maintained in 1 mM calcium throughout. Additional organisms were studied in parallel in the presence of EDTA (5 mM). After aggregation, the organisms were cultured for an additional 6 h with alveolar macrophages, and organism uptake was determined as described in Materials and Methods. P. carinii aggregated with dodecameric SP-D exhibited significantly lower uptake by macrophages than control P. carinii. In contrast, RrSP-Dser15,20 did not alter P. carinii uptake by macrophages. Divalent cations are necessary for P. carinii uptake, as exhibited by impaired organism uptake in the presence of EDTA. Shown are means ± SEM for three separate determinations. *, P < 0.05 for comparison to control P. carinii.
DISCUSSION
Prior studies in immune-suppressed rodents demonstrated that SP-D accumulates in the lower respiratory tract during P. carinii pneumonia and that SP-D interacts with the mannose-rich glycoprotein gpA complex and also with cell surface β-glucans on the organisms (3, 42, 55, 56). The interactions of SP-D with P. carinii ligands are largely mediated through its CRD and are further enhanced by the multimeric structure of natural SP-D, predominantly composed of cruciform dodecameric forms (55). The present study demonstrates for the first time that SP-D levels are also higher in the lower respiratory tracts of patients with P. carinii pneumonia than in those of other immunocompromised patients without this infection. These studies also suggest that airspace SP-D is an important factor contributing to the observed agglutination of P. carinii trophic forms and cysts in the infected lung. Our investigations further reveal that the dodecameric structure of SP-D facilitates aggregation of the organisms, again through interaction of CRDs present on dodecameric SP-D with glycosylated ligands on the surface of the organism.
The data further indicate that the aggregation of P. carinii by SP-D impairs phagocytic uptake of the organism by alveolar macrophages. The present observations obtained using glutathione-stripped organisms are compatible with earlier studies using directly isolated organisms in the presence of SP-D neutralizing antibodies, and in the presence and absence of exogenous SP-D (42). Those studies revealed that SP-D impairs internalization despite augmented binding of P. carinii to the surfaces of macrophages, likely by the formation of aggregates too large to be engulfed by the phagocytes (42). Suppression of macrophage uptake of the organism is potentially a serious breach of host defense, since alveolar macrophages have been shown to be necessary for optimal clearance of P. carinii and are responsible for generation of tumor necrosis factor alpha, oxidants, and other essential inflammatory mediators during the course of this infection (9, 25, 33, 40, 53). The impact of SP-D-induced P. carinii aggregation on other mechanisms of organism clearance, such as through bulk mucociliary transport, will require additional investigation.
SP-D monomers exhibit general structural similarities to SP-A, and the respective CRDs exhibit overlapping sugar specificities (29). However, the unique tertiary assembly of SP-D largely into cruciform dodecamers and that of SP-A predominantly into an octadecameric floret configuration predict discordant functions of these two proteins. Striking differences have been noted between the interactions of SP-D and SP-A with pathogens. For instance, while SP-A acts as an opsonin increasing the phagocytosis of J5 E. coli and influenza A virus by alveolar macrophages, SP-D does not promote macrophage uptake (5, 45). The aggregation effects of SP-D on P. carinii in the present study do appear to be somewhat less potent than that previously reported for E. coli (28). E. coli showed roughly 50% measurable aggregation by use of 1 μg of SP-D/ml for 2 h, whereas P. carinii required 10 μg of SP-D/ml to produce similar aggregation over comparable incubation periods (28).
The present study further documents a substantial reduction in macrophage phagocytosis of SP-D-treated P. carinii. Other investigations have revealed that SP-D also binds to M. tuberculosis and similarly reduces the phagocytosis of this important pulmonary pathogen (15). In contrast, recent studies have shown that SP-D can increase the phagocytosis and killing of unencapsulated strains of Klebsiella pneumoniae and Pseudomonas aeruginosa by alveolar macrophages (41, 48). Distinct differences in bacterial killing and inflammatory responses have been noted for mice genetically deficient in SP-A and SP-D that were challenged with either group B streptococci or Haemophilus influenzae (31). Thus, the net impact of SP-A and SP-D on infection is quite diverse and varies not only with the relative availability of the two surfactant proteins but also with the microbial species involved.
SP-A is also known to interact with P. carinii and also consists of multimers of trimeric CRDs (26, 57, 60). However, our findings suggest that it does not contribute significantly to P. carinii aggregation by lung proteins recovered by BAL. In particular, adsorption of the BAL proteins with immobilized maltosyl supports, which are quite selective for SP-D, consistent with its known saccharide specificities, reversed almost all of the aggregating properties of BAL proteins. These findings are consistent with other studies that have shown that SP-D is a much more effective agglutinin of certain organisms such as influenza A virus (18). This has been attributed to much longer collagenous arms of SP-D, which permit bridging interactions over distances as great as 100 nm.
SP-D has previously been shown to interact with other fungal organisms including Aspergillus fumigatus, Candida albicans, and Saccharomyces cerevisiae (1, 2, 52). The effects of SP-D binding on the phagocytosis of A. fumigatus organisms are somewhat controversial (1, 2). However, interactions of SP-D with C. albicans cause agglutination of the organism and decrease fungal growth in culture (52). Similarly to the present findings, SP-D inhibits the phagocytosis of C. albicans by macrophages (52). The binding of SP-D to S. cerevisiae cell wall components further appears to involve interactions between the CRD and β-1,6 side chains of the glucan polymer (1).
A number of mechanisms likely contribute to the accumulation of SP-D in the alveolar spaces during P. carinii pneumonia. The well-characterized binding activity of P. carinii suggests that the organisms themselves may act as affinity substrates to trap secreted SP-D within the alveoli (42). Furthermore, SP-D gene expression has also been shown to increase dramatically during P. carinii pneumonia (3). Other studies suggest that type II cell function is also impaired during this infection (49, 51). Thus, it is possible that abnormal surfactant protein clearance contributes to the accumulation of SP-D during infection. Interestingly, in certain immune suppression conditions, such as chronic steroid use, the accumulation of SP-D may indeed precede the development of infection (42). Therefore, it is possible that the accumulation of SP-D itself contributes to the development of P. carinii pneumonia.
Under normal conditions, alveolar epithelial lining fluid contains significant levels of the reducing agent glutathione, which may under basal conditions reduce the extent of cross-linking of SP-D in the lung (8). However, during Pneumocystis pneumonia the lower respiratory tract is subjected to an excess oxidant burden, which limits the availability of such reducing agents (32). Indeed, our group actually has recovered increased fractions of dodecameric and higher-order SP-D aggregates associated with organisms in the setting of P. carinii pneumonia, further indicating that multimeric SP-D is present in the lungs during pneumonia to facilitate the aggregation of this organism (55).
Available information indicates that SP-D interacts with at least two prominent components on P. carinii, namely, the gpA surface complex and cell wall β-glucans, through interaction of its CRDs (42, 55, 56). Accumulating evidence demonstrates that P. carinii also binds a diverse array of other host proteins including fibronectin, vitronectin, laminin, collagen 1, and immunoglobulin (32, 37, 38, 58). The binding of SP-D through cell wall-associated ligands promotes aggregation of the organism. Optimal aggregation required dodecameric forms of SP-D. It should be noted, however, that P. carinii stripped of host proteins also exhibits a low level of autoaggregation that was abolished by EDTA chelation of divalent cations. Thus, the organism itself may contain additional lectins or molecules capable of self-association.
The net impact of SP-D interaction with P. carinii on the overall course of infection is not yet known. Investigations of P. carinii pneumonia in SP-D knockout mice are complicated by the accumulation of lipids and other surfactant components in the lungs as these animals mature (6, 16). The present study, however, suggests that the accumulation of SP-D is of potential benefit to the organism through impairment of macrophage uptake and subsequent modulation of inflammatory responses associated with P. carinii infection (40).
Acknowledgments
This work was supported by NIH grants R01-HL55934, R01-HL57125, and R01-HL62150 (to A. H. Limper) and NIH grants R01-HL044015 and P01-HL029594 (to E. C. Crouch). The studies were also supported by funds from Yonsei University (to S.-J. Yong).
We thank Theodore Kottom for technical assistance and advice and Charles Thomas and Robert Vassallo for many helpful discussions. Finally, we appreciate the help of Kathy Stanke in the final preparation of the manuscript.
Editor: J. M. Mansfield
REFERENCES
- 1.Allen, M. J., R. Harbeck, B. Smith, D. R. Voelker, and R. J. Mason. 1999. Binding of rat and human surfactant proteins A and D to Aspergillus fumigatus conidia. Infect. Immun. 67:4563-4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen, M. J., D. R. Voelker, and R. J. Mason. 2001. Interactions of surfactant proteins A and D with Saccharomyces cerevisiae and Aspergillus fumigatus. Infect. Immun. 69:2037-2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atochina, E. N., J. M. Beck, S. T. Scanlon, A. M. Preston, and M. F. Beers. 2001. Pneumocystis carinii pneumonia alters expression and distribution of lung collectins SP-A and SP-D. J. Lab. Clin. Med. 137:429-439. [DOI] [PubMed] [Google Scholar]
- 4.Bartlett, M. S., J. A. Fishman, S. A. Queener, M. M. Durkin, M. A. Jay, and J. W. Smith. 1988. New rat model of Pneumocystis carinii pneumonia. J. Clin. Microbiol. 26:1100-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benne, C. A., B. Benaissa-Trouw, J. A. van Strijp, C. A. Kraaijeveld, and J. F. van Iwaarden. 1997. Surfactant protein A, but not surfactant protein D, is an opsonin for influenza A virus phagocytosis by rat alveolar macrophages. Eur. J. Immunol. 27:886-890. [DOI] [PubMed] [Google Scholar]
- 6.Botas, C., F. Poulain, J. Akiyama, C. Brown, L. Allen, J. Goerke, J. Clements, E. Carlson, A. M. Gillespie, C. Epstein, and S. Hawgood. 1998. Altered surfactant homeostasis and alveolar type II cell morphology in mice lacking surfactant protein D. Proc. Natl. Acad. Sci. USA 95:11869-11874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown-Augsburger, P., K. Hartshorn, D. Chang, K. Rust, C. Fliszar, H. G. Welgus, and E. Crouch. 1996. Site-directed mutagenesis of Cys-15 and Cys-20 of pulmonary surfactant protein-D. Expression of a trimeric protein with altered anti-viral properties. J. Biol. Chem. 271:13724-13730. [DOI] [PubMed] [Google Scholar]
- 8.Cantin, A. M., S. L. North, R. C. Hubbard, and R. G. Crystal. 1987. Normal alveolar epithelial lining fluid contains high levels of glutathione. J. Appl. Physiol. 63:152-157. [DOI] [PubMed] [Google Scholar]
- 9.Castro, M., T. I. Morgenthaler, O. A. Hoffman, J. E. Standing, M. S. Rohrbach, and A. H. Limper. 1993. Pneumocystis carinii induces the release of arachidonic acid and its metabolites from alveolar macrophages. Am. J. Respir. Cell Mol. Biol. 9:73-81. [DOI] [PubMed] [Google Scholar]
- 10.Crouch, E., A. Persson, D. Chang, and J. Heuser. 1994. Molecular structure of pulmonary surfactant protein-D (SP-D). J. Biol. Chem. 269:17311-17319. [PubMed] [Google Scholar]
- 11.Crouch, E. C. 1998. Collectins and pulmonary host defense. Am. J. Respir. Cell Mol. Biol. 19:177-201. [DOI] [PubMed] [Google Scholar]
- 12.Crouch, E. C., D. Chang, K. Rust, A. Persson, and J. Heuser. 1994. Recombinant pulmonary surfactant protein D. Post-translational modification and molecular assembly. J. Biol. Chem. 269:15808-15813. [PubMed] [Google Scholar]
- 13.Crouch, E. C., A. Persson, D. Chang, and D. Parghi. 1991. Surfactant protein D (SP-D): increased accumulation in silica-induced lipoproteinosis. Am. J. Pathol. 139:765-776. [PMC free article] [PubMed] [Google Scholar]
- 14.Ericson, T., K. Pruitt, and H. Wedel. 1975. The reaction of salivary substances with bacteria. J. Oral Pathol. 4:307-323. [DOI] [PubMed] [Google Scholar]
- 15.Ferguson, J. S., D. R. Voelker, F. X. McCormack, and L. S. Schlesinger. 1999. Surfactant protein D binds to Mycobacterium tuberculosis bacilli and lipoarabinomannan via carbohydrate-lectin interactions resulting in reduced phagocytosis of the bacteria by macrophages. J. Immunol. 163:312-321. [PubMed] [Google Scholar]
- 16.Fisher, J. H., V. Sheftelyevich, Y. S. Ho, S. Fligiel, F. X. McCormack, T. R. Korfhagen, J. A. Whitsett, and M. Ikegami. 2000. Pulmonary-specific expression of SP-D corrects pulmonary lipid accumulation in SP-D gene-targeted mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 278:L365-L373. [DOI] [PubMed] [Google Scholar]
- 17.Hartshorn, K. L., E. C. Crouch, M. R. White, P. Eggelton, A. I. Tauber, D. Chang, and K. Sastry. 1994. Evidence for a protective role of pulmonary surfactant protein D (SP-D) against influenza A viruses. J. Clin. Investig. 94:311-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hartshorn, K. L., K. Reid, M. R. White, J. C. Jensenius, S. M. Morris, A. I. Tauber, and E. Crouch. 1996. Neutrophil deactivation by influenza A viruses: mechanism of protection after viral opsonization with collectins and hemagglutination-inhibiting antibodies. Blood 87:3450-3461. [PubMed] [Google Scholar]
- 19.Hartshorn, K. L., M. R. White, D. R. Voelker, J. Coburn, K. Zaner, and E. C. Crouch. 2000. Mechanism of binding of surfactant protein D to influenza A viruses: importance of binding to haemagglutinin to antiviral activity. Biochem. J. 351:449-458. [PMC free article] [PubMed] [Google Scholar]
- 20.Hawgood, S., and F. R. Poulain. 2001. The pulmonary collectins and surfactant metabolism. Annu. Rev. Physiol. 63:495-519. [DOI] [PubMed] [Google Scholar]
- 21.Holmskov, U., R. Malhotra, R. B. Sim, and J. C. Jensenius. 1994. Collectins: collagenous C-type lectins of the innate immune defense system. Immunol. Today 15:67-74. [DOI] [PubMed] [Google Scholar]
- 22.Kaneshiro, E. S., M. A. Wyder, L. H. Zhou, J. E. Ellis, D. R. Voelker, and S. G. Langreth. 1993. Characterization of Pneumocystis carinii preparations developed for lipid analysis. J. Eukaryot. Microbiol. 40:805-815. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan, J. E., D. Hanson, M. S. Dworkin, T. Frederick, J. Bertolli, M. L. Lindegren, S. Holmberg, and J. L. Jones. 2000. Epidemiology of human immunodeficiency virus-associated opportunistic infections in the United States in the era of highly active antiretroviral therapy. Clin. Infect. Dis. 30:S5-S14. [DOI] [PubMed] [Google Scholar]
- 24.Kottom, T. J., and A. H. Limper. 2000. Cell wall assembly by Pneumocystis carinii. Evidence for a unique gsc-1 subunit mediating beta-1,3-glucan deposition. J. Biol. Chem. 275:40628-40634. [DOI] [PubMed] [Google Scholar]
- 25.Koziel, H., X. Li, M. Y. Armstrong, F. F. Richards, and R. M. Rose. 2000. Alveolar macrophages from human immunodeficiency virus-infected persons demonstrate impaired oxidative burst response to Pneumocystis carinii in vitro. Am. J. Respir. Cell Mol. Biol. 23:452-459. [DOI] [PubMed] [Google Scholar]
- 26.Koziel, H., D. S. Phelps, J. A. Fishman, M. Y. Armstrong, F. F. Richards, and R. M. Rose. 1998. Surfactant protein-A reduces binding and phagocytosis of Pneumocystis carinii by human alveolar macrophages in vitro. Am. J. Respir. Cell Mol. Biol. 18:834-843. [DOI] [PubMed] [Google Scholar]
- 27.Krombach, F., S. Münzing, A. M. Allmeling, J. T. Gerlach, J. Behr, and M. Dörger. 1997. Cell size of alveolar macrophages: an interspecies comparison. Environ. Health Perspectives 105(Suppl. 5):1261-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuan, S.-F., K. Rust, and E. Crouch. 1992. Interactions of surfactant protein D with bacterial lipopolysaccharides. Surfactant protein D is an Escherichia coli-binding protein in bronchoalveolar lavage. J. Clin. Investig. 90:97-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuroki, Y., and D. R. Voelker. 1994. Pulmonary surfactant proteins. J. Biol. Chem. 269:25943-25946. [PubMed] [Google Scholar]
- 30.Lebron-Ruiz, F., Z. Vuk-Pavlovic, R. Vassallo, and A. H. Limper. 2000. Surfactant protein D modulates lipopolysaccharide-induced tumor necrosis factor-alpha release from alveolar macrophages. FASEB J. 14:A1162. [Google Scholar]
- 31.LeVine, A. M., J. A. Whitsett, J. A. Gwozdz, T. R. Richardson, J. H. Fisher, M. S. Burhans, and T. R. Korfhagen. 2000. Distinct effects of surfactant protein A or D deficiency during bacterial infection on the lung. J. Immunol. 165:3934-3940. [DOI] [PubMed] [Google Scholar]
- 32.Limper, A. H. 1998. Alveolar macrophage and glycoprotein responses to Pneumocystis carinii. Semin. Respir. Infect. 13:339-347. [PubMed] [Google Scholar]
- 33.Limper, A. H., J. S. Hoyte, and J. E. Standing. 1997. Alveolar macrophages mediate Pneumocystis carinii degradation and organism clearance from the lung. J. Clin. Investig. 99:2110-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Limper, A. H., and W. J. Martin. 1990. Pneumocystis carinii: inhibition of lung cell growth mediated by parasite attachment. J. Clin. Investig. 85:391-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Limper, A. H., K. P. Offord, T. F. Smith, and W. J. Martin II. 1989. Pneumocystis carinii pneumonia. Differences in lung parasite number and inflammation in patients with and without AIDS. Am. Rev. Respir. Dis. 140:1204-1209. [DOI] [PubMed] [Google Scholar]
- 36.Limper, A. H., U. Specks, W. M. Brutinel, W. J. Rochester II, and M. S. Rohrbach. 1993. Interlobar variation in the recovery of bronchoalveolar lavage fluid, cell populations, and angiotensin-converting enzyme in normal volunteers. J. Lab. Clin. Med. 121:785-791. [PubMed] [Google Scholar]
- 37.Limper, A. H., J. E. Standing, O. A. Hoffman, M. Castro, and L. W. Neese. 1993. Vitronectin binds to Pneumocystis carinii and mediates organism attachment to cultured lung epithelial cells. Infect. Immun. 61:4302-4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Merali, S., U. Frevert, J. H. Williams, K. Chin, R. Bryan, and A. B. Clarkson, Jr. 1999. Continuous axenic cultivation of Pneumocystis carinii. Proc. Natl. Acad. Sci. USA 96:2402-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morris, A. M., M. Swanson, H. Ha, and L. Huang. 2000. Geographic distribution of human immunodeficiency virus-associated Pneumocystis carinii pneumonia in San Francisco. Am. J. Respir. Crit. Care Med. 162:1605-1606. [DOI] [PubMed] [Google Scholar]
- 40.Neese, L. W., J. E. Standing, E. J. Olson, M. Castro, and A. H. Limper. 1994. Vitronectin, fibronectin, and gp120 antibody enhance macrophage release of TNF-α in response to Pneumocystis carinii. J. Immunol. 152:4549-4556. [PubMed] [Google Scholar]
- 41.Ofek, I., A. Mesika, M. Kalina, Y. Keisari, R. Podschun, H. Sahly, D. Chang, D. McGregor, and E. Crouch. 2001. Surfactant protein D enhances phagocytosis and killing of unencapsulated phase variants of Klebsiella pneumoniae. Infect. Immun. 69:24-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O'Riordan, D. M., J. E. Standing, K. Y. Kwon, D. Chang, E. C. Crouch, and A. H. Limper. 1995. Surfactant protein D interacts with Pneumocystis carinii and mediates organism adherence to macrophages. J. Clin. Investig. 95:2699-2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Persson, A., D. Chang, and E. Crouch. 1990. Surfactant protein D is a divalent cation-dependent carbohydrate-binding protein. J. Biol. Chem. 265:5755-5760. [PubMed] [Google Scholar]
- 44.Phelps, D. S., and R. M. Rose. 1991. Increased recovery of surfactant protein A in AIDS-related pneumonia. Am. Rev. Respir. Dis. 143:1072-1075. [DOI] [PubMed] [Google Scholar]
- 45.Pikaar, J. C., W. F. Voorhout, L. M. van Golde, J. Verhoef, J. A. Van Strijp, and J. F. van Iwaarden. 1995. Opsonic activities of surfactant proteins A and D in phagocytosis of gram-negative bacteria by alveolar macrophages. J. Infect. Dis. 172:481-489. [DOI] [PubMed] [Google Scholar]
- 46.Pottratz, S. T., and W. J. Martin II. 1990. Mechanism of Pneumocystis carinii attachment to cultured rat alveolar macrophages. J. Clin. Investig. 86:1678-1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pottratz, S. T., J. Paulsrud, J. S. Smith, and W. J. Martin. 1991. Pneumocystis carinii attachment to cultured lung cells by Pneumocystis gp120, a fibronectin binding protein. J. Clin. Investig. 88:403-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Restrepo, C. I., Q. Dong, J. Savov, W. I. Mariencheck, and J. R. Wright. 1999. Surfactant protein D stimulates phagocytosis of Pseudomonas aeruginosa by alveolar macrophages. Am. J. Respir. Cell Mol. Biol. 21:576-585. [DOI] [PubMed] [Google Scholar]
- 49.Rice, W. R., F. M. Singleton, M. J. Linke, and P. D. Walzer. 1993. Regulation of surfactant phosphatidylcholine secretion from alveolar type II cells during Pneumocystis carinii pneumonia in the rat. J. Clin. Investig. 92:2778-2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomas, C. F., R. A. Anders, M. P. Gustafson, E. B. Leof, and A. H. Limper. 1998. Pneumocystis carinii contains a functional cell-division-cycle Cdc2 homologue. Am. J. Respir. Cell Mol. Biol. 18:297-306. [DOI] [PubMed] [Google Scholar]
- 51.Tran Van Nhieu, J., A. M. Vojtek, J. F. Bernaudin, E. Escudier, and J. Fleury-Feith. 1990. Pulmonary alveolar proteinosis associated with Pneumocystis carinii. Ultrastructural identification in bronchoalveolar lavage in AIDS and immunocompromised non-AIDS patients. Chest 98:801-805. [DOI] [PubMed] [Google Scholar]
- 52.van Rozendaal, B. A., A. B. van Spriel, J. G. van De Winkel, and H. P. Haagsman. 2000. Role of pulmonary surfactant protein D in innate defense against Candida albicans. J. Infect. Dis. 182:917-922. [DOI] [PubMed] [Google Scholar]
- 53.Vassallo, R., T. J. Kottom, J. E. Standing, and A. H. Limper. 2001. Vitronectin and fibronectin function as glucan binding proteins augmenting macrophage responses to Pneumocystis carinii. Am. J. Respir. Cell Mol. Biol. 25:203-211. [DOI] [PubMed] [Google Scholar]
- 54.Vassallo, R., C. F. Thomas, Jr., Z. Vuk-Pavlovic, and A. H. Limper. 2000. Mechanisms of defence in the lung: lessons from Pneumocystis carinii pneumonia. Sarcoidosis Vasc. Diffuse Lung Dis. 17:130-139. [PubMed] [Google Scholar]
- 55.Vuk-Pavlovic, Z., J. E. Standing, E. C. Crouch, and A. H. Limper. 2001. The carbohydrate recognition domain of surfactant protein D mediates interactions with Pneumocystis carinii glycoprotein A. Am. J. Respir. Cell Mol. Biol. 24:475-484. [DOI] [PubMed] [Google Scholar]
- 56.Vuk-Pavlovic, Z., J. E. Standing, and A. H. Limper. 2000. Glycoprotein A and beta-glucan from Pneumocystis carinii cell wall interact with alveolar macrophages and surfactant protein-D. FASEB J. 14:A1032. [Google Scholar]
- 57.Williams, M. D., J. R. Wright, K. L. March, and W. J. Martin II. 1996. Human surfactant protein A enhances attachment of Pneumocystis carinii to rat alveolar macrophages. Am. J. Respir. Cell Mol. Biol. 14:232-238. [DOI] [PubMed] [Google Scholar]
- 58.Wisniowski, P., and W. J. Martin. 1995. Interaction of vitronectin with Pneumocystis carinii: evidence for binding via the heparin binding domain. J. Lab. Clin. Med. 125:38-45. [PubMed] [Google Scholar]
- 59.Yale, S. H., and A. H. Limper. 1996. Pneumocystis carinii pneumonia in patients without acquired immunodeficiency syndrome: associated disorders and prior corticosteroid therapy. Mayo Clin. Proc. 71:5-13. [DOI] [PubMed] [Google Scholar]
- 60.Zimmerman, P. E., D. R. Voelker, F. X. McCormack, J. R. Paulsrud, and W. J. Martin II. 1992. 120-kD surface glycoprotein of Pneumocystis carinii is a ligand for surfactant protein A. J. Clin. Investig. 89:143-149. [DOI] [PMC free article] [PubMed] [Google Scholar]