Abstract
Tuberculosis (TB) remains an enormous global health problem, and a new vaccine against TB more potent than the current inadequate vaccine, Mycobacterium bovis BCG, is urgently needed. We describe a recombinant BCG vaccine (rBCG30) expressing and secreting the 30-kDa major secretory protein of Mycobacterium tuberculosis, the primary causative agent of TB, that affords greater survival after challenge than parental BCG in the highly demanding guinea pig model of pulmonary TB. Animals immunized with rBCG30 and then challenged by aerosol with a highly virulent strain of M. tuberculosis survived significantly longer than animals immunized with conventional BCG. The parental and recombinant vaccine strains are comparably avirulent in guinea pigs, as they display a similar pattern of growth and clearance in the lung, spleen, and regional lymph nodes. The pMTB30 plasmid encoding the 30-kDa protein is neither self-transmissible nor mobilizable to other bacteria, including mycobacteria. The pMTB30 plasmid can be stably maintained in Escherichia coli but is expressed only in mycobacteria. The recombinant and parental strains are sensitive to the same antimycobacterial antibiotics. rBCG30, the first vaccine against TB more potent than nearly century-old BCG, is being readied for human clinical trials.
Tuberculosis continues as a major global health problem, especially in the developing world where 1 in 6 adults between the ages of 15 and 59 dies from this disease (10, 22). The AIDS epidemic, whose victims are severalfold more susceptible to tuberculosis than the general population, and the worldwide emergence of drug-resistant strains of Mycobacterium tuberculosis, the primary causative agent of tuberculosis, compound the problem (8, 14). A better vaccine against tuberculosis is urgently needed (11, 23). The current vaccine, Mycobacterium bovis BCG, a live attenuated vaccine derived from the bovine tuberculosis bacillus in the early 1900s by Calmette and Guérin, while protective against disseminated forms of tuberculosis such as meningitis and miliary tuberculosis, is of inconsistent efficacy against pulmonary tuberculosis, the dominant form (9, 12).
In a previous study (20), two recombinant BCG vaccines (rBCG30) overexpressing the major secretory protein of M. tuberculosis, a 30-kDa mycolyl transferase (2, 27), were described. This protein is not only the major secretory protein of M. tuberculosis in broth culture (19) but it is also among the major proteins of all M. tuberculosis proteins expressed in human macrophages (15, 21). Derived from the commercially available Connaught (Conn) and Tice strains of BCG, the recombinant vaccines were tested in the demanding guinea pig model of pulmonary tuberculosis. In contrast to other small animals used for models of tuberculosis, guinea pigs are much more susceptible to tuberculosis than humans, yet they develop disease that closely mimics human disease clinically, immunologically, and pathologically. Guinea pigs immunized with the recombinant vaccines and then challenged by aerosol with a high dose of the highly virulent Erdman strain of M. tuberculosis had 0.5 log fewer M. tuberculosis bacteria in their lungs and 1 log fewer bacteria in their spleens 10 weeks after challenge than guinea pigs immunized with the parental BCG vaccines, differences that statistically were highly significant. Moreover, the rBCG30-immunized animals had significantly less lung pathology and fewer tubercles in their lungs, livers, and spleens than the animals immunized with the parental BCG vaccines. However, aside from these laboratory and pathological findings, a superior effect of the recombinant vaccines on the well-being of the animals was not demonstrated.
In the present study, we examine the impact of recombinant vaccines on the survival of guinea pigs challenged with M. tuberculosis. We demonstrate that animals immunized with the recombinant rBCG30 vaccines survive significantly longer than animals immunized with the parental conventional BCG vaccines after aerosol challenge with virulent M. tuberculosis. We also show that the recombinant and parental vaccines initially multiply in guinea pig lungs, spleens, and regional lymph nodes and are subsequently cleared from these tissues at the same rate, although, interestingly, low levels of organisms persist for at least a half of a year after immunization.
MATERIALS AND METHODS
Strains.
The M. bovis BCG strains Conn (Connaught Laboratories), Tice (Organon), Copenhagen (ATCC 27290), Glaxo (ATCC 35741), Japanese (ATCC 35737), and Pasteur (ATCC 35734) and the M. tuberculosis Erdman strain (ATCC 35801) were used.
The following strains were used for bacterial mating experiments: Escherichiacoli strains DH5α [recA1 endA1 gyrA96 thi-1 hsdR17(rK−mK+) supE44 relA1 lacZα+], Y1090 {[D(lac)U169 Δlon araD139 strA supF mcrA trpC22::Tn10(Tetr)]}(pMC9AmprTetr), and XL1-Blue {recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac[F′ proAB lacIqZΔM15 Tn10(Tetr)]}, all obtained from Stratagene; E. coli strains S17(λpir+ recA thi pro hsdR−M+)(pLC28StrpsCamr) and SM10 (λpir+ Strpr Cams), both gifts from Olaf Schneewind, University of Chicago, Chicago, Ill. (7); M. bovis BCG Tice, purchased from Organon; Mycobacterium smegmatis 1-2c [sodA::Tn5(Kanr)], generated by the chromosomal insertion of a resistance marker (M. Tullius, G. Harth, and M. A. Horwitz, unpublished data) into wild-type M. smegmatis 1-2c, originally obtained from Peadar O'Gaora, Imperial College, London, United Kingdom (17); and M. tuberculosis Erdman [glnA1::Tn5(Kanr)], generated from the wild type (see strains listed above) in the same way as M. smegmatis 1-2c [sodA::Tn5(Kanr)].
Plasmids.
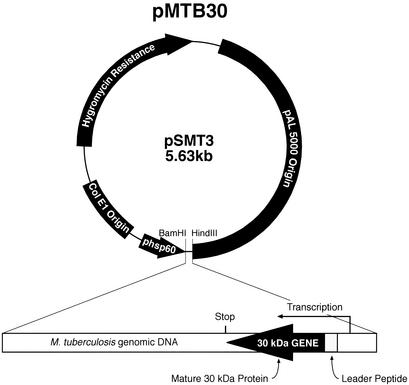
The following plasmids were studied in bacterial mating experiments: pLC28, a conjugative R6K derivative containing the mob region of plasmid RP4 and a mutant oriR6K that requires a functional π protein in trans for replication (provided by E. coli S17's λpir lysogen); pGB9, a derivative of the cryptic Mycobacterium fortuitum plasmid pMF1 conferring kanamycin resistance that was obtained from K. G. Papavinasasundaram, National Institute for Medical Research, London, United Kingdom (1); pMTB30 (Fig. 1); and pVK173T, an E. coli-mycobacterium shuttle plasmid conferring resistance to ampicillin, apramycin, and hygromycin, that was obtained from Julian Davies, University of British Columbia, Vancouver, Canada (24).
FIG. 1.
Plasmid pMTB30. Derived from the mycobacterium-E. coli shuttle vector pSMT3 (13), the plasmid contains a full-length copy of the M. tuberculosis 30-kDa major secretory protein gene and flanking regions, including the 5′ promoter region. The insert is placed into the HindIII and BamHI restriction enzyme sites in the pSMT3 vector in an orientation opposite that of the vector-encoded promoter of heat shock protein 60 (15).
Guinea pig immunization.
Specific-pathogen-free 250- to 300-g outbred male Hartley strain guinea pigs from Charles River Breeding Laboratories were injected intradermally with 103 CFU of parental BCG or rBCG30 Conn or Tice (n = 20 or 21/group) prepared as described previously (20) or sham immunized with buffer (phosphate-buffered saline) only.
Cutaneous delayed-type hypersensitivity (DTH).
Guinea pigs were shaved over the back and injected intradermally with 10 μg of purified recombinant M. tuberculosis 30-kDa major secretory protein (r30) in 100 μl of phosphate-buffered saline. r30 was purified as described previously (15, 19). The diameter of hard induration was measured using a blunt instrument. (A separate group of sham-immunized animals from the one used in the challenge studies was used for skin testing. Sham-immunized animals used in challenge studies were not skin tested, to eliminate the possibility that the skin test itself might influence the outcome).
Guinea pig challenge.
Guinea pigs were challenged with an aerosol generated from a 10-ml single-cell suspension containing a total of 5 × 104 CFU of M. tuberculosis Erdman strain, a dose that delivers ∼20 live bacteria to the lungs of each animal, as described previously (19). Afterwards, guinea pigs were individually housed in stainless steel cages contained within a laminar flow biohazard safety enclosure and allowed free access to standard laboratory food and water.
Bacterial mating experiments.
Donor and recipient bacteria were mixed on an Amicon YM3-43 filter at ratios of 1 × 108:1 × 108 or 1 × 108:1 × 109 and incubated either for 4 or 16 h at 37°C. Bacteria were washed with 1 to 2 ml of medium (Luria-Bertani or 7H9), and exconjugants were assayed by plating on selective medium and incubation for 24 h at 37°C (E. coli), 3 to 5 days at 37°C in 5% CO2-95% air (M. smegmatis), or 2 weeks at 37°C in 5% CO2-95% air (BCG and M. tuberculosis).
Assay for pMTB30 expression in a nonmycobacterium.
The recombinant plasmid pMTB30 was transformed into E. coli DH5α, and 2 clones were analyzed in detail for the expression of r30. The clones were grown for 16 h at 37°C in 10 ml of Luria-Bertani medium containing 250 μg of hygromycin/ml to a density of ∼1 × 109 to 2 × 109 bacteria/ml. The cultures were centrifuged at 3,500 × g for 15 min to separate bacteria from the culture medium. The bacteria were resuspended in 250 μl of polyacrylamide gel sample buffer, boiled for 5 min, and centrifuged at 10,000 × g for 3 min to pellet the insoluble material. The culture supernate was filtered and concentrated with a Centricon 10 unit to 250 μl. Both the pellet and supernate fractions were adjusted so as to reflect the equivalent of approximately 5 × 109 cells in 50 μl of soluble material and then electrophoresed on a 12.5% denaturing polyacrylamide gel. The proteins were transferred electrophoretically to a nitrocellulase membrane and incubated with polyvalent rabbit mycolyl transferase-specific antibodies (raised to the purified M. tuberculosis 30-kDa protein) at a dilution of 1:2,500 for 24 h at 4°C. After incubation of the membrane with secondary goat anti-rabbit, alkaline phosphatase-conjugated antibodies at a dilution of 1:2,500 for 24 h at 4°C, the immunoblot was developed with the alkaline phosphatase-specific substrates 5-bromo-4-chloro-3-indolyl-phosphate-p-toluidine and nitroblue tetrazolium for 30 min at room temperature. As positive controls, culture supernates obtained from the equivalent of 5 × 109 rBCG30 Tice (harboring the plasmid pMTB30) and 1 μg of purified 30-kDa protein (r30) were electrophoresed on the same gel, immunoblotted, and probed with the antibodies on the same membrane.
Antibiotic susceptibility.
The two parental BCG strains (BCG Tice and BCG Conn) and the two recombinant strains (rBCG30 Tice and rBCG30 Conn) were tested by the UCLA Clinical Microbiology Laboratory (Microbiology Reference Laboratory, Cypress, Calif.) for sensitivity to the following antibiotics (concentrations are given in parentheses): ethambutol (2.5 μg/ml), isoniazid (0.1 and 0.4 μg/ml), pyrazinamide (100 μg/ml), rifampin (2.0 μg/ml), streptomycin (2.0 μg/ml), ethionamide (5.0 μg/ml), capreomycin (5.0 μg/ml), ciprofloxacin (2.0 μg/ml), amikacin (5.0 μg/ml), and p-aminosalicylic acid (4.0 or 5.0 μg/ml).
RESULTS
Production of the 30-kDa protein by BCG strains used in this study relative to other BCG strains.
In a previous study, we determined that the parental BCG Conn and BCG Tice strains used in our study produce comparable amounts of the 30-kDa protein (1.00 and 1.10 relative units, respectively), which is substantially less than the recombinant rBCG30 strains, which produce twofold (Conn) and sixfold (Tice) more 30-kDa protein. To determine if the study strains are typical of other BCG strains with respect to production of the 30-kDa mycolyl transferase, we assayed the production of the 30-kDa protein by four other widely used BCG strains (Copenhagen, Glaxo, Japanese, and Pasteur) at the same time as BCG Tice. We cultured the five strains under identical conditions, subjected the culture filtrates to sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis, and measured the production of the 30-kDa protein by densitometry as described previously (20). The densitometry measurements were normalized to a BCG Tice level of 1.10 to allow comparison with the results of the previous study. As shown in Table 1, all parental BCG strains secreted comparably low amounts of the 30-kDa protein.
TABLE 1.
Production of 30-kDa protein by various strains of BCG
Survival after challenge.
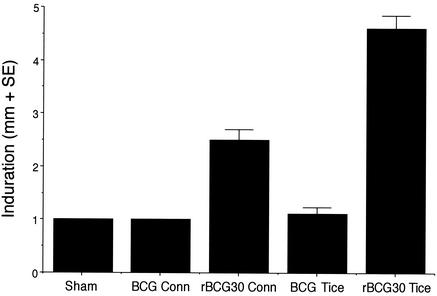
To determine the impact of the rBCG30 vaccines on survival after M. tuberculosis challenge, we immunized guinea pigs, in groups of 20 or 21 animals, intradermally with 103 CFU of the parental or recombinant rBCG30 Conn and Tice vaccines. The rBCG30 vaccines contained plasmid pMTB30 encoding the M. tuberculosis 30-kDa major secretory protein (Fig. 1). Nine weeks after immunization, we tested 10 animals in each group and 12 sham-immunized animals for cutaneous DTH to the purified recombinant M. tuberculosis 30-kDa major secretory protein (r30). The parental BCG vaccines, which each express comparably low levels of the endogenous 30-kDa protein, did not induce responses above the baseline level (Fig. 2). As previously observed (20), the rBCG30 vaccines, which express twofold (Conn)- or sixfold (Tice)-more 30-kDa protein than the parental BCG vaccines (Table 1), induced significantly greater DTH responses to r30 than the parental vaccines (P = 8 × 10−10 for all rBCG30-immunized animals versus all BCG-immunized animals, Kruskal-Wallis method). Moreover, the higher-producing rBCG30 Tice strain induced a significantly greater DTH response to r30 than the lower-producing rBCG30 Conn strain (P = 0.0001).
FIG. 2.
rBCG30-immunized animals exhibit strong cutaneous DTH to r30. Guinea pigs were sham immunized (Sham) or immunized with parental BCG or rBCG30 (Conn or Tice, as indicated), and skin tested with an intradermal injection of r30. The extent of induration was measured after 24 h. Data are the mean diameters (in millimeters) + standard errors (SE). The differences between BCG Conn and rBCG30 Conn were significant at a P value of 0.000007, differences between BCG Tice and rBCG30 Tice were significant at a P value of 0.00001, and differences between rBCG30 Conn and rBCG30 Tice were significant at a P value of 0.0001 by the Kruskal-Wallis nonparametric method. (By analysis of variance, P is <0.0001 for all three comparisons.)
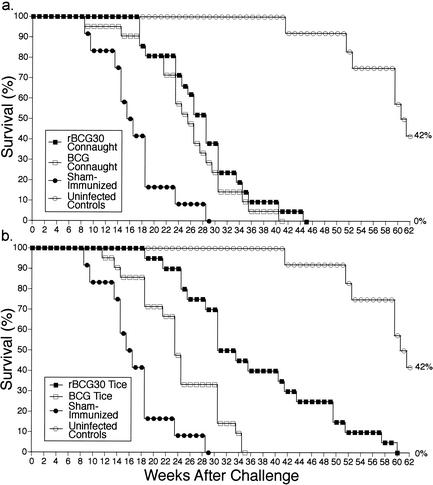
At 10 weeks after immunization, the BCG and rBCG30-immunized animals and sham-immunized animals that had not been skin tested were challenged by aerosol with the M. tuberculosis Erdman strain and monitored for survival along with uninfected animals for 62 weeks (Fig. 3). As expected, sham-immunized animals died most rapidly after challenge. BCG-immunized animals (median survival, 24 weeks) survived significantly longer than sham-immunized animals (median survival, 15.5 weeks). Most importantly, the rBCG30-immunized animals (median survival, 30 weeks) survived significantly longer than the BCG-immunized animals (P = 0.0004 for all rBCG30-immunized animals versus all BCG-immunized animals, log rank test). Animals immunized with rBCG30 Conn (median survival, 28 weeks), which overexpresses relatively low amounts of r30, survived only slightly longer than animals immunized with the parental BCG Conn counterpart (median survival, 25 weeks; difference not statistically significant) (Fig. 3a). However, animals immunized with the higher-producing rBCG30 Tice (median survival, 31.5 weeks) survived significantly longer than animals immunized with the parental BCG Tice vaccine (median survival, 23 weeks) (P = 0.0003) (Fig. 3b). At 35 weeks after challenge, by which time all animals immunized with BCG Tice had died, 45% of rBCG30 Tice-immunized animals remained alive. Moreover, animals immunized with the higher-producing rBCG30 Tice strain survived significantly longer than animals immunized with the rBCG30 Conn strain (P = 0.007). By 41 weeks after challenge, by which time all of the sham- and parental BCG-immunized animals had died, only 4.8% of the rBCG30 Conn-immunized animals remained alive, whereas 35% of the rBCG30 Tice-immunized animals remained alive. Beginning at 42 weeks after challenge, the uninfected control animals began to die off. At this time point, 35% of the rBCG30 Tice-immunized animals remained alive. By the time all of the rBCG30 Tice-immunized animals had died, half of the uninfected controls had also died. Although the death rate after 41 weeks of the cohort of rBCG30 Tice-immunized animals that survived to this point was somewhat greater than that of the uninfected controls, the recombinant vaccine allowed this cohort of infected animals to nearly reach full life expectancy under laboratory conditions.
FIG. 3.
rBCG30-immunized animals survive longer than BCG-immunized animals. Animals immunized with parental or recombinant BCG Conn (a) or Tice (b) were challenged with virulent M. tuberculosis and monitored for survival (n = 20 or 21/group). Sham-immunized animals and uninfected animals served as additional controls (n = 12/group). Differences in survival between sham-immunized animals and animals immunized with either one of the parental BCG strains were statistically significant (P = 0.0005 for BCG Conn and P = 0.0009 for BCG Tice, by the log rank test). Differences between animals immunized with BCG Conn and rBCG30 Conn were not statistically significant. However, differences between animals immunized with BCG Tice and rBCG30 Tice were statistically significant (P = 0.0003), as were differences between animals immunized with rBCG30 Tice and rBCG30 Conn (P = 0.007). When all rBCG30-immunized animals (Conn and Tice) were compared with all BCG-immunized animals, differences in survival were also statistically significant (P = 0.0004). Within groups, there was no difference in survival between animals that were skin tested before challenge and those that were not.
Virulence of the vaccine.
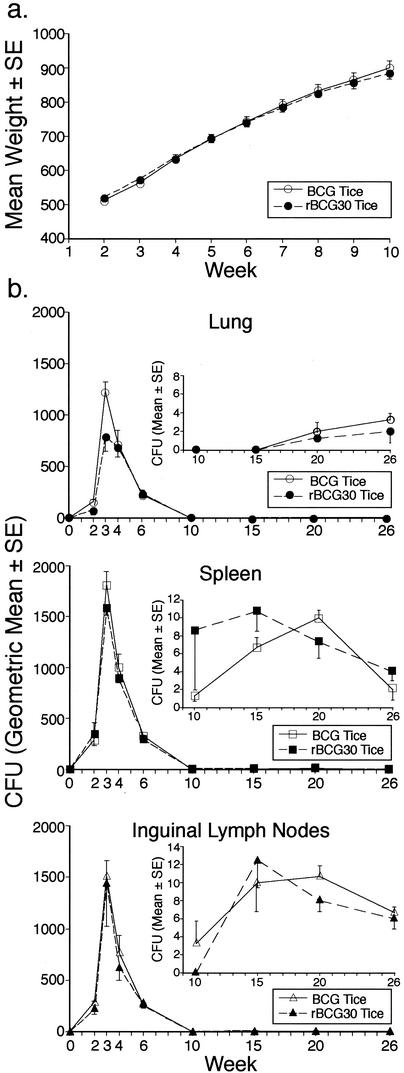
Preliminary to human trials of the rBCG30 Tice strain, we examined the virulence of this strain in the guinea pig model in comparison with the parental BCG Tice vaccine, which has a well-established safety profile in humans. First, we examined the effect of the vaccine on the general health status of the animals, including weight gain. In guinea pigs immunized intradermally with 103 CFU of the recombinant and parental strains, no adverse health effects were observed for either vaccine, and animals in both groups gained weight at the normal rate, including during the first 10 weeks after immunization, when bacterial levels in animal organs were highest (Fig. 4a). Second, we investigated the capacity of guinea pigs to clear the vaccine strains. Guinea pigs immunized with either the parental or recombinant vaccine were euthanized at various intervals after challenge, after which the lungs, spleens, and regional (inguinal) lymph nodes were assayed for CFU of BCG or rBCG30. In animals immunized with either strain, CFU peaked at 3 weeks after immunization in all three tissue sites and then rapidly declined to levels near zero by 10 weeks (Fig. 4b). Both the parental and recombinant vaccines were cleared at the same rate. Extensive culturing of specimens (50% of the organ homogenate on 20 agar plates) obtained 10 weeks or later after immunization revealed low levels of persistent organisms at all three sites (Fig. 4b, inserts). rBCG30 isolated from the three sites (50 colonies per site) at 6 weeks after immunization were hygromycin resistant, indicating that the recombinant bacteria retain the pMTB30 plasmid in vivo in the absence of selective pressure just as they do when subcultured monthly for at least 1 year in broth medium lacking hygromycin. These studies demonstrated that the recombinant and parental Tice strains are comparably avirulent in an animal that is highly susceptible to mycobacterial infection.
FIG. 4.
Parental and recombinant BCG Tice strains are comparably avirulent in guinea pigs. (a) Weight gain after immunization. Guinea pigs were immunized intradermally with 103 CFU of BCG or rBCG30 Tice and weighed weekly. Both groups (n = 12/group) gained weight normally after immunization. (b) Clearance of vaccine strains. Guinea pigs were immunized intradermally as described for panel a and then euthanized 2 to 26 weeks later (n = 3/group/timepoint), as indicated, and CFUs per organ were assayed in the right lung, spleen, and inguinal lymph nodes. The insets show total CFU per organ at 10 to 26 weeks after immunization plotted against a vertical scale appropriate for low bacterial counts. SE, standard error.
Additional safety studies.
In preparation for human trials of the rBCG30 Tice vaccine, we have performed several additional studies of potential relevance to the safety of the vaccine in humans, as follows.
(i) Assay for self-transmissibility of plasmid pMTB30.
We first investigated whether the plasmid pMTB30 was capable of self-transmissibility from one bacterium to another and especially from the vaccine strain to other bacteria. We tested the self-transmissibility of plasmid pMTB30 (Hygr) from (i) the recombinant E. coli strain DH5α to E. coli XL1-Blue, (ii) the recombinant M. bovis rBCG30 Tice strain to the E. coli strains DH5α and Y1090 (Tetr), and (iii) the recombinant M. bovis rBCG30 Tice strain to the kanamycin-resistant mycobacteria M. smegmatis 1-2c [sodA::Tn5(Kanr)] and M. tuberculosis Erdman [glnA1::Tn5(Kanr)]. We observed no hygromycin (250 μg/ml)- or hygromycin (250 μg/ml)- and tetracycline (12.5 μg/ml)-resistant E. coli colonies, no hygromycin (50 μg/ml)- and kanamycin (20 μg/ml)-resistant M. smegmatis, and no hygromycin (50 μg/ml)- and kanamycin (20 μg/ml)-resistant M. tuberculosis (M. tuberculosis plates were also supplemented with 20 mM l-glutamine to compensate for the glutamine synthetase deficiency). In a parallel series of experiments, we tested the self-transmissibility of the E. coli-mycobacterium shuttle plasmid pVK173T. We replicated the mating experiments described for pMTB30, and again we observed no apramycin (50 μg/ml)- or apramycin (50 μg/ml)- and tetracycline (12.5 μg/ml)-resistant E. coli colonies and no apramycin (30 μg/ml)- and kanamycin (20 μg/ml)-resistant mycobacterial colonies.
In a set of control experiments, we assayed the transfer of a conjugative plasmid (pLC28StrpsCamr) between E. coli strains under the same conditions. We mixed E. coli S17 (harboring the plasmid) with E. coli SM10 (Strpr Cams) and screened for streptomycin- and chloramphenicol (both at 30 μg/ml)-resistant exconjugants. Exconjugates were obtained at a frequency of 9.5 × 10−7.
These studies demonstrated that plasmid pMTB30 is not self-transmissible to E. coli or mycobacteria.
(ii) Assay for mobilization of plasmid pMTB30.
Although the above studies showed that plasmid pMTB30 was not self-transmissible, it remained possible that the plasmid could be mobilizable by a helper plasmid and thus transferred to another bacterial species. We tested this possibility by first introducing pMTB30 into E. coli S17 harboring the conjugative plasmid pLC28StrpsCamr and then mating this strain with E. coli SM10 (Strpr Cams). No streptomycin (30 μg/ml)- and hygromycin (250 μg/ml)-resistant exconjugants were obtained. More importantly, when this same E. coli S17 strain was mated with the mycobacterial strains M. smegmatis 1-2c [sodA::Tn5(Kanr)] and M. tuberculosis Erdman [glnA1::Tn5(Kanr)], no hygromycin (50 μg/ml)- and kanamycin (20 μg/ml)-resistant mycobacterial colonies were obtained (M. tuberculosis Erdman plates were again supplemented with 20 mM l-glutamine). As a positive control, we introduced the mobilizable E. coli-mycobacterium shuttle plasmid pVK173T into E. coli S17 (containing pLC28StrpsCamr) and then mated this strain with the mycobacterial strains M. smegmatis 1-2c [sodA::Tn5(Kanr)] and M. tuberculosis Erdman [glnA1::Tn5(Kanr)]. These matings resulted in hygromycin (50 μg/ml)-, apramycin (30 μg/ml)-, and kanamycin (20 μg/ml)-resistant M. smegmatis colonies at a frequency of 7.7 × 10−7 and M. tuberculosis colonies with the same resistance markers at a frequency of 1.3 × 10−7, demonstrating that this plasmid can be mobilized to other mycobacteria.
Although these studies showed that pMTB30 was not mobilizable with an E. coli-based helper plasmid, it remained possible that the plasmid was mobilizable to other mycobacteria with a mycobacterial helper plasmid. We investigated this possibility by introducing plasmid pGB9(Kanr) into the wild-type strains M. smegmatis 1-2c and M. tuberculosis Erdman and then mating these strains with the recombinant rBCG30 Tice[pMTB30(Hygr)]. In both cases, hygromycin (50 μg/ml)- and kanamycin (20 μg/ml)-resistant exconjugant BCG Tice colonies were obtained at frequencies of ∼8.0 × 10−7. However, when recombinant BCG Tice[pMTB30(Hygr), pGB9(Kanr)] bacteria were mixed with M. smegmatis 1-2c or M. tuberculosis Erdman wild-type bacteria, no hygromycin (50 μg/ml)- and kanamycin (20 μg/ml)-resistant colonies were obtained, except, of course, for the donor bacteria. (M. smegmatis is differentiated from BCG and M. tuberculosis by its much faster growth [visible colonies after an incubation period of 3 to 4 days versus 10 to 12 days for BCG and M. tuberculosis], and BCG is differentiated from M. tuberculosis by replating the bacteria on plates containing 50 μg of 2-thiophene carboxylic acid hydrazide/ml, which allows growth of only M. tuberculosis.) Thus, pGB9 is self-transmissible but cannot mobilize pMTB30 into mycobacteria.
These studies showed that pMTB30 cannot be mobilized with the help of a conjugative plasmid, a not-unexpected result, since the sequence of pMTB30 lacks both a mob locus and an oriT element.
(iii) Assay for pMTB30 expression in a nonmycobacterium.
To determine if the plasmid pMTB30 could be expressed in a nonmycobacterium, we transformed the plasmid by electroporation into E. coli DH5α, cultured the transformed organism in the presence of hygromycin to maintain the plasmid, and assayed expression of the 30-kDa protein in both the bacterial pellet and the culture medium by immunoblot analysis by using polyvalent mycolyl transferase-specific antibodies. The antibodies did not react with either of the E. coli-derived protein fractions, demonstrating that E. coli cannot express the 30-kDa protein gene as contained in the plasmid pMTB30. In contrast, the antibodies reacted strongly with both the recombinant protein expressed in rBCG30 Tice and the purified 30-kDa protein (r30). This analysis showed that even when pMTB30 is forced into E. coli by electroporation, it does not express the M. tuberculosis 30-kDa major secretory protein, a result consistent with nonrecognition of the mycobacterial promoter by E. coli.
(iv) Antibiotic susceptibility.
Both parent BCG strains and recombinant BCG strains were resistant to pyrazinamide, as is typical of M. bovis. All were sensitive to the other nine antibiotics tested. However, the recombinant rBCG30 Tice strain was slightly less sensitive to isoniazid (resistant to a concentration of 0.1 μg/ml but sensitive to a concentration of 0.4 μg/ml) than the parental BCG Tice, the recombinant rBCG30 Conn, and the parental BCG Conn (all sensitive to concentrations of both 0.1 and 0.4 μg/ml). Possibly, the rBCG30 Tice strain's much greater expression of the 30-kDa mycolyl transferase, a protein involved in the synthesis of the cell wall component trehalose dimycolate, rendered this strain slightly more resistant to isoniazid, an inhibitor of mycolic acid synthesis.
DISCUSSION
This study demonstrates that the recombinant rBCG30 vaccines enhance survival after M. tuberculosis challenge. The magnitude of both protection and the cutaneous DTH response to r30 was correlated with the amount of the 30-kDa protein produced by the vaccine strain, i.e., animals immunized with the higher-producing rBCG30 Tice strain survived longer and had a greater DTH response than animals immunized with the lower-producing rBCG30 Conn strain, and the rBCG30 vaccines induced greater survival and DTH than the parental BCG strains, which produced the least amount of 30-kDa major secretory protein.
The clear superiority of the higher-producing rBCG30 Tice strain compared with the lower-producing rBCG30 Conn strain in this study was not demonstrated in the predecessor study in which the two recombinant vaccines induced comparable reductions in bacterial load in the lungs and spleen (20). Hence, the assessment of survival but not bacterial load after immunization and challenge allowed for differentiation between the vaccines in potency. However, even in the predecessor study, the rBCG30 Tice-immunized animals had less organ pathology than the rBCG30 Conn-immunized animals.
Many different strains of BCG have been used as vaccines throughout the world, reflecting a lack of evidence that any one of them is superior to any other. Colditz et al. (9), in their meta-analysis of the published literature on BCG trials, investigated the impact of BCG pedigree on between-study variability and found no impact. They additionally concluded that trials with Mycobacterium microti (vole bacillus), a strain less-closely related to M. tuberculosis than M. bovis BCG, gave protection equal to that of BCG. Recently, the Tice and Conn BCG strains were compared for immunogenicity and reactogenicity in a human clinical trial, and no difference was detected between them (18). Similarly, in this and our previous study, no significant difference in efficacy was observed between the parental BCG Conn and Tice strains. These and four other BCG strains secreted comparably low amounts of the 30-kDa protein.
This study lends further support to the extracellular protein hypothesis for vaccines against intracellular pathogens, which holds that proteins secreted or otherwise released into the intracellular compartment of such pathogens are potentially potent immunoprotective molecules (3-6, 16, 19, 20, 25). Such proteins are available for processing and presentation to the immune system as major histocompatibility complex-peptide complexes on host cells. These complexes serve to alert the host immune system to the presence of a pathogen sequestered within the host cell, allowing it to mount an appropriate antimicrobial response. As in previous studies (3-5), in which immunization with either of two major extracellular proteins of Legionella pneumophila, the agent of Legionnaires' disease, was demonstrated to enhance the survival of guinea pigs after challenge by aerosol with L. pneumophila, this study shows that immunization with a major extracellular protein of M. tuberculosis, albeit via a live vector rather than as a protein in an adjuvant, enhances survival after challenge by aerosol with M. tuberculosis in a highly susceptible animal model.
Prior to this study, an alternative explanation for the enhanced efficacy of the recombinant BCG vaccine compared with that of the parental BCG vaccine was that the recombinant vaccine was more virulent in the guinea pig model and, for this reason, it induced a stronger immune response. However, the parental and recombinant BCG vaccines were found to be equally avirulent for guinea pigs, ruling out this possibility.
Of the small-animal models of tuberculosis, the guinea pig model most closely resembles human disease. Like humans, but unlike mice and rats, guinea pigs are susceptible to low doses of aerosolized M. tuberculosis, exhibit a high sensitivity to tuberculin, develop a cutaneous DTH response characterized by a dense mononuclear cell infiltrate, and exhibit Langhans giant cells and caseation in their lung lesions. A major difference between humans and guinea pigs, however, is the susceptibility to disease after infection with M. tuberculosis. Whereas only about 10% of humans develop active disease after infection, some soon after and some after a period of latency, 100% of guinea pigs develop active rapidly progressive disease to which they quickly succumb. The high susceptibility of guinea pigs to the development of active rapidly fatal disease renders this model especially suitable for assessing the capacity of a vaccine against tuberculosis to enhance survival after M. tuberculosis challenge.
Assessing survival in guinea pigs after respiratory challenge with M. tuberculosis is a time-consuming and expensive undertaking. While not often done, our study is not the first to do so. In a study conducted over three decades ago, Wiegeshaus et al. (26), using experimental conditions very different from ours (lower challenge dose, less-virulent challenge strain, shorter immunization-challenge interval, and different housing conditions, etc.), found that guinea pigs immunized with the BCG vaccine survived significantly longer than sham-immunized animals after aerosol challenge with M. tuberculosis, which was similar to our results with the parental BCG vaccines. Coincidentally, the BCG vaccine studied by Wiegeshaus et al. originated at Tice Laboratory at the University of Illinois, as did one of our parental vaccines.
Thus far, our studies of the rBCG30 Tice vaccine have demonstrated that, in the highly susceptible guinea pig model of pulmonary tuberculosis, immunization with the vaccine results in a marked reduction in bacterial burden, number of tubercles, and pathology in the lungs and other organs, and in enhanced survival—all to an extent greater than that achieved by immunization with parental BCG Tice. To what extent this effect of the rBCG30 vaccine will translate to humans and whether the vaccine will have an impact on reactivation tuberculosis remains to be determined in forthcoming clinical studies. However, given the relatively high innate resistance of even unvaccinated humans to M. tuberculosis, it seems reasonable to postulate that an immunologic intervention such as the rBCG30 Tice vaccine that is likely to shift the balance of power between the host and pathogen further toward the host will have a beneficial impact on human disease incidence. Even a modest reduction in disease incidence would translate into hundreds of thousands of lives saved.
Acknowledgments
We are grateful to Barbara Jane Dillon, Saša Masleša-Galić, and Chalermchai Chaloyphian for technical assistance, Jeffrey Gornbein and Lena Ting for assistance with statistical analyses, David Bruckner for assistance with antibiotic susceptibility testing, and the Sequella Global Tuberculosis Foundation for encouragement of these studies.
This work was supported by National Institutes of Health grant AI31338.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Bachrach, G., M. J. Colston, H. Bercovier, D. Bar-Nir, C. Anderson, and K. G. Papavinasasundaram. 2000. A new single-copy mycobacterial plasmid, pMF1, from Mycobacterium fortuitum which is compatible with the pAL5000 replicon. Microbiology 146:297-303. [DOI] [PubMed] [Google Scholar]
- 2.Belisle, J. T., V. D. Vissa, T. Sievert, K. Takayama, P. J. Brennan, and G. S. Besra. 1997. Role of the major antigen of Mycobacterium tuberculosis in cell wall biogenesis. Science 276:1420-1422. [DOI] [PubMed] [Google Scholar]
- 3.Blander, S. J., and M. A. Horwitz. 1989. Vaccination with the major secretory protein of Legionella pneumophila induces cell-mediated and protective immunity in a guinea pig model of Legionnaires' disease. J. Exp. Med. 169:691-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blander, S. J., and M. A. Horwitz. 1991. Vaccination with the major secretory protein of Legionella induces humoral and cell-mediated immune responses and protective immunity across different serogroups of Legionella pneumophila and different species of Legionella. J. Immunol. 147:285-291. [PubMed] [Google Scholar]
- 5.Blander, S. J., and M. A. Horwitz. 1993. The major cytoplasmic membrane protein of Legionella pneumophila, a genus common antigen and member of the hsp 60 family of heat shock proteins, induces protective immunity in a guinea pig model of Legionnaires' disease. J. Clin. Investig. 91:717-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breiman, R. F., and M. A. Horwitz. 1987. The major secretory protein of Legionella pneumophila stimulates proliferation of splenic lymphocytes from immunized guinea pigs. Clin. Res. 35:469A.
- 7.Cheng, L. W., D. M. Anderson, and O. Schneewind. 1997. Two independent type III secretion mechanisms for YopE in Yersinia enterocolitica. Mol. Microbiol. 24:757-765. [DOI] [PubMed] [Google Scholar]
- 8.Cohn, D. L., F. Bustreo, and M. C. Raviglione. 1997. Drug-resistant tuberculosis: review of the worldwide situation and the W. H. O./IUATLD Global Surveillance Project. Clin. Infect. Dis. 24:S121-S130. [DOI] [PubMed] [Google Scholar]
- 9.Colditz, G. A., T. F. Brewer, C. S. Berkey, M. E. Wilson, E. Burdick, H. V. Fineberg, and F. Mosteller. 1994. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA 271:698-702. [PubMed] [Google Scholar]
- 10.Dye, C., S. Scheele, P. Dolin, V. Pathania, and M. C. Raviglione. 1999. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. W. H. O. Global Surveillance and Monitoring Project. JAMA 282:677-686. [DOI] [PubMed] [Google Scholar]
- 11.Ensernik, M. 2001. Driving a stake into resurgent TB. Science 293:234-235. [DOI] [PubMed] [Google Scholar]
- 12.Fine, P. E. M. 1989. The BCG story: lessons from the past and implications for the future. Rev. Infect. Dis. 11:(Suppl. 2):S353-S359. [DOI] [PubMed] [Google Scholar]
- 13.Garbe, T. R., J. Barathi, S. Barnini, Y. Zhang, C. Abou-Zeid, D. Tang, R. Mukherjee, and D. B. Young. 1994. Transformation of mycobacterial species using hygromycin resistance as a selectable marker. Microbiology 140:133-138. [DOI] [PubMed] [Google Scholar]
- 14.Haas, D. W., and R. M. Des Prez. 1995. Mycobacterium tuberculosis, p. 2213-2243. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases. Churchill Livingstone, Inc., New York, N.Y.
- 15.Harth, G., B.-Y.Lee, J. Wang, D. L. Clemens, and M. A. Horwitz. 1996. Novel insights into the genetics, biochemistry, and immunocytochemistry of the 30-kilodalton major extracellular protein of Mycobacterium tuberculosis. Infect. Immun. 64:3038-3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harth, G., B-Y. Lee, and M. A. Horwitz. 1997. High-level heterologous expression and secretion in rapidly growing nonpathogenic mycobacteria of four major Mycobacterium tuberculosis extracellular proteins considered to be leading vaccine candidates and drug targets. Infect. Immun. 65:2321-2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herrmann, J. L., P. O'Gaora, A. Gallagher, J. E. R. Thole, and D. B. Young.1996. Bacterial glycoproteins: a link between glycosylation and proteolytic cleavage of a 19 kDa antigen from Mycobacterium tuberculosis. EMBO J. 15:3547-3554. [PMC free article] [PubMed] [Google Scholar]
- 18.Hoft, D. F., E. B. Kemp, M. Marinaro, O. Cruz, H. Kiyono, J. R. McGhee, J. T. Belisle, T. W. Milligan, J. P. Miller, and R. B. Belshe. 1999. A double-blind, placebo-controlled study of Mycobacterium-specific human immune responses induced by intradermal bacille Calmette-Guerin vaccination. J. Lab. Clin. Med. 134:244-252. [DOI] [PubMed] [Google Scholar]
- 19.Horwitz, M. A., B.-W. E. Lee, B. J. Dillon, and G. Harth. 1995. Protective immunity against tuberculosis induced by vaccination with major extracellular proteins of Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 92:1530-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horwitz, M. A., G. Harth, B. J. Dillon, and S. Malesa-Galic. 2000. Recombinant BCG vaccines expressing the Mycobacterium tuberculosis 30 kDa major secretory protein induce greater protective immunity against tuberculosis than conventional BCG vaccines in a highly susceptible animal model. Proc. Natl. Acad. Sci. USA 97:13853-13858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee, B,-Y., and M. A. Horwitz. 1995. Identification of macrophage and stress induced proteins of Mycobacterium tuberculosis. J. Clin. Investig. 96:245-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murray, C. J. L., K. Styblo, and A. Rouillon. 1990. Tuberculosis in developing countries: burden, intervention, and cost. Bull. Int. Union Tuberc. Lung Dis. 65:6-24. [PubMed] [Google Scholar]
- 23.Orme, I. M., D. N. McMurray, and J. T. Belisle. 2001. Tuberculosis vaccine development: recent progress. Trends Microbiol. 9:115-118. [DOI] [PubMed] [Google Scholar]
- 24.Paget, E., and J. Davies. 1996. Apramycin resistance as a selective marker for gene transfer in mycobacteria. J. Bacteriol. 178:6357-6360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pal, P. G., and M. A. Horwitz. 1992. Immunization with extracellular proteins of Mycobacterium tuberculosis induces cell-mediated immune responses and substantial protective immunity in a guinea pig model of pulmonary tuberculosis. Infect. Immun. 60:4781-4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiegeshaus, E. H., D. N. McMurray, A. A. Grover, G. E. Harding, and D. W. Smith. 1970. Host-parasite relationships in experimental airborne tuberculosis. III. Relevance of microbial enumeration to acquired resistance in guinea pigs. Am. Rev. Respir. Dis. 102:422-429. [DOI] [PubMed] [Google Scholar]
- 27.Wiker, H. G., and M. Harboe. 1992. The antigen 85 complex: a major secretion product of Mycobacterium tuberculosis. Microbiol. Rev. 56:648-661. [DOI] [PMC free article] [PubMed] [Google Scholar]