Abstract
Intralesional Th2 responses preceded the development of Th1 responses in localized cutaneous leishmaniasis due to Leishmania guyanensis. Although the number of parasites increased in Th2 lesions, no correlation was found between the levels of cytokine expression and the number of parasites. In contrast, the decreased number of parasites in Th1 lesions is negatively correlated to gamma interferon expression.
Leishmania spp. can induce diseases ranging from self-curing cutaneous lesions to noncuring disseminated cutaneous, mucocutaneous, or visceral disease. The identification of two functionally distinct CD4+-T-cell subsets, T helper 1 (Th1), which produces interleukin 2 (IL-2) and gamma interferon (IFN-γ), and Th2, which produces IL-4, IL-5, IL-10, and IL-13, made it possible to characterize the CD4+ T cells involved in the pathological processes of various diseases (1, 8). In the murine model of infection with L. major, development of CD4+ Th1 and Th2 cells conferred resistance and susceptibility to infection, respectively (12). Although peripheral T-cell responses in human leishmaniasis involve a mixture of Th1 and Th2 cells (11), the Th responses elicited at the lesion site are not yet fully elucidated. In localized cutaneous leishmaniasis (LCL) caused by L. braziliensis or L. major, Th1 cytokines (IL-2 and IFN-γ) predominate over Th2 cytokines (IL-4) (4, 7, 10, 15). IL-4, used as a marker of Th2 response, has been detected indisputably only in cases of diffuse and mucocutaneous leishmaniasis, which are the more severe forms of American cutaneous leishmaniasis (4, 10). Recent data from our laboratory on patients with cutaneous leishmaniasis due to L. guyanensis showed, however, that Th2 cytokines, and particularly IL-13, are produced locally at the site of infection (3). Since Louzir et al. (7), analyzing the local response in patients infected with L. major, noted that IL-4 was detected in an early stage of lesion evolution, we therefore hypothesized that Th2 cytokines, and particularly IL-13, that were found in LCL lesions could play a transient immunoregulatory role in early infection. To address this issue, we studied the association between intralesional cytokine expression and disease evolution in patients with leishmaniasis due to L. guyanensis.
First, the ratio of Th2 (IL-4 and/or IL-13 mRNA expression) to Th1 (IFN-γ mRNA expression) cytokines was analyzed in biopsy samples from 76 patients with active LCL due to L. guyanensis by semiquantitative RT-PCR as previously described (3). We found a predominance of Th2 cytokines (ratio, >1) in 39 biopsy samples labeled as Th2 biopsies, a predominance of Th1 response (ratio, <1) in 28 samples labeled as Th1 biopsies, and equivalent Th2 and Th1 cytokine expression in nine samples (ratio, 1).
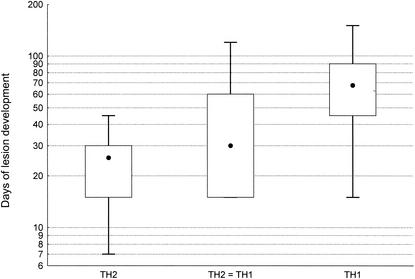
When we analyzed the correlation between Th1 and Th2 responses with the clinical data, we found no correlation between Th responses and the number of lesions, location of lesions, or presence of adenopathy or lymphadenitis. However, the Th responses correlated with the number of days required for development of lesions. As shown in Fig. 1, the duration of development of lesions was statistically shorter (P < 0.0001) in Th2 biopsies (median, 25.5 days) than in Th1 biopsies (median, 67.5 days). For biopsies that developed equivalent Th1 and Th2 responses, the time of development of lesions was intermediate (30 days) between that in Th1 biopsies and that in Th2 biopsies. Thus, in patients with LCL due to L. guyanensis, a Th2 response transiently predominated during the early phase of infection and was followed by the development of a Th1 response during the late course of lesion development.
FIG.1.
Duration of evolution of lesions in various biopsy samples. Results are given as median, range, and interquantile of days of the development of lesions for Th1, Th2, and equivalent Th1 and Th2 (TH2 = TH1) expression.
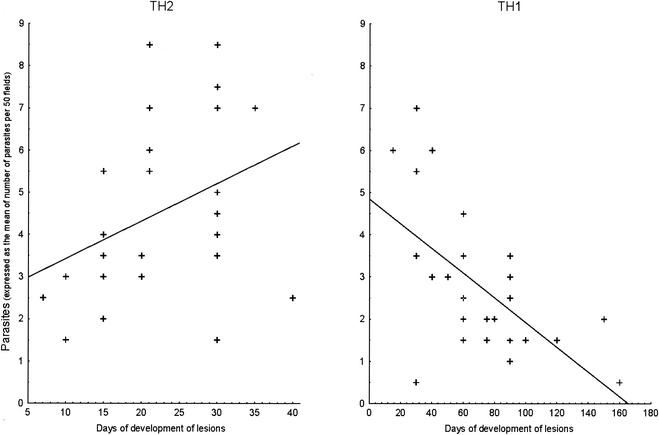
Next we reevaluated the correlation between the duration of lesion development and the clinical parameters for Th1 and Th2 biopsies. The only clear association was found for the number of parasites within the lesions, determined by immunofluorescence staining with specific monoclonal antibody (G2D10; specific for L. guyanensis) (5). Figure 2 shows a positive correlation between the duration of development of lesions and the number of parasites in Th2 biopsies (r = 0.44; P = 0.01 [Spearman rank correlation]). In contrast, a negative correlation was found between the duration of development of lesions and the number of parasites in Th1 biopsies (Fig. 2) (r = −0.59; P = 0.013 [Spearman rank correlation]). These results are in accordance with the finding that healing of lesions requires a T-cell-mediated immune response with IFN-γ production that can activate macrophages harboring parasites to make them microbicidal (14, 16). Indeed, the level of IFN-γ was negatively associated with the number of parasites within Th1 lesions (Table 1). However, Th2 cytokines counter the activation of macrophages triggered by IFN-γ (6, 13). Thus, we postulated that the increase in the number of parasites during Th2 cell development is a consequence of Th2 cytokine deactivation. However, and as shown in Table 1, there was no association between the number of parasites within lesions and the level of any cytokine in Th2 biopsies, even if IL-13, found in LCL due to L. guyanensis, is the cytokine responsible for the inability of specific T cells to respond to IL-12 by inhibiting the IL-12 receptor β2 expression on these cells (3). Nonetheless, we cannot rule out the possibility that Th2 cytokines other than IL-4, IL-10, and IL-13, which act on nitric oxide production, influence parasite growth. The possible role of a well-known deactivating factor, such as transforming growth factor beta, is under investigation.
FIG.2.
Correlation of parasite burden with the duration of lesion development in Th1 and Th2 biopsies. The number of parasites was evaluated by counting fluorescent parasites as previously described (5), and the results are expressed as the mean of the number of parasites in 50 oil immersion fields (×100). The regression lines are only indicative, since the results of the statistical analysis given in the text are nonparametric (Spearman correlation).
TABLE 1.
Spearman rank correlation between intralesional cytokine expression and number of parasites in lesions in relation to local Th response
| Cytokine | Correlation for no. of parasites in:
|
|
|---|---|---|
| Th2 lesions | Th1 lesions | |
| IL-4 | 0.07 | 0.15 |
| IL-13 | −0.08 | −0.18 |
| IL-10 | 0.07 | −0.10 |
| IFN-γ | −0.12 | −0.60a |
P < 0.001.
The precise role of Th2 cytokines in the development of leishmaniasis must therefore be determined. First, Th2 cytokines, with their anti-inflammatory properties, might limit the development of lesions to allow better elimination of parasites when a Th1 response develops (1, 9). In a murine model of infection with L. major, it has also been recently shown that IL-4 can instruct the polarization of CD4+ T cells towards the Th1 phenotype (2). Thus, in human LCL, Th2 cytokines might prepare the microenvironment, favoring the development of a Th1 response.
In conclusion, we demonstrated that a Th2 response precedes the development of a Th1 response at the local site of infection in LCL patients. The exact role of this Th2 response in the development of the disease is not yet well understood, but it might be involved in the development of an effective Th1 immune response. Therefore, the precocious treatment of patients might need reconsideration, since the development of Th2 responses during early phases of Leishmania infection might lead to the development of recall immunity. The early treatment might, therefore, prevent the consequent development of a Th1 response capable of inducing protection against reinfection. To address this issue, epidemiological studies are strongly suggested.
Acknowledgments
We are specially grateful to J. A. Louis (University of Lausanne, Lausanne, Switzerland) and G. Milon (Institut Pasteur de Paris, Paris, France) for helpful comments during this project.
The study was supported by grants from the Institut Pasteur and the French Ministry of Research
Editor: J. M. Mansfield
REFERENCES
- 1.Abbas, A. K., K. M. Murphy, and A. Sher. 1996. Functional diversity of helper T lymphocytes. Nature 383:787-793. [DOI] [PubMed] [Google Scholar]
- 2.Biedermann, T., S. Zimmermann, H. Himmelrich, A. Gumy, O. Egeter, A. K. Sakrauski, I. Seemüller, H. Voigt, P. Launois, A. D. Levine, H. Wagner, K. Heeg, J. A. Louis, and M. Röcken. 2001. IL-4 instructs Th1 responses and resistance to Leishmania major in susceptible BALB/c mice. Nat. Immunol. 2:1054-1060. [DOI] [PubMed] [Google Scholar]
- 3.Bourreau, E., G. Prévot, R. Pradinaud, and P. Launois. 2001. Interleukin(IL)-13 is the predominant Th2 cytokine in localized cutaneous leishmaniasis lesions and renders specific CD4+ T cells unresponsive to IL-12. J. Infect. Dis. 158:953-959. [DOI] [PubMed] [Google Scholar]
- 4.Caceres-Dittmar, G., F. J. Tapia, M. A. Sanchez, M. Yamuara, K. Uyemera, R. L. Modlin, B. R. Bloom, and J. Convit. 1993. Determination of the cytokine profile in American cutaneous leishmaniasis using the polymerase chain reaction. Clin. Exp. Immunol. 91:500-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chico, M. E., R. H. Guderian, P. J. Cooper, R. Armijos, and M. Grogl. 1995. Evaluation of a direct immunofluorescent antibody (DIMA) test using Leishmania genus-specific monoclonal antibody in the routine diagnosis of cutaneous leishmaniasis. Rev. Soc. Bras. Med. Trop. 28:99-103. [DOI] [PubMed] [Google Scholar]
- 6.Liew, F. Y., S. Millott, Y. Li, R. Lelchuck, W. L. Chan, and H. Ziltener. 1989. Macrophage activation by interferon-γ from host-protective T cells is inhibited by interleukin (IL) 3 and IL4 produced by disease-promoting T cells in leishmaniasis. Eur. J. Immunol. 19:1227-1232. [DOI] [PubMed] [Google Scholar]
- 7.Louzir, H. L., P. C. Melby, A. B. Salah, H. Marrakchi, K. Aoun, R. Ben Ismail, and K. Dellagi. 1998. Immunologic determinants of disease evolution in localized cutaneous leishmaniasis due to Leishmania major. J. Infect. Dis. 177:1987-1995. [DOI] [PubMed] [Google Scholar]
- 8.Mosmann, T. R., and R. L. Coffman. 1989. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 7:145-173. [DOI] [PubMed] [Google Scholar]
- 9.Paul, W. E., and R. A. Seder. 1994. Lymphocyte responses and cytokines. Cell 76:241-251. [DOI] [PubMed] [Google Scholar]
- 10.Pirmez, C., M. Yamamura, K. Uyemura, M. Paes-Oliveira, F. Conceicao-Silva, and R. L. Modlin. 1993. Cytokine patterns in the pathogenesis of human leishmaniasis. J. Clin. Investig. 91:1390-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reed, S. G., and P. A. Scott. 1993. T-cell and cytokine responses in leishmaniasis. Curr. Opin. Immunol. 5:524-531. [DOI] [PubMed] [Google Scholar]
- 12.Reiner, S. L., and R. M. Locksley. 1995. The regulation of immunity to Leishmania major. Annu. Rev. Immunol. 13:151-177. [DOI] [PubMed] [Google Scholar]
- 13.Stenger, S., H. Thüring, M. Röllinghoff, and C. Bogdan. 1994. Tissue expression of inducible nitric oxide synthase is closely associated with resistance to Leishmania major. J. Exp. Med. 180:783-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swihart, K., U. Fruth, N. Messmer, K. Hug, R. Behin, S. Huang, G. Del Giudice, M. Aguet, and J. A. Louis. 1995. Mice from a genetically resistant background lacking the interferon gamma receptor are susceptible to infection with Leishmania major but mount a polarized T helper cell 1-type CD4+ T cell response. J. Exp. Med. 181:961-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tapia, F. J., G. Caceres-Dittmar, M. A. Sanchez, A. E. Fernandez, and J. A. Convit. 1993. The cutaneous lesion in American leishmaniasis: leukocyte subsets, cellular interaction and cytokine production. Biol. Res. 26:239-247. [PubMed] [Google Scholar]
- 16.Wang, Z.-E., S. L. Reiner, S. Zheng, D. K. Dalton, and R. M. Locksley. 1994. CD4+ effector cells default to the Th2 pathway in interferon γ-deficient mice infected with Leishmania major. J. Exp. Med. 179:1367-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]