Abstract
Yersinia enterocolitica mutant strains, including mutants deficient in the chaperone SycH resulting in a functional deficiency in tyrosine phosphatase (YopH), Mn-cofactored superoxide dismutase (SodA), iron-repressive protein 1 (IRP-1), and Yersinia adhesin A (YadA), were demonstrated to be highly attenuated in wild-type C57BL/6 mice. TNFRp55−/−, IL-12p40−/−, and IL-18−/− mutant mice, in which the Yersinia wild-type strain causes severe systemic infections, were used to investigate whether these Yersinia mutant strains would be attenuated in immunodeficient hosts. A plasmid-cured Yersinia mutant strain was unable to colonize any of the mutant mice tested. A SycH-deficient mutant strain colonized intestinal tissues of these mice but was attenuated for systemic infection in all of the mutant mice. Both YadA- and Irp-1-deficient Yersinia mutants were still attenuated in IL-12−/− and IL-18−/− mice but were pathogenic in TNFRp55−/− mice. By contrast, a Yersinia sodA mutant was highly pathogenic for TNFRp55−/− and IL-12p40−/− mice while interleukin-18 (IL-18) was dispensable. This finding demonstrates that certain virulence factors enable yersiniae to compete with distinct cytokine-dependent host defense mechanisms. Moreover, while gamma interferon mRNA expression did not reflect protective host responses in cytokine-deficient mice, IL-10 expression coincided with a heavy splenic bacterial load and was associated with progressive infection courses. We can thus segregate minor (SodA), intermediate (YadA and IRP-1), and major (YopH) virulence factors of Y. enterocolitica. Finally, we demonstrate that, even in immunocompromised hosts, Yersinia sycH and, with some restrictions, irp-1 mutants may be suitable for use as live carrier vaccines.
In the past decade, many of the molecular events operating during infections with enteropathogenic bacteria such as Salmonella and Yersinia spp. have been elucidated. It has been clearly established that some virulence factors mediate, e.g., intestinal fluid and ion secretion or cytotoxicity, giving rise to diarrhea, while others mediate colonization and invasion of intestinal tissue. Furthermore, certain virulence factors cause subversion of innate or acquired immune responses and are required for replication and dissemination of the pathogens within host tissues. Targeted disruption of such virulence genes in enteric bacteria has facilitated the development of attenuated immunogenic and protective vaccine vectors (19, 52). However, the immune mechanisms involved in the control of attenuated vaccine strains have not been fully elucidated (55). This issue, however, is of considerable interest because recent studies have demonstrated that vaccination with attenuated live vaccines may give rise to severe and fatal infection courses in patients with previously unknown or minor immunodeficiency (23).
Yersinia enterocolitica is a gram-negative, extracellularly located pathogen that causes a wide range of clinical manifestations, including enteritis, enterocolitis, mesenteric lymphadenitis, and septicemia, as well as immunopathological sequelae such as reactive arthritis (22). The pathogenicity of Y. enterocolitica depends on both plasmid-encoded pathogenicity factors such as secreted proteins (Yops) or outer membrane proteins (YadA) and chromosome-encoded factors including, e.g., yersiniabactin, invasin, SodA, and Irp-1 (19). In particular, the yop virulon enables yersiniae to overcome the innate immune system of their host and to survive in lymphoid tissues. This virulence apparatus enables extracellularly located yersiniae to translocate toxic effector proteins (Yops) via a type III secretion system directly into the cytosol of the host cells.
There are at least six effector Yops that are translocated into host cells (20). YopE, YopT, and YpkA/YopO cause destruction of the actin microfilament structures (34-35, 49). YopH is a protein tyrosine phosphatase that acts on eukaryotic proteins such as the focal adhesion kinase, paxillin, and p130Cas (10). YopM is mostly distributed in the cell within the nucleus (32, 53), but its function remains unclear. YopJ/YopP affects eukaryotic cells by inhibiting NF-κB signaling pathways, resulting in inhibition of cytokine production and/or apoptosis (24, 39, 50). Another important plasmid-encoded virulence factor is YadA, a nonfimbrial adhesin. YadA mediates adherence to epithelial cells, phagocytes, and extracellular matrix proteins, as well as resistance to defensins, phagocytosis, and complement lysis (9, 26).
The chromosome-encoded virulence factor irp-1 encodes for high-molecular-weight-protein 1 which is part of the yersiniabactin biosynthesis apparatus. Yersiniabactin is a siderophore that enables uptake of iron by yersiniae (18, 43). The Mn-cofactored superoxide dismutase (SodA) provides resistance to oxygen radicals derived from phagocytes (47).
In a mouse infection model, Y. enterocolitica was shown to be taken up by M cells located within the follicle-associated epithelium of Peyer's patches (PP) (4, 28-29). Subsequently, yersiniae colonize the PP and may eventually disseminate via the lymphatics and the bloodstream to the lymph nodes, liver, lungs, and spleen. Histological and immunohistological analyses suggest that innate host defense mechanisms, including polymorphonuclear leukocytes and macrophages, are involved in Yersinia control in PP (5). In addition, clearance of infection involves NK cells and the activation of an adaptive immune response including CD8+ and CD4+ Th1 cells (7-8, 13). By administration of neutralizing antibodies in vivo or by infection of cytokine-deficient mice, we showed that tumor necrosis factor alpha (TNF-α), interleukin-12 (IL-12), IL-18, and gamma interferon (IFN-γ) are essential for clearance of Yersinia infections, suggesting that T-cell-activated macrophages are possibly the final effector components in pathogen control (6, 13, 15-16).
In previous studies, various attenuated Y. enterocolitica mutant strains have been used and analyzed for the ability to confer protective immune responses in murine infection models (43, 47-48). Recently, we showed that attenuated Y. enterocolitica mutant strains have a significant potential to act as live oral vaccine carriers (33). Thus, Yersinia mutant strains that were deficient in YadA, SodA, or Irp-1 were tested for their potential as live carrier vaccines. Vaccination of C57BL/6 mice with these strains induced both humoral and cellular immune responses and mediated protection against a challenge with virulent wild-type yersiniae, suggesting that these mutant strains have great potential as efficacious live carrier vaccines.
We extended this study by investigating whether these mutant strains would be safe live carrier vaccines in compromised hosts. A second goal of this study was to determine the remaining pathogenicity level of attenuated vaccine strains and clarify the relative importance of particular immune pathways involved in the control of such strains. Our data show that cytokine-deficient mice are sensitive models that clearly reveal the actual attenuation of a given mutated bacterial vaccine carrier strain. In addition, the differential attenuation of Yersinia mutant strains in various cytokine-deficient mice gives new insights into the immune subversion strategies of enteric yersiniae.
MATERIALS AND METHODS
Mice.
The mouse strains used were TNFRp55−/− (45), IL-12p40−/− (38), and IL-18−/− (54) mutants on a C57BL/6 background and corresponding C57BL/6 wild-type mice. The mice were kept under specific-pathogen-free conditions in positive-pressure cabinets (Techniplast) and provided with sterile food and water ad libitum. Female mice 6 to 8 weeks old were used for the experiments.
Bacteria and infection of animals.
The wild-type and mutant bacterial strains used in this study are summarized in Table 1. Bacteria were grown in Luria broth at 27°C, harvested during the log phase, and frozen in 1-ml aliquots at −80°C. Prior to each experiment, an aliquot was thawed, washed, and resuspended in sterile phosphate-buffered saline (PBS), pH 7.4.
TABLE 1.
Bacterial strains used in this study
| Bacterial strain | Description | Reference |
|---|---|---|
| WA-314 | Yersinia enterocolitica serotype O8 clinical isolate, virulent wild-type strain; pYV O8+ (YadA+ Yops+) Irp1+ SodA+ | 31 |
| WA-C | Plasmidless derivative of WA-314; pYV− (Yad A− Yops−) Irp1+ SodA+; attenuated in wild-type mice | 31 |
| WA-yadA | YadA-deficient mutant of WA-314; pYV+ (Yops+ YadA−) Irp1+ SodA+ Kmr; attenuated in wild-type mice | 30 |
| WA-sycH | SycH-deficient mutant of WA-314; pYV+ (YadA+ Yops+) Spectr; loss of function of YopH; attenuated in wild-type mice | 51 |
| WA-sodA | SodA-deficient mutant of WA-314; pYV+ (YadA+ Yops+) Kmr; attenuated in wild-type mice | 47 |
| WA-irp-1 | Irp1-deficient mutant of WA-314; pYV+ (YadA+ Yops+) Kmr | 43 |
Mice were starved for 8 h, and 200 μl of 5% NaHCO3 in PBS was given prior to infection. Mice were orogastrically infected with a gastric tube. A volume of 200 μl of a suspension containing 1 × 108 to 5 × 108 yersiniae was injected. The actual number of bacteria administered was controlled for each experiment by plating serial dilutions of the suspension inoculated on Mueller-Hinton agar and counting the CFU after incubation at 27°C for 48 h. At 5 days after infection, mice were sacrificed and the spleen and PP of each mouse were aseptically removed. The number of bacteria present in the PP and spleen was determined by homogenization of these organs in PBS containing 0.1% Tergitol TMN 10 (Fluka, Buchs, Switzerland) and 0.1% bovine serum albumin (Merck, Darmstadt, Germany) and plating of serial dilutions of the homogenates on cefsulodin-irgasan-novobiocin or Mueller-Hinton agar, respectively (5). The limit of detection was 25 CFU (log10 of 25 = 1.4). Spleen bacterial numbers determined after orogastric infection were compared with those obtained after parenteral infection with 104 yersiniae.
Determination of cytokine production by ELISA.
For determination of cytokine production, 2 × 106 splenocytes were cultured in the presence of either 10 μg of heat-killed yersiniae (HKY) per ml or 3 μg of concanavalin A (ConA) per ml or without antigenic stimulation. Supernatants were removed after 48 h and tested for IFN-γ, TNF-α, and IL-10 production by using a capture enzyme-linked immunosorbent assay (ELISA).
For determination of IFN-γ, microtiter plates (Greiner, Solingen, Germany) were coated with anti-IFN-γ monoclonal antibody (MAb) AN-18.17.24. After blocking of nonspecific binding sites, supernatants were added to the wells in duplicate and incubated overnight. After several wash steps, biotin-conjugated anti-IFN-γ MAb R4-6A2 was added. Finally, an avidin-alkaline phosphatase complex (Strep ABC-AP kit; Dako, Glostrup, Denmark) was added. The signal was developed with p-nitrophenyl phosphate disodium (Sigma, St. Louis, Mo.) and the optical density was determined at wavelengths of 405 and 490 nm with an ELISA reader.
TNF-α levels were determined by using rat anti-mouse TNF-α MAb G281-2626 and biotin-conjugated anti-TNF-α MAb MPG-XT3 (PharMingen, San Diego, Calif.) as described above for IFN-γ. Levels of IL-10 were measured by using anti-mouse IL-10 MAb MAB417 (R&D Systems) and biotin-conjugated goat anti-mouse IL-10 antibody BAF417 (R&D Systems).
RT-PCR.
Liver RNA was isolated as described previously (14). PCR was performed on cDNA for a total of 22 (β-actin) and 30 (IFN-γ and TNF-α) cycles at 94°C for 30 s, 60°C for 45 s, and at 72°C for 60 s. Taq Gold (Perkin-Elmer, Überlingen, Germany) was used for amplification of IL-10 cDNA by using 30 cycles at 94°C for 45 s, 64°C for 60 s, and 72°C for 90 s. Reverse transcription (RT)-PCR products were visualized by agarose gel electrophoresis. Semiquantitative mRNA levels were determined by digital scanning and calculating of the intensity of each cytokine band with a FluoroS MultiImager (Bio-Rad, Munich, Germany) and the Multi-Analyst 1.1 program (Bio-Rad). Values were expressed in arbitrary units as the ratio of the cytokine mRNA level to the corresponding β-actin mRNA level. The sequences of the sense and antisense primers used in this study are as follows (5′ to 3′): β-actin sense, TGGAATCCTGTGGCATCCATGAAAC; antisense, TAAAACGCAGCTCAGTAACAGTCCG (348-bp product); IFN-γ sense, TGAACGCTACACACTGCATCTTGG; antisense, TGACTCCTTTTCCGCTTCCTGAG (460-bp product); TNF-α sense, GGCAGGTCTACT; TTGGAGTCATTGC; antisense, ACATTCGAGGCTCCAGTGAATTCGG (307-bp product); IL-10 sense, ACCTGGTAGAAGTGATGCCCCAGGCA; antisense, CTATGCAGTTGATGAAGAAGATGTCAAA (237-bp product).
Panning of B cells, CD4 T cells, and macrophages.
To investigate the cells producing IL-10 during Y. enterocolitica infection, B-cell-, CD4+ T-cell-, or macrophage-depleted fractions were obtained from a pool of four spleens by using the panning technique (57) and the following antibodies: rabbit anti-rat immunoglobulin solution (DAKO, Glostrup, Denmark), anti-mouse CD4 MAb E191, and anti-mouse Mac-1 antibody 5C6/α. The purity of each specific depleted fraction was determined by flow cytometry with fluorescein isothiocyanate (FITC)-conjugated anti-mouse CD3 (14.5 2C11), phycoerythrin-conjugated anti-mouse CD4 (PharMingen, San Diego, Calif.), FITC-conjugated anti-mouse CD8 (PharMingen), FITC-conjugated goat anti-mouse immunoglobulins (Becton Dickinson, San Jose, Calif.), and FITC-conjugated anti-Mac-1 antibody M1.70.
Statistics.
Differences between mean values were analyzed with Student's t test. The Mann-Whitney U test was used to assess differences between two groups. Nonparametric testing among three or more groups was performed by Kruskal-Wallis one-way analysis of variance. A P value of <0.05 was considered statistically significant. All experiments were repeated al least twice and revealed comparable results.
RESULTS
Susceptibility of cytokine-deficient mice to infection with attenuated Y. enterocolitica mutants.
Previous work revealed that mutants of Y. enterocolitica O8 wild-type strain WA-314 with a disrupted yadA, sycH, irp-1, or sodA gene or plasmid-cured derivative strain WA-C, which lacks YadA and the effector Yops, are highly attenuated in immunocompetent C57BL/6 mice (25, 33, 43, 46-48). To determine the safety of infection with these attenuated Y. enterocolitica mutant strains, TNFRp55−/−, IL-12p40−/−, IL-18−/− mutant and wild-type C57BL/6 mice were inoculated orogastrically with the various Y. enterocolitica mutants. Five days after infection, the mice were killed and the numbers of bacteria present in their PP and spleens were assessed. As shown in Fig. 1A, determination of bacterial counts in PP demonstrated that Y. enterocolitica mutant strains deficient in SycH, SodA, or IRP-1 can colonize the intestinal tissue and persist for at least 5 days in PP of C57BL/6 mice. By contrast, the Y. enterocolitica YadA mutant strain and plasmid-cured Y. enterocolitica strain WA-C showed an impaired ability to persist within intestinal tissue (Fig. 1A). These results confirm previous data indicating that YadA is required for persistence of Y. enterocolitica in PP (44), while deficiency in expression of sycH, irp-1, and sodA has no significant influence on the colonization of PP. Furthermore, while Y. enterocolitica sodA mutant-infected IL-12p40−/− mice showed slightly increased bacterial numbers in their PP, a comparable Yersinia load was detected in IL-12p40−/−, IL-18−/−, and wild-type mice infected with Y. enterocolitica WA-314, WA-sycH, or WA-irp-1 (Fig. 1A). These data indicate that both IL-12 and IL-18 play only a minor role in protective host responses in PP tissue. The intestinal bacterial load in TNFRp55−/− mice could not be determined because PP are only rudimentarily developed in these mice.
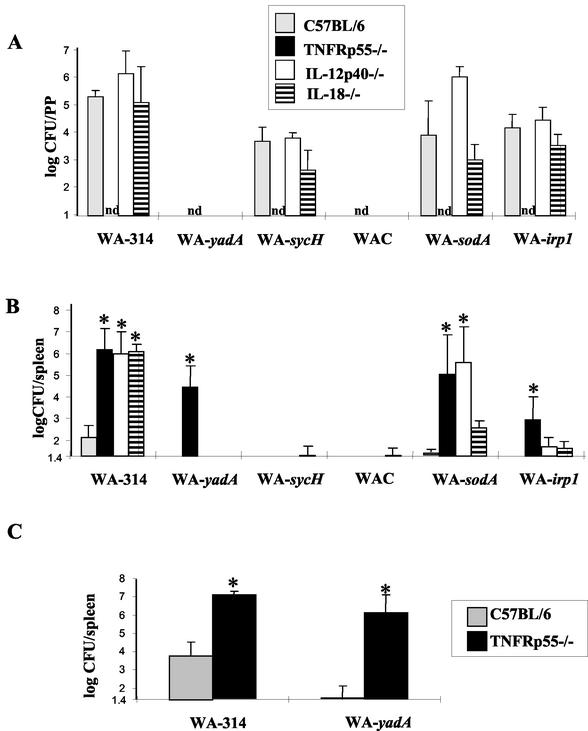
FIG. 1.
Susceptibility of normal and immunodeficient mice to infection with various Y. enterocolitica mutants. TNFRp55−/−, IL-12p40−/−, IL-18−/−, and wild-type C57BL/6 mice were orogastrically infected with 108 wild-type or mutant Y. enterocolitica bacteria. Five days postinfection, the numbers of bacteria (CFU) present in the PP (A) and spleen (B) were assessed. (C) Bacterial numbers in the spleen 5 days after intravenous infection with 104 Y. enterocolitica WA-314 or Y. enterocolitica WA-yadA bacteria. Values represent the means of at least four animals with standard deviations. An asterisk indicates a statistically significant difference (P < 0.05) from control mice. nd, not determined.
The data depicted in Fig. 1B show that in wild-type C57BL/6 mice, all of the Yersinia mutants were highly attenuated as they did not cause significant infection of the spleen, liver, or lungs (data not shown). In contrast, Y. enterocolitica WA-314 was disseminated to the spleen and caused a systemic infection with high bacterial splenic counts in immunodeficient mice. According to previous data (13, 15-16), lack of TNFRp55, IL-12p40, and IL-18 expression in mice led to higher bacterial numbers in the spleen after infection than in wild-type mice, which confirms that these factors are essential for an adequate immune response to Yersinia infection. Y. enterocolitica WA-yadA and plasmid-cured Y. enterocolitica WA-C, both of which did not persist in PP, were not disseminated to the spleens of wild-type or IL-12p40- or IL-18-deficient C57BL/6 mice, indicating that successful colonization of the PP is a prerequisite for dissemination to the spleen. SycH- and Irp-1-deficient Yersinia mutants, which were able to colonize the PP of IL-12p40 −/− and IL-18 −/− mice, were not detectable in the spleen after oral infection. This demonstrates that, for protection against systemic dissemination of SycH- and Irp-1-deficient Yersinia strains, IL-12p40- and IL-18-dependent defense mechanisms are no longer required. In contrast, oral infection with Y. enterocolitica sodA, which also colonizes the PP of IL-12p40 −/− and IL-18 −/− mice, led to high bacterial numbers in the spleens of IL-12 −/− mice but to only a light bacterial load in the spleens of IL-18−/− mice. This shows that IL-12, rather than IL-18, is still required for protection against systemic dissemination or for clearance of Y. enterocolitica WA-sodA from the spleen, respectively.
In the spleens of TNFRp55−/− mice, high bacterial numbers could be detected upon oral infection with the Yersinia mutants deficient in YadA, SodA, and Irp-1 but not upon infection with Y. enterocolitica WA-sycH or plasmid-cured strain Y. enterocolitica WA-C. This reflects the fact that TNFRp55-dependent host responses are required for protection against YadA-, SodA-, and Irp1-deficient Yersinia strains but not for protection against the SycH-deficient or plasmid-cured Yersinia strain. In addition, parenteral infection of C57BL/6 and TNFRp55−/− mice with the Y. enterocolitica WA-yadA mutant strain produced splenic bacterial loads comparable to those obtained by oral infection (Fig. 1C).
Taken together, these results indicate a general requirement for TNFRp55, IL-12p40, and IL-18 for host resistance to wild-type Y. enterocolitica and mutant Yersinia strain infections. Furthermore, these data argue against SodA-, YadA-, and, to a lesser degree, Irp-1-deficient Yersinia mutants as candidates for a safe live carrier vaccine.
Cytokine responses in immunodeficient mice upon infection with attenuated Y. enterocolitica mutants.
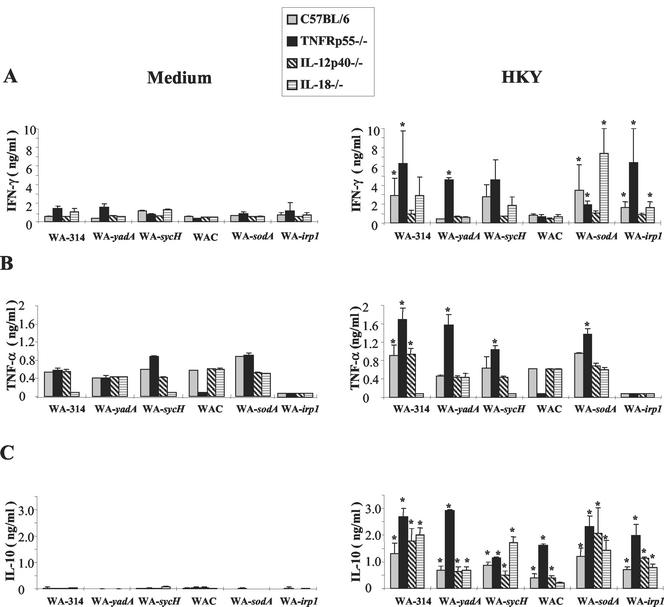
Both T cells and macrophages, including the cytokines TNF-α, IFN-γ, IL-18, and IL-12, are essential components of protective host responses against Y. enterocolitica in immunocompetent mice (3, 13, 15). To elucidate whether this is also the case for Yersinia mutant strains and immunocompromised mice, we analyzed whether there is an association between cytokine induction in vitro and bacterial elimination in vivo. Splenocytes from infected mice were cultured in medium with or without yersiniae. After oral infection of IL-18-, IL-12p40-, and TNFRp55-deficient C57BL/6 mice with wild-type or mutant Y. enterocolitica, IFN-γ production in Yersinia-stimulated splenocytes derived from C57BL/6 mice was detectable upon infection with all of the Yersinia strains (Fig. 2A) that were able to colonize PP, such as wild-type and SycH-, SodA-, and Irp-1-deficient Yersinia mutant strains, respectively, indicating that either the bacteria were disseminated to the spleen earlier or immune cells had migrated from the PP to the spleen. However, in contrast to infection of mice with wild-type Yersinia strains, there is no clear-cut association between the amount of Yersinia-triggered IFN-γ production by splenocytes and bacterial numbers detected in the spleens of cytokine-deficient mice infected with Yersinia mutants. In Yersinia-stimulated splenocytes derived from Yersinia-infected IL-12p40−/− mice, the level of IFN-γ detected was low, which can be explained by the requirement of IL-12 for IFN-γ production. In cultures of splenocyte from IL-18−/− and TNFRp55−/− mice, IFN-γ levels were variable upon infection with the various Yersinia mutants. However, after infection with plasmid-cured strain Y. enterocolitica WA-C, cytokine levels were very low, suggesting that infection with totally apathogenic yersiniae does not induce significant cytokine responses in mice.
FIG. 2.
IFN-γ, TNF-α, and IL-10 production by spleen cells of mice after oral infection with wild-type and mutant Y. enterocolitica strains. Mice were orogastrically infected with various bacterial strains as stated in the legend to Fig. 1. Spleen cells were cultured with either 10 μg of HKY per ml or without antigen. After 48 h, supernatants were used in an IFN-γ (A)-, TNF-α (B)-, or IL-10 (C)-specific ELISA. Results are the mean values ± the standard deviations of at least four animals. An asterisk indicates a statistically significant difference (P < 0.05) from nonstimulated spleen cells.
Investigation of TNF-α levels produced in spleen cell cultures revealed that TNF-α was already produced without restimulation in vitro (Fig. 2B). Yersinia stimulation increased TNF-α levels in splenocytes derived from Yersinia-infected C57BL/6, IL-12p40−/−, and TNFRp55−/− mice (Fig. 2B). Furthermore, TNF-α levels were increased in Yersinia-stimulated splenocytes from TNFRp55−/− mice infected with YadA-, SycH-, and SodA-deficient mutants, respectively (Fig. 2B). However, there was no clear-cut relationship between TNF-α levels and splenic bacterial counts upon infection with the various mutant Yersinia strains.
IL-10 production was found to be significantly increased in the culture supernatants of Yersinia-stimulated splenocytes after infection with all of the Yersinia strains tested (Fig. 2C). However, IL-10 levels in spleen cell cultures were particularly increased after infection with those Yersinia strains that proved to be more pathogenic and in those mouse strains that turned out to be highly susceptible to Yersinia infection. Thus, the highest IL-10 levels were found in cultures from Yersinia-infected TNFRp55−/− mice although IL-10 levels were increased in these mice also upon infection with plasmid-cured Y. enterocolitica WA-C, which did not cause splenic infection. Nevertheless, IL-10 may represent a marker for the severity of Yersinia infection.
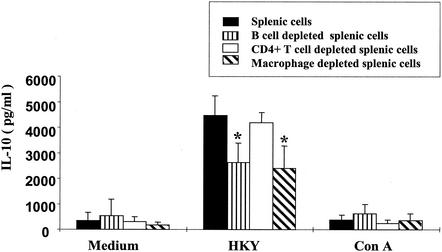
IL-10 responses induced by yersiniae were significantly higher than those induced by ConA stimulation in both C57BL/6 and TNFRp55−/− mice (Fig. 3), suggesting that cells other than T cells might account for IL-10 production (38). In order to show which cell type is involved in IL-10 production in TNFRp55−/− mice, spleen cell cultures were depleted of B cells, CD4+ T cells, or macrophages by panning. With this procedure, more than 95% of the CD4+ T cells, 90% of the B cells, or 70% of the macrophages were eliminated from spleen cell populations, as determined by flow cytometry analysis (date not shown). Stimulation of these cell populations with yersiniae revealed that IL-10 levels were significantly reduced in B-cell- and macrophage-depleted spleen cell cultures but not in CD4 T-cell-depleted spleen cell cultures (Fig. 3). These results suggest that macrophages and B cells, rather than T cells, were the main source of IL-10.
FIG. 3.
IL-10 production by normal or B cell-, CD4+ T cell-, or macrophage-depleted spleen cells. Spleen cells from Y. enterocolitica WA-yadA-infected TNFRp55−/− mice were depleted of B cells, CD4+ T cells, or macrophages by panning as described in Materials and Methods. Cell suspensions were either stimulated with HKY or ConA or not stimulated (medium), and IL-10 levels in culture supernatants were determined by ELISA. The values shown are the means and standard deviations of three separate experiments. An asterisk indicates a statistically significant difference (P < 0.05) from spleen cells.
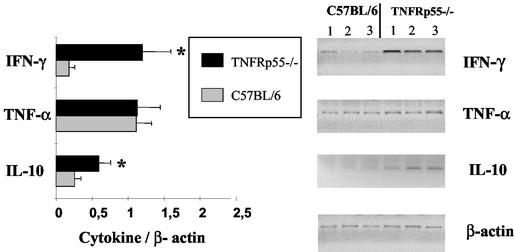
To determine cytokine production in infected mice in vivo, mRNA expression in the liver was determined by RT-PCR after infection with the various Y. enterocolitica wild-type and mutant strains. Figure 4 shows a representative experiment for IFN-γ, TNF-α, and IL-10 mRNA expression levels in TNFRp55−/− and control mice after infection with Y. enterocolitica WA-yadA. After normalization of cytokine mRNA expression with β-actin mRNA expression levels, we found a greater increase in the expression of IFN-γ and IL-10 mRNAs in the livers of TNFRp55−/− mice than in those of normal C57BL/6 mice. Thus, the cytokine mRNA expression levels in vivo closely correlated with the cytokine production levels of spleen cell cultures in vitro. This was also the case for Yersinia infections in IL-12p40−/− and IL-18−/− mice (data not shown).
FIG. 4.
Cytokine mRNA expression in the livers of TNFRp55−/− and C57BL/6 mice infected with Y. enterocolitica WA-yadA. Five days after Y. enterocolitica WA-yadA infection, mice were sacrificed and total RNA was isolated from their livers and assayed for expression of cytokine mRNA by RT-PCR. Semiquantitative mRNA levels were determined by digitally scanning the bands and calculating the intensity of each cytokine band by fluoroimager. Values are expressed in arbitrary units as the ratio of the cytokine mRNA level to that of the corresponding β-actin mRNA (means of samples of three mice ± standard deviations). An asterisk indicates a statistically significant difference (P < 0.05) from control mice.
DISCUSSION
A number of virulence factors of Y. enterocolitica mediate functions such as (i) resistance to phagocytosis and complement lysis and (ii) iron uptake and thus promote extracellular survival of Y. enterocolitica in infected host tissue (19). By targeted disruption of these genes, attenuated Y. enterocolitica mutant strains have been generated (43, 47-48) that might be suitable for use as live carrier vaccine strains. Yersiniae have not met universal acceptance as candidates for use as live carrier vaccines because complications such as reactive arthritis may occur after vaccination. However, because of their interesting features, e.g., M-cell targeting, extracellular replication, and a type III secretion system, it is tempting to speculate that attenuated enteric Yersinia mutants might be as suitable as Salmonella vaccine strains. Moreover, Salmonella vaccine strains have also been reported to account for reactive arthritis even in HLA-B27-negative individuals (1, 17). In the presented study, we addressed the question of whether these Yersinia mutant strains are safe in gene-targeted compromised hosts because recent studies demonstrated that vaccination with attenuated live vaccines may result in fatal infections in patients with minor, previously unknown immunodeficiency (23). In fact, studies of patients with fatal infections caused by poorly pathogenic mycobacteria or Salmonella species have revealed novel human immunodeficiencies in type 1 cytokine or cytokine receptors, indicating that the study of attenuated strains under immunodeficient conditions can have important implications for future vaccination strategies. Furthermore, we wanted to identify host effector mechanisms that are necessary for protection against a certain Yersinia pathogenicity factor. Herein, we demonstrate that TNFRp55, IL-12, and IL-18 are essentially involved in control of wild-type Y. enterocolitica strains upon oral infection. Infection of gene-targeted compromised mice with attenuated Yersinia mutant strains revealed a differential role of these cytokines and cytokine receptors, respectively. Table 2 summarizes the virulence of the various Yersinia mutant strains in cytokine-deficient mice. All of these cytokines are dispensable upon infection with plasmid-cured Y. enterocolitica WA-C. Both IL-12 and IL-18 are dispensable upon infection with SycH-, YadA-, or Irp-1-deficient Yersinia mutants but not upon infection with a SodA-deficient Yersinia mutant. In general, IL-12 or IL-18 deficiency did not affect colonization of PP by all of the Yersinia strains tested compared to that of wild-type mice, which leads to the suggestion that these cytokines play no prominent role in the defense against yersiniae in PP. TNFRp55-dependent mechanisms are dispensable only upon infection with SycH-deficient strains and not upon infection with a YadA-deficient Yersinia strain.
TABLE 2.
Virulence of Y. enterocoltica mutant strains in cytokine-deficient mice
| Mouse strain |
Y. enterocolitica straina
|
|||||
|---|---|---|---|---|---|---|
| WA-314 | YadA− | SycH− | WA-C | SodA− | Irp-1− | |
| C57BL/6 | + | − | (+) | − | (+) | (+) |
| TNFRp55−/− | ++ | ++ | − or (+)b | − or (+)b | ++ | + |
| IL-12p40−/− | ++ | − | (+) | − | ++ | + |
| IL-18−/− | ++ | − | (+) | − | + | + |
−, avirulent, no dissemination to the spleen, light bacterial load in PP; (+), low or high bacterial counts in PP, no dissemination to spleen; +, virulent, high bacterial counts in PP, low bacterial counts in spleen; ++, virulent, high bacterial counts in PP and spleen.
Bacterial counts in PP could not be determined because TNFRp55−/− mice have only rudimentary PP.
YadA mediates adhesion to several cell types, such as epithelial cells and phagocytes, and mediates resistance against complement lysis and defensins (26, 48, 56). These effects may contribute to the fact that YadA enables yersiniae to colonize PP. Consequently, the Y. enterocolitica WA-yadA mutant was not able to colonize the PP of C57BL/6 wild-type mice as well as those of IL-12p40−/− and IL-18−/− mice.
Interestingly, this mutant caused a severe infection in TNFRp55−/− mice, with bacterial counts similar to those seen upon infection with the wild-type Y. enterocolitica strain. It is known that TNF-α plays an essential role in the local defense mechanism in the intestinal tissues, possibly by activation of phagocytes (5). Unfortunately, we could not determine bacterial colonization in PP of TNFRp55−/− mice because the PP are only incompletely developed and markedly reduced in number in these mice (42). Nevertheless, histological analysis revealed Yersinia-induced abscesses in rudimentary PP tissue, indicating that despite the altered intestinal tissue morphology of TNFRp55−/− mice, yersiniae invade at the same tissue sites as in C57BL/6 mice (data not shown). However, these data do not totally rule out the possibility that dissemination of yersiniae from PP to the spleen is due to the change in the microarchitecture of the gastrointestinal tract in TNFRp55−/− mice. Parenteral infection of wild-type and TNFRp55−/− mice with Y. enterocolitica WA-yadA caused an outcome similar to that achieved by orogastric infection. Together, these results demonstrate that TNFRp55-dependent host mechanisms may be required for control of YadA-independent virulence factors involved in bacterial dissemination.
YopH is involved in the antiphagocytic effect of Y. enterocolitica by dephosphorylation of host cell proteins required for actin polymerization (11). In the present study, Y. enterocolitica WA-sycH, a functional YopH mutant, was able to colonize the PP but did not cause systemic infection of the spleen, indicating that yersiniae lacking YopH function can be cleared by mechanisms that do not require an IL-12-, IL-18-, and TNFRp55-dependent immune defense. Vice versa, the presence of YopH in Yersinia strains helps them to compete with immune response mechanism of the innate immunity mediated by TNF-α and to compete with immune responses mediated by factors that link innate immunity and adaptive immunity, such as IL-18 and IL-12. In terms of the safety aspects of their potential use as live carrier vaccines, Y. enterocolitica sycH mutants seem to be the most appropriate mutants described in this study.
Y. enterocolitica WA-irp-1 is deficient in the siderophore yersiniabactin, which is a high-affinity ferric iron uptake system that significantly contributes to the virulence of yersiniae (43). This strain was able to colonize PP but was impaired in the ability to cause systemic infection in immunocompetent mice. TNFRp55 seems to play a role in the attenuation of this strain, as mice deficient in this cytokine receptor were susceptible to systemic infection by this mutant strain, indicating that lack of Irp-1 is only partially sufficient to overcome the requirement for TNFRp55-mediated immune defense mechanisms. Therefore, one can assume that Irp-1 helps to enable yersiniae to compete with immune mechanisms that link adaptive and innate immunity, such as IL-12 and IL-18.
Attenuation of a SodA-deficient Yersinia mutant is due to its reduced ability to detoxify metabolites and exogenous oxygen radicals produced by phagocytes (47). In keeping with previous reports (33, 47), we showed that Y. enterocolitica sodA colonized PP after orogastric infection. The survival of this mutant in the spleen was markedly reduced, compared with that of the wild-type Yersinia strain, in immunocompetent mice. However, TNFRp55−/− and IL-12p40−/− mice were unable to control this mutant strain and IL-18−/− mice were partially susceptible to this infection. Thus, this mutant strain still bears pathogenicity factors that require TNF-α- and IL-12-dependent defense mechanisms. The lack of SodA is sufficient to attenuate this Yersinia strain, although IL-18-dependent defense is no longer present. Therefore, we conclude that additional virulence functions mediated by SodA enable yersiniae to compete with a more complex host defense in which IL-18 is essential in concert with other factors, such as, e.g., IL-12 and TNF-α.
The cellular immune response, in particular, production of IFN-γ by Yersinia-specific T cells, is associated with resistance of mice to Y. enterocolitica (16). This study demonstrates that IFN-γ production occurs in TNFRp55−/− mice after Y. enterocolitica infection, which indicates that unlike in immunocompetent mice, Yersinia-induced IFN-γ production does not necessarily correlate with clearance of the infection. Levels of TNF-α and IFN-γ production in TNFRp55−/− mice even exceeded those in control mice. Similar findings on Listeria monocytogenes-infected TNFRp55−/− mice have been reported by Endres et al. (27).
Moreover, we found increased levels of IFN-γ production only in mice infected with Yersinia strains that were able to colonize and persist in PP and therefore had the potential to disseminate in the spleen. In IL-12−/− mice, IFN-γ production was not increased because of the lack of IL-12, as described before. It has been reported that IL-18−/− mice display a phenotype largely similar to that of IL-12-deficient mice, exhibiting reductions in IFN-γ production, NK cell activity, and Th1 response (2). However, in the present study, IL-18−/− mice infected with Yersinia strains produced levels of IFN-γ similar to those of wild-type mice. This finding is in keeping with recent findings showing that in IL-18−/− mice that produce high levels of IL-12 upon Leishmania infection, high levels of IFN-γ can be observed (41). The increased levels of IFN-γ in our study reflect the fact that dissemination of bacteria to the spleen occurred.
IL-10, a Th2 cytokine that is antagonistic to the Th1 pathway and downregulates the function of macrophages (12), was found to be increased in the culture supernatants of TNFRp55−/− mouse splenocytes. Also, IL-10 mRNA expression in the liver was higher in Yersinia-infected TNFRp55−/− mice than in C57BL/6 mice. The levels of this cytokine were increased more after infections with attenuated Yersinia mutants that turned out to be pathogenic in TNFRp55−/− mice. This relationship was also the case in the other mouse strains tested, suggesting an association between Yersinia-triggered IL-10 production and bacterial pathogenicity. Thus, low bacterial numbers in the spleen coincided with low IL-10 production in Yersinia-stimulated splenocytes. It is important to note that in gene-targeted mice, cytokine production is even more complicated to interpret because, e.g., lack of certain cytokines, such as IL-12, could positively upregulate IL-10 levels. Dissection of IL-10 production in vitro by elimination of certain IL-10-producing cell types showed that macrophages and B cells rather than T cells are the dominant IL-10 producers.
Finally, our results suggest that cytokine-deficient mice can be used to characterize whether and on which level bacterial virulence factors might interfere with the host defense. Y. enterocolitica sycH may be the most appropriate mutant in terms of safety, as this mutant invades and persists in PP but is not able to establish a persistent infection in the spleen of an immunocompromised host. Although this mutant induced cytokine responses in spleen cell cultures, the immunogenicity of this mutant needs to be further investigated because an optimal attenuated vaccine strain should be both safe and immunogenic.
Acknowledgments
We thank K. Pfeffer and S. Akira for cytokine-deficient mouse strains and J. Heesemann for Yersinia mutant strains.
This work was supported by grants from the Bayerische Forschungsstiftung, the Deutsche Forschungsgemeinschaft, the European Union, the Alexander-von-Humboldt Stiftung, and the Consejo Nacional de Investigaciones Cientificas y Tecnicas (CONICET).
Editor: J. T. Barbieri
REFERENCES
- 1.Adachi, J. A., F. R. Dálessio, and C. D. Ericsson. 2000. Reactive arthritis associated with typhoid vaccination in travelers: report of two cases with negative HLA-B27. J. Travel Med. 7:35-36. [DOI] [PubMed] [Google Scholar]
- 2.Akira, S. 2000. The role of IL-18 in innate immunity. Curr. Opin. Immunol. 12:59-63. [DOI] [PubMed] [Google Scholar]
- 3.Autenrieth, I. B., M. Beer, E. Bohn, S. H. E. Kaufmann, and J. Heesemann. 1994. Immune responses to Yersinia enterocolitica in susceptible BALB/c and resistant C57BL/6 mice: an essential role for gamma interferon. Infect. Immun. 62:2590-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Autenrieth, I. B., and R. Firsching. 1996. Penetration of M cells and destruction of Peyer's patches by Yersinia enterocolitica: an ultrastructural and histological study. J. Med. Microbiol. 44:285-294. [DOI] [PubMed] [Google Scholar]
- 5.Autenrieth, I. B., V. Kempf, T. Sprinz, S. Preger, and A. Schnell. 1996. Defense mechanisms in Peyer′s patches and mesenteric lymph nodes against Yersinia enterocolitica involve integrins and cytokines. Infect. Immun. 64:1357-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Autenrieth, I. B., and J. Heesemann. 1992. In vivo neutralization of tumor necrosis factor alpha and interferon-gamma abrogates resistance to Yersinia enterocolitica in mice. Med. Microbiol. Immunol. 181:333-338. [DOI] [PubMed] [Google Scholar]
- 7.Autenrieth, I. B., A. Tingle, A. Reske Kunz, and J. Heesemann. 1992. T lymphocytes mediate protection against Yersinia enterocolitica in mice: characterization of murine T-cell clones specific for Y. enterocolitica. Infect. Immun. 60:1140-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Autenrieth, I. B., U. Vogel, S. Preger, B. Heymer, and J. Heesemann. 1993. Experimental Yersinia enterocolitica infection in euthymic and T-cell-deficient athymic nude C57BL/6 mice: comparison of time course, histomorphology, and immune response. Infect. Immun. 61:2585-2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balligand, G., Y. Laroche, and G. Cornelis. 1985. Genetic analysis of virulence plasmid from a serogroup 9 Yersinia enterocolitica strain: role of outer membrane protein P1 in resistance to human serum and autoagglutination. Infect. Immun. 48:782-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Black, D. S., and J. B. Bliska. 1997. Identification of p130Cas as a substrate of Yersinia YopH (Yop51), a bacterial protein tyrosine phosphatase that translocates into mammalian cells and targets focal adhesions. EMBO J. 16:2730-2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bliska, J. B., J. C. Clemens, J. E. Dixon, and S. Falkow. 1992. The Yersinia tyrosine phosphatase: specificity of a bacterial virulence determinant for phosphoproteins in the J774A.1 macrophage. J. Exp. Med. 176:1625-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bogdan, C., Y. Vodovotz, and C. Nathan. 1991. Macrophage deactivation by interleukin 10. J. Exp. Med. 174:1549-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bohn, E., and I. B. Autenrieth. 1996. IL-12 is essential for resistance against Yersinia enterocolitica by triggering IFN-gamma production in NK cells and CD4+ T cells. J. Immunol. 156:1458-1468. [PubMed] [Google Scholar]
- 14.Bohn, E., J. Heesemann, S. Ehlers, and I. B. Autenrieth. 1994. Early gamma interferon mRNA expression is associated with resistance of mice against Yersinia enterocolitica. Infect. Immun. 62:3027-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bohn, E., E. Schmitt, C. Bielfeldt, A. Noll, R. Schulte, and I. B. Autenrieth. 1998. Ambiguous role of interleukin-12 in Yersinia enterocolitica infection in susceptible and resistant mouse strains. Infect. Immun. 66:2213-2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bohn, E., A. Sing, R. Zumbihl, C. Bielfeldt, H. Okamura, M. Kurimoto, J. Heesemann, and I. B. Autenrieth. 1998. IL-18 (IFN-γ-inducing factor) regulates early cytokine production in, and promotes resolution of, bacterial infection in mice. J. Immunol. 160:299-307. [PubMed] [Google Scholar]
- 17.Calin, A., N. Goulding, and D. Brewerton. 1987. Reactive arthropathy following Salmonella vaccination. Arthritis Rheum. 30:1197.. [DOI] [PubMed] [Google Scholar]
- 18.Carniel, E. 2001. The Yersinia high-pathogenicity island: an iron-uptake island. Microbes Infect. 3:561-569. [DOI] [PubMed] [Google Scholar]
- 19.Cornelis, G. R. 2000. Molecular and cell biology aspects of plague. Proc. Natl. Acad. Sci. USA 97:8778-8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cornelis, G. R., and H. Wolf-Watz. 1997. The Yersinia Yop virulon: a bacterial system for subverting eukaryotic cells. Mol. Microbiol. 23:861-867. [DOI] [PubMed] [Google Scholar]
- 21.Corthésy-Theulaz, I. E., S. Hopkins, D. Bachmann, P. F. Saldinger, N. Porta, R. Haas, Y. Zheng-Xin, T. Meyer, H. Bouzourène, A. L. Blum, and J.-P. Kraehenbuhl. 1998. Mice are protected from Helicobacter pylori infection by nasal immunization with attenuated Salmonella typhimurium phoPc expressing urease A and B subunits. Infect. Immun. 66:581-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cover, T. L., and R. C. Aber. 1989. Yersinia enterocolitica. N. Engl. J. Med. 321:16-24. [DOI] [PubMed] [Google Scholar]
- 23.de Jong, R., F. Altare, I. A. Haagen, D. G. Elferink, T. Boer, P. J. Breda Vriesman, P. J. Kabel, J. M. Draaisma, J. T. van Dissel, F. P. Kroon, J. L. Casanova, and T. H. Ottenhoff. 1998. Severe mycobacterial and Salmonella infections in interleukin-12 receptor-deficient patients. Science 280:1435-1438. [DOI] [PubMed] [Google Scholar]
- 24.Denecker, G., W. Declercq, C. A. Geuijen, A. Boland, R. Benabdillah, M. van Gurp, M. P. Sory, P. Vandenabeele, and G. R. Cornelis. 2001. Yersinia enterocolitica YopP-induced apoptosis of macrophages involves the apoptotic signaling cascade upstream of bid. J. Biol. Chem. 276:19706-19714. [DOI] [PubMed] [Google Scholar]
- 25.Denis, M., A. Forget, A. C. Miailhe, M. Pelletier, and E. Skamene. 1985. Evolution of cell types and T-cell subsets in the spleens of Mycobacterium bovis BCG-resistant and M. bovis BCG-susceptible strains of mice after infection with M. bovis BCG. Infect. Immun. 49:253-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.el Tahir, Y., and M. Skurnik. 2001. YadA, the multifaceted Yersinia adhesin. Int. J. Med. Microbiol. 291:209-218. [DOI] [PubMed] [Google Scholar]
- 27.Endres, R., A. Luz, H. Schulze, H. Neubauer, A. Futterer, S. M. Holland, H. Wagner, and K. Pfeffer. 1997. Listeriosis in p47(phox−/−) and TRp55−/− mice: protection despite absence of ROI and susceptibility despite presence of RNI. Immunity 7:419-432. [DOI] [PubMed] [Google Scholar]
- 28.Grutzkau, A., C. Hanski, H. Hahn, and E. O. Riecken. 1990. Involvement of M cells in the bacterial invasion of Peyer's patches: a common mechanism shared by Yersinia enterocolitica and other enteroinvasive bacteria. Gut 31:1011-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanski, C., U. Kutschka, H. P. Schmoranzer, M. Naumann, A. Stallmach, H. Hahn, H. Menge, and E. O. Riecken. 1989. Immunohistochemical and electron microscopic study of interaction of Yersinia enterocolitica serotype O8 with intestinal mucosa during experimental enteritis. Infect. Immun. 57:673-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heesemann, J., U. Gross, and L. Gruter. 1987. Genetic manipulation of virulence of Yersinia enterocolitica. Contrib. Microbiol. Immunol. 9:312-316. [PubMed] [Google Scholar]
- 31.Heesemann, J., C. Keller, R. Morawa, N. Schmidt, H. J. Siemens, and R. Laufs. 1983. Plasmids of human strains of Yersinia enterocolitica: molecular relatedness and possible importance for pathogenesis. J. Infect. Dis. 147:107-115. [DOI] [PubMed] [Google Scholar]
- 32.Hines, J., E. Skrzypek, A. V. Kajava, and S. C. Straley. 2001. Structure-function analysis of Yersinia pestis YopM's interaction with alpha-thrombin to rule on its significance in systemic plague and to model YopM's mechanism of binding host proteins. Microb. Pathog. 30:193-209. [DOI] [PubMed] [Google Scholar]
- 33.Igwe, E. I., H. Russmann, A. Roggenkamp, A. Noll, I. B. Autenrieth, and J. Heesemann. 1999. Rational live oral carrier vaccine design by mutating virulence-associated genes of Yersinia enterocolitica. Infect. Immun. 67:5500-5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iriarte, M., and G. R. Cornelis. 1998. YopT, a new Yersinia Yop effector protein, affects the cytoskeleton of host cells. Mol. Microbiol. 29:915-929. [DOI] [PubMed] [Google Scholar]
- 35.Juris, S. J., A. E. Rudolph, D. Huddler, K. Orth, and J. E. Dixon. 2000. A distinctive role for the Yersinia protein kinase: actin binding, kinase activation, and cytoskeleton disruption. Proc. Natl. Acad. Sci. USA 97:9431-9436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lahesmaa Rantala, R., K. Granfors, R. Kekomaki, and A. Toivanen. 1987. Circulating yersinia specific immune complexes after acute yersiniosis: a follow up study of patients with and without reactive arthritis. Ann. Rheum. Dis. 46:121-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lalani, I., K. Bhol, and A. R. Ahmed. 1997. Interleukin-10: biology, role in inflammation and autoimmunity. Ann. Allergy Asthma Immunol. 79:469-483. [Erratum, 80(3):A-6, 1998.] [DOI] [PubMed]
- 38.Magram, J., S. E. Connaughton, R. R. Warrier, D. M. Carvajal, C. Y. Wu, J. Ferrante, C. Stewart, U. Sarmiento, D. A. Faherty, and M. K. Gately. 1996. IL-12-deficient mice are defective in IFN gamma production and type 1 cytokine responses. Immunity 4:471-481. [DOI] [PubMed] [Google Scholar]
- 39.Monack, D. M., J. Mecsas, N. Ghori, and S. Falkow. 1997. Yersinia signals macrophages to undergo apoptosis and YopJ is necessary for this cell death. Proc. Natl. Acad. Sci. USA 94:10385-10390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Monteforte, G. M., K. Takeda, M. Rodriguez-Sosa, S. Akira, J. R. David, and A. R. Satoskar. 2000. Genetically resistant mice lacking IL-18 gene develop Th1 response and control cutaneous Leishmania major infection. J. Immunol. 164:5890-5893. [DOI] [PubMed] [Google Scholar]
- 41.Mosmann, T. R., and K. W. Moore. 1991. The role of IL-10 in cross-regulation of TH1 and TH2 responses. Immunol. Today 12:A49-A53. [DOI] [PubMed]
- 42.Pasparakis, M., L. Alexopoulou, M. Grell, K. Pfizenmaier, H. Bluethmann, and G. Kollias. 1997. Peyer's patch organogenesis is intact yet formation of B lymphocyte follicles is defective in peripheral lymphoid organs of mice deficient for tumor necrosis factor and its 55-kDa receptor. Proc. Natl. Acad. Sci. USA 94:6319-6323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pelludat, C., A. Rakin, C. A. Jacobi, S. Schubert, and J. Heesemann. 1998. The yersiniabactin biosynthetic gene cluster of Yersinia enterocolitica: organization and siderophore-dependent regulation. J. Bacteriol. 180:538-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pepe, J. C., M. R. Wachtel, E. Wagar, and V. L. Miller. 1995. Pathogenesis of defined invasion mutants of Yersinia enterocolitica in a BALB/c mouse model of infection. Infect. Immun. 63:4837-4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pfeffer, K., T. Matsuyama, T. M. Kundig, A. Wakeham, K. Kishihara, A. Shahinian, K. Wiegmann, P. S. Ohashi, M. Kronke, and T. W. Mak. 1993. Mice deficient for the 55 Kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell 73:457-467. [DOI] [PubMed] [Google Scholar]
- 46.Robins-Browne, R. M., C. S. Still, M. D. Miliotis, and H. J. Koornhof. 1979. Mechanism of action of Yersinia enterocolitica enterotoxin. Infect. Immun. 25:680-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roggenkamp, A., T. Bittner, L. Leitritz, A. Sing, and J. Heesemann. 1997. Contribution of the Mn-cofactored superoxide dismutase (SodA) to the virulence of Yersinia enterocolitica serotype O8. Infect. Immun. 65:4705-4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roggenkamp, A., H. R. Neuberger, A. Flugel, T. Schmoll, and J. Heesemann. 1995. Substitution of two histidine residues in YadA protein of Yersinia enterocolitica abrogates collagen binding, cell adherence and mouse virulence. Mol. Microbiol. 16:1207-1219. [DOI] [PubMed] [Google Scholar]
- 49.Rosqvist, R., A. Forsberg, and H. Wolf-Watz. 1991. Intracellular targeting of the Yersinia YopE cytotoxin in mammalian cells induces actin microfilament disruption. Infect. Immun. 59:4562-4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ruckdeschel, K., O. Mannel, K. Richter, C. A. Jacobi, K. Trulzsch, B. Rouot, and J. Heesemann. 2001. Yersinia outer protein P of Yersinia enterocolitica simultaneously blocks the nuclear factor-kappa B pathway and exploits lipopolysaccharide signaling to trigger apoptosis in macrophages. J. Immunol. 166:1823-1831. [DOI] [PubMed] [Google Scholar]
- 51.Ruckdeschel, K., A. Roggenkamp, S. Schubert, and J. Heesemann. 1996. Differential contribution of Yersinia enterocolitica virulence factors to evasion of microbicidal action of neutrophils. Infect. Immun. 64:724-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schafer, R., D. A. Portnoy, S. A. Brassell, and Y. Paterson. 1992. Induction of a cellular immune response to a foreign antigen by a recombinant Listeria monocytogenes vaccine. J. Immunol. 149:53-59. [PubMed] [Google Scholar]
- 53.Skrzypek, E., C. Cowan, and S. C. Straley. 1998. Targeting of the Yersinia pestis YopM protein into HeLa cells and intracellular trafficking to the nucleus. Mol. Microbiol. 30:1051-1065. [DOI] [PubMed] [Google Scholar]
- 54.Takeda, K., H. Tsutsui, T. Yoshimoto, O. Adachi, N. Yoshida, T. Kishimoto, H. Okamura, K. Nakanishi, and S. Akira. 1998. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity 8:383-390. [DOI] [PubMed] [Google Scholar]
- 55.VanCott, J. L., S. N. Chatfield, M. Roberts, D. M. Hone, E. L. Hohmann, D. W. Pascual, M. Yamamoto, H. Kiyono, and J. R. McGhee. 1998. Regulation of host immune responses by modification of Salmonella virulence genes. Nat. Med. 4:1247-1252. [DOI] [PubMed] [Google Scholar]
- 56.Visser, L. G., P. S. Hiemstra, M. T. van den Barselaar, P. A. Ballieux, and R. van Furth. 1996. Role of YadA in resistance to killing of Yersinia enterocolitica by antimicrobial polypeptides of human granulocytes. Infect. Immun. 64:1653-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wysocki, L. J., and V. L. Sato. 1978. “Panning” for lymphocytes: a method for cell selection. Proc. Natl. Acad. Sci. USA 75:2844-2848. [DOI] [PMC free article] [PubMed] [Google Scholar]