Abstract
The mouse strains BALB/cHeA (BALB/c) and STS/A (STS) are susceptible and resistant to Leishmania major-induced disease, respectively. We analyzed this difference using recombinant congenic (RC) BALB/c-c-STS/Dem (CcS/Dem) strains that carry different random subsets of 12.5% genes of the strain STS in a BALB/c background. Previously, testing the resistant strain CcS-5, we found five novel Lmr (Leishmania major response) loci, each associated with a different combination of pathological and immunological reactions. Here we analyze the response of RC strain CcS-16, which is even more susceptible to L. major than BALB/c. In the (CcS-16 × BALB/c)F2 hybrids we mapped three novel loci that influence cutaneous or visceral pathology. Lmr14 (chromosome 2) controls splenomegaly and hepatomegaly. On the other hand Lmr15 (chromosome 11) determines hepatomegaly only, and Lmr13 (chromosome 18) determines skin lesions only. These data confirm the complex control of L. major-induced pathology, where cutaneous and visceral pathology are controlled by different combinations of genes. It indicates organ-specific control of antiparasite responses. The definition of genes controlling these responses will permit a better understanding of pathways and genetic diversity underlying the different disease phenotypes.
Leishmania major is an obligate intracellular protozoan parasite that infects mononuclear phagocytes of vertebrate hosts (23). The parasite is transmitted to vertebrates by phlebotomine sand flies (16). The type of pathology which develops following a bite from an infected sand fly is genetically controlled. In most mouse strains L. major infection causes no or only transient pathological changes, whereas in some strains parasites disseminate from the lymph nodes draining the site of the original cutaneous infection, and these mice develop a systemic disease with spleno- and hepatomegaly, anemia, hypergammaglobulinemia, and skin lesions (17). The nonhealer phenotype is controlled by multiple genes (3, 5a, 14, 18, 19), suggesting that different genetic factors contribute to susceptibility by different mechanisms (5a, 14).
To dissect the multigenic control of response to L. major infection, we used a special tool for genetic analysis of multigenically controlled biological traits, the recombinant congenic (RC) strains (5, 27). A series of 20 RC strains is derived from two inbred strains, a background strain and a donor strain. Each of the 20 RC strains contains a different random set of about 12.5% genes from the donor strain and 87.5% genes from the background strain. As a consequence, the individual donor strain genes contributing to the control of a biological trait (e.g., resistance to infection) were distributed among different RC strains. In our study we have used the RC strains of the BALB/c-c-STS/Dem (CcS/Dem) mouse series which were derived from the susceptible background strain BALB/cHeA (BALB/c) and the resistant donor strain STS/A (STS).
By this approach, we have dissected the genetic and functional aspects of susceptibility to leishmaniasis using, in place of two contrasting inbred strains, the set of 20 CcS/Dem RC strains. Strain CcS-5/Dem turned out to be highly resistant (5a), whereas RC strain CcS-16/Dem (CcS-16) is highly susceptible. The latter developed skin lesions earlier and had a tendency to develop larger skin lesions than BALB/c (13). By linkage analysis of the F2 hybrids between the most-resistant RC strain CcS-5 and the susceptible strain BALB/c, we mapped five novel loci, Lmr3 to Lmr7 (for L. major response), each of which appears to be associated with a different combination of pathological symptoms and immunological reactions (14). In the present study we have analyzed the control of skin lesions and visceral pathology in the most-susceptible strain, CcS-16.
MATERIALS AND METHODS
Mice.
Females of strain BALB/c and RC strain CcS-16 were obtained from the breeding colony of P. Demant. When used for these experiments, the CcS-16 was in generation 37 of inbreeding and therefore highly homozygous. The parts of CcS-16 genome inherited from the BALB/c or STS parents were defined (24). Female F2 hybrids between CcS-16 and BALB/c (age at the time of infection, 14 to 17 weeks) were produced at the Institute of Molecular Genetics. They were tested in four independent experimental groups.
Parasites.
L. major LV 561 (MHOM/IL/67/LRC-L137 JERICHO II) was maintained in rump lesions of BALB/c females. Amastigotes were transformed to promastigotes using SNB-9 (6). A total of 107 promastigotes from 6-day- old subculture 2 were inoculated in 50 μl of sterile saline subcutaneously into mouse rump.
Disease phenotype.
The size of the primary skin lesions was measured weekly using a vernier caliper gauge. The mice were killed 8 weeks after infection and body, spleen, and liver weights were recorded.
Genotyping of F2 mice by PCR.
DNA was isolated from tails using a standard proteinase procedure. The strain CcS-16 differs from BALB/c at STS-derived segments on nine chromosomes (24). These differential segments were typed in the F2 hybrid mice between CcS-16 and BALB/c using 23 microsatellite markers (Research Genetics, Huntsville, Fla.): D2Mit156, D2Mit389, D2Mit102, D2Nds3, D2Mit283, D2Mit51, D3Mit25, D3Mit11, D4Mit153, D6Mit48, D6Mit320, D10Mit67, D10Mit103, D11Mit139, D11Mit242, D11Mit37, D16Mit126, D17Mit38, D17Mit130, D18Mit120, D18Mit35, D18Mit40, and D18Mit49. The maximum distance between any two markers in the chromosomal segments derived from the strain STS or from the nearest BALB/c-derived markers was 20 centimorgans (cM). The PCR genotyping was performed as described elsewhere (10).
Statistical analysis.
The role of genetic factors in control of skin lesions, splenomegaly, and hepatomegaly induced by L. major infection was examined by analysis of variance (ANOVA) (Number Cruncher Statistical System [NCSS]). Marker, gender, and age were fixed factors, and the experiment was considered a random parameter. In order to obtain normal distribution required for ANOVA, the obtained values were transformed as shown in the footnotes to Table 1. The normality of data was tested by Martinez-Iglewitz and Kolmogorov-Smirnov normality tests in the NCSS. Markers and interactions with a P of <0.05 were combined in a single comparison. The time course of skin lesion development was evaluated on the basis of weekly measurements of lesion size in each mouse in week 4 to 8 after infection. Covariance analysis (general linear model ANOVA [GLM ANOVA]) (NCSS) with marker as the fixed variable and the week of observation as the covariate has been used to evaluate the linkage. To obtain whole genome significance values (corrected P) the observed P (αT) values were adjusted according to the method of Lander and Kruglyak (11) using the formula αT* ≈ [C + 2ρFh(T)]αT, where G = 2 M (the length of the segregating part of the genome: 12.5% of 16 M); C = 9 (number of chromosomes segregating in cross between CcS-16 and BALB/c); ρ = 1.5 for F2 hybrids; and h(T) = the observed statistics (F ratio).
TABLE 1.
Linkage analysis of visceral disease in L. major-infected F2 hybrids between BALB/c and CcS-16a
| Marker | Splenomegaly
|
Hepatomegaly
|
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genotype
|
P | Corrected P | Genotype
|
P | Corrected P | |||||||||||||||||
| CC
|
CS
|
SS
|
CC
|
CS
|
SS
|
|||||||||||||||||
| Mean relative wt (102) | Mean ANOVA-transformed wt ± SE | n | Mean relative wt (102) | Mean ANOVA-transformed wt ± SE | n | Mean relative wt (102) | Mean ANOVA-transformed wt ± SE | n | Mean relative wt (102) | Mean ANOVA-transformed wt ± SE | n | Mean relative wt (102) | Mean ANOVA-transformed wt ± SE | n | Mean relative wt (102) | Mean ANOVA-transformed wt ± SE | n | |||||
| D2Mit389 | 1.326 | 1.073 ± 0.006 | 138 | 1.340 | 1.076 ± 0.004 | 285 | 1.475 | 1.102 ± 0.006 | 139 | 0.000303 | 0.0177 | 6.876 | 5.680 ± 0.050 | 139 | 7.037 | 5.800 ± 0.040 | 285 | 7.294 | 5.990 ± 0.050 | 139 | 0.000286 | 0.0168 |
| D2Mit102 | 1.296 | 1.067 ± 0.006 | 144 | 1.350 | 1.078 ± 0.004 | 289 | 1.480 | 1.103 ± 0.006 | 132 | 0.0000397 | 0.00282 | 6.768 | 5.600 ± 0.050 | 145 | 7.051 | 5.810 ± 0.040 | 289 | 7.375 | 6.050 ± 0.050 | 133 | 0.00000003 | 0.00000348 |
| D2Nds3 | 1.306 | 1.069 ± 0.005 | 150 | 1.340 | 1.076 ± 0.004 | 281 | 1.513 | 1.109 ± 0.006 | 130 | 0.00000076 | 0.0000728 | 6.755 | 5.590 ± 0.050 | 151 | 7.078 | 5.830 ± 0.040 | 281 | 7.402 | 6.070 ± 0.050 | 130 | 0.000000001 | 0.000000137 |
| D2Mit283 | 1.321 | 1.072 ± 0.005 | 159 | 1.366 | 1.081 ± 0.004 | 305 | 1.448 | 1.097 ± 0.007 | 100 | 0.0138 | 0.303 | 6.822 | 5.640 ± 0.050 | 160 | 7.105 | 5.850 ± 0.040 | 305 | 7.307 | 6.000 ± 0.060 | 100 | 0.0000286 | 0.00209 |
| D2Mit51 | 1.321 | 1.072 ± 0.006 | 136 | 1.366 | 1.081 ± 0.004 | 308 | 1.427 | 1.093 ± 0.006 | 118 | 0.0438 | 0.808 | 6.809 | 5.630 ± 0.050 | 137 | 7.091 | 5.840 ± 0.040 | 308 | 7.240 | 5.950 ± 0.060 | 119 | 0.000245 | 0.0146 |
| D11Mit139 | 1.427 | 1.093 ± 0.006 | 128 | 1.366 | 1.081 ± 0.004 | 285 | 1.335 | 1.075 ± 0.006 | 140 | 0.0833 | 1.374 | 7.129 | 5.858 ± 0.060 | 128 | 7.173 | 5.890 ± 0.040 | 285 | 7.293 | 5.680 ± 0.050 | 141 | 0.0049 | 0.123 |
| D11Mit242 | 1.448 | 1.097 ± 0.006 | 132 | 1.371 | 1.082 ± 0.004 | 291 | 1.316 | 1.071 ± 0.006 | 136 | 0.0084 | 0.197 | 7.199 | 5.920 ± 0.050 | 132 | 7.145 | 5.880 ± 0.040 | 291 | 6.809 | 5.630 ± 0.050 | 137 | 0.000115 | 0.00741 |
| D11Mit37 | 1.443 | 1.096 ± 0.006 | 132 | 1.360 | 1.080 ± 0.004 | 282 | 1.335 | 1.075 ± 0.006 | 147 | 0.0267 | 0.533 | 7.186 | 5.910 ± 0.050 | 132 | 7.145 | 5.880 ± 0.040 | 282 | 6.836 | 5.650 ± 0.050 | 148 | 0.000456 | 0.0255 |
In order to normalize the distribution of the observed values, the relative spleen and liver weight were transformed to the power of 0.25 and 0.9, respectively. C and S indicate the presence of BALB/c and STS allele, respectively. The table gives both the relative spleen and liver weight × 100 attributed by ANOVA to individual genotypes as well as (in italics) the means ± SE of the transformed values computed by ANOVA. P values that were significant after correction for genome-wide significance (corrected P < 0.05) are underlined. The average spleen weight ± standard error and the relative spleen weight [(mean ± standard error) × 100] were 0.38 ± 0.03 g and 1.52 ± 0.16 g in strain BALB/c (n = 13), 0.27 ± 0.02 g and 1.17 ± 0.09 g in strain CcS-16 (n = 14), and 0.32 ± 0.09 g and 1.36 ± 0.02 g in all the F2 hybrids (n = 572). In the F2 hybrids in the individual experiments, the relative spleen weight [(mean ± standard error) × 100] was 1.32 ± 0.02 g (n = 137), 1.46 ± 0.03 g (n = 140), 1.34 ± 0.03 g (n = 147), and 1.46 ± 0.04 g (n = 148) in experiments 1, 2, 3, and 4, respectively. The absolute spleen weights (mean ± standard error) were 0.29 ± 0.07 g, 0.33 ± 0.09 g, 0.31 ± 0.09 g, and 0.34 ± 0.11 g, respectively. The average liver weight ± standard error and the relative liver weight [(mean ± standard error) × 100] were 1.67 ± 0.05 g and 7.36 ± 0.01 g in strain BALB/c (n = 13), 1.49 ± 0.04 g and 7.28 ± 0.13 g in strain CcS-16 (n = 16), and 1.61 ± 0.22 g and 7.02 ± 0.02 g in all the F2 hybrids (n = 574). In the F2 hybrids in the individual experiments, the relative liver weight [(mean ± standard error) × 100] was 7.28 ± 0.08 g (n = 137), 7.08 ± 0.07 g (n = 140), 6.99 ± 0.06 g (n = 149), and 6.82 ± 0.08 g (n = 148) in experiments 1, 2, 3, and 4, respectively. The absolute liver weights (mean ± standard error) were 1.61 ± 0.22 g, 1.63 ± 0.23 g, 1.60 ± 0.19 g, and 1.62 ± 0.25 g, respectively.
RESULTS
Novel loci that influence skin lesions or visceral pathology. We examined 576 F2 hybrids between BALB/c and CcS-16. This analysis revealed three novel loci that influence skin lesion development (Fig. 1) and/or the visceral disease (Table 1).
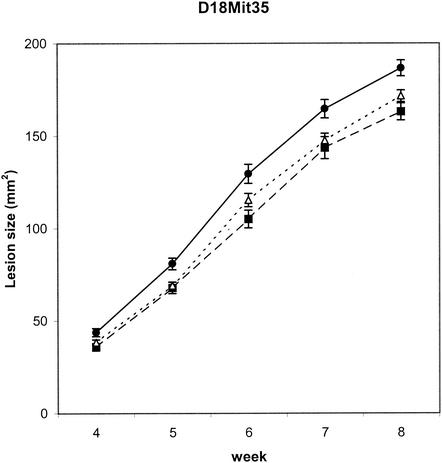
FIG. 1.
Effects of genotype at Lmr13 on size of cutaneous lesions weeks 4 to 8 after infection. The means and standard errors of means (error bars) for each of the three genotypes at different time points are given (n = 565). C and S indicate the presence of BALB/c and STS allele, respectively. The numbers of mice of individual D18Mit35 genotypes are as follows: for CC (▪), n = 144; for CS (▵), n = 285; for SS (•), n = 136. The P corrected for multiple testing is 0.00000125. Lesion sizes at week 8 after infection (mean ± standard deviation) were 158.34 ± 11.92 mm2 in strain BALB/c (n = 19), 220 ± 11.61 mm2 in strain CcS-16 (n = 20), and 171.84 ± 2.19 mm2 in the all F2 hybrids (n = 576); in the F2 hybrids, means ± standard deviations in the individual experiments were 152.20 ± 4.35 mm2 (n = 137), 170.56 ± 4.12 mm2 (n = 140), 175.49 ± 4.33 mm2 (n = 150), and 189.12 ± 4.67 mm2 (n = 149) in the experiment 1, 2, 3, and 4, respectively.
(i) Lmr13 on chromosome 18 controls development of the skin lesions.
Lmr13 is linked to the markers D18Mit120 and D18Mit35 (both corrected P values < 0.00000125), and its STS allele is associated with larger lesions.
(ii) Lmr14 on chromosome 2 controls splenomegaly and hepatomegaly.
Splenomegaly is linked with the markers on chromosome 2 (D2Mit389, D2Mit102, and D2Nds3 [corrected P < 0.0177, 0.00282, and 0.0000728, respectively]), and hepatomegaly is linked with the markers D2Mit389, D2Mit102, D2Nds3, D2Mit283, and D2Mit51 (corrected P < 0.0168, 0.00000348, 0.000000137, 0.00209, and 0.0146, respectively). The STS allele is associated with larger spleno- and hepatomegaly. A weak influence on size of the skin lesions linked to the marker D2Mit283 was observed (data not shown).
(iii) Lmr15 on chromosome 11 controls hepatomegaly.
Lmr15 is linked to the markers D11Mit242 and D11Mit37 (corrected P < 0.00741 and 0.0255, respectively), and the STS allele is associated with less pronounced hepatomegaly.
The influence of the experimental group on skin lesions was pronounced (P < 10−7 for Lmr13), and it was obvious for splenomegaly (P < 0.0008 for Lmr14) and hepatomegaly (P < 0.017 and 0.0086 for Lmr14 and Lmr15, respectively). However, there is no interaction between the experiment (or age) and any of the markers exhibiting linkage, indicating that the differences between the experimental groups do not affect the observed linkage and that the observed linkages are not artifacts due to unequal distribution of phenotypes and markers in different experiments, or to other untraceable causes.
DISCUSSION
We have studied the differences in the L. major-induced pathology between the susceptible strain BALB/c and the resistant STS. Part of the STS genome, represented in the RC strain CcS-16, contains genes responsible for even higher susceptibility of CcS-16 mice than BALB/c. This finding is not unique; susceptibility alleles originating from resistant strains were found for example in studies of liver and colon tumor susceptibility (12, 28). Analysis of a number of disease symptoms and immunological parameters in F2 hybrids between BALB/c and CcS-16 strains revealed three Leishmania major response (Lmr) loci Lmr13, -14, and -15, with differential effects on cutaneous and visceral components of the disease (Fig. 2, Table 2).
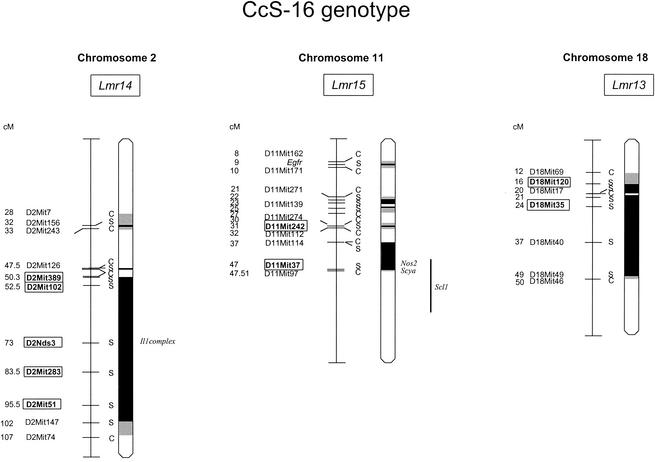
FIG. 2.
Position of the loci that control cutaneous or visceral leishmaniasis in CcS-16. The regions of STS origin are represented as dark, the regions of undetermined origin are shaded. Only the BALB/c markers at the position between BALB/c and STS markers and STS markers that were used for typing are shown. The markers that exhibit significant P values (corrected P < 0.05) are shown in boxes.
TABLE 2.
Summary of Lmr loci that control pathological parameters in CcS-16 and potential candidate genes
| Chromosome | Locus | Marker | Position (cM) | Phenotypic effect of allelesb | Parameter(s) controlled | Candidate gene(s) |
|---|---|---|---|---|---|---|
| 2 | Lmr14 | D2Mit389 | 50.3 | C < S | Hepatomegaly, splenomegaly | |
| D2Mit102 | 52.5 | C < S | Hepatomegaly, splenomegaly | |||
| D2Nds3 | 73.0 | C < S | Hepatomegaly, splenomegaly | Il1 complex (IL-1 complex) | ||
| D2Mit283 | 83.5 | C < S | Hepatomegaly, splenomegaly | |||
| D2Mit51 | 95.5 | C < S | Hepatomegaly, splenomegaly | |||
| 11 | Lmr15 | D11Mit242 D11Mit37 | 31.0 47.0 | C > S C > S | Hepatomegaly Hepatomegaly | nos2 (nitric oxide synthase 2) scya (small inducible cytokine) |
| 18 | Lmr13 | D18Mit120 | 16.0 | C < S | Lesion size | ?a |
| D18Mit35 | 24.0 | C < S | Lesion size |
?, unknown.
C < S, effect of STS allele aggravates pathology; C > S, effect of STS allele alleviates pathology.
The locus Lmr13 on chromosome 18 affects the cutaneous disease only, whereas the loci Lmr14 and Lmr15 on chromosomes 2 and 11, respectively, affect only the visceral pathology (splenomegaly and/or hepatomegaly). CcS-16 carries on chromosome 18 two STS-derived segments (Fig. 2). Lmr13 maps to the proximal part of the chromosome. In the close vicinity of this locus, there is no obvious candidate gene. We have also found that Lmr13 influences immunoglobulin E synthesis after L. major infection (1). Lmr13 is situated in the part of mouse chromosome 18 that is homologous with the human segment 5q31-q33, which is known to be involved in the control of atopy (2) and defense against pathogens. Locus SM1 in the region 5q31-q33 determines the intensity of infection by Schistosoma mansoni (15) and may also influence numbers of Plasmodium falciparum in blood (7). These findings may stress the importance of this chromosomal segment in defense against different infections.
Locus Lmr14 on chromosome 2 controls the visceral symptoms (splenomegaly and hepatomegaly) (Table 1; Fig. 2). However, as the mapping of these effects appears to be spread over a broader region of chromosome 2 (>25 cM) with a peak of linkage at D2Nds3, it cannot be ruled out that the visceral pathologies are caused by two or more linked loci. Near D2Nds3 are candidate genes in the Il1 complex. Interleukin-1 (IL-1) was shown to play an important role in the development of cutaneous leishmaniasis (26).
CcS-16 has on chromosome 11 four short STS-derived regions. Lmr15 is linked to two of them, which are localized on the central part. The most distal one contains genes involved in defense against infection with Nos2 (which codes for nitric oxide synthase 2) and a cluster of several Scya (small inducible cytokine) genes. Nos2 was described as playing a central role in defense against L. major (4). Lmr15, which influences visceral pathology in our experiments, overlaps with Scl1, which was described to control cutaneous leishmaniasis (20). More experiments are necessary to establish the relationship and precise position of Lmr15 and Scl1. In the central part of chromosome 11 are located the candidate gene encoding interferon regulatory factor 1 and locus Tmp1, which were reported to influence in vitro IL-12 responsiveness and Th1 versus Th2 development, respectively (8, 9). However, no role of these loci in response to L. major in vivo has been demonstrated. The locus influencing skin lesions described by Beebe et al. (3) maps to the centromeric part of chromosome 11 that is not polymorphic between the two strains tested here and therefore cannot play a role in the effects we describe.
The present observations of the distinct gene effects on cutaneous and visceral components of leishmaniasis extend our previous data on the heterogeneous effects on pathology and immunology of the differential loci (Lmr3 to Lmr12) (1, 14). For example, the locus Lmr3 (chromosome 5) predominantly affects the visceral manifestations, Lmr4 (chromosome 6) affects the cutaneous lesions, and Lmr5 (chromosome 10) affects both cutaneous and visceral pathology. Organ-specific control of antiparasite response to L. major were also observed in functional analysis of inducible nitric oxide synthase control, where inhibition of the Nos2 product, inducible nitric oxide synthase, in resistant C57BL/6 mice led to parasite reactivation in the skin and draining lymph nodes but not in the spleen (25). Similar genetic heterogeneity was observed in response to another pathogen, the bacterium Borrelia burgdorferi, in which different loci controlled swelling of the ankle, tendon sheath thickness, and the amount of B. burgdorferi in heart tissue (21). Our data also indicate that the extent of pathological changes in organs does not directly correlate with the L. major parasite load but that the relation between the two is to a large degree strain-dependent, i.e., related to the host genome (J. Badalová et al., unpublished data).
Our results are compatible with the extensive genetic effects on the clinical heterogeneity of the disease caused by L. major revealed by the RC strains (13). Whereas the strain BALB/c develops both the cutaneous and visceral manifestations and the strain STS develops barely any signs of disease, the 20 RC strains, each carrying a different subset of 12.5% of STS genes on a BALB/c genetic background, differ widely in both clinical and immunological effects of L. major infection.
Collectively, these findings show that the effects of host genotype on the disease are complex and heterogeneous but also that they can be successfully dissected into the effects of individual genes that can be further characterized in detail. This genetic and functional dissection of the infectious disease will ultimately lead to the understanding of the specific effects of genes of the host on the pathogenesis and prognosis of the disease in the affected individuals, thus providing the possibilities for individualized medicine (22).
Acknowledgments
M. Lipoldová received financial support as an International Research Scholar of the Howard Hughes Medical Institute. This investigation also received financial support from the Grant Agency of the Czech Republic (grants 310/00/0760 and 310/03/1381), the Ministry of Education of the Czech Republic (grants OK394 and MSM-111200002), and the European Commission (contracts ERBI-C15-CT98-0317 and BIO-4-CT98-0445).
We acknowledge the permission of P. Kodym and K. Zitek (State Institute for Health, Prague) to use their laboratory and animal facilities for part of these experiments.
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Badalová, J., M. Svobodová, H. Havelková, V. Vladimirov, J. Vojtíšková, J. Engová, T. Pilčík, P. Volf, P. Demant, and M. Lipoldová. 2002. Separation and mapping of multiple genes that control IgE level in Leishmania major infected mice. Genes Immun. 3:187-195. [DOI] [PubMed] [Google Scholar]
- 2.Baldini, M., I. C. Lohman, M. Halonen, R. P. Erickson, P. G. Holt, and F. D. Martinez. 1999. A polymorphism in the 5′ flanking region of the CD14 gene is associated with circulating soluble CD14 levels and with total serum immunoglobulin E. Am. J. Respir. Cell Mol. Biol. 20:976-983. [DOI] [PubMed] [Google Scholar]
- 3.Beebe, A. M., S. Mauze, N. J. Schork, and R. L. Coffman. 1997. Serial backcross mapping of multiple loci associated with resistance to Leishmania major in mice. Immunity 6:551-557. [DOI] [PubMed] [Google Scholar]
- 4.Bogdan, C., M. Röllinghoff, and A. Diefenbach. 2000. The role of nitric oxide in innate immunity. Immunol. Rev. 173:17-26. [DOI] [PubMed] [Google Scholar]
- 5.Demant, P., and A. A. M. Hart. 1986. Recombinant congenic strains: a new tool for analyzing genetic traits determined by more than one gene. Immunogenetics 24:416-422. [DOI] [PubMed] [Google Scholar]
- 5a.Demant, P., M. Lipoldová, and M. Svobodová. 1996. Resistance to Leishmania major in mice. Science 274:1392-1393. [PubMed] [Google Scholar]
- 6.Diamond, L. S., and C. M. Herman. 1954. Incidence of trypanosomes in the Canada goose as revealed by bone marrow culture. J. Parasitol. 40:195-202. [Google Scholar]
- 7.Garcia, A., S. Marquet, B. Bucheton, D. Hillaire, M. Cot, N. Fievet, A. J. Dessein, and L. Abel. 1998. Linkage analysis of blood Plasmodium falciparum levels: interest of the 5q31-q33 chromosome region. Am. J. Trop. Med. Hyg. 58:705-709. [DOI] [PubMed] [Google Scholar]
- 8.Gorham, J. D., M. L. Güler, R. G. Steen, R. J. Mackey, M. I. Daly, K. Frederick, W. F. Dietrich, and K. M. Murphy. 1996. Genetic mapping of a murine locus controlling development of T helper 1/T helper 2 type responses. Proc. Natl. Acad. Sci. USA 93:12467-12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Güler, M. L., J. D. Gorham, C.-S. Hsieh, A. J. Mackey, R. G. Steen, W. F. Dietrich, and K. M. Murphy. 1996. Genetic susceptibility to Leishmania: IL-12 responsiveness in Th1 cell development. Science 271:984-987. [DOI] [PubMed] [Google Scholar]
- 10.Krulová, M., H. Havelková, M. Kosarová, V. Holán, A. A. M. Hart, P. Demant, and M. Lipoldová. 1997. IL-2-induced proliferative response is controlled by loci Cinda1 and Cinda2 on mouse chromosomes 11 and 12: a distinct control of the response induced by different IL-2 concentrations. Genomics 42:11-15. [DOI] [PubMed] [Google Scholar]
- 11.Lander, E., and L. Kruglyak. 1995. Genetic dissection of complex traits: guidelines for interpreting and reporting results. Nat. Genet. 11:241-247. [DOI] [PubMed] [Google Scholar]
- 12.Lee, G. H., L. M. Benett, R. A. Carabeo, and N. R. Drinkwater. 1995. Identification of hepatocarcinogen resistance genes in DBA/2 mice. Genetics 193:387-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lipoldová, M., M. Svobodová, H. Havelková, M. Krulová, J. Badalová, E. Nohýnková, A. A. M. Hart, D. Schlegel, P. Volf, and P. Demant. 2002. Mouse genetic model for clinical and immunological heterogeneity of leishmaniasis. Immunogenetics 54:174-183. [DOI] [PubMed] [Google Scholar]
- 14.Lipoldová, M., M. Svobodová, M. Krulová, H. Havelková, J. Badalová, E. Nohýnková, V. Holán, A. A. M. Hart, P. Volf, and P. Demant. 2000. Susceptibility to Leishmania major infection in mice: multiple loci and heterogeneity of immunopathological phenotypes. Genes Immun. 1:200-206. [DOI] [PubMed] [Google Scholar]
- 15.Marquet, S., L. Abel, D. Hillaire, H. Dessein, J. Kalil, J. Feingold, J. Weissenbach, and A. J. Dessein. 1996. Genetic localization of a locus controlling the intensity of infection by Schistosoma mansoni on chromosome 5q31-q33. Nat. Genet. 14:181-184. [DOI] [PubMed] [Google Scholar]
- 16.Molyneux, D. H., and R. Killick-Kendrick. 1987. Morphology, ultrastructure and life cycles, p. 121-176. In W. Peters and R. Killick-Kendrick (ed.), The leishmaniases in biology and medicine, vol. I. Academic Press, London, United Kingdom
- 17.Reiner, S. L., and R. M. Locksley. 1995. The regulation of immunity to Leishmania major. Annu. Rev. Immunol. 13:151-177. [DOI] [PubMed] [Google Scholar]
- 18.Roberts, L. J., T. M. Baldwin, J. M. Curtis, E. Handman, and S. J. Foote. 1997. Resistance to Leishmania major is linked to H2 region on chromosome 17 and to chromosome 9. J. Exp. Med. 9:1705-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberts, L. J., T. M. Baldwin, T. P. Speed, E. Handman, and S. J. Foote. 1999. Chromosomes X, 9 and the H2 locus interact epistatically to control Leishmania major infection. Eur. J. Immunol. 29:3047-3050. [DOI] [PubMed] [Google Scholar]
- 20.Roberts, M., B. A. Mock, and J. M. Blackwell. 1993. Mapping of genes controlling Leishmania major infection in CXS recombinant inbred mice. Eur. J. Immunogenet. 20:349-362. [DOI] [PubMed] [Google Scholar]
- 21.Roper, R. J., J. J. Weis, B. A. McCracken, C. B. Green, Y. Ma, K. S. Weber, D. Fairbairn, R. J. Butterfield, M. R. Potter, J. F. Zachary, R. W. Doerge, and C. Teuscher. 2001. Genetic control of susceptibility to experimental Lyme arthritis is polygenic and exhibits consistent linkage to multiple loci on chromosome 5 in four independent mouse crosses. Genes Immun. 2:388-397. [DOI] [PubMed] [Google Scholar]
- 22.Sander, C. 2000. Genomic medicine and the future of health care. Science 287:1977-1978. [DOI] [PubMed] [Google Scholar]
- 23.Solbach, W., and T. Laskay. 1996. Evasion strategies of Leishmania parasites, p. 25-47. In F. J. Tapia, G. Cáceres-Dittmar, and M. A. Sánchez (ed.), Molecular and immune mechanisms in the pathogenesis of cutaneous leishmaniasis. R. G. Landes Company, Austin, Tex.
- 24.Stassen, A. P. M., P. C. Groot, J. T. Eppig, and P. Demant. 1996. Genetic composition of the recombinant congenic strains. Mamm. Genome 7:55-58. [DOI] [PubMed] [Google Scholar]
- 25.Stenger, S., N. Donhauser, H. Thüring, M. Röllinghoff, and C. Bogdan. 1996. Reactivation of latent leishmaniasis by inhibition of inducible nitric oxide synthase. J. Exp. Med. 183:1501-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Theodos, C. M., A. Shankar, A. L. Glasebrook, W. D. Roeder, and R. G. Titus. 1994. The effect of treating with anti-interleukin-1 receptor antibody on the course of experimental murine cutaneous leishmaniasis. Parasite Immunol. 16:571-577. [DOI] [PubMed] [Google Scholar]
- 27.van Wezel, T., M. Lipoldová, and P. Demant. 2001. Identification of disease susceptibility genes (modifiers) in mouse models: cancer and infectious diseases, p. 107-130. In S. Malcolm and J. Goodship (ed.), Genotype to phenotype, 2nd ed. BIOS Scientific Publishers Ltd., Oxford, United Kingdom
- 28.van Wezel, T., A. P. M. Stassen, C. J. A. Moen, A. A. M. Hart, M. A. van der Valk, and P. Demant. 1996. Gene interaction and single gene effects in colon tumour susceptibility in mice. Nat. Genet. 14:468-470. [DOI] [PubMed] [Google Scholar]