Abstract
Streptococcus pneumoniae is a major cause of illness and death in children in developing countries. In these children, zinc deficiency is associated with an increased risk of acute respiratory tract infections, which can be reduced by daily zinc administration. Severe infections decrease zinc levels in plasma and may thereby move individuals with preexisting low zinc stores into a vicious cycle of infection and unavailable zinc. Pneumococcal surface protein A (PspA) has emerged as a promising vaccine candidate, and immunization with this antigen protects animals from pneumococcal infection. In an animal experiment, we measured the effect of zinc depletion on the immune response to parenterally administrated PspA and assessed the effect of this PspA vaccination and zinc depletion on the severity of pneumococcal infection and on zinc status. Mice were kept on different diets for 5 weeks, immunized twice 14 days apart, and challenged intranasally with S. pneumoniae. Mice on the zinc-deficient diet showed substantially reduced immune responses to PspA, more extensive pneumococcal colonization in the nasal mucosa, more severe infections, and an increased risk of death. PspA immunization reduced the risk of severe disease, and the reduction in severity was reflected in substantially reduced zinc depletion from bones.
Approximately 2.5 million children die annually from acute lower respiratory tract infections, and the mortality is particularly high in developing countries (2, 13). Streptococcus pneumoniae is the causal agent in more than one third of bacterial pneumonias in these children (4, 13). Young children are at high risk because of their naïve immune system and the reduced ability to mount an antipolysaccharide immune response (3, 10). The pneumococci reside in the nasopharynx, usually without causing disease, but may spread locally and cause otitis media, sinusitis, and lower respiratory tract infections and can reach the bloodstream to cause meningitis, infection in other organs, and sepsis. Untreated pneumococcal pneumonia has a high case fatality ratio, especially in children, the elderly, and immunocompromised and splenectomized patients (1). Infection with S. pneumoniae is among the predominant causes of death among children with sickle cell anemia (14, 15). In addition to their lack of a functional spleen, these children have low zinc levels in plasma and share many other features of children with nutritional zinc deficiency such as impaired growth, reduced gonad function, and impaired immunity (14, 15).
Zinc deficiency is prevalent among children in developing countries (20), and daily zinc administration reduces the incidence and severity of acute lower respiratory tract infections in these children (5, 6, 17). Several animal and human experiments have shown that even mild zinc deficiency affects many facets of the immune system (18, 20). The effect of zinc in reducing the incidence of acute respiratory tract infections may thus be due in part to the replenishment of depleted zinc stores, thereby preventing an impairment of the immune system.
Resistance to respiratory tract infections, such as those caused by S. pneumoniae, depends on appropriate cellular linings in the respiratory tract, normal phagocytosis, and adequate humoral and cellular immune responses. Pneumococcal surface protein A (PspA) is present on all virulent strains of S. pneumoniae (11). Immunization with this protein protects animals against sepsis, intratracheal infections, and, when administered mucosally, carriage of S. pneumoniae (7, 21, 22). Natural pneumococcal infection in humans induces an immune response against PspA (16), and PspA is considered a promising vaccine candidate for prevention of pneumococcal disease in humans (8).
In a previous mouse experiment, we demonstrated that zinc deprivation resulted in an increased risk of invasive pneumococcal infection and death (19). In the present study, we used a factorial design with zinc depletion and PspA immunization as the experimental variables to determine the effect of zinc deprivation on the immune response to PspA and on pharyngeal carriage of pneumococci. This design enabled us to simultaneously assess whether parenteral PspA immunization could protect mice from severe infection and whether any such protection would decrease inflammation-associated zinc depletion (9).
MATERIALS AND METHODS
Overall design.
We used a factorial design to assess the effect of zinc deficiency and PspA immunization on mice that were challenged intranasally with virulent pneumococci. Experimental feeding and immunization were done over a 4-week period. The mice were challenged and then observed for 1 week until sacrifice. Outcomes were the presence or counts of pneumococci in blood, lungs, and nasal washes, zinc levels in bone and serum, and death. We also assessed the effect of zinc depletion on the immune response to PspA.
Animals.
Female BALB/c mice (Bomholtgård Ltd., Bomholt, Denmark) obtained at 5 weeks of age were allowed to acclimate for 3 days before the experiment started. They were then matched for weight and randomized into four experimental groups, which had the same average weights. All mice were housed individually. The local officer of the experimental animal board under the Norwegian Ministry of Agriculture approved the study protocol, and the experiment was in conformity with the laws and regulations controlling experiments with live animals in Norway.
Feeding.
Experimental and control diets and the pair feeding procedure have been described in detail elsewhere (19). Briefly, as zinc intake has marked effects on growth and appetite, precautions were taken to avoid differences in the intake of other nutrients by pair feeding the zinc-replete mice. Furthermore, the amount of food offered to the pair-fed mice was corrected for body weight because of the retarded growth of the zinc-depleted animals (19). The zinc-deficient and control diets contained 2.0 μg and 25 μg of elemental zinc per g, respectively. Deionized water was given ad libitum.
Immunization.
Recombinant PspA was PspA/Rx1 (amino acids 1 to 302) expressed from pUAB055 and purified by nickel affinity chromatography as previously described (7). PspA/Rx1 belongs to clade 2 and family 1 of the PspA molecule family (12). The immunized mice were injected subcutaneously twice with 100 μl of a phosphate-buffered saline (PBS) solution containing 1 μg of recombinant PspA and 50 μg of alum (Imject; Pierce, Rockford, Ill.). The placebo recipients were injected with 100 μl of PBS containing 50 μg of alum. The doses were administered 2 weeks apart on days 1 and 14 after randomization.
Bacteria and culture conditions.
Strain DBL2 of S. pneumoniae serotype 2 produces carriage and/or invasive infections in mice (19) and contains PspA that belongs to clade 2. DBL2 bacteria from a stock culture kept at −70°C were grown in Todd-Hewitt broth (Difco Laboratories, Detroit, Mich.) to the mid-logarithmic growth phase and stored frozen at −70°C in 1-ml aliquots. The aliquots were thawed immediately prior to challenge. The inoculum dose was confirmed by quantitative plating before and after challenge; there were no substantial differences in the concentration of viable bacteria, indicating that there was no loss of bacteria between thawing and challenge. Bacteria were confirmed to be S. pneumoniae by colony morphology and optochin sensitivity. The number of viable bacteria from the infection batch, lung homogenate, blood, and nose washes was determined by quantitative culture of serial dilutions on blood agar with 5 μg of gentamicin per ml. The agar plates were incubated overnight at 37°C in an 8% CO2 atmosphere. The bacterial counts were expressed as median and interquartile range CFU/100 μl of nasal wash.
Challenge.
After 28 days of experimental feeding, i.e., 14 days after the second immunization, the mice were anaesthetized with Isoflurane (Forene; Abbot Laboratories, Chicago, Ill.) in a double plenum induction chamber for small animals (International Marketing Services, North Andover, Mass.). Immediately after induction, when breathing was slow and deep, a suspension containing 1.2 × 109 bacteria per ml was introduced intranasally. Because the average weight in the zinc-depleted group was lower than in the zinc-replete group, the inoculum size was adjusted by giving 2 μl of suspension per g of body weight.
Blood sampling.
The mice were bled in the hind leg vein at the start of the experiment, immediately prior to each of the two immunizations, on alternate days after pneumococcal challenge, and by cardiac puncture after sacrifice. Until cardiac puncture, the 20-μl sample was diluted in 80 μl of PBS in a 1.5-ml microcentrifuge tube (Eppendorf, Hinz, Germany). Then 20 μl of the suspension was used for bacterial quantification immediately after the mice had been bled. The tube was centrifuged, and the supernatant was kept frozen at −70°C until the immunoassay was performed. On day 7 after challenge, the blood was collected by heart puncture after killing the mice with a subcutaneous overdose of Hypnorm (fenytyl citrate and fluanisone; Janssen Pharmaceutica, Beerse, Belgium) and Vival (diazepam; Alpharma, Oslo, Norway). The throat and thorax were opened aseptically, and sterile PBS was injected into the epipharynx through an incision in the proximal larynx. The larynx was clamped to avoid contamination between the lungs and the epipharynx. The first 100-μl wash from the nostrils was collected and inoculated on blood agar. The lungs were dissected free, and the left lung was homogenized and cultured on blood agar.
Immunoassay.
Microtiter plates (Nunc Maxisorp; P.G.C. Scientific, Fair Lawn, N.J.) were coated with 100 μl of 0.5-μg/ml PspA in PBS for 2 h at 37°C and then overnight at 4°C. They were then blocked for 30 min with PBS-Tween 20 (PBST) with 1% skimmed milk. Between each step, we washed the plates four times with PBST. The supernatant of the diluted mouse blood was diluted through five consecutive fivefold dilutions in the microtiter plates. A standard with PspA-positive pooled reference serum was diluted threefold eight times. The standard and the samples were incubated for 90 min at 37°C. Biotin-labeled rabbit anti-mouse immunoglobulin antibodies (Southern, Birmingham, Ala.) diluted 1:5,000 in the blocking buffer were then added and incubated in 37°C for 90 min. Streptavidin-alkaline phosphatase diluted 1:4,000 was added and incubated for another 90 min before adding the conjugate [100 μl of 104 p-nitrophenyl phosphate per 2 ml of 9.6% dietolamin-0.5 mol of MgCl2 (pH 9.8) per liter]. After 30 min, the reaction was stopped by adding 10 μl of 3-mol/liter NaOH to each well. The optical density was measured at 490 nm in a spectrophotometer (Molecular Devices Thermomax Reader, Sunnyvale, Calif.). The results were analyzed and interpreted with Softmax data analysis software version 2.3.5 (Molecular Devices). The optical density was converted to an arbitrary concentration scale by interpolation from a calibration curve made from the pooled serum. Each sample was examined in duplicate, and each microtiter plate contained samples from one zinc-deficient and one zinc-replete mouse.
Zinc analysis.
The left femoral bone was dissected free from the surrounding tissues with stainless steel scissors and stored at −20°C before zinc determination. Prior to element analysis, tissue and feed samples were wet digested in a Milestone microwave laboratory system (Milestone, Sorisole, Italy) by the addition of 2 ml of 65% (wt/vol) nitric acid and 0.5 ml of 30% (wt/vol) H2O2 to samples of approximately 0.2 g of dry matter. The concentration of zinc was determined on a Perkin Elmer 3300 atomic absorption spectrometer (Perkin Elmer, Norwalk, Conn.), equipped with a high-sensitivity nebulizer. The concentration of zinc was calculated with an external calibration procedure, and the accuracy and precision were assessed by concomitant analysis of reference material. Bovine liver 1577 b from the National Institute of Standards and Technology, Oslo, Norway, and Seronorm from Sero AS, Billingstad, Norway, were used for zinc determinations in femur and serum samples, respectively.
Statistical analyses.
Means, standard deviations, differences of means, and 95% confidence intervals (95% CI) and t tests were used to describe and compare continuous variables. Immune responses were log transformed to achieve symmetrial distributions, and t tests assuming unequal variances of the log-transformed values were used in the analyses. Bacterial counts and their log transformations were not normally distributed, and medians and interquartile ranges were calculated, while the Wilcoxon rank-sum (Mann-Whitney) test was used for comparisons. Proportions were compared with Fisher's exact test, and relative risks (RR) were calculated to describe the differences between the experimental groups. If no event was observed in a cell, the value of that cell was set to 1 to calculate an underestimated RR. Statistical analyses were undertaken with Stata, version 6 (StataCorp, College Station, Tex.). A P value of <0.05 was considered to represent statistical significance.
RESULTS
Growth and zinc levels.
The mean weight on the day before immunization was 15.8 g (standard deviation, 1.19 g) in the mice that were to be zinc-depleted and 15.7 g (standard deviation, 1.14 g) in the mice that would be given a zinc-adequate diet. At the time of challenge (day 28), the mean weight of the zinc-deficient mice was 14.0 g, while the zinc-replete mice weighed an average of 16.7 g, a difference in the mean weight change of 2.8 g (95% CI, 2.0 to 3.5 g). One mouse in the zinc-deficient-placebo-vaccinated group died immediately after randomization but before immunization because animal fodder had blocked the water supply. The mouse was accordingly excluded from the analyses.
The zinc content of the diet was reflected in the zinc concentration in bone and plasma at the end of the study (Table 1). Compared to the sham-immunized mice, PspA-immunized mice had a substantially and significantly higher zinc concentration in bone. Overall, the same tendency was seen for zinc in serum. It should be noted, however, that fewer mice were assessed for serum zinc in the zinc-deficient animals because blood for zinc analysis was collected only from those that survived until the end of the observation period. Among the zinc-replete mice, all of which could be assessed for zinc levels in plasma at the end of the study, there was a significantly lower zinc concentration in plasma in the unimmunized than in the immunized mice.
TABLE 1.
Zinc levels in a mouse experiment to assess the effect of zinc deprivation and PspA immunization on pneumococcal colonization of the nasal mucosa, severe infection, and death after pneumococcal challengea
| Group | Zinc in femur
|
Zinc in plasma
|
||||||
|---|---|---|---|---|---|---|---|---|
| No. of samples | Mean ± SD (μg/g) | Difference between groups (μg/g) | P | No. of samples | Mean ± SD (mg/liter) | Difference between groups (mg/liter) | P | |
| Zinc adequate | 24 | 60.1 ± 11.1 | 6.1 | 0.11 | 24 | 0.82 ± 0.3 | 0.39 | <0.0005 |
| Zinc depleted | 22 | 54.0 ± 14.2 | 13 | 0.43 ± 0.2 | ||||
| Immunized | 23 | 61.6 ± 13.6 | 8.7 | 0.02 | 18 | 0.77 ± 0.4 | 0.18 | 0.08 |
| Unimmunized | 23 | 52.9 ± 10.9 | 19 | 0.59 ± 0.2 | ||||
| Zinc adequate | ||||||||
| Immunized | 12 | 62.6 ± 10.9 | 4.9 | 0.29 | 12 | 0.93 ± 0.3 | 0.23 | 0.03 |
| Unimmunized | 12 | 57.7 ± 11.2 | 12 | 0.70 ± 0.2 | ||||
| Zinc depleted | ||||||||
| Immunized | 11 | 60.4 ± 16.5 | 12.8 | 0.04 | 6 | 0.46 ± 0.2 | 0.05 | 0.72 |
| Unimmunized | 11 | 47.6 ± 7.9 | 7 | 0.41 ± 0.2 | ||||
Means and mean differences are presented; Student's t tests assuming unequal variances were used to estimate the level of significance for the differences between the experimental groups. Thirty-seven mice survived until sacrifice, when blood for analysis of zinc in plasma was collected.
Immune response.
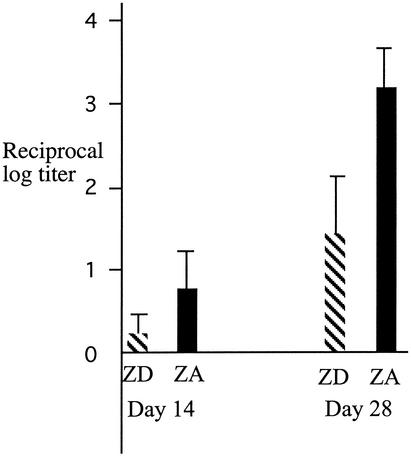
The zinc-depleted mice had substantially lower mean titers of anti-PspA immunoglobulin G after immunization compared to the zinc-replete mice (Fig. 1).
FIG. 1.
Immune response to PspA in zinc-deficient and zinc-replete mice after one and two immunizations. ZD, mice on low-zinc diet, ZA, mice on zinc-adequate diet. Values are means and the upper 95% CI of log-transformed titers of anti-PspA immunoglobulin G. Day 14 is after the first immunization, while day 28 is after the second immunization with PspA. Significance levels of the differences: day 14, P = 0.052; day 28, P < 0.001. The preimmune anti-PspA titers were zero.
Pneumococci in nasal washes.
The zinc-deficient mice had substantially more pneumococci in their nasal washes than did the zinc-replete mice (P = 0.03, Table 2). For this outcome, there was no significant difference between the immunization groups.
TABLE 2.
Number of pneumococci recovered from nasal mucosa in an experiment to assess the effect of zinc deprivation and PspA immunization on intranasal pneumococcal challenge in micea
| Group | No. of samples | Median no. of pneumococci (IQR) | P | |
|---|---|---|---|---|
| Zinc adequate | 24 | 12,960 (6,480-19,643) | 0.03 | |
| Zinc depleted | 13 | 29,160 (17,820-52,245) | ||
| Immunized | 18 | 14,985 (10,125-53,460) | 0.40 | |
| Unimmunized | 19 | 13,770 (6,075-23,490) | ||
| Zinc adequate | ||||
| Immunized | 12 | 13,568 (9,315-23,692) | 0.49 | |
| Unimmunized | 12 | 11,542 (5,130-18,630) | ||
| Zinc depleted | ||||
| Immunized | 6 | 52,852 (29,160-55,890) | 0.87 | |
| Unimmunized | 7 | 19,845 (16,200-36,450) |
Thirty-seven mice survived until the scheduled sacrifice and could be assessed for pneumococci in nasal washes. The central tendency and spread of the pneumococcal counts are presented as the median and the interquartile range (IQR), while the significance level of the differences in these counts was estimated with the Wilcoxon rank sum test.
Bacteremia.
All 10 mice that tested positive for pneumococci in the blood belonged to the groups that were given the zinc-deficient diet (Table 3). Six of these belonged to the nonimmunized group, while four of the mice in the immunized group had bacteremia (P = 0.7). Only one mouse with bacteremia survived until sacrifice.
TABLE 3.
Outcomes in a mouse experiment to assess the effect of zinc deprivation and PspA immunization on pneumococcal colonization of the nasal mucosa, severe infection, and death after pneumococcal challengea
| Group | Death during observation
|
Bacteremia
|
Severe infection
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| No. dead/no. in group | RR | P | No. with pneumococci in blood/no. in group | RR | P | No. with bacteria in lungs or blood/no. in group | RR | P | |
| Zinc adequate | 0/24 | >10.4 | <0.001 | 0/24 | >10.4 | <0.001 | 4/24 | 3.1 | 0.01 |
| Zinc depleted | 10/23 | 10/23 | 12/23 | ||||||
| Immunized | 5/23 | 1.0 | 1.00 | 4/23 | 1.4 | 0.72 | 4/23 | 2.9 | 0.03 |
| Unimmunized | 5/24 | 6/24 | 12/24 | ||||||
| Zinc adequate | |||||||||
| Immunized | 0/12 | 0/12 | 0/12 | >3 | 0.09 | ||||
| Unimmunized | 0/12 | 0/12 | 4/12 | ||||||
| Zinc depleted | |||||||||
| Immunized | 5/11 | 0.9 | 1.00 | 4/11 | 1.4 | 0.68 | 4/11 | 1.8 | 0.22 |
| Unimmunized | 5/12 | 6/12 | 8/12 | ||||||
Unadjusted relative risks (RR) are shown with the corresponding significance levels (P), which were calculated with Fisher’s two-sided exact test. Adjusting for the effect of diet or immunization in a generalized linear model with binomial variability and a logarithmic link function increased the RR for zinc depletion during severe infection to 4.0 (P = 0.002) and the RR for sham immunization to 3.6 (P = 0.004).
Severe pneumococcal infection and death.
Sixteen of the 47 infected mice had severe pneumococcal disease, defined as having detectable pneumococci in the blood at any time point or in the lungs at sacrifice. Compared to the zinc-replete mice (4 of 24), a substantially higher proportion of the zinc-deficient mice (12 of 23) had severe pneumococcal infection, corresponding to a 3.1-fold increased risk (P = 0.01, Table 3). Moreover, although PspA immunization did not protect the zinc-deficient mice from dying during the observation period (Table 3), it did confer a substantial protection against severe infection in that the risk of such infection was 2.9 times higher in the sham-immunized mice, corresponding to a protective efficacy of 66% (95% CI, 8% to 87%, P = 0.03), calculated as 100 × (1 − 1/RR). Of the mice that died during observation, only one did not yield bacteria from the blood or the lung tissue. All 10 mice that died were in the zinc-depleted group (P < 0.001).
DISCUSSION
This experiment demonstrates that zinc depletion results in a substantially reduced immune response to parenterally administered PspA and leads to a more extensive mucosal colonization following pneumococcal challenge in mice. Moreover, PspA immunization conferred a substantial protection against severe pneumococcal disease and reduced infection-induced zinc depletion. The experiment also confirms our previous finding that zinc-depleted mice have an increased risk of lethal pneumococcal infection (19).
All mice that were sacrificed and thereby could undergo the nasal wash procedure had detectable levels of pneumococci in the nose. The nasal washes from the zinc-deficient mice had substantially and significantly higher bacterial concentrations than the nasal washes from the zinc-replete mice. The estimated difference is likely to be conservative because zinc-deficient mice that were dead and thereby most probably had had a more severe illness could not be included in the analysis. If zinc has an effect on mucosal immunity, it may in turn affect pharyngeal carriage of S. pneumoniae, as seen in this experiment. Although we are reluctant to extrapolate the findings of this animal study to human beings, increased carriage and thereby incidence of pneumonia in children in developing countries may in part be due to an altered immune response which may be caused by zinc deficiency. Conversely, the reduction in the incidence of pneumonia following zinc supplementation (5, 6, 17) may in part reflect an augmentation of the mucosal resistance to infection. The effects of zinc deficiency and zinc administration on mucosal immunity need further clarification. The failure of subcutaneous immunization with PspA to reduce mucosal colonization is consistent with the findings of an earlier study in which intranasal but not parenteral immunization of mice offered some protection against nasal carriage (21).
We failed to recover pneumococci from the lungs in only 1 of the 10 mice that had bacteremia. There were six that had pneumococci in the lungs but not in the blood. All six mice survived until the end of the observation period; the lungs were processed and examined for pneumococci immediately after sacrifice and could be used to define severe infection. The zinc-depleted mice faced a 3.1-fold (P = 0.01) higher risk of severe infection than the zinc-replete mice. When adjusting for the effect of immunization in a generalized linear model with binomial variability and a logarithmic link function, the relative risk increased to 4.0 (P = 0.002), showing that our unadjusted estimate was conservative. Immunization with PspA induced a substantial (66%) and significant (P = 0.03) protection against severe pneumococcal infection. This unadjusted effect of immunization is also conservative because adjusting for the effect of zinc as described above increased the protection to 72% (P = 0.004). This adjustment is valid because there was no interaction between the diet and PspA immunization. Pneumococcal colonization in lung tissue as well as bacteremia may reflect hematogenous spread of the pneumococci, and the observed protection from PspA immunization may be via an enhanced resistance to generalized pneumococcal infection.
Compared to in the zinc-replete mice, a borderline significantly (P = 0.052) lower immune response was observed in the zinc-deficient animals even after the first immunization (Fig. 1). Even larger and highly significant (P < 0.001) effects of zinc deficiency on anti-PspA responsiveness was observed after the second injection of the vaccine. The substantially reduced response reflects an impaired capacity to mount adequate T-cell-dependent immune responses that again may have contributed to the increased morbidity. However, the increased mortality ascribed to zinc depletion was similar across the immunization groups, indicating a general impairment of host immunity and/or mucosal integrity. Thus, zinc deficiency affected the specific antibody response to PspA and the severity of pneumococcal infection, as reflected in increased mucosal colonization and in the increased risk of invasive infection and death.
As expected, zinc levels were lower in the zinc-deficient animals than in those given a zinc-adequate diet. Systemic infections affect the plasma and tissue zinc concentration (9, 20). During the acute-phase response, secretion of cytokines activates the intracellular synthesis of metallothionein, which increases zinc absorption in the liver, while the uptake of zinc in the bone is decreased (9). This redistribution of zinc from the plasma and the bones increases with the severity the infection (9). The zinc content in the tissues in this experiment was therefore a function not only of the diet, but also of the severity of the infection and the acute-phase response. The average zinc concentration in bone was substantially and significantly higher in the immunized than in the nonimmunized animals. The fact that zinc depletion was less pronounced in the immunized animals indicates that PspA immunization reduced the severity of the disease. This effect, however, was not matched by a statistically significant reduction in the proportion of mice with pneumococci in the blood or a reduced risk of death, nor did the immunization have any measurable effect on pneumococcal colonization of the pharyngeal mucosa. Immunization did, however, substantially reduce the risk of severe disease.
Zinc supplementation reduces the incidence of pneumonia in children in developing countries (5, 6, 17). The present study shows that, in mice, zinc depletion reduces the immune response to PspA, while PspA immunization decreases the severity of pneumococcal infection, including the associated impairment of zinc status. The study also confirmed that zinc depletion leads to increased severity and fatality of pneumococcal infection. Many children in developing countries have suboptimal zinc nutriture and may therefore have an increased risk of entering the vicious cycle of severe pneumococcal infection and detrimental reduction of available zinc. If the effect of PspA immunization in mice is mirrored in children, such immunization, along with measures to improve zinc nutrition, may contribute to breaking the vicious cycle of zinc depletion and severe infection.
Acknowledgments
We thank the staff at the experimental animal facilities at the Vivarium, University of Bergen, for their help and flexibility and the Department of Microbiology and Immunology, University of Bergen, for providing excellent laboratory facilities. We are also grateful for input from and assistance of Flora Gathof, Amy Swift, and Robert Fulgham, Department of Microbiology, University of Alabama at Birmingham.
This study was supported by the Norwegian Agency for Development Cooperation (Ind-040), the Norwegian Universities' Committee for Development Research and Education (PRO 52-53/96), and NIH grant AI21548.
Editor: V. J. DiRita
REFERENCES
- 1.Anonymous. 1998. Acute respiratory infections: the forgotten pandemic. Communique from the International Conference on Acute Respiratory Infections, held in Canberra, Australia, 7-10 July 1997. Int. J. Tuberc. Lung Dis. 2:2-4. [PubMed] [Google Scholar]
- 2.Anonymous. 2000. World Health Report 2000, Health systems: improving performance. World Health Organization, Geneva, Switzerland.
- 3.Barrett, D. J., J. W. Sleasman, D. A. Schatz, and M. Steinitz. 1992. Human anti-pneumococcal polysaccharide antibodies are secreted by the CD5-B cell lineage. Cell. Immunol. 143:66-79. [DOI] [PubMed] [Google Scholar]
- 4.Berman, S. 1991. Epidemiology of acute respiratory infections in children of developing countries. Rev. Infect. Dis. 13(Suppl. 6):S454-S462. [DOI] [PubMed] [Google Scholar]
- 5.Bhandari, N., R. Bahl, S. Taneja, T. Strand, K. Molbak, R. J. Ulvik, H. Sommerfelt, and M. K. Bhan. 2002. Effect of routine zinc supplementation on pneumonia in children aged 6 months to 3 years: randomised controlled trial in an urban slum. Br. Med. J. 324:1358.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhutta, Z. A., R. E. Black, K. H. Brown, J. M. Gardner, S. Gore, A. Hidayat, F. Khatun, R. Martorell, N. X. Ninh, M. E. Penny, J. L. Rosado, S. K. Roy, M. Ruel, S. Sazawal, and A. Shankar. 1999. Prevention of diarrhea and pneumonia by zinc supplementation in children in developing countries: pooled analysis of randomized controlled trials. Zinc Investigators' Collaborative Group. J. Pediatr. 135:689-697. [DOI] [PubMed] [Google Scholar]
- 7.Briles, D. E., E. Ades, J. C. Paton, J. S. Sampson, G. M. Carlone, R. C. Huebner, A. Virolainen, E. Swiatlo, and S. K. Hollingshead. 2000. Intranasal immunization of mice with a mixture of pneumococcal proteins PsaA and PspA is highly protective against nasopharyngeal carriage of Streptococcus pneumoniae. Infect. Immun. 68:796-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Briles, D. E., S. Hollingshead, A. Brooks-Walter, G. S. Nabors, L. Ferguson, M. Schilling, S. Gravenstein, P. Braun, J. King, and A. Swift. 2000. The potential to use PspA and other pneumococcal proteins to elicit protection against pneumococcal infection. Vaccine 18:1707-1711. [DOI] [PubMed] [Google Scholar]
- 9.Brown, K. H. 1998. Effect of infections on zinc levels in plasma concentration and implications for zinc status assessment in low-income countries. Am. J. Clin. Nutr. 68:425S-429S. [DOI] [PubMed] [Google Scholar]
- 10.Brussow, H., M. Baensch, and J. Sidoti. 1992. Seroprevalence of immunoglobulin M (IgM) and immunoglobulin G antibodies to polysaccharides of Streptococcus pneumoniae in different age groups of Ecuadorian and German children. J. Clin. Microbiol. 30:2765-2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crain, M. J., W. D. d. Waltman, J. S. Turner, J. Yother, D. F. Talkington, L. S. McDaniel, B. M. Gray, and D. E. Briles. 1990. Pneumococcal surface protein A (PspA) is serologically highly variable and is expressed by all clinically important capsular serotypes of Streptococcus pneumoniae. Infect. Immun. 58:3293-3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hollingshead, S. K., R. Becker, and D. E. Briles. 2000. Diversity of PspA: mosaic genes and evidence for past recombination in Streptococcus pneumoniae. Infect. Immun. 68:5889-5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leowski, J. 1986. Mortality from acute respiratory infections in children under 5 years of age: global estimates. World Health Stat. Q. 39:138-144. [PubMed] [Google Scholar]
- 14.Overturf, G. D., D. Powars, and L. J. Baraff. 1977. Bacterial meningitis and septicemia in sickle cell disease. Am. J. Dis. Child. 131:784-787. [DOI] [PubMed] [Google Scholar]
- 15.Prasad, A. S., E. B. Schoomaker, J. Ortega, G. J. Brewer, D. Oberleas, and F. J. Oelshlegel, Jr. 1975. Zinc deficiency in sickle cell disease. Clin. Chem. 21:582-587. [PubMed] [Google Scholar]
- 16.Rapola, S., V. Jantti, R. Haikala, R. Syrjanen, G. M. Carlone, J. S. Sampson, D. E. Briles, J. C. Paton, A. K. Takala, T. M. Kilpi, and H. Kayhty. 2000. Natural development of antibodies to pneumococcal surface protein A, pneumococcal surface adhesin A, and pneumolysin in relation to pneumococcal carriage and acute otitis media. J. Infect. Dis. 182:1146-1152. [DOI] [PubMed] [Google Scholar]
- 17.Sazawal, S., R. E. Black, S. Jalla, S. Mazumdar, A. Sinha, and M. K. Bhan. 1998. Zinc supplementation reduces the incidence of acute lower respiratory infections in infants and preschool children: a double-blind, controlled trial. Pediatrics 102:1-5. [DOI] [PubMed] [Google Scholar]
- 18.Shankar, A. H., and A. S. Prasad. 1998. Zinc and immune function: the biological basis of altered resistance to infection. Am. J. Clin. Nutr. 68:447S-463S. [DOI] [PubMed] [Google Scholar]
- 19.Strand, T. A., D. E. Briles, H. K. Gjessing, A. Maage, M. K. Bhan, and H. Sommerfelt. 2001. Pneumococcal pulmonary infection, septicaemia and survival in young zinc-depleted mice. Br. J. Nutr. 86:301-306. [DOI] [PubMed] [Google Scholar]
- 20.Walsh, C. T., H. H. Sandstead, A. S. Prasad, P. M. Newberne, and P. J. Fraker. 1994. Zinc: health effects and research priorities for the 1990s. Environ. Health Perspect. 2:5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu, H. Y., M. H. Nahm, Y. Guo, M. W. Russell, and D. E. Briles. 1997. Intranasal immunization of mice with PspA (pneumococcal surface protein A) can prevent intranasal carriage, pulmonary infection, and sepsis with Streptococcus pneumoniae. J. Infect. Dis. 175:839-846. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto, M., L. S. McDaniel, K. Kawabata, D. E. Briles, R. J. Jackson, J. R. McGhee, and H. Kiyono. 1997. Oral immunization with PspA elicits protective humoral immunity against Streptococcus pneumoniae infection. Infect. Immun. 65:640-644. [DOI] [PMC free article] [PubMed] [Google Scholar]