Abstract
We determined the immunohistochemical distributions of orexin-A and orexin-B, hypothalamic peptides that function in the regulation of feeding behavior and energy homeostasis. Orexin-A and -B neurons were restricted to the lateral and posterior hypothalamus, whereas both orexin-A and -B nerve fibers projected widely into the olfactory bulb, cerebral cortex, thalamus, hypothalamus, and brainstem. Dense populations of orexin-containing fibers were present in the paraventricular thalamic nucleus, central gray, raphe nuclei, and locus coeruleus. Moderate numbers of these fibers were found in the olfactory bulb, insular, infralimbic and prelimbic cortex, amygdala, ventral, and dorsolateral parts of the suprachiasmatic nucleus, paraventricular nucleus except the lateral magnocellular division, arcuate nucleus, supramammillary nucleus, nucleus of the solitary tract, and dorsal motor nucleus of the vagus. Small numbers of orexin fibers were present in the perirhinal, motor and sensory cortex, hippocampus, and supraoptic nucleus, and a very small number in the lateral magnocellular division of the paraventricular nucleus. Intracerebroventricular injections of orexins induced c-fos expression in the paraventricular thalamic nucleus, locus coeruleus, arcuate nucleus, central gray, raphe nuclei, nucleus of the solitary tract, dorsal motor nucleus of the vagus, suprachiasmatic nucleus, supraoptic nucleus, and paraventricular nucleus except the lateral magnocellular division. The unique neuronal distribution of orexins and their functional activation of neural circuits suggest specific complex roles of the peptides in autonomic and neuroendocrine control.
The lateral hypothalamus (LH) is a region classically implicated in the central regulation of feeding behavior and energy homeostasis (1–3). Feeding behavior is regulated by a large number of substances, including peptides, whereas, until the discovery of orexins, melanin-concentrating hormone (MCH) was the only neuropeptide known to be synthesized specifically in the LH and zona incerta and to stimulate food intake (4, 5). Very recently, two novel hypothalamic peptides named orexin-A and orexin-B (from the Greek word for appetite, orexis) were discovered in an intracellular calcium influx assay on multiple cells expressing individual “orphan” G protein-coupled receptors (6). Orexin-A is a 33-residue peptide with two intramolecular disulfide bonds in the N-terminal region, and orexin-B is a linear 28-residue peptide. These peptides, encoded by a single mRNA transcript, have a 46% amino acid sequence identity. This mRNA also was found in rat hypothalamus by another group of researchers using the directional tag PCR subtraction method (7). Bolus injections of orexin-A and -B to rat lateral ventricle stimulated food intake dose dependently (6). prepro-orexin mRNA was restricted to the LH and adjacent areas, and its mRNA level up-regulated on fasting (6). Orexins therefore are thought to participate in the hypothalamic regulation of feeding behavior. Better understanding of the physiological functions of orexins requires detailed analyses of their distributions and neuronal networks in the brain.
We prepared three antisera specific for individual rat orexins and here show complete maps of the projection of orexin neurons in the brain. We also investigated immunohistochemically the c-fos expression induced by intracerebroventricular injections of orexins to clarify which neurons are activated by these peptides.
MATERIALS AND METHODS
Tissue Preparation.
Two groups of male Wistar rats, untreated (n = 3) and colchicine-treated (n = 2), weighing 250–275 g, were used in the immunohistochemical study of orexin-A and -B. Colchicine (100 μg per rat) was injected into the lateral ventricle 30 h before perfusion to increase the immunostaining of orexin neurons (8). Fos, the protein product of the c-fos gene, was studied immunohistochemically in three groups of male Wistar rats weighing 200–225 g: orexin-A-injected (n = 3), orexin-B-injected (n = 3), and saline-injected (n = 3). Orexin-A (3 μg or 30 μg/10 μl of 0.9% saline), orexin-B (3 μg or 30 μg/10 μl of 0.9% saline), or 0.9% saline (10 μl) was injected into the lateral ventricle 90 min before perfusion. Rats were housed individually in plastic cages at constant room temperature and a 12:12-h light-dark cycle with standard rat chow and water available ad libitum. They were anesthetized by intraperitoneal injection of sodium pentobarbital (75 mg per kg body weight) and then were perfused transcardially for 10 min with 100 ml of 0.1 M phosphate buffer (pH 7.4) containing heparin (100 units/100 ml) for 15 min with 150 ml of fixative containing 4% paraformaldehyde and 0.2% picric acid in 0.1 M phosphate buffer. Brains were removed, postfixed with the same fixative for 3 days at 4°C, then kept for 24 h in 0.1 M phosphate buffer containing 20% sucrose. Proper placement of the cannulae was verified at the end of the experiments by the injection of 10 μl of dye, removal of the brain, and visual examination of coronal brain slices (9). All procedures were done in accordance with the Japanese Physiological Society’s guidelines for animal care.
Antisera.
Rat orexin-A (15 mg) was conjugated with bovine thyroglobulin (15 mg) by the carbodiimide method, and a C-terminal fragment (positions 17 to 33) of rat orexin-A (orexin-A[17 to 33]) (10 mg) and rat orexin-B (12 mg) were conjugated with bovine thyroglobulin (15 mg) by the glutaraldehyde method. Each conjugate was dialyzed against saline then emulsified with Freund’s complete adjuvant and used to immunize New Zealand white rabbits. Specificity of these three antisera was confirmed by the detection of orexin-immunoreactive molecules in rat hypothalamus extract by RP-HPLC coupled with separate RIAs for orexin-A and -B. The hypothalamus was boiled at 95–100°C for 10 min in a 10-fold volume of water to inactivate intrinsic proteases. After cooling the sample to 4°C, CH3COOH and HCl were added to the respective final concentrations of 1 M and 20 mM. The tissue then was homogenized and the homogenate centrifuged. A portion of the supernatant, equivalent to 10 mg wet weight, was loaded on a TSK ODS SIL 120 A (Tosoh, Toyko) (4.6 × 150 mm) RP-HPLC column. RP-HPLC was done for 40 min at the rate of 1.0 ml/min with a linear gradient of acetonitrile (10–60%) in 0.1% trifluoroacetic acid. All the fractions were assayed by RIAs for orexins. In brief, a sample or a standard peptide solution (100 μl) was incubated for 24 h with 100 μl of the antiserum diluent (final dilutions 1/720,000 for orexin-A antiserum A211, 1/180,000 for orexin-A[17–33] antiserum A052, and 1/1,050,000 for orexin-B antiserum B311). Rat orexin-A and Tyr0-orexin-B were radioiodinated by the lactoperoxidase method. The tracer solution (16,000 cpm/100 μl) was added and the mixture incubated for 24 h. Bound and free ligands were separated by the second antibody method. All procedures were done at 4°C. Samples were assayed in duplicate. Half-maximum inhibition by rat orexin-A on the standard RIA curve for orexin-A was 7.8 fmol/tube and that for orexin-A[17–33] was 27.4 fmol/tube. Half-maximum inhibition by rat orexin-B on the standard RIA curve for orexin-B was 4.2 fmol/tube. Neither of the antisera for orexin-A or orexin-A[17–33] recognized orexin-B, and the orexin-B antiserum did not recognize orexin-A or orexin-A[17–33].
Immunohistochemistry.
Frozen serial sections, 40-μm thick, were treated with 0.3% hydrogen peroxide for 1 h to inactivate endogenous peroxidases then incubated for 5 days at 4°C with antiorexin-A antiserum diluted 1:5,000, antiorexin-A[17–33] antiserum diluted 1:500, or antiorexin-B antiserum diluted 1:5,000 in 0.3% Triton X/PBS (PBS, pH 7.4); or for 3 days at 4°C with anti-c-Fos antiserum diluted 1:100 (Santa Cruz Biotechnology) in 0.3% Triton X/PBS. After the sections were washed for 30 min with 0.3% Triton X/PBS, they were incubated for 2 h with biotinylated secondary antibody solution diluted 1:250 then for 2 h with an avidin–biotin peroxidase complex (Vectastain ABC kit, Vector Laboratories), after which they were stained for 10 min at room temperature with 0.02% 3–3′-diaminobenzidine and 0.05% hydrogen peroxide in Tris buffer (pH 7.6). Absorption tests were done with antiorexin-A and antiorexin-A[17–33] antisera that had been absorbed with 10 μg of orexin-A and with orexin-B antiserum that had been absorbed with 10 μg of orexin-B.
RESULTS
Specificity of Antisera.
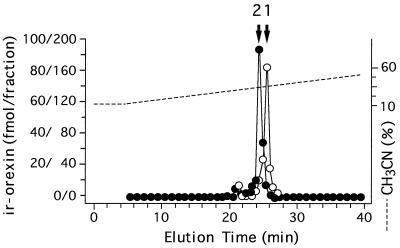
We analyzed immunoreactive orexin-A and -B molecules in the hypothalamus by RP-HPLC. More than 95% of the orexin-A immunoreactivity in the tissue was eluted at the position of authentic orexin-A (Fig. 1, open circles). A very minor immunoreactive peak was present 4 min before the elution position of orexin-A. More than 95% of the orexin-B immunoreactivity was detected at the elution position of authentic orexin-B (Fig. 1, closed circles). In addition, a very minor orexin-B immunoreactive peak was found 3.5 min before the position of orexin-B. The HPLC profile detected by the RIA with the orexin-A[17–33] antiserum showed similar findings (data not shown).
Figure 1.
Representative RP-HPLC profile of orexin-A (○) and -B (•) immunoreactivities in rat hypothalamus. Orexin-A content is represented by the left scale and orexin-B content by the right scale. Fraction volumes are 0.5 ml. Arrows indicate the elution positions of orexin-A (1) and orexin-B (2).
Immunohistochemistry of Orexin-A and -B.
Neurons and fibers immunostained with antisera for orexin-A, orexin-A[17–33], and orexin-B were extensively colocalized throughout the brain. Neuronal cell bodies immunoreactive for orexins were restricted to the lateral and posterior hypothalamus, being most abundant in the perifornical region of the LH (Figs. 2 and 3 A–C). They had various shapes, and their sizes ranged from small to medium (Fig. 3D). The immunoreactivities for orexin-A and -B were both stronger in colchicine-treated rats than in untreated ones (Fig. 3 D and E). Orexin-containing nerve fibers were abundant in the lateral and posterior hypothalamic areas (Fig. 3 A–C), the paraventricular thalamic nucleus (PVT) (Fig. 3F), the dorsal raphe nucleus and central gray (Fig. 3G), the median raphe nucleus (data not shown), and locus coeruleus (LC) (Fig. 3H). Moderate numbers of orexin fibers were present in the olfactory bulb (not shown), insular, prelimbic and infralimbic cortex (Fig. 3I), amygdala (not shown), ventral and dorsolateral parts of the suprachiasmatic nucleus (SCN) (Fig. 3J), paraventricular nucleus (PVN) except the lateral magnocellular division (Fig. 3K), arcuate nucleus (Fig. 3L), supramammillary nucleus (not shown), nucleus of the solitary tract (NTS) (Fig. 3M), and dorsal motor nucleus of the vagus (Fig. 3M). Small numbers of orexin fibers were present in the perirhinal, sensory and motor cortex (Fig. 3N), hippocampus (not shown), and supraoptic nucleus (SON) (Fig. 3O), and a very small number in the lateral magnocellular division of the PVN (Fig. 3K). There were few orexin fibers in the cerebellum (not shown). No immunoreativities for orexin-A, orexin-A[17–33], or orexin-B were detected in the tissues when normal rabbit serum or antisera absorbed with excessive orexin-A and -B were used (not shown).
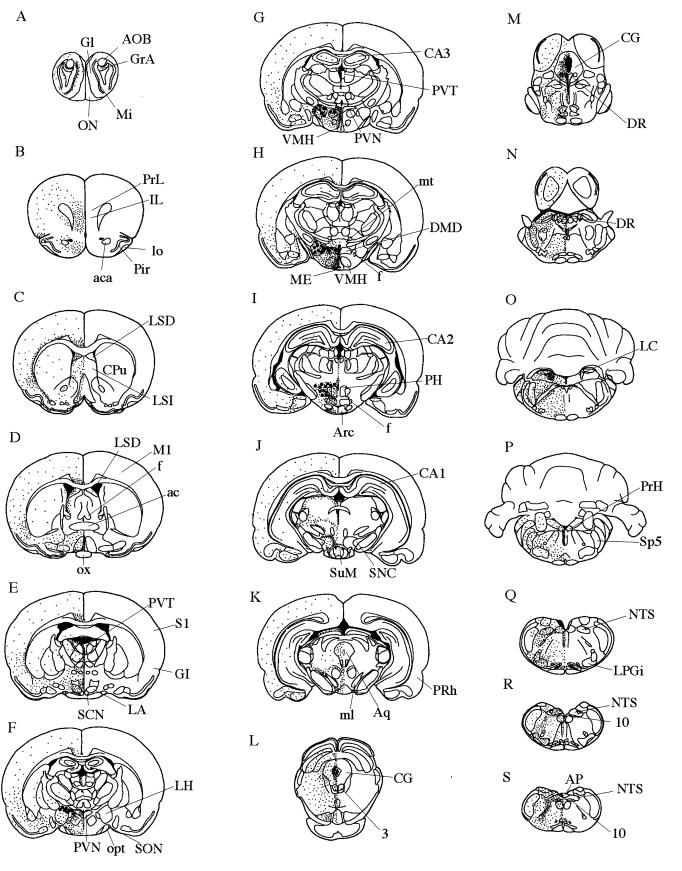
Figure 2.
Localization of orexin-A and -B from rostal (A) to caudal (S) in rat brain. Orexin neurons: large filled circles. Orexin nerve fibers: small dots. 3, oculomotor nucleus; 10, dorsal motor nucleus of vagus; ac, anterior commissure; aca, anterior commissure, anterior part; AOB, accessory olfactory bulb; AP, area postrema; Aq, aqueduct; Arc, arcuate hypothalamic nucleus; CA1–3, fields CA1–3 of hippocampus; CG, central gray; CPu, caudate putamen; DMD, dorsomedial hypothalamic nucleus, dorsal part; DR, dorsal raphe nucleus; f, fornix; GI, granular insular cortex; Gl, glomerular layer of the olfactory bulb; GrA, granule cell layer of the accessory olfactory bulb; IL, infralimbic cortex; LA, lateroanterior hypothalamic nucleus; LC, locus coeruleus; LH, lateral hypothalamus; lo, lateral olfactory tract; LPGi, lateral paragigantocellular nucleus; LSD, lateral septal nucleus, dorsal part; LSI, lateral septal nucleus, intermediate part; M1, primary motor cortex; ME, median eminence; Mi, mitral cell layer of the olfactory bulb; ml, medial lemniscus; mt, mammillothalamic tract; NTS, nucleus of the solitary tract; ON, olfactory nerve layer; opt, optic tract; ox, optic chiasm; PH, posterior hypothalamus; Pir, piriform cortex; PRh, perirhinal cortex; PrH, prepositus hypoglossal nucleus; PrL, prelimbic cortex; PVN, paraventricular hypothalamic nucleus; PVT, paraventricular thalamic nucleus; S1, primary somatosensory cortex; SCN, suprachiasmatic nucleus; SNC, substantia nigra, compact part; SON, supraoptic nucleus; Sp5, spinal trigeminal nucleus; SuM, supramammillary nucleus; VMH, ventromedial hypothalamic nucleus.
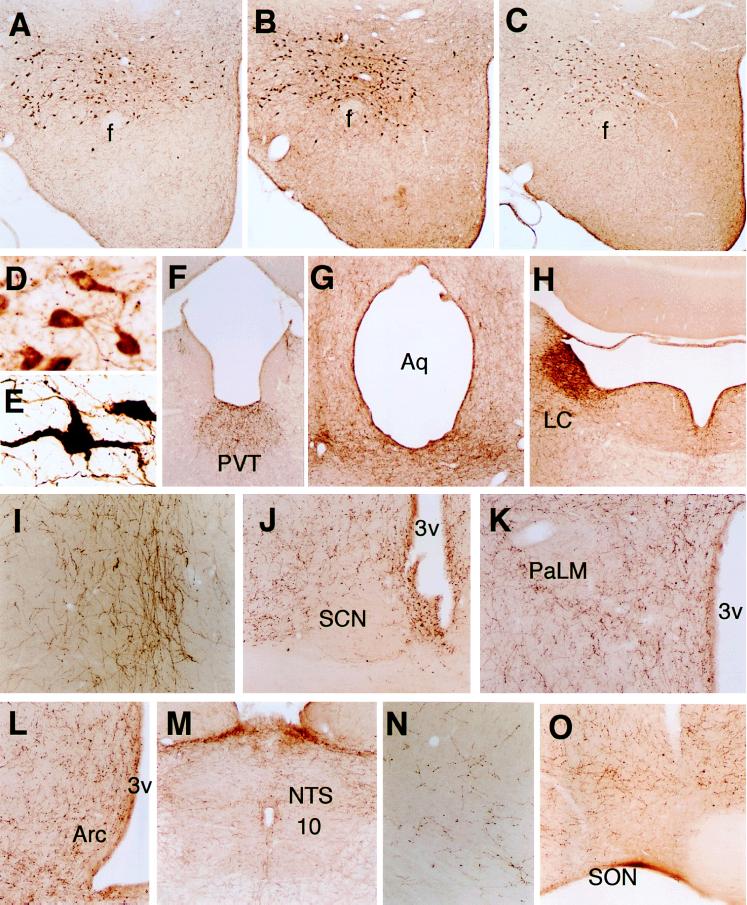
Figure 3.
Immunohistochemical localizations of orexin-A, orexin-A[17–33], and orexin-B. Neurons immunoreactive for orexin-A (A) and orexin-A[17–33] (B) are present in the perifornical region of the LH and posterior hypothalamic area. Orexin-B-immunoreactive neurons are present in the same areas (C). Orexin-A immunoreactive neurons in the LH at high magnification. Control (D), colchicine-treated rats (E). Orexin-A nerve fibers in the PVT (F), central gray and dorsal raphe nucleus (G), LC (H), infralimbic cortex (I), SCN (J), paraventricular nucleus (K), arcuate nucleus (Arc) (L), NTS, and dorsal motor nucleus of the vagus (10) (M), motor cortex (N), or SON (O). Abbreviations: f, fornix; Aq, aqueduct; 3v, third ventricle; PaLM, lateral magnocellular division of the paraventricular nucleus. Original magnification (A–C, F–H ×30, D, E ×280, I–L, N, O ×70, M ×35.)
c-fos Expression.
Fos distributions were similar in the orexin-A- and -B-injected rats as well as in the 3- and 30-μg orexin-injected rats (Fig. 4). Fos was induced in nuclei to which orexin fibers projected, and Fos immunoreactive neurons were prominent in the arcuate nucleus (Fig. 5 A), PVT (Fig. 5B), LC (Fig. 5C), central gray and dorsal raphe nucleus (Fig. 5D), NTS, and dorsal motor nucleus of the vagus (Fig. 5E), ventral and dorsolateral parts of the SCN (Fig. 5F), SON (Fig. 5G), and PVN except the lateral magnocellular division (Fig. 5H). In the control rats, no Fos immunoreactivity was present in any of the regions observed (Fig. 5 I–L, other regions not shown).
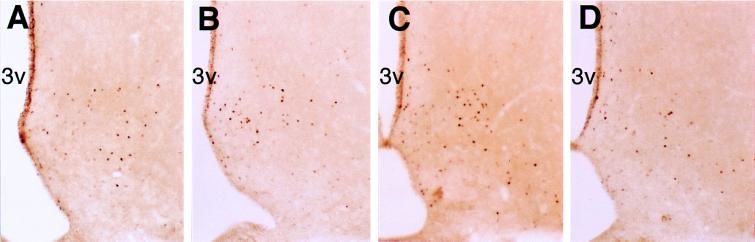
Figure 4.
Fos immunoreactivity in the arcuate nucleus of the (A) 30-μg orexin-A-, (B) 3-μg orexin-A-, (C) 30-μg orexin-B-, and (D) 3- μg orexin-B-injected rats. 3v, third ventricle. Original magnification (A–D ×70.)
Figure 5.
Fos immunoreactivity in the arcuate nucleus (A and I), paraventricular thalamic nucleus (B and J), locus coeruleus (C and K), central gray and dorsal raphe nucleus (D and L), NTS and dorsal motor nucleus of the vagus (10) (E), suprachiasmatic nucleus (F), supraoptic nucleus (G), and paraventricular nucleus (H). Sections shown are from a rat given an intracerebroventricular injection of 30 μg orexin-A (A–H) and one given saline (I–L). Abbreviations: 3v, third ventricle; D3v, dorsal third ventricle; Aq, aqueduct; PaLM, lateral magnocellular division of the paraventricular nucleus. Original magnification (A–D, F–L ×70, E ×35.)
DISCUSSION
The LH is considered an evolutionary anatomic development of the reticular formation that is involved in catecholaminergic and serotonergic feeding systems (3). It has a role in circadian feeding, sex differences in feeding, and spontaneous activity. Orexin neurons are restricted to lateral and posterior hypothalamic areas, an immunohistochemical finding in accord with the distribution of hypocretins, another name for orexins (7), and the localization of prepro-orexin mRNA as detected by in situ hybridization histochemistry (6, 7). The localization of orexin neurons differs completely from the localization of the other known neurotransmitters or neuropeptides, except for MCH, which also is a potent stimulant of food intake and whose neurons are coextensive but not colocalized with orexin neurons in the LH (J. K. Elmquist and M.Y., unpublished observations).
Numerous physiological events originating throughout the body result in afferent signals being sent to the hypothalamus. After processing the information according to the degree of deprivation or satiation and the status of the environment, the hypothalamus integrates the results and mediates interactions with efferent pathways, thereby regulating energy intake through feeding behavior and energy expenditure (1, 3, 10). Feeding behavior is a complicated activity composed of a large number of learning, memory, cognitive, emotional, somatosensorimotor, and autonomic events related to the search for food and its handling as well as mastication, deglutition, and digestion. Orexins form broad projections in the brain in patterns that generally conform to projections that have been described as arising from the lateral hypothalamic area (11–13). MCH fibers also diffusely innervate the brain, including the entire cerebral cortex and brainstem (4). The MCH neuronal system is fully defined and appears to be ideally positioned to coordinate feeding behavior and to participate in integration of complex processes associated with emotional responses, cognition, and general arousal. The distribution of orexin fibers is similar to that of MCH fibers, but there are some differences in relative densities of the projections of these peptides. Relatively to MCH, orexins are densely accumulated in the PVT, LC, NTS, dorsal motor nucleus of the vagus, and the autonomic part of the PVN, suggesting that the orexin system is more in a position to modulate the autonomic functions. To examine this possibility, we investigated the systemic effects of centrally administered orexins on the autonomic nervous system. Bolus intracerebroventricular injections of orexins dose dependently increased heart rate, blood pressure, and renal sympathetic nerve activity in conscious unrestrained rats, and these effects lasted for 20–40 min (H. Kannan and M.N., unpublished observations). The orexin system is probably involved in the central regulation of autonomic functions. Orexin fibers are frequent in the SON and PVN except the lateral magnocellular division, as compared with MCH. In addition, orexins induced Fos expression in these nuclei. The PVN contains neuroendocrine parvocellular neurons that project to the median eminence and influence the secretion of anterior lobe hormones. The magnocellular neurons in the SON and PVN synthesize vasopressin and oxytocin and transport these peptides to the neural lobe of the hypophysis. Although the neurons that orexins activate in the SON and PVN are unidentified, orexins may affect the neuroendocrine system by way of orexin-responsive neurons in these nuclei.
Central injections of orexins produced a characteristic anatomical pattern of Fos, an immediate early gene product that is correlated with the functional activation of neural circuits (14, 15), although we cannot exclude the possibility that Fos data may be secondary to peripheral consequences of orexins administered intracerebroventricularly. Orexins induced Fos in nuclei to which orexin fibers project. The LC, the source of the brain’s major noradrenergic system, is thought to have a crucial role in the control of the behavioral state, including vigilance, attention, memory, and sleep (16). It receives the afferents from many brain structures, including the perifornical hypothalamic nucleus and the area of the posterior hypothalamus (17). We have shown that orexins induce vigilance and locomotor behaviors in addition to feeding behavior when administered intracerebroventricularly (submitted elsewhere). Orexin fibers that project into the LC may participate in the control of various types of behavior through the noradrenergic system. The major serotonergic neurons are located in the raphe nuclei, central gray, and the surrounding reticular formation of the brainstem. Serotonin is implicated in the central regulation of many autonomic functions (blood pressure, sodium and glucose balance, and body fluid homeostasis), motor behaviors (locomotion, sex, and feeding), nociception, cognition, arousal, and affective behaviors (depression and anxiety) (18). Orexin projections and Fos expression in the raphe nuclei and central gray suggest that orexins may function in serotonin-mediated behavior. The arcuate nucleus in the mediobasal hypothalamus is critical to feeding and body-weight regulation because it has neurons that express leptin receptors, leptin-receptive neuropeptide Y, and the melanocortin receptor ligands α-melanin stimulating hormone and agouti-related peptide (refs. 10 and 19 and references therein). The anatomical connection between orexin neurons and the arcuate nucleus indicates that orexins may influence the activities of neurons in the arcuate nucleus to regulate feeding behavior. Orexin-induced Fos expression in the dorsal motor nucleus of the vagus implies that orexins act in the regulation of orogastrointestinal, hepatic, cardiac, and other visceral organ functions. The PVT receives orexin fibers and many afferents from some hypothalamic nuclei, including the lateral hypothalamic area, but its role is largely unknown (13). The distribution of orexin fibers and Fos expression in the SCN suggest that orexins activate many neurons that contain the master circadian pacemaker.
The extensive distribution of orexins and their induction of Fos in various regions of the brain suggest that they function as neuromodulators and/or neurotransmitters in a wide variety of neural circuitry. This neuroanatomical study lays the groundwork for a rational approach to the further investigation of their possible interactions with other neuronal systems.
Acknowledgments
We thank Ms. Yuko Hara for her technical assistance. This research was supported by a grant from the Ministry of Health and Welfare, Japan, to M.N.
ABBREVIATIONS
- LH
lateral hypothalamus
- MCH
melanin-concentrating hormone
- PVT
paraventricular thalamic nucleus
- LC
locus coeruleus
- SCN
suprachiasmatic nucleus
- PVN
paraventricular nucleus
- NTS
nucleus of the solitary tract
- SON
supraoptic nucleus
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Oomura Y. In: Handbook of the Hypothalamus. Morgane P J, Panksepp J, editors. New York: Marcel Dekker; 1980. pp. 557–620. [Google Scholar]
- 2.Bernardis L L, Bellinger L L. Neurosci Biobehav Rev. 1993;17:141–193. doi: 10.1016/s0149-7634(05)80149-6. [DOI] [PubMed] [Google Scholar]
- 3.Bernardis L L, Bellinger L L. Neurosci Biobehav Rev. 1996;20:189–287. doi: 10.1016/0149-7634(95)00015-1. [DOI] [PubMed] [Google Scholar]
- 4.Bittencourt J C, Presse F, Arias C, Peto C, Vaughan J, Nahon J-L, Vale W, Sawchenko P E. J Comp Neurol. 1992;319:218–245. doi: 10.1002/cne.903190204. [DOI] [PubMed] [Google Scholar]
- 5.Qu D, Ludwig D S, Gammeltoft S, Piper M, Pelleymounter M A, Cullen M J, Mathes W F, Przypek J, Kanarek R, Maratos-Flier E. Nature (London) 1996;380:243–247. doi: 10.1038/380243a0. [DOI] [PubMed] [Google Scholar]
- 6.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli R M, Tanaka H, Williams S C, Richardson J A, Kozlowski G P, Wilson S, et al. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 7.de Lecea L, Kilduff T S, Peyron C, Gao X-B, Foye P E, Danielson P E, Fukuhara C, Battenberg E L F, Gautvik V T, Bartlett F S, II, et al. Proc Natl Acad Sci USA. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alonso G. Brain Res. 1988;453:191–203. doi: 10.1016/0006-8993(88)90158-8. [DOI] [PubMed] [Google Scholar]
- 9.Jezová D, Bartanusz V, Westergren I, Johansson B B, Rivier J, Vale W, Rivier C. Endocrinology. 1992;130:1024–1029. doi: 10.1210/endo.130.2.1310274. [DOI] [PubMed] [Google Scholar]
- 10.Rosenbaum M, Leibel R L, Hirsch J. N Engl J Med. 1997;337:396–407. doi: 10.1056/NEJM199708073370606. [DOI] [PubMed] [Google Scholar]
- 11.Hosoya Y, Matsushita M. Neurosci Lett. 1981;24:111–116. doi: 10.1016/0304-3940(81)90232-9. [DOI] [PubMed] [Google Scholar]
- 12.Villalobos J, Ferssiwi A. Neurosci Lett. 1987;81:95–99. doi: 10.1016/0304-3940(87)90346-6. [DOI] [PubMed] [Google Scholar]
- 13.Saper C B. J Comp Neurol. 1985;237:21–46. doi: 10.1002/cne.902370103. [DOI] [PubMed] [Google Scholar]
- 14.Sagar S M, Sharp F R, Curran T. Science. 1988;240:1328–1331. doi: 10.1126/science.3131879. [DOI] [PubMed] [Google Scholar]
- 15.Chan R K W, Brown E R, Ericsson A, Kovács K J, Sawchenko P E. J Neurosci. 1993;13:5126–5138. doi: 10.1523/JNEUROSCI.13-12-05126.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aston-Jones G, Shipley M T, Grzanna R. In: The Rat Nervous System. Paxinos G, editor. San Diego: Academic; 1994. pp. 183–213. [Google Scholar]
- 17.Luppi P-H, Aston-Jones G, Akaoka H, Chouvet G, Jouvet M. Neuroscience. 1995;65:119–160. doi: 10.1016/0306-4522(94)00481-j. [DOI] [PubMed] [Google Scholar]
- 18.Halliday G, Harding A, Paxinos G. In: The Rat Nervous System. Paxinos G, editor. San Diego: Academic; 1994. pp. 929–974. [Google Scholar]
- 19.Schwartz M W, Seeley R J. N Engl J Med. 1997;336:1802–1811. doi: 10.1056/NEJM199706193362507. [DOI] [PubMed] [Google Scholar]