Abstract
Helicobacter pylori infection of the gastric mucosa is a significant cause of morbidity and mortality because of its etiologic role in symptomatic gastritis, peptic ulcer disease, and gastric adenocarcinoma. Infection occurs in young children; therefore, a prophylactic vaccine would have to be administered within the first year of life, a period thought to be immunologically privileged. We investigated vaccine formulations administered by different routes to confer protective anti-H. pylori immunity in neonatal mice. Neonatal mice immunized with a single dose of vaccine in complete Freund's adjuvant (CFA) generated antigen-specific gamma interferon-, interleukin-2 (IL-2)-, IL-4-, and IL-5-secreting T cells in numbers similar to those in immunized adult mice, while vaccine administered to neonates in incomplete Freund's adjuvant (IFA) induced such cells in reduced numbers compared to those in adult mice. Both IFA and CFA, however, provided partial protection from a challenge with infectious H. pylori when the vaccine was administered subcutaneously. Neonatal immunized mice also had reduced bacterial loads when immunized intraperitoneally with CFA. In all cases, protection was equivalent to that achieved when adult counterparts were immunized. These studies suggest that an efficacious vaccine might be successfully administered to very young children to prevent perinatal infection of H. pylori.
The neonatal period is thought to predispose infants to immune tolerance because the first wave of negative selection of autoreactive T cells occurs at this time (6). This is when the immune system also learns not to respond to ubiquitous environmental antigens. Early exposure in life to infectious agents can also lead to tolerance, resulting in the inability of adults to clear the infection (8).
Immunization of neonatal mice has also historically been associated with the induction of immune tolerance (neonatal tolerance) to injected antigen (6). However, it has been demonstrated that immunization of neonates under appropriate conditions can result in the induction of pronounced and long-lasting antigen-specific immune responses (2, 4, 5, 12, 17, 31, 33, 34). Most of these studies have primarily focused on antibody responses, CTL activity, and cytokine profiles of activated T cells. However, some laboratories have employed models of neonatal immunization for the induction of protective immunity to an infectious challenge (17, 33, 34).
Although human infants generate strong Th-1 responses following bacillus Calmette-Guérin vaccination (22, 37) and some childhood vaccines are administered as early as 2 months of age, with the exception of a recent report employing a mouse model of Bordetella pertussis infection (32), few studies have addressed the protective efficacy of neonatal immunization against bacterial pathogens. The bacterium Helicobacter pylori is a ubiquitous gastric pathogen in most of the world and in developing nations in particular. Essentially everyone infected with H. pylori develops histologic gastritis (9), but many infected individuals also develop chronic gastric diseases such as symptomatic gastritis, peptic ulcer disease, and gastric adenocarcinoma (11, 23, 26, 29, 30, 38). Although these afflictions typically manifest themselves in adults, recent studies have demonstrated that infection occurs within the first 5 years of life and often within the first year (18, 27, 35, 36, 39). The ability of H. pylori to persist in the gastric mucosa may be due to the induction of neonatal immune tolerance. In any event, a successful vaccine for H. pylori would need to be administered very early in life, prior to initial natural exposure in a manner capable of inducing protective immunity.
Most Helicobacter vaccine strategies have employed mucosal vaccination. We and others, however, have demonstrated that protective immunity to chronic infection can be induced in adult mice by systemic immunization (14-16, 40). Protection could be achieved by the induction of either a Th2 or a Th1 cytokine-mediated immune response as generated through the use of aluminum hydroxide (alum) and complete Freund's adjuvant (CFA), respectively (14).
The aims of this study were to determine if protective immunity can be achieved by systemic vaccination of neonatal mice and to characterize the immune response with regard to T-cell cytokine signatures. We demonstrate that while immunization of adult mice results in frequencies of H. pylori-specific cytokine-producing memory T cells greater than those in similarly immunized neonates, significant bacterial load reductions can be induced in neonates with both type 1 and 2 polarizing adjuvants.
MATERIALS AND METHODS
Mice.
C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, Maine) and then bred and raised at Case Western Reserve University under specific-pathogen-free conditions in microisolator units. The Case Western Reserve University animal facility is fully accredited by the American Association for Accreditation of Animal Care.
Bacteria.
H. pylori strain SS1 was used for Helicobacter antigen generation and for challenge of mice (20). Bacteria were grown on Columbia agar supplemented with 7% horse blood and antibiotics (trimethoprim, 20 μg/ml; vancomycin, 6 μg/ml; amphotericin B, 2.5 μg/ml; cefsulodin, 16 μg/ml) at 37°C in a microaerobic environment. For experimental challenge of mice, H. pylori bacteria were resuspended in Brucella broth supplemented with 10% fetal bovine serum and antibiotics and grown in 60-ml static cultures at 37°C in 5% CO2.
Antigen preparation.
H. pylori lysate was prepared as previously described (7). Briefly, confluent plates of H. pylori SS1 were harvested in sterile phosphate-buffered saline (PBS), concentrated by centrifugation at 5,000 × g for 20 min, and resuspended in a minimal volume of PBS. Cells were lysed by four 60-s bursts of power from a probe sonicator (Sonics and Materials Inc., Danbury, Conn.) set at a 50% duty cycle and a power setting of 5. Whole cells were removed by centrifugation at 5,000 × g for 20 min, and the supernatant was filtered through a 0.45-μm-pore-size filter. The protein concentration of the filtrate was determined by the Lowry assay (21). Ovalbumin grade V (OVA) was purchased from Sigma Chemical Co. (St. Louis, Mo.).
Immunization and challenge of mice.
Antigens were emulsified in either CFA or incomplete Freund's adjuvant (IFA; Sigma). Antigen solutions in PBS were mixed 1 to 1 with the respective adjuvant until a stiff emulsion was achieved. Adult mice were immunized intraperitoneally (i.p.) or subcutaneously (s.c.) by injection of 100 μl of a 1-mg/ml emulsion. Neonatal mice were immunized with 50 μl within 24 h of birth. All mice were immunized with a single dose of antigen.
Bacterial challenge.
Immunized and control mice were challenged 28 days postimmunization with 107 CFU of H. pylori SS1 in 0.5 ml of Brucella broth. The inoculum was administered by gastric intubation with flexible tubing on an 18-gauge needle and 1-ml syringe. Bacterial numbers for challenge were determined by optical density at 450 nm by using a previously established H. pylori SS1 growth curve.
Bacterial load determination by culture.
Diagnosis by culture was accomplished as previously described (14). Briefly, a narrow strip of tissue encompassing the length of the greater curvature of the stomach was homogenized in 200 μl of Columbia broth, and serial dilutions were plated on Columbia agar supplemented with 7% horse blood and antibiotics. Plates were sealed in bell jars in a microaerobic environment at 37°C for 7 days. Bacteria were confirmed as H. pylori on the basis of colony morphology, Gram staining, and production of urease, catalase, and oxidase.
Assessment of inflammation.
Strips of gastric mucosa ranging from the pyloric-duodenal junction to the gastric cardia were surgically removed and fixed in 10% buffered formalin. Tissues were paraffin embedded, sectioned (7 μm), and stained with hematoxylin and eosin by Histology Consultation Services (Oak Harbor, Wash.). Gastric tissue was evaluated blindly for the extent, depth, and character of inflammation at the antral-fundic junction. Inflammation was scored on a scale of 0 to 5, and the mean ± the standard deviation was calculated for each separate group.
ELISPOT assay.
Assays were performed with 96-well ELISPOT plates (ImmunoSpot200; Cellular Technologies Ltd., Cleveland, Ohio) that had been precoated overnight with monoclonal capture antibodies specific for gamma interferon (IFN-γ) (R46-A2; 5 μg/ml), interleukin-2 (IL-2) (JES6-1A12; 2 μg/ml), IL-4 (11B11; 4 μg/ml), or IL-5 (TRFK5; 2 μg/ml) in PBS. Prior to addition of spleen cells, the plates were blocked with 1% bovine serum albumin in PBS for 1 h at room temperature and washed four times with PBS. Spleen cell suspensions were prepared and distributed at 106 cells per well and maintained for 24 to 48 h in serum-free HL-1 medium (BioWhittaker, Walkersville, Md.) supplemented with l-glutamine at 1 mM with or without Helicobacter antigens added at a final concentration of 5 μg/ml. The cells were then removed by washing, and the detection antibody (XMG1.2-biotin at 2 μg/ml for IFN-γ, JES6-5H4-biotin at 3 μg/ml for IL-2, BVD6-24G2-biotin at 4 μg/ml for IL-4, or TRFK4-biotin at 4 μg/ml for IL-5) was added and the mixture was incubated overnight. On the following day, streptavidin-horseradish peroxidase (Dako A/S, Glostrup, Denmark) was added at a 1:2,000 dilution and the plates were left for 2 h at room temperature. The plate-bound antibody was then visualized by adding the horseradish peroxidase substrate 3-amino-9-ethylcarbozole (Pierce, Rockford, Ill.). To evaluate the results, we used a series 1 ImmunoSpot image analyzer (Cellular Technologies Ltd.).
Statistical analysis.
Differences between experimental groups in the number of cytokine-secreting T cells, the bacterial load of gastric tissue samples to determine vaccine efficacy, and the degree of histologic gastritis were evaluated for significance by analysis of variance.
RESULTS
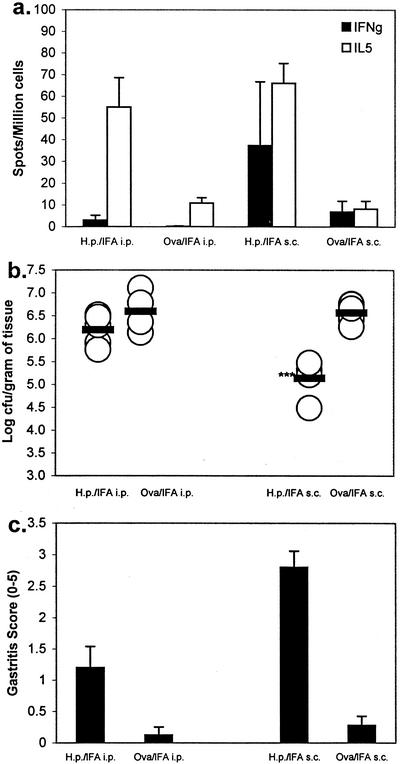
We have previously demonstrated that adult mice can be protectively immunized against H. pylori infection of the gastric mucosa by systemic immunization (14). To determine if systemic immunization can also induce protective H. pylori immunity in neonatal mice, we performed a pilot study with newborn mice, H. pylori antigen, and IFA. Twenty-eight days after antigen injection, the spleen cells of three mice from each group were examined by ELISPOT assay to determine the frequency of IL-5- and IFN-γ-producing, H. pylori-specific T cells. Antigenic stimulation of spleen cells from these mice induced equivalent numbers of IL-5-producing T cells when administered by either s.c. or i.p. injection (Fig. 1a). Spleen cells from mice immunized with OVA and IFA did not produce IL-5 when challenged with H. pylori antigen in vitro. Unlike i.p. immunization, which induced a unipolar type 2 response consisting of IL-5-producing cells, injection by the s.c. route induced a mixed type 1-type 2 immune response consisting of antigen-specific IL-5- and IFN-γ-producing T cells.
FIG. 1.
Induction of anti-H. pylori (H.p.) immunity by systemic immunization of neonatal mice. (a) Spleen cells collected 28 days after a single i.p. or s.c. immunization with H. pylori lysate or OVA emulsified in IFA were stimulated in vitro with H. pylori lysate and tested by ELISPOT assay for IFN-γ or IL-5. Each value shown is the mean of three individual mice (tested in triplicate) ± the standard deviation. (b) Groups of mice given each vaccine formulation were challenged with infectious H. pylori on day 28 and sacrificed on day 48 for bacterial load assessment. Each symbol represents an individual mouse, and each bar represents the geometric mean of each respective group. A statistical significance of greater than 99.9% relative to OVA-immunized mice is indicated by the triple asterisks. (c) Gastric biopsy samples of the greater curvature were hematoxylin and eosin stained and graded for inflammation. Each value shown is the mean of each immunized-and-challenged group of mice ± the standard deviation.
The remaining mice in each group (four to nine mice, depending on the size of the respective litters) were challenged 28 days postimmunization with 107 CFU of H. pylori. Mice were sacrificed 28 days postchallenge, and gastric biopsy samples were removed for bacterial culture and histologic examination to determine the protective efficacy of the H. pylori vaccination. Subcutaneous immunization with H. pylori antigen resulted in a significant reduction in the bacterial load (average of log 5.14 CFU/g) compared to that of control mice immunized with OVA (average of log 6.57 CFU/g; Fig. 1b; P < 0.0001). No significant reduction in bacteria was observed in mice immunized by i.p. injection, the route typically used to induce neonatal tolerance. All H. pylori antigen-immunized mice demonstrated histologic postimmune gastritis, as has been frequently observed in animal models used for Helicobacter vaccine trials (Fig. 1c) (7, 10, 13, 24). As expected, this postimmune gastritis was not observed in OVA-IFA-immunized mice that had no exposure to H. pylori antigens prior to challenge.
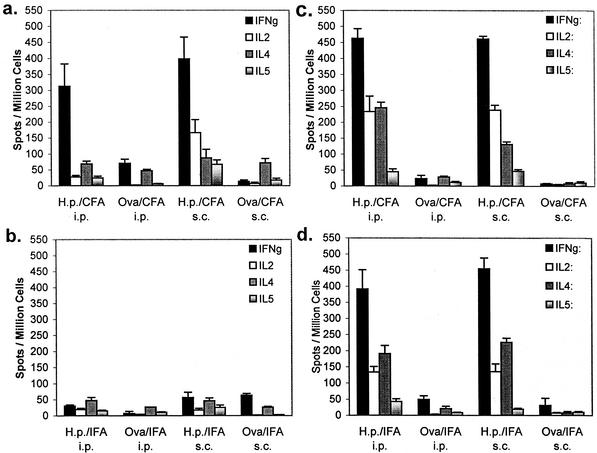
We next expanded the study to include the use of type 1 polarizing CFA and to compare the efficacy of systemic immunization of neonatal mice to that of adult mice. Neonatal and adult mice were immunized either s.c. or i.p. with H. pylori antigen in combination with either CFA or IFA, and control mice were immunized with OVA and the respective adjuvants. Four to six mice from each group were examined 28 days postimmunization to evaluate the T-cell cytokine signature induced by each immunization protocol. Similar to our pilot study described above, immunization of neonates with H. pylori antigen in IFA by either route resulted in a low frequency of cytokine-producing cells in our recall ELISPOT assay (Fig. 2b). Although both IL-4- and IL-5-producing T cells were observed, OVA-immunized mice had similar numbers of T cells capable of responding to in vitro exposure to H. pylori antigen. However, immunization by both the s.c. and i.p. routes with CFA resulted in a pronounced induction of antigen-specific IFN-γ-producing T cells (Fig. 2a; 398 and 313 spot-producing cells/106 spleen cells, respectively). The TH1-inducing nature of this adjuvant was evident in mice immunized s.c. as IL-2 was also specifically elevated (167 spot-producing cells/106 spleen cells). Comparison of the spleen cells of adult mice with those of neonatally immunized mice revealed that both IFA and CFA immunizations by either i.p. or s.c. injection induced more potent cytokine responses in adults (Fig. 2c and d). Both IFA and CFA induced strong TH1 (IFN-γ and IL-2) and TH2 (IL-4) responses relative to those of neonatally immunized mice.
FIG. 2.
Prechallenge cytokine profiles of spleen cells from mice immunized as neonates (a and b) or adults (c and d) with a single dose of H. pylori (H.p.) antigen and CFA (a and c) or IFA (b and d). Spleen cells were tested by ELISPOT assay for IFN-γ, IL-2, IL-4, and IL-5. Each value shown is the mean of each group (four to six mice each, tested in triplicate) ± the standard deviation.
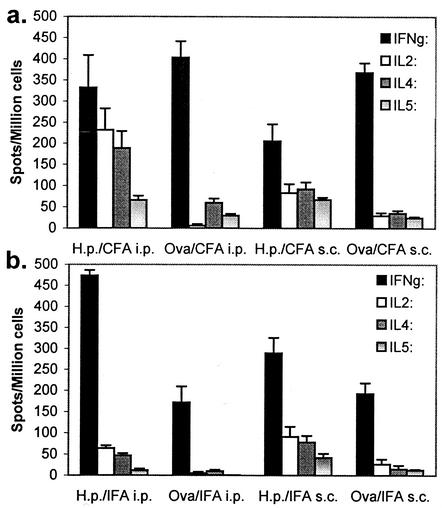
The remaining mice were challenged with 107 CFU of H. pylori and sacrificed 28 days postchallenge. Challenge of both OVA- and H. pylori antigen-immunized mice resulted in a significant number of H. pylori-specific IFN-γ-producing T cells (Fig. 3a and b). Overall, the frequency of antigen-specific IFN-γ-producing T cells from H. pylori antigen-immunized mice did not exceed the number of spot-forming cells from OVA-immunized mice and in some cases was actually lower (Fig. 3a). Previous exposure to H. pylori antigen due to immunization, however, uniformly resulted in elevated levels of IL-2, IL-4, and IL-5 compared to those of the corresponding OVA-immunized controls. CFA administered i.p. was the most potent inducer of these other cytokines.
FIG. 3.
Cytokine profiles of spleen cells from mice immunized as neonates with a single dose of H. pylori (H.p.) antigen and CFA (a) or IFA (b) and challenged with infectious H. pylori 28 days after immunization. Spleen cells were tested on day 56 by ELISPOT assay for IFN-γ, IL-2, IL-4, and IL-5. Each value shown is the mean of each group (five to seven mice each, tested in triplicate) ± the standard deviation.
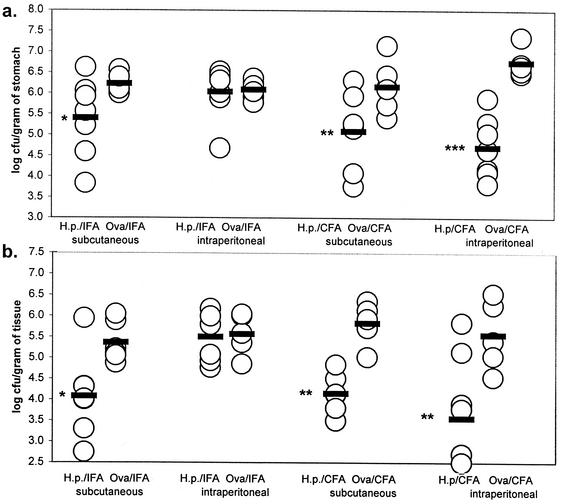
H. pylori-challenged mice in this experiment demonstrated significant reductions in gastric colonization; this was true of both neonates and adults immunized by the s.c. route with either IFA (P = 0.032 and 0.017, respectively) or CFA (P = 0.006 and 0.008, respectively) and also by the i.p. route when immunized with CFA (P = 0.001 and P < 0.0001, respectively; Fig. 4). In agreement with the pilot study (Fig. 1b), neither neonates nor adults showed any evidence of protection from H. pylori colonization when immunized i.p. with IFA. These results were confirmed in a third experiment that showed a significant bacterial load reduction in H. pylori-challenged adult mice immunized i.p. with CFA but no reduction in either adult or neonatal animals immunized i.p. with IFA (data not shown).
FIG. 4.
Bacterial loads in the gastric mucosae of mice immunized as neonates (a) or adults (b) with a single dose of H. pylori (H.p.) antigen and CFA or IFA and challenged on day 28. Gastric biopsy samples were examined on day 56 by quantitative culture. Each symbol represents an individual mouse, and each bar represents the geometric mean of each respective group. Statistical significance of differences relative to the respective OVA-immunized control groups is indicated by single (>95% significant), double (>99% significance), or triple (>99.9% significance) asterisks.
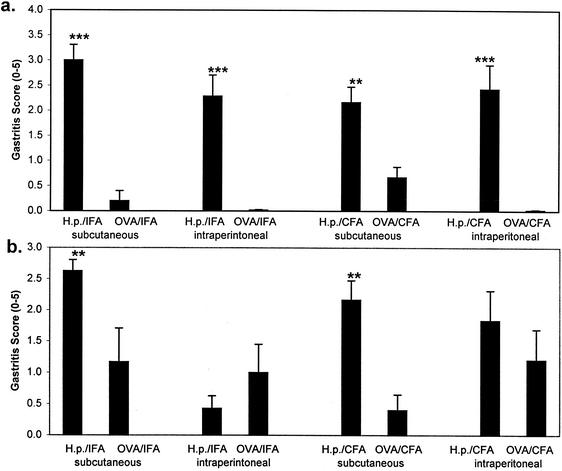
Challenge of mice immunized as neonates against Helicobacter antigens resulted in histologic gastritis in all four groups (Fig. 5a). Postimmunization gastritis was primarily restricted to the antral fundic junction of the gastric mucosa, which is the predominant site of colonization by H. pylori in this mouse model. The degree of inflammation did not differ appreciably between neonatal groups but in all cases was significantly greater (range of 2.2 to 3.0) than that of mice immunized with OVA prior to the challenge (range of 0.1 to 0.67; P < 0.005). Similarly, a challenge of mice immunized as adults with H. pylori antigen and adjuvant resulted in histologic gastritis that was significantly greater than that of the corresponding OVA-immunized control groups immunized by the s.c. route (Fig. 5b). H. pylori antigen immunization performed i.p. with IFA failed to generate an inflammatory infiltrate. Postimmune gastritis for the three immunization strategies that achieved immunity (s.c. CFA, i.p. CFA, and s.c. IFA) was not statistically different between neonatal and adult immunize mice.
FIG. 5.
Mucosal inflammation in the stomachs of mice immunized as neonates (a) or adults (b) with a single dose of H. pylori (H.p.) antigen and challenged with infectious H. pylori on day 28. Strips of the greater curvature of the stomach were surgically removed on day 56, hematoxylin and eosin stained, and graded for inflammation. Each value shown is the mean of each immunized-and-challenged group of mice ± the standard deviation. Statistical significance of differences relative to the respective OVA-immunized control groups is indicated by double (>99% significance) or triple (>99.9% significance) asterisks.
DISCUSSION
Classic protocols for the induction of neonatal tolerance have relied on i.p. injection of antigen with IFA. Because of their delicate size, s.c. injection of neonatal mice has not been pursued to the same extent. In our study, i.p. immunization of neonatal mice with IFA did not induce protective immunity. This observation is consistent with classical models. However, the present study demonstrates that a single s.c. injection of neonates (the route relevant for human systemic immunization) with bacterial antigen in CFA or IFA induced a significant degree of protective immunity in mice subsequently challenged with H. pylori as adults. Since adult mice immunized i.p. with IFA also failed to develop protective immunity, lack of immunity does not represent immune privilege of the neonates. These results suggest that neonates are not inherently unresponsive to H. pylori immunization. In fact, no difference in the degree of protection was observed between mice immunized as neonates and mice immunized as adults. This protective immunity could be induced in neonates after only a single immunization.
While only a fraction of H. pylori-infected individuals develop symptomatic clinical disorders such as gastritis (23, 26), peptic ulcer disease, and gastric adenocarcinoma (11, 29, 30), the prevalence of H. pylori infection worldwide makes these diseases a significant burden. The most recent available data indicate that in the year 2000 in the United States, where prevalence has dropped below 50%, H. pylori infections cost approximately $10 billion, including the direct costs for health care and drugs and the indirect costs due to loss of work (3). The cost to developing nations and parts of the world where more than 80 to 90% of the general population is infected constitutes a significant drain on health care resources, particularly in those nations where gastric cancer remains a leading cause of death due to cancer. Many studies suggest that H. pylori infection is actually acquired in early childhood (18, 27, 35, 36, 39). Thus, a prophylactic vaccine against H. pylori would have to be administered to very young children to be successful. Our data suggest that such a vaccine might be feasible.
Previous neonatal immunity challenge models involved the use of a viral challenge. Recovery from infection in viral models requires Th1-dependent CTL responses. Since neonatal mice favor the development of Th2 immunity, success in those previous studies relied on the ability of specific adjuvants to generate a mixed Th1-Th2 immune response (4) or the use of Th1 immunity-inducing immunizations (17, 33). We attempted to polarize the T helper cell response through the use CFA and IFA for the induction of Th1 and Th2 immune responses, respectively, because we have accomplished this previously with lysates and purified-protein antigens (12, 14). In general, we achieved mixed Th1-Th2 responses with a strong IFN-γ component. Our inability to induce a dominant IL-4 or IL-5 response with IFA may be due to our use of a whole bacterial lysate as the antigen. Yip et al. demonstrated that addition of bacterial components such as lipopolysaccharide or CpG motifs converts IFA to a Th1-inducing adjuvant, and similar components may be present in our lysate preparation (41). The inability to polarize the neonatal response, however, was not detrimental to our efforts to induce protective immunity.
While the use of CFA induced the most significant protection, its use cannot be extended to humans. It is likely that aluminum hydroxide (alum) or new proprietary adjuvants would be looked at more favorably than IFA as an adjuvant for new human vaccines (19). Protection, even with IFA, always included IFN-γ induction. Therefore, selection of an appropriate adjuvant with IFN-γ-inducing properties might enhance the desired adjuvant function. The distinction could be critical because in our study, replacement of CFA with IFA resulted in a loss of efficacy by the i.p. route, although IFA did work well for s.c. immunizations.
Alum is an obvious candidate for testing, and we have reported the use of alum in achieving protection against H. pylori in adult mice (14). However, in the present study, repeated attempts to use alum with whole-cell lysates administered s.c. resulted in the death of all injected neonatal mice (data not shown). No adverse symptoms were displayed by the adult mice. When neonatal mice were immunized with purified H. pylori urease and alum s.c., however, no deaths resulted. Our limited supply of purified urease prevented us from pursuing the use of alum in the present studies. These experiments indicate that whole-cell lysates of H. pylori in combination with alum contain a component that induces a state of toxic shock in neonates when delivered s.c. but not in adult mice. One explanation for the lack of IFA toxicity is that IFA, being an oil, may release lipopolysaccharide and other lipophilic toxins more slowly than alum. As stated above, our previous experiments have demonstrated that certain bacterial components are sufficient to drive a Th1 response (41). The same components that prevented polarization to Th2 immunity may also provide a level of toxicity to neonatal mice when combined with alum.
Regardless of the clonal sizes induced by systemic immunization, both adjuvants were capable of inducing an immune response in neonates that significantly reduced the bacterial load upon challenge. Our observations may seem surprising because immunization with IFA resulted in cytokine responses that were significantly weaker in neonatal immunized mice than in the corresponding adult groups, but the ability to induce protective immunity via neonatal immunization and high frequencies of cytokine-producing Th1 cells in recall assays provides further evidence that neonatal T-cells are fully capable of adult level cytokine production upon secondary exposure to antigen (1, 2, 17).
Notably, i.p. injection with IFA, which failed to induce protective immunity in both neonatal and adult mice, did induce IL-5-producing Th2 cells. Therefore, this classic tolerization protocol was immunogenic even if the type of effector class engaged was not protective. These results are consistent with the report by Guy et al., who demonstrated that varying the location of a systemically (s.c.) administered H. pylori therapeutic vaccine could have a profound effect on its efficacy (16). Nevertheless, as our i.p. immunizations with H. pylori and CFA demonstrated in both neonatal and adult mice, we were able to overcome the lack of immunity associated with the route of administration by using a different adjuvant.
Finally, these studies are consistent with the widespread observation that a challenge of Helicobacter-immunized mice results in significant inflammation even when protective immunity is achieved (7, 13, 14, 24, 25, 28). Since the gastric mucosa of healthy mice (and humans) lacks organized or diffuse immune tissue, recruitment of inflammatory and immune cells to the gastric mucosa is likely necessary for eradication of H. pylori to occur. Thus, similar to chronic H. pylori infection, challenge of immunized mice with H. pylori results in colonization that leads to a localized inflammatory response. However, if the immune system has been appropriately stimulated, this white blood cell infiltration takes on a protective character, either because of its increased magnitude or because of an as yet unidentified effector mechanism. Appropriate stimulation of the immune system is critical, as shown when neonatal mice were immunized with IFA i.p. Significant postimmune gastritis was stimulated following a challenge with H. pylori, but bacterial load reductions were only marginal.
Although the degree of postimmunization gastritis in neonatal mice was comparable to that of adult mice, the inflammatory response of control OVA-immunized neonatal mice to H. pylori infection was considerably weaker than that of infected adult groups and in some cases was absent. Evaluation of gastritis and protection was performed when mice were 8 weeks old, 28 days following a challenge. Thus, although fully mature at sacrifice, the degree of H. pylori-induced gastritis was significantly lower than that of mice in the adult immunization group challenged at 12 weeks of age. These observations suggest distinct differences in the ability of the young immune system to respond to H. pylori infection. It is possible that, as they aged, these mice developed H. pylori-associated gastritis, similar to mice infected at 12 weeks of age. These differences, however, did not hinder the ability to stimulate a protective immune response through appropriate immunization within hours of birth.
These observations may represent an important advance in the ongoing development of a human vaccine against H. pylori. While prototype H. pylori vaccines can be efficacious when administered orally to mice, similar success has not been achieved in human clinical trials. Lack of a potent, safe mucosal adjuvant for humans has limited the development of an H. pylori vaccine. The ability to employ the types of systemic immunizations successfully used for numerous other human vaccines would circumvent the need for a mucosal adjuvant. Immunization of very young children prior to environmental exposure to H. pylori may be a feasible strategy with which to prevent H. pylori acquisition, and adjuvants such as IFA may prove to be appropriate for the induction of protective immunity in children. However, since the induction of immunity in mice was invariably associated with IFN-γ-producing cells and gastric inflammation upon challenge, it is likely that addition of a safe, type 1 polarizing substance will be necessary to obtain acceptable efficacy. A well-designed vaccine would enhance the adjuvant-guided differentiation of the immune response so as to engage the desired class of immunity.
Acknowledgments
This research was supported by National Institutes of Health research grants DK57756 (T.G.B. and S.J.C.), DK-46461 (S.J.C.), and AI-36359 (T.G.B., S.J.C., and J.G.N.). J.C.E., W.C.B., and J.M.G. are fellows of the Studienstifung des Deutschen Volkes.
Editor: J. D. Clements
REFERENCES
- 1.Adkins, B., Y. Bu, E. Cepero, and R. Perez. 2000. Exclusive Th2 primary effector function in spleens but mixed Th1/Th2 function in lymph nodes of murine neonates. J. Immunol. 164:2347-2353. [DOI] [PubMed]
- 2.Adkins, B., and R.-Q. Du. 1998. Newborn mice develop balanced Th1/Th2 primary effector responses in vivo but are biased to Th2 secondary responses. J. Immunol. 160:4217-4224. [PubMed] [Google Scholar]
- 3.American Gastroenterological Association. 2001. Acid-related gastrointestinal disorders, p. 18-26. In The burden of gastrointestinal diseases. American Gastroenterological Association, Bethesda, Md.
- 4.Barrios, C., C. Brandt, M. Berney, P.-H. Lambert, and C.-A. Siegrist. 1996. Partial correction of the TH2/TH1 imbalance in neonatal murine responses to vaccine antigens through selective adjuvant effects. Eur. J. Immunol. 26:2666-2670. [DOI] [PubMed] [Google Scholar]
- 5.Barrios, C., P. Brawand, M. Berney, C. Brandt, P.-H. Lambert, and C.-A. Siegrist. 1996. Neonatal and early life immune responses to various forms of vaccine antigens qualitatively differ from adult responses: predominance of a Th2-biased pattern which persists after adult boosting. Eur. J. Immunol. 26:1489-1496. [DOI] [PubMed] [Google Scholar]
- 6.Billingham, R. E., L. Brent, and P. B. Medawar. 1953. Actively acquired tolerance of foreign cells. Nature 172:603.. [DOI] [PubMed] [Google Scholar]
- 7.Blanchard, T. G., S. J. Czinn, R. W. Redline, N. Sigmund, G. Harriman, and J. G. Nedrud. 1999. Antibody-independent protective mucosal immunity to gastric Helicobacter infection in mice. Cell. Immunol. 191:74-80. [DOI] [PubMed] [Google Scholar]
- 8.Cihak, J., and F. Lehmann-Grube. 1978. Immunological tolerance to lymphocytic choriomeningitis virus in neonatally infected virus carrier mice: evidence supporting a clonal inactivation mechanism. Immunology 34:265-275. [PMC free article] [PubMed] [Google Scholar]
- 9.Dooley, C. P., H. Cohen, P. L. Fitzgibbons, M. Bauer, M. D. Appleman, G. I. Perez-Perez, and M. J. Blaser. 1989. Prevalence of Helicobacter pylori infection and histologic gastritis in asymptomatic persons. N. Engl. J. Med. 321:1562-1566. [DOI] [PubMed] [Google Scholar]
- 10.Ermak, T. H., R. Ding, B. Ekstein, J. Hill, G. A. Myers, C. K. Lee, J. Pappo, H. K. Kleanthous, and T. P. Monath. 1997. Gastritis in urease-immunized mice after Helicobacter felis challenge may be due to residual bacteria. Gastroenterology 113:1118-1128. [DOI] [PubMed] [Google Scholar]
- 11.Eurogast Study Group. 1993. An international association between Helicobacter pylori infection and gastric cancer. Lancet 341:1359-1362. [PubMed] [Google Scholar]
- 12.Forsthuber, T., H. C. Yip, and P. V. Lehmann. 1996. Induction of TH1 and TH2 immunity in neonatal mice. Science 271:1728-1730. [DOI] [PubMed] [Google Scholar]
- 13.Goto, T., A. Nishizono, T. Fujioka, J. Ikewaki, K. Mifune, and M. Nasu. 1999. Local secretory immunoglobulin A and postimmunization gastritis correlate with protection against Helicobacter pylori infection after oral vaccination of mice. Infect. Immun. 67:2531-2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gottwein, J. M., T. G. Blanchard, O. S. Targoni, J. C. Eisenberg, B. M. Zagorski, R. W. Redline, J. G. Nedrud, M. Tary-Lehmann, P. V. Lehmann, and S. J. Czinn. 2001. Protective anti-Helicobacter immunity is induced with aluminum hydroxide or complete Freund's adjuvant by systemic immunization. J. Infect. Dis. 184:308-314. [DOI] [PubMed] [Google Scholar]
- 15.Guy, B., C. Hessler, S. Fourage, J. Haensler, E. Vialon-Lafay, B. Rokbi, and M.-J. Millet. 1998. Systemic immunization with urease protects mice against Helicobacter pylori infection. Vaccine 16:850-856. [DOI] [PubMed] [Google Scholar]
- 16.Guy, B., C. Hessler, S. Fourage, B. Rokbi, and M.-J. Millet. 1999. Comparison between targeted and untargeted systemic immunizations with adjuvanted urease to cure Helicobacter pylori infection in mice. Vaccine 17:1130-1135. [DOI] [PubMed] [Google Scholar]
- 17.Hassett, D. E., J. Zhang, M. Slifka, and J. L. Whitton. 2000. Immune responses following neonatal DNA vaccination are long-lived, abundant, and qualitatively similar to those induced by conventional immunization. J. Virol. 74:2620-2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holcombe, C., B. A. Omotara, J. Eldridge, and D. M. Jones. 1992. H. pylori, the most common bacterial infection in Africa: a random serological study. Am. J. Gastroenterol. 87:28-30. [PubMed] [Google Scholar]
- 19.Kovarik, J., and C.-A. Siegrist. 1998. Optimization of vaccine responses in early life: the role of delivery systems and immunomodulators. Immunol. Cell Biol. 76:222-236. [DOI] [PubMed] [Google Scholar]
- 20.Lee, A., J. O'Rourke, M. C. De Ungria, B. Robertson, G. Daskalopoulos, and M. F. Dixon. 1997. A standardised mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology 112:1386-1397. [DOI] [PubMed] [Google Scholar]
- 21.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 22.Marchant, A., T. Goetghebuer, M. O. Ota, I. Wolfe, S. J. Ceesay, D. De Groote, T. Corrah, S. Bennet, J. Wheeler, K. Huygen, P. Aaby, K. P. W. J. McAdam, and M. J. Newport. 1999. Newborns develop a Th1-type immune response to Mycobacterium bovis bacillus Calmette-Guérin vaccination. J. Immunol. 163:2249-2255. [PubMed] [Google Scholar]
- 23.Marshall, B. J., J. A. Armstrong, and D. B. McGechie. 1985. Attempt to fulfill Koch's postulate for pyloric Campylobacter. Med. J. Aust. 142:436-439. [DOI] [PubMed] [Google Scholar]
- 24.Michetti, P., I. Corthesy-Theulaz, C. Davin, R. Haas, A.-C. Vaney, M. Heitz, J. Bille, J. P. Kraehenbuhl, E. Saraga, and A. L. Blum. 1994. Immunization of BALB/c mice against Helicobacter felis infection with Helicobacter pylori urease. Gastroenterology 107:1002-1011. [DOI] [PubMed] [Google Scholar]
- 25.Mohammadi, M., S. Czinn, R. Redline, and J. Nedrud. 1996. Helicobacter-specific cell-mediated immune responses display a predominant TH1 phenotype and promote a DTH response in the stomachs of mice. J. Immunol. 156:4729-4738. [PubMed] [Google Scholar]
- 26.NIH Consensus Conference. 1994. Helicobacter pylori in peptic ulcer disease. JAMA 272:65-69. [PubMed] [Google Scholar]
- 27.Oliveira, A. M. R., D. M. Queiroz, G. A. Rocha, and E. N. Mendes. 1994. Seroprevalence of Helicobacter pylori infection in children of low socioeconomic level in Belo Horizonte, Brazil. Am. J. Gastroenterol. 89:2201-2204. [PubMed] [Google Scholar]
- 28.Pappo, J., W. D. Thomas, Z. Kabok, N. S. Taylor, J. C. Murphy, and J. G. Fox. 1995. Effect of oral immunization with recombinant urease on murine Helicobacter felis gastritis. Infect. Immun. 63:1246-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parsonnet, J., G. D. Friedman, D. P. Vandersteen, Y. Chang, J. H. Vogelman, N. Orentreich, and R. K. Sibley. 1991. Helicobacter pylori infection and the risk of gastric carcinoma. N. Engl. J. Med. 325:1127-1131. [DOI] [PubMed] [Google Scholar]
- 30.Parsonnet, J., D. Vandersteen, J. Goates, R. K. Silbey, J. Pritkin, and Y. Chang. 1991. Helicobacter pylori infection in intestinal- and diffuse-type gastric adenocarcinomas. J. Natl. Cancer Inst. 83:640-643. [DOI] [PubMed] [Google Scholar]
- 31.Ridge, J. P., E. J. Fuchs, and P. Matzinger. 1996. Neonatal tolerance revisited: turning on newborn T cells with dendritic cells. Science 271:1723-1730. [DOI] [PubMed] [Google Scholar]
- 32.Roduit, C., P. Bozzotti, N. Mielcarek, P. H. Lambert, G. del Giudice, C. Locht, and C.-A. Siegrist. 2002. Immunogenicity and protective efficacy of neonatal vaccination against Bordetella pertussis in a murine model: evidence for early control of pertussis. Infect. Immun. 70:3521-3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sarzotti, M., D. S. Robbins, and P. M. Hoffman. 1996. Induction of protective CTL responses in newborn mice by a murine retrovirus. Science 271:1726-1728. [DOI] [PubMed] [Google Scholar]
- 34.Siegrist, C.-A., H. Plotnicky-Gilquin, M. Cordova, M. Berney, J. Y. Bonnefoy, T. N. Nguyen, P. H. Lambert, and U. F. Power. 1999. Protective efficacy against respiratory syncytial virus following murine neonatal immunization with BBG2Na vaccine: influence of adjuvants and maternal antibodies. J. Infect. Dis. 179:1326-1333. [DOI] [PubMed] [Google Scholar]
- 35.Thomas, J. E., A. Dale, M. Harding, W. A. Coward, T. J. Cole, and L. T. Weaver. 1999. Helicobacter pylori colonization in early life. Pediatr. Res. 45:218-223. [DOI] [PubMed] [Google Scholar]
- 36.Thomas, J. E., A. M. Whatmore, M. R. Barer, E. J. Eastham, and M. A. Kehoe. 1990. Serodiagnosis of Helicobacter infection in childhood. J. Clin. Microbiol. 28:2141-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vekemans, J., A. Amedei, M. O. Ota, M. M. D'Elios, T. Goetghebuer, J. Ismaili, M. J. Newport, G. Del Prete, M. Goldman, K. P. McAdam, and A. Marchant. 2001. Neonatal bacillus Calmette-Guérin vaccination induces adult-like IFN-γ production by CD4+ T lymphocytes. Eur. J. Immunol. 31:1531-1535. [DOI] [PubMed] [Google Scholar]
- 38.Warren, J. R., and B. J. Marshall. 1983. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet i:1273-1275. [PubMed] [Google Scholar]
- 39.Weaver, T. 1995. Aspects of Helicobacter pylori infection in the developing and developed world. Helicobacter pylori infection, nutrition and growth of West African infants. Trans. R. Soc. Trop. Med. Hyg. 89:347-350. [DOI] [PubMed] [Google Scholar]
- 40.Weltzin, R., B. Guy, W. D. Thomas, Jr., P. J. Giannasca, and T. P. Monath. 2000. Parental adjuvant activities of Escherichia coli heat-labile toxin and its B subunit for immunization of mice against gastric Helicobacter pylori infection. Infect. Immun. 68:2775-2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yip, H. C., A. Y. Karulin, M. Tary-Lehmann, M. D. Hesse, H. Radeke, P. S. Heeger, R. P. Trezza, F. P. Heinzel, T. Forsthuber, and P. V. Lehmann. 1999. Adjuvant-guided type 1 and type 2 immunity: infectious/noninfectious dichotomy defines the class of response. J. Immunol. 162:3942-3949. [PubMed] [Google Scholar]