Abstract
Syrian hamsters become anemic and exhibit delayed growth following oral infection with third-stage Ancylostoma ceylanicum hookworm larvae. Here we describe experiments designed to determine the feasibility of adult worm transfer (AWT) between hosts, a technique that would facilitate the specific study of bloodfeeding hookworms in vivo without prior exposure of the host to larva-specific antigens, permit the ex vivo manipulation of adult parasites prior to reimplantation, and also allow for cross-species transfer of worms. Weanling hamsters given an oral AWT of 40 or 60 mixed-sex A. ceylanicum worms rapidly developed anemia; in the higher-dose group, hemoglobin levels declined from prechallenge levels by 44% within 4 days following AWT. Long-term survival of transferred worms was demonstrated by recovery of parasites from the intestines 42 days after AWT. AWT hamsters acquired humoral immune responses against soluble adult hookworm extracts and excretory-secretory products that were comparable in magnitude to those of animals given a typical infection with larvae. In AWT experiments employing the nonpermissive murine model, C57BL/6 mice given adult worms rapidly became anemic and lost weight in a manner similar to AWT hamsters. Infection of additional mouse strains demonstrated that while C57BL/10 and CD-1 mice also developed anemia following AWT, BALB/c mice were resistant. The technique of AWT to mice may further our understanding of hookworm pathogenesis by allowing the study of adult hookworm infections in a species with well-characterized genetics and an abundance of available reagents.
Hundreds of millions of persons worldwide suffer from disease caused by bloodfeeding hookworms (12, 13), with children and pregnant women bearing a particularly heavy pathological burden (14, 34-37, 40). Effective anthelmintic agents for hookworm are available (1); however, reinfection typically occurs quickly following treatment (2, 28), and drug resistance has been documented in human isolates (16, 33). Accordingly, novel strategies to control hookworm disease, such as vaccines, may offer a welcome addition to currently available treatment and control options.
Over the past 30 years, the Syrian golden hamster (Mesocricetus auratus) has been utilized as a rodent model for studies of hookworm pathology, immunology, and vaccination. Unlike mice, which do not generally permit the development of adult Ancylostoma sp. hookworms upon larval infection (9, 30), immunocompetent hamsters are fully permissive hosts for the human hookworm Ancylostoma ceylanicum (29, 31, 38). Our group (5) and others (17, 25) have found that, when infected with A. ceylanicum larvae, hamsters exhibit the major clinical features observed in children, namely, anemia and delayed growth. It has been demonstrated that immunization with soluble extracts or secretory products from adult A. ceylanicum worms leads to reduced worm burdens (20) or reduced pathology (5) in hamsters following challenge infection, establishing the utility of this model for vaccine development.
In most studies to date, hamsters have been infected with A. ceylanicum third-stage larvae (L3) via oral gavage. This approximates a naturally acquired infection in which the host is exposed sequentially to larval and adult worm proteins. Here we describe experiments employing the alternative technique of adult worm transfer (AWT), in which a previously naïve host is given an oral infection consisting of mature bloodfeeding parasites. Although AWT has been reported previously for hamsters (32) and Plasmodium berghei-infected mice (15), until the present study the pathological and immunological aspects of such an infection have not been described for either species. The studies presented here were designed to confirm the feasibility of AWT and to establish the utility of the method for the evaluation of host responses to bloodfeeding adult parasites without the influence of prior larval exposure.
MATERIALS AND METHODS
Parasites and hosts.
The A. ceylanicum life cycle was maintained as previously described (17). Twenty-one-day-old male Syrian golden hamsters of the Lak:LVG(SYR)BR outbred strain (Charles River Laboratories) were infected by oral gavage with 200 A. ceylanicum L3 so that they could serve as donors for adult worm transfer (AWT). Fourteen days after infection, the donor hamsters were euthanized and the bloodfeeding adult hookworms were recovered manually from the intestinal mucosa. Recovered parasites were rinsed and incubated at room temperature (RT) in Dulbecco's phosphate-buffered saline (PBS) with 5% normal hamster serum until transfer to recipient hamsters (transfer occurred on the same day as worm recovery). Naïve hamsters were then infected orally with recovered hookworms, receiving a mixed-sex infection of 0, 40, or 60 live adult parasites. For experiments involving AWT to mice, female C57BL/6, C57BL/10, BALB/c, and CD-1 mice (approximately 3 to 4 weeks of age) were infected orally with 20 adult worms recovered from donor hamsters as above. In each experiment, normalized postinfection weights were determined by calculating the percentage of the preinfection value for each animal. Animal research protocols employed in this study were approved by the Yale University Animal Care and Use Committee and complied with all relevant federal guidelines.
Hemoglobin assay.
Blood was collected from the orbital plexus of hamsters and mice into heparinized capillary tubes (Fisher Scientific, Pittsburgh, Pa.) and was assayed within 4 h of collection. Hemoglobin was measured by using a Total Hemoglobin assay kit (Sigma Diagnostics, St. Louis, Mo.) as previously described (5).
Hookworm antigens.
Soluble whole-hookworm extract (HEX) was prepared by homogenizing previously frozen adult A. ceylanicum hookworms (recovered 21 to 28 days after infection) in 50 mM Tris-HCl, pH 7.5, by using a glass homogenizer (6). Homogenates were briefly sonicated and then centrifuged for 30 min at 12,000 × g and 4°C. The supernatant (HEX) was removed, and its protein content was assayed by using the bicinchoninic acid system (Pierce Chemical Co., Rockford, Ill.) with a bovine serum albumin standard curve. Larval extract (LEX) was prepared from A. ceylanicum L3 by following a similar protocol. Excretory-secretory (ES) products were prepared by incubating live adult A. ceylanicum worms in sterile PBS (10 worms per ml) for 6 h at 37°C. The worms were removed, and the raw ES products were centrifuged at 3,300 × g for 15 min to remove particulates. The ES products were then concentrated by using a spin concentrator with a molecular weight cutoff of 5,000 (Millipore Corp., Bedford, Mass.). The protein content of the concentrated ES product was determined as described above. HEX, LEX, and ES aliquots were stored at −80°C until use.
Analysis of antibody responses by enzyme-linked immunosorbent assay (ELISA).
Immulon-2 microtiter plates (Dynex, Chantilly, Va.) were incubated overnight at 4°C with 100 μl of ES or HEX, diluted to 1 μg/ml in sterile PBS, per well. The plates were then incubated for 1 h at 37°C, decanted, and rinsed four times with PBS containing 0.05% Tween 20 (PBS-T). They were blocked for 1 h at RT with 1% nonfat dry milk in PBS. Plates were rinsed four times with PBS-T after blocking and between all subsequent steps. Serum samples in duplicate were serially diluted in PBS-T to a final volume of 100 μl per well and incubated for 2 h at 37°C. Antigen-specific antibodies were then detected by using 100 μl of horseradish peroxidase-labeled goat anti-hamster or anti-mouse immunoglobulin G (IgG) (ICN Biochemicals, Irvine, Calif.) per well, diluted 1:1,000 in PBS-T and incubated for 30 min at 37°C. Bound secondary antibody was detected by addition of 100 μl of ABTS substrate solution [0.1% 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) (Sigma) in 0.1 M citrate buffer (pH 5.0)-0.03% H2O2] to each well. After a 30-min incubation at RT, the A405 was recorded by using a microplate reader, and antigen-specific titers were calculated by interpolating the dilution giving an A405 of 0.2 following subtraction of background. All values were normalized to a positive standard (5) which was included in each assay to control for day-to-day variation.
Analysis of antibody responses by Western immunoblotting.
LEX, HEX, and ES samples (2 μg per well) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on a 10% acrylamide, Tricine-buffered minigel, and separated proteins were blotted onto 0.45-μm-pore-size nitrocellulose membranes (Bio-Rad, Hercules, Calif.). The membranes were blocked overnight by using 5% milk in PBS-T at 4°C. Membranes were incubated for 2 h at room temperature with pooled serum diluted in 5% milk-PBS-T. Membranes were washed three times with PBS-T, followed by a 1-h incubation with horseradish peroxidase-conjugated goat anti-hamster or anti-mouse IgG, diluted 1:5,000 in 5% milk-PBS-T. Membranes were again washed three times in PBS-T, and bound secondary antibody was detected by using Super Signal West Pico reagents (Pierce Chemical). The resulting chemiluminescent signal was detected by exposure to BioMax film (Kodak, Rochester, N.Y.).
Statistical analysis of data.
Data are presented in the text and figures as means ± standard errors of the means (SEM). Significance testing was conducted by using the StatView, version 4.51, statistical analysis software package (Abacus Concepts, Inc., Berkeley, Calif.). Pairwise comparisons were conducted using Student's t test. For multiple group comparisons, analysis of variance was performed followed by Fisher's protected least significant difference as a posttest. P values of <0.05 were considered significant.
RESULTS
Adult hookworm transfer leads to anemia in hamsters.
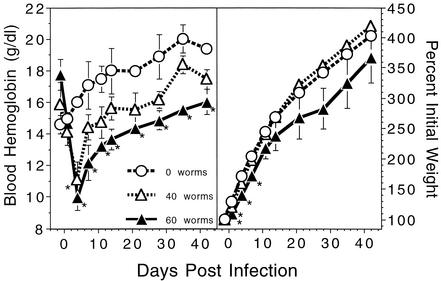
Weanling hamsters were orally infected with 40 or 60 A. ceylanicum adults on day 0 and were compared to uninfected control hamsters for 42 days. As shown in Fig. 1, blood hemoglobin levels in uninfected hamsters gradually increased from an initial mean of 14.6 ± 0.6 g/dl, approaching 18 g/dl by day 14 and remaining between 18 and 20 g/dl throughout the observation period. In contrast, both groups of hamsters infected orally with adult A. ceylanicum showed a marked and rapid decline in hemoglobin levels that reached a nadir on day 4 postinfection. Hemoglobin levels in hamsters infected with 40 adult worms (AWT:40) decreased from an initial mean of 15.8 ± 1.4 g/dl to 11.1 ± 1.1 g/dl by day 4, representing a 30% decline. This value was also 30% lower than that of the uninfected group at this time point (P < 0.02). Likewise, hemoglobin levels in the 60-worm (AWT:60) group decreased from an initial mean of 17.7 ± 1.0 g/dl to 10.0 ± 0.8 g/dl on day 4, representing a 44% decline and a value 38% lower than that of the uninfected group (P < 0.008). Hemoglobin levels began to rise in both infected groups after day 4. Levels in the AWT:40 group returned to preinfection values by day 14, and while they remained somewhat lower than those for uninfected animals at all subsequent time points, this difference was not statistically significant. By contrast, despite a gradual increase after day 4, hemoglobin levels of the AWT:60 group remained significantly lower than those of uninfected controls at all subsequent time points. Moreover, levels in this group did not return to preinfection values before the end of the trial. Long-term survival of transferred worms was demonstrated by the recovery of viable eggs from the AWT:60 group on day 35. Furthermore, live parasites (approximately 20% of the inoculum) were recovered on day 42 from the intestines of a group of hamsters given 20 adult worms (data not shown).
FIG. 1.
Blood hemoglobin levels (left panel) and weight gain (right panel) in the hamster AWT experiment. Twenty-four-day-old male hamsters (initial weight range, 19 to 33 g) were either infected with 40 (n = 3) or 60 (n = 3) adult worms each or left uninfected (n = 2). All values are means ± SEM. Asterisks indicate statistical significance versus the 0-worm group.
Adult hookworm transfer leads to delayed weight gain in hamsters.
As can be seen in Fig. 1, uninfected control hamsters gained weight rapidly throughout the observation period, exceeding 200% of initial weight by day 7, 300% by day 21, and 400% by day 42. AWT hamsters also gained weight throughout the trial; however, as early as 1 day postinfection, a statistically significant growth delay could be observed. This effect was modest in the AWT:40 group and was not significant after day 1. However, in the AWT:60 group, the effect on growth was somewhat more pronounced and was statistically significant for the first week after AWT. The mean weight of the AWT:60 group remained consistently lower than that of the uninfected group for the remainder of the experiment, although the difference was not significant after the first week due to high variance in the AWT:60 group.
AWT induces hookworm-specific humoral immune responses in hamsters.
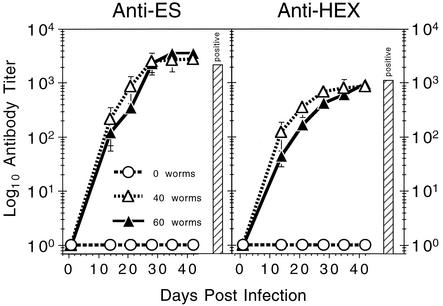
Figure 2 presents hookworm-specific humoral immune responses in AWT hamsters as measured by ELISA. Antibodies to hookworm ES products and to HEX were first detected in both groups of infected hamsters at day 14. Anti-ES responses increased after day 14, with titers for both AWT groups becoming equivalent to that for the positive-control serum (5) by day 28 and continuing to increase moderately until the end of the trial at day 42. Anti-HEX titers also increased in each AWT group throughout the trial (although somewhat more gradually than the anti-ES titers), becoming equivalent to that of the control serum by day 42. Titers of antibody mounted against ES by the AWT animals were consistently higher than those against HEX, a finding which is in agreement with the results for the positive-control serum. No significant differences in antibody titer were observed between the AWT:40 and AWT:60 groups for either antigen preparation. No hookworm-specific antibody titers were detectable in uninfected hamsters at any time point assayed.
FIG. 2.
Kinetics of hookworm-specific antibody responses in hamsters following oral infection with adult A. ceylanicum. ELISAs were performed using adult worm ES products (left panel) and soluble HEX (right panel). All values are means ± SEM. For reference, the titer of the positive-control standard used to normalize experimental values is indicated by the vertical bar in each panel.
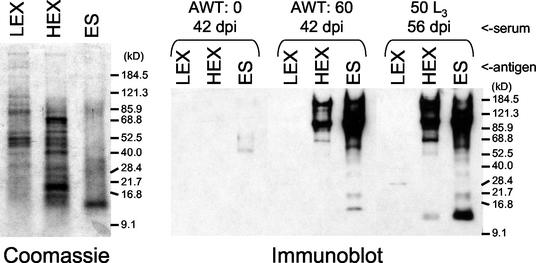
In order to characterize the antigen recognition profile of sera from AWT animals, immunoblotting was conducted using pooled sera collected 42 days postinfection from the AWT:60 group. Serum samples from the uninfected (AWT:0) group served as an age-matched negative control, and samples from animals infected with L3 served as a positive control for a standard laboratory infection (4). The positive-control serum was prepared 56 days postinfection; this time (i.e., 14 days later) was chosen to approximate the 42-day exposure of the AWT:60 group to adult parasites. As shown in Fig. 3, the reaction pattern exhibited by the AWT:60 serum against the adult antigen fractions HEX and ES was quite similar to that of the positive-control serum, especially for antigens above 50 kDa. Conspicuously absent from the AWT:60 serum, however, was recognition of a band of approximately 12 kDa in HEX and ES products. This band was prominently recognized in ES products by the positive-control serum, which recognized it to a lesser extent in HEX. Also absent from AWT:60 serum was reactivity against a band of approximately 29 kDa in LEX which was recognized by the positive-control serum.
FIG. 3.
Analysis of hookworm-specific humoral immune responses in hamsters by immunoblotting. (Left) Coomassie-stained SDS-PAGE gel of LEX, HEX, and ES products, each at 2 μg of protein per well. (Right) Immunoblot of LEX, HEX, and ES products (2 μg per well) probed with pooled sera from AWT animals (infected with 0 or 60 worms) collected 42 days postinfection (dpi) or with sera, collected 56 dpi, from animals infected with 50 L3. All sera were diluted 1:1,000. Molecular size markers are indicated on the right of each panel.
Adult hookworm transfer causes anemia and weight loss in mice.
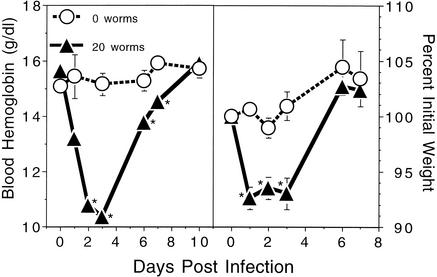
In order to examine the feasibility of adult worm transfer in a nonpermissive species, female C57BL/6 mice were orally infected with 20 hamster-derived A. ceylanicum worms. As shown in the left panel of Fig. 4, following AWT the mice became acutely anemic, with hemoglobin levels declining from a prechallenge mean of 15.6 ± 0.3 g/dl to 13.2 ± 0.4 g/dl on day 1 and 10.4 ± 0.3 g/dl on day 3, the latter representing a 34% decline at the nadir of disease and a value 31% lower than that of the uninfected group (P < 0.00004) at that time point. The presence of attached bloodfeeding worms in the intestine during the period of acute anemia was confirmed by the sacrifice of two representative AWT mice at day 2, yielding parasite burdens of 4 and 14 live adults. Hemoglobin levels in AWT mice began to rise after day 4 and became equivalent to those in uninfected controls by day 10. Hemoglobin levels remained at or above 15 g/dl in control mice at all time points assayed. The nonpermissiveness of C57BL/6 mice for a standard larval infection was confirmed by infection of an additional group of five animals with 200 L3; anemia did not develop (data not shown), and no adult worms were recovered when the mice were sacrificed on day 21.
FIG. 4.
Blood hemoglobin levels (left) and weight change (right) in mice after AWT. Data are representative of two experiments. Female C57BL/6 mice (initial weight range, 15.7 to 19.0 g) were either infected with 20 adult worms (n = 5) or left uninfected (n = 3). All values are means ± SEM. Asterisks indicate statistical significance versus the 0-worm group.
The right panel of Fig. 4 shows that C57BL/6 mice exhibited significant weight loss, concurrent with acute anemia, after AWT. To illustrate, by day 1, normalized weights for these animals had declined to a mean of 92.6% of prechallenge values (P < 0.0007 for comparison with the uninfected control group). The weights of the AWT mice remained significantly reduced until day 3 and then began to increase rapidly, becoming statistically equivalent with those of uninfected controls by day 6. The sharp increase in weight was concomitant with the recovery of hemoglobin levels in these animals. Normalized weights of control mice remained at or above 99% of prechallenge values at all time points assayed.
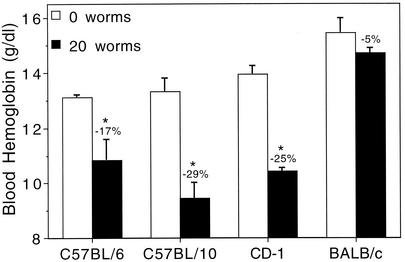
To examine the possibility of strain differences in the susceptibility to anemia following AWT, an experiment was performed in which C57BL/6, C57BL/10, BALB/c, and outbred CD-1 mice were either infected with 20 A. ceylanicum worms or left uninfected (n = 3 for each group). Figure 5 shows that, like C57BL/6 mice, C57BL/10 and CD-1 mice exhibited statistically significant reductions in hemoglobin levels relative to uninfected controls of the same strain within 3 days of AWT. Conversely, BALB/c mice appeared to be relatively resistant to anemia following AWT, showing only a slight, nonsignificant reduction in hemoglobin levels at day 3.
FIG. 5.
Comparison of blood hemoglobin levels in various mouse strains 3 days after AWT. Female mice of each strain (n = 3 per group) were either infected with 20 adult worms or left uninfected. All values are means ± SEM. Asterisks indicate statistical significance (P < 0.02) versus the 0-worm group for each strain.
Restricted hookworm-specific humoral immune responses in mice infected with adult hookworms.
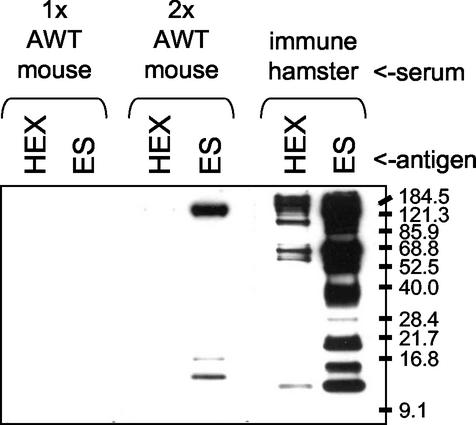
Although humoral immune responses to HEX or ES products by C57BL/6 AWT mice examined 42 days after a single exposure to worms could not be detected by immunoblotting (data not shown), reactivity to a limited number of ES antigens could be observed 14 days after a second challenge. As shown in Fig. 6, while singly exposed AWT mice failed to react with any HEX or ES proteins at a detectable level, antibodies from twice-exposed AWT mice recognized three bands of approximately 150, 16, and 14 kDa in ES products. Antibodies from twice-exposed AWT mice also reacted faintly with a 150-kDa band in HEX. This reaction profile was considerably more restricted and required more-concentrated serum as well as a longer exposure time to visualize the bands than was needed for immune hamster serum (Fig. 6) or AWT hamster serum (Fig. 3).
FIG. 6.
Analysis of hookworm-specific humoral immune responses in mice by immunoblotting. HEX and ES products (1 μg per well) were probed with pooled sera collected on day 56 from AWT animals. Twice-infected (2×) mice were infected on days 0 and 42 with 20 adult worms. Singly infected (1×) mice were infected on day 42. Mouse sera were diluted 1:200, and the blot was exposed to film for 45 min; immune hamster serum was diluted 1:1,000, and the blot was exposed for 2 min. Molecular size markers, in kilodaltons, are indicated on the right.
DISCUSSION
Hookworms continue to infect hundreds of millions of people (12, 13) and remain a leading cause of anemia and growth delay in children (14, 35-37, 40). High rates of reinfection following chemotherapy in areas of endemicity (2, 28) and the worrisome potential for drug resistance (16, 33) suggest that additional strategies are needed to augment existing hookworm control methods. Consequently, much interest in the development of hookworm vaccines has been generated (19). The apparent lack of naturally occurring protective immunity in humans (reviewed in reference 23), however, suggests that innovative approaches will be required for vaccine development. AWT may represent one such approach, as it allows the decoupling of host exposure to adult parasites from that to larvae, thus facilitating the study of pathological and immunological responses specific to the bloodfeeding stage. AWT may therefore serve as a unique model for identification of novel vaccine antigens and/or evaluation of candidate vaccines aimed at reducing pathology caused by putative adult worm “virulence factors” (4, 7, 8, 11, 26). In addition, AWT will permit the ex vivo manipulation of adult parasites by immunological, genetic, or pharmacological means prior to reimplantation, make possible the study of single-sex infections, and, as described here, allow for cross-species transfer of worms. Such cross-species AWT will extend the study of hookworm pathogenesis into traditionally nonpermissive models and may also allow the basis of permissiveness to be characterized.
Following AWT, hamsters rapidly exhibit a profound diminution in hemoglobin levels, which reach a nadir on day 4 in both dosage groups (Fig. 1). This observation suggests that a substantial proportion of the transferred worms were able to quickly resume bloodfeeding in the recipient hamsters. The subsequent rapid improvement in the hemoglobin status of the AWT animals contrasts somewhat with previous findings for hamsters infected with 50 L3, whose hemoglobin levels remained stably depressed for approximately 2 weeks beyond day 21 postinfection (5). Possible explanations for this disparity may include early attrition of worms inadvertently damaged during the processes of recovery and transfer, or enhanced host-mediated expulsion of parasites in AWT hamsters. The latter possibility is particularly intriguing and warrants further study, as it may illuminate a role for larvae in modulating the host's response to adult hookworms. However, regardless of the cause of the improved hemoglobin status of AWT animals, long-term survival of clinically significant worm burdens in the AWT:60 group was indicated by the finding that hemoglobin levels remained depressed relative to those of uninfected controls for the remainder of the trial. Continued survival of a portion of the transferred parasites was also demonstrated by the isolation of viable eggs from AWT:60 hamsters at day 35 and by the recovery of live worms from another group of AWT animals at day 42. This is in agreement with the results of Ray and Shrivastava, who found viable eggs and live parasites on day 51 following AWT (32).
We report here that, concordant with anemia, AWT was associated with negative effects on weight gain in hamsters (Fig. 1). These findings are in general agreement with data previously published by our group for larval infection (5), although in the present study the effects were more modest and transient. However, like the rapid rebound in hemoglobin levels observed in this study, this may be explained by enhanced worm attrition and/or expulsion in the AWT hamsters compared to that of adult parasites resulting from a typical larval infection. The variability observed in weight responses of the AWT:60 group also prevented an apparent long-term growth delay from achieving statistical significance. Further studies employing larger groups of animals and additional parasite dosage levels will be necessary to more thoroughly characterize effects on pathology in hamsters and to examine changes in parasite burden following AWT.
Analysis of hookworm-specific humoral immune responses by ELISA demonstrated that following AWT, hamsters acquired vigorous serum IgG responses to soluble HEX and ES products (Fig. 2). Adult-worm-specific responses were induced to comparable levels and with similar kinetics to responses generated by a larval infection (5), indicating that the transferred worms retained considerable immunogenicity. Evaluation of AWT serum by immunoblotting (Fig. 3) demonstrated an adult antigen recognition profile that was generally similar to that of larva-infected hamsters, although AWT animals failed to respond to a 12-kDa protein present in ES products. This intriguing result is under further study to determine the identity of the antigen in question. AWT hamsters also did not react appreciably with the 29-kDa larval protein recognized by larva-infected controls. This result is not unexpected given the lack of exposure of AWT animals to larvae, although the dearth of reactivity to larval antigens in the controls is interesting given that larvae and adult A. ceylanicum worms share numerous antigens (27). However, a possible explanation for this disparity may lie in the fact that the previous study employed sera from rabbits immunized with parasite extracts as opposed to the hamster infection serum utilized here. It would appear from the immunoblot data presented here that a relatively restricted profile of “naturally” immunogenic adult antigens exists and that few of these are present at detectable levels in larvae.
Having established AWT as a viable technique for the evaluation of pathological and immunological responses to adult hookworms in hamsters, we next sought to determine if AWT could be used to overcome the “permissiveness block” in mice. Like hamsters, C57BL/6 mice given an AWT quickly became anemic and exhibited negative effects on weight (Fig. 4). The presence of bloodfeeding adult worms was confirmed by the recovery of live parasites from representative AWT mice on day 2. These findings demonstrate for the first time the establishment of adult hookworms in immunocompetent mice with ensuing pathology. The complete resolution of anemia and weight loss by day 10, however, suggests rapid attrition of the transferred worms. Rapid parasite attrition is further supported by the finding that hookworms could not be recovered from a representative AWT mouse sacrificed on day 14, despite the fact that this animal had exhibited profound anemia on day 3 (44% reduction in baseline hemoglobin levels [data not shown]). Further studies will be necessary to characterize the kinetics of adult hookworm attrition in mice; however, these preliminary results suggest that an additional barrier to permissiveness exists that prevents long-term survival of adult hookworms in the mouse. It has been suggested that the permissiveness of hosts for the various species of hookworms may be affected by the degree of specificity of parasite proteases for molecules that may serve as food sources, such as hemoglobin (39). Other host physiological factors, such as hormones (as has been proposed for schistosomes [21, 22]), may influence permissiveness in hookworm infections. Lamina propria mast cells, goblet cells, and eosinophils are induced in the intestinal mucosa by A. ceylanicum larvae (10); although their protective role has not been definitively established in the murine model, it is conceivable that these cell types may be involved in the expulsion of adult worms. Moreover, the existence of strain-dependent susceptibility to disease in mice following AWT (Fig. 5) provides compelling evidence for an immunological influence on the permissive state, as various mouse strains with varying degrees of susceptibility to other helminth infections are known to differ in their immune responses (reviewed in references 3, 18, and 24). Additional studies are planned to evaluate worm attrition in the various strains following AWT and to examine the role of certain host responses that vary by strain, such as cytokine response phenotype, in order to define the basis of resistance and susceptibility.
Examination of humoral immune responses by immunoblotting revealed that in contrast to AWT hamsters (Fig. 3), AWT mice failed to produce appreciable hookworm-specific antibody responses following a single exposure to adult parasites (Fig. 6). Moreover, the adult worm antigen recognition profile in mice was rather limited following a second AWT. Enhanced parasite attrition with subsequently reduced antigen exposure in mice relative to that in hamsters may provide some explanation for this finding. Alternatively, mice may be less immunologically competent to respond to adult hookworm antigens. Further studies will be necessary to characterize the immune responses in AWT mice.
Based on the data presented here, we believe that the AWT technique will allow for the study of acute disease caused by adult hookworms in the previously inaccessible murine model. The development and characterization of the murine AWT model will further our understanding of hookworm pathogenesis by allowing the study of adult hookworm infections in a species with well-characterized genetics and an abundance of available immunological reagents. Furthermore, comparison of AWT in various species will facilitate our understanding of the basis of permissiveness, which may in turn provide a novel approach for identification of parasite vulnerabilities.
Acknowledgments
This research was supported by NIH grants T32 AI07404 (to R.D.B.) and R01 AI47929 (to M.C.) and by a New Investigator Award in Molecular Parasitology from the Burroughs Wellcome Fund (to M.C.).
We acknowledge the ongoing support of the Yale Child Health Research Center. We thank Paul M. Knopf for thoughtful discussions of this work.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Albonico, M., D. W. Crompton, and L. Savioli. 1999. Control strategies for human intestinal nematode infections. Adv. Parasitol. 42:277-341. [DOI] [PubMed] [Google Scholar]
- 2.Albonico, M., P. G. Smith, E. Ercole, A. Hall, H. M. Chwaya, K. S. Alawi, and L. Savioli. 1995. Rate of reinfection with intestinal nematodes after treatment of children with mebendazole or albendazole in a highly endemic area. Trans. R. Soc. Trop. Med. Hyg. 89:538-541. [DOI] [PubMed] [Google Scholar]
- 3.Bancroft, A. J., and R. K. Grencis. 1998. Th1 and Th2 cells and immunity to intestinal helminths. Chem. Immunol. 71:192-208. [DOI] [PubMed] [Google Scholar]
- 4.Bungiro, R. D., L. M. Harrison, and M. Cappello. 2002. Ancylostoma ceylanicum excretory/secretory protein 1: purification and molecular cloning of a major secretory protein from adult hookworms. Mol. Biochem. Parasitol. 119:147-151. [DOI] [PubMed] [Google Scholar]
- 5.Bungiro, R. D., Jr., J. Greene, E. Kruglov, and M. Cappello. 2001. Mitigation of hookworm disease by immunization with soluble extracts of Ancylostoma ceylanicum. J. Infect. Dis. 183:1380-1387. [DOI] [PubMed] [Google Scholar]
- 6.Cappello, M., L. P. Clyne, P. McPhedran, and P. J. Hotez. 1993. Ancylostoma factor Xa inhibitor: partial purification and its identification as a major hookworm-derived anticoagulant in vitro. J. Infect. Dis. 167:1474-1477. [DOI] [PubMed] [Google Scholar]
- 7.Cappello, M., J. M. Hawdon, B. F. Jones, W. P. Kennedy, and P. J. Hotez. 1996. Ancylostoma caninum anticoagulant peptide: cloning by PCR and expression of soluble, active protein in E. coli. Mol. Biochem. Parasitol. 80:113-117. [DOI] [PubMed] [Google Scholar]
- 8.Cappello, M., G. P. Vlasuk, P. W. Bergum, S. Huang, and P. J. Hotez. 1995. Ancylostoma caninum anticoagulant peptide: a hookworm-derived inhibitor of human coagulation factor Xa. Proc. Natl. Acad. Sci. USA 92:6152-6156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carroll, S. M., D. I. Grove, H. J. Dawkins, G. F. Mitchell, and L. K. Whitten. 1983. Infections with a Malaysian dog strain of Ancylostoma ceylanicum in outbred, inbred and immunocompromised mice. Parasitology 87:229-238. [DOI] [PubMed] [Google Scholar]
- 10.Carroll, S. M., D. I. Grove, and P. J. Heenan. 1986. Kinetics of cells in the intestinal mucosa of mice following oral infection with Ancylostoma ceylanicum. Int. Arch. Allergy Appl. Immunol. 79:26-32. [DOI] [PubMed] [Google Scholar]
- 11.Chadderdon, R. C., and M. Cappello. 1999. The hookworm platelet inhibitor: functional blockade of integrins GPIIb/IIIa (αIIbβ3) and GPIa/IIa (α2β1) inhibits platelet aggregation and adhesion in vitro. J. Infect. Dis. 179:1235-1241. [DOI] [PubMed] [Google Scholar]
- 12.Chan, M. S., G. F. Medley, D. Jamison, and D. A. Bundy. 1994. The evaluation of potential global morbidity attributable to intestinal nematode infections. Parasitology 109:373-387. [DOI] [PubMed] [Google Scholar]
- 13.Crompton, D. W. 1999. How much human helminthiasis is there in the world? J. Parasitol. 85:397-403. [PubMed] [Google Scholar]
- 14.Crompton, D. W., and M. C. Nesheim. 2002. Nutritional impact of intestinal helminthiasis during the human life cycle. Annu. Rev. Nutr. 22:35-59. [DOI] [PubMed] [Google Scholar]
- 15.Datta, A. K. 1984. Establishment of Ancylostoma ceylanicum adult infection in Plasmodium berghei-infected albino mice (Mus musculus). Trans. R. Soc. Trop. Med. Hyg. 78:415-416. [DOI] [PubMed] [Google Scholar]
- 16.De Clercq, D., M. Sacko, J. Behnke, F. Gilbert, P. Dorny, and J. Vercruysse. 1997. Failure of mebendazole in treatment of human hookworm infections in the southern region of Mali. Am. J. Trop. Med. Hyg. 57:25-30. [DOI] [PubMed] [Google Scholar]
- 17.Garside, P., and J. M. Behnke. 1989. Ancylostoma ceylanicum in the hamster: observations on the host-parasite relationship during primary infection. Parasitology 98:283-289. [DOI] [PubMed] [Google Scholar]
- 18.Garside, P., M. W. Kennedy, D. Wakelin, and C. E. Lawrence. 2000. Immunopathology of intestinal helminth infection. Parasite Immunol. 22:605-612. [DOI] [PubMed] [Google Scholar]
- 19.Hotez, P. J., K. Ghosh, J. M. Hawdon, S. Narasimhan, B. Jones, X. Shuhua, L. Sen, Z. Bin, X. Haechou, R. Hainan, W. Heng, and R. A. Koski. 1999. Experimental approaches to the development of a recombinant hookworm vaccine. Immunol. Rev. 171:163-171. [DOI] [PubMed] [Google Scholar]
- 20.Khan, A. M., S. Gupta, J. C. Katiyar, and V. K. Srivastava. 1996. Correlation between degree of protection and humoral antibody response in hamsters immunized with somatic and excretory-secretory antigens of Ancylostoma ceylanicum. Indian J. Exp. Biol. 34:1015-1018. [PubMed] [Google Scholar]
- 21.Knopf, P. M., and T. Linden. 1985. Completion of Schistosoma mansoni life cycle in thyroidectomized rats and effects of thyroid hormone replacement therapy. J. Parasitol. 71:422-426. [PubMed] [Google Scholar]
- 22.Knopf, P. M., and M. Soliman. 1980. Effects of host endocrine gland removal on the permissive status of laboratory rodents to infection by Schistosoma mansoni. Int. J. Parasitol. 10:197-204. [DOI] [PubMed] [Google Scholar]
- 23.Loukas, A., and P. Prociv. 2001. Immune responses in hookworm infections. Clin. Microbiol. Rev. 14:689-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacDonald, A. S., M. I. Araujo, and E. J. Pearce. 2002. Immunology of parasitic helminth infections. Infect. Immun. 70:427-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menon, S., and M. K. Bhopale. 1985. Ancylostoma ceylanicum (Looss, 1911) in golden hamsters (Mesocricetus auratus): pathogenicity and humoral immune response to a primary infection. J. Helminthol. 59:143-146. [DOI] [PubMed] [Google Scholar]
- 26.Milstone, A. M., L. M. Harrison, R. D. Bungiro, P. Kuzmic, and M. Cappello. 2000. A broad spectrum Kunitz type serine protease inhibitor secreted by the hookworm Ancylostoma ceylanicum. J. Biol. Chem. 275:29391-29399. [DOI] [PubMed] [Google Scholar]
- 27.Mohan, S., N. A. Kaushal, A. Misra, D. C. Kaushal, J. C. Katiyar, and S. Ghatak. 1988. Ancylostoma ceylanicum. I. Protein and antigenic composition of adult and larval stages. Immunol. Investig. 17:295-307. [DOI] [PubMed] [Google Scholar]
- 28.Quinnell, R. J., A. F. Slater, P. Tighe, E. A. Walsh, A. E. Keymer, and D. I. Pritchard. 1993. Reinfection with hookworm after chemotherapy in Papua New Guinea. Parasitology 106:379-385. [DOI] [PubMed] [Google Scholar]
- 29.Ray, D. K., and K. K. Bhopale. 1972. Complete development of Ancylostoma ceylanicum (Looss, 1911) in golden hamsters, Mesocricetus auratus. Experientia 28:359-361. [DOI] [PubMed] [Google Scholar]
- 30.Ray, D. K., K. K. Bhopale, and V. B. Shrivastava. 1975. Development of Ancylostoma ceylanicum Looss, 1911 (hamster strain) in the albino mouse, Mus musculus, with and without cortisone. Parasitology 71:193-197. [DOI] [PubMed] [Google Scholar]
- 31.Ray, D. K., K. K. Bhopale, and V. B. Shrivastava. 1972. Migration and growth of Ancylostoma ceylanicum in golden hamsters, Mesocricetus auratus. J. Helminthol. 46:357-362. [DOI] [PubMed] [Google Scholar]
- 32.Ray, D. K., and V. B. Shrivastava. 1981. The infectivity of ingested adult hookworms. Trans. R. Soc. Trop. Med. Hyg. 75:566-567. [DOI] [PubMed] [Google Scholar]
- 33.Reynoldson, J. A., J. M. Behnke, L. J. Pallant, M. G. Macnish, F. Gilbert, S. Giles, R. J. Spargo, and R. C. Thompson. 1997. Failure of pyrantel in treatment of human hookworm infections (Ancylostoma duodenale) in the Kimberley region of north west Australia. Acta Trop. 68:301-312. [DOI] [PubMed] [Google Scholar]
- 34.Santiso, R. 1997. Effects of chronic parasitosis on women's health. Int. J. Gynaecol. Obstet. 58:129-136. [DOI] [PubMed] [Google Scholar]
- 35.Savioli, L., D. Gundy, and A. Tomkins. 1992. Intestinal parasitic infections: a soluble public health problem. Trans. R. Soc. Trop. Med. Hyg. 86:353-354. [DOI] [PubMed] [Google Scholar]
- 36.Smillie, W. G., and D. L. Augustine. 1926. Hookworm infestation: the effect of varying intensities on the physical condition of school children. Am. J. Dis. Child. 31:151-168. [Google Scholar]
- 37.Stoltzfus, R. J., H. M. Chwaya, J. M. Tielsch, K. J. Schulze, M. Albonico, and L. Savioli. 1997. Epidemiology of iron deficiency anemia in Zanzibari schoolchildren: the importance of hookworms. Am. J. Clin. Nutr. 65:153-159. [DOI] [PubMed] [Google Scholar]
- 38.Visen, P. K., J. C. Katiyar, and A. B. Sen. 1984. Studies on infectivity, longevity and fecundity of Ancylostoma ceylanicum in golden hamsters. J. Helminthol. 58:159-163. [DOI] [PubMed] [Google Scholar]
- 39.Williamson, A. L., P. J. Brindley, G. Abbenante, P. Prociv, C. Berry, K. Girdwood, D. I. Pritchard, D. P. Fairlie, P. J. Hotez, J. P. Dalton, and A. Loukas. 2002. Cleavage of hemoglobin by hookworm cathepsin D aspartic proteases and its potential contribution to host specificity. FASEB J. 16:1458-1460. [DOI] [PubMed] [Google Scholar]
- 40.World Health Organization. 1996. Report of the WHO: informal consultation on hookworm infection and anaemia in girls and women. WHO/CTD/SIP/96.1. World Health Organization, Geneva, Switzerland.