Abstract
Vaccines that induce mosquito-killing (mosquitocidal) activity could substantially reduce the transmission of certain mosquito-borne diseases, especially vaccines against African malaria vectors, such as the mosquito Anopheles gambiae. To generate and characterize antimosquito immunity we immunized groups of mice with two individual A. gambiae midgut cDNAs, Ag-Aper1 (a secreted peritrophic matrix protein) and AgMuc1 (a midgut-bound mucin), and an A. gambiae midgut cDNA library from blood-fed mosquitoes. We observed significantly increased mortality among mosquitoes that fed on either the AgMuc1- or the cDNA library-immunized mice compared to that of controls, but no differences were observed among those fed on Ag-Aper1-immunized mice. Analysis of the humoral and cellular immune responses from mice showed that the induced mosquitocidal effect was associated with immune profiles characterized by elevated tumor necrosis factor alpha and gamma interferon cytokine levels and very low antibody titers. Furthermore, an additional immunization of cDNA library-immunized mice with midgut protein shifted immunity toward a Th2-type immune response, characterized by elevated antibody titers and high interleukin-5 and interleukin-10 cytokine levels; importantly, mosquitoes feeding on these mice exhibited no undo mortality. Finally, when immune sera was ingested by mosquitoes through a membrane feeder, no effect on mosquito mortality was observed, indicating that serum factors alone were not responsible for the mosquitocidal effect. Our results demonstrate that mosquitocidal immunity in mice can be consistently generated by midgut cDNA immunization and suggest this cDNA-induced mosquitocidal immunity is cell mediated.
Most successful attempts at controlling vector-borne disease have involved killing or repelling vector populations with insecticides (13, 15). Unfortunately, classic vector control methods are failing due to insecticide resistance, costs, and environmental concerns (11, 20, 24, 42). Accordingly, there is an urgent need to develop alternative strategies to control vector-borne disease transmission. Hematophagous vectors, such as mosquitoes, take blood meals for both nutrition and egg production. The midgut of these vectors functions to store, digest, diurese, and adsorb the blood meal (30); hence, the midgut plays a critical role in the biology of the vector. From a disease control perspective, the midgut is critical because it serves as the initial barrier for pathogen infection of the insect (23). Identification of molecular targets in the midgut that are critical for homeostatic physiology and for vector infection with human pathogens is crucial to the development of alternative vector control strategies. Ultimately, these antigens could be targets of vaccination control strategies because cellular and humoral immune effectors present in the blood are in direct contact with midgut antigens upon blood meal uptake. Inclusion of mosquitocidal antigens or epitopes into developing malaria subunit vaccines may complement vaccine strategies that target many facets of the Plasmodium life cycle (21, 44).
We have previously proposed that a mosquitocidal vaccine targeting malaria vectors may be particularly effective in controlling malaria transmission, especially in Africa (14). This potential vulnerability stems from the life cycle characteristics of both Anopheles gambiae vectors and the human Plasmodium parasite. Human malaria is transmitted only laterally, there are no zoonotic reservoirs, and sporogonic development in the mosquito takes at least 10 days (6). Furthermore, members of the A. gambiae complex are highly anthropophilic (6), they often take 2 to 3 blood meals per gonotrophic cycle (5), and they can survive for more than a month; therefore, they have the potential to feed on more than 10 different humans in their lifetime (12). Ultimately, most malaria transmission is carried out by A. gambiae that have taken between 4 and 12 blood meals (26). If a mosquitocidal vaccine were developed, these life cycle characteristics reveal that any A. gambiae mosquito would have a high probability of ingesting a lethal blood meal from a vaccinated person before transmitting the parasite. Mathematical models suggest such a vaccine could radically reduce malaria prevalence with only modest vaccine coverage (8, 14). By comparison, transmission-blocking vaccines may require almost complete coverage to be effective (14).
Anti-vector immunity was first demonstrated by Trager against the tick Dermacentor variabilis by animal immunization with homogenized tick extracts (50). Since then, only a few specific antivector molecular targets have been identified, and most of these targets are from ticks. Immunological targeting of tick midgut antigens has culminated with the commercial development of a recombinant protein vaccine against the cattle tick Boophilus microplus (54). However, the identification of one target tick antigen alone took 4 years to accomplish through the biochemical fractionation of kilograms of ticks down to microgram quantities of protein for serial vaccination and tick challenge studies (55). Such methods are nearly impossible when dealing with smaller vectors, such as mosquitoes. Experimentation involving immunization with mosquito and other insect antigens to generate anti-insect immunity has proven far less successful than that against ticks. Preliminary success has been achieved by immunization with crude insect antigens, but the published literature as a whole has been ambiguous and the experimental variability has been high (2, 14, 22, 54).
We hypothesize that the previously reported variability in generating mosquitocidal immunity by immunization with midgut antigens stems in part from the form of immunized antigen and its purity, which in turn can effect the type of immune response generated (e.g., Th1 or Th2). Immunization of midgut antigens in the form of cDNA offers the possibility of generating consistent immunity, because the purity of DNA can be easily controlled and different midgut antigens (as cDNA) can be easily separated for immunization. Furthermore, DNA immunization often stimulates potent cellular immunity in addition to humoral immunity against the immunogen, while protein immunization responses are often dominated by a humoral response (16, 27, 43). This enhanced immunity may increase the likelihood of generating a mosquitocidal immune response. Finally, it has been shown that immunization of whole DNA libraries from pathogens can elicit a protective immune response against the pathogen (4, 34, 35). These libraries can then be easily fractionated and serially immunized as smaller and smaller library pools in order to eventually identify novel individual genes that stimulate immune protection. Immunization with an insect cDNA library may eventually allow for the identification of undiscovered vector antigen targets through such reductive immunization screening of the library.
MATERIALS AND METHODS
Preparation of DNA.
The individual cDNAs enhanced green fluorescent protein (GFP) (EGFP), A. gambiae mucin 1 (AgMuc1), and A. gambiae peritrophic matrix 1 (PM1) (Ag-Aper1) were subcloned from their parental vectors into the pcDNA3.1+ plasmid vector (Invitrogen) and then were sequenced prior to immunization to ensure proper orientation and translation. AgMuc1 (mucin) is a member of a large family of mucin glycoproteins and is attached to the cellular membrane via a glycosylphosphatidyl inositol (GPI) anchor (45); thus, it is exposed on the lumenal surface of midgut cells. Ag-Aper1 (PM1) is a secreted PM protein that helps to envelop the ingested blood meal (46), possibly by cross-linking chitin components of the PM. The midgut cDNA library of blood-fed A. gambiae was originally constructed from mRNA that was harvested from the midguts of A. gambiae 0 to 24 h after feeding on blood and is described in detail elsewhere (31). Importantly, during library construction blood was removed from the midguts prior to harvesting RNA in order to limit the number of mouse transcripts. Sequencing of more than 200 random clones from this library confirmed the lack of mouse transcripts, as less than 1% of these sequences showed only low homology to cataloged mouse genes. The library was excised into pBluescript II SK(+) by using the Stratagene ExAssist kit, the cDNAs were subcloned into the pcDNA3.1+ plasmid vector, and the electroporated library was harvested from bacterial colonies on large agar plates. Random colonies from the subcloned library were picked and plasmids were isolated, restriction digested, and examined by gel electrophoresis in order to confirm the high diversity of the library. Some of these clones were then sequenced for further examination of the library diversity, orientation, and proper translation. Calculations made following subcloning of the library indicate that the final immunized library contained approximately 2.1 × 104 clones.
All plasmid DNA used for mice immunization was harvested from bacteria by using endotoxin-free Gigaprep kits (Qiagen) and was diluted to a final concentration of 4 μg/μl in endotoxin-free phosphate-buffered saline. Final plasmid preparations were verified to have <0.1 U of endotoxin/μg of DNA by using the Chromogenic Limulus Amoebocyte Lysate test (BioWhittaker).
Immunizations.
Groups of BALB/c mice (seven per group) were immunized intradermally in both hind footpads (25 μl/footpad); the naive group consisted of four mice. A total of 200 μg of DNA was injected into each mouse per immunization. All groups received four DNA immunizations administered in 10-day intervals. Each group of mice was immunized with one of five different DNA preparations: the pcDNA3.1+ plasmid vector alone (vector), plasmid vector with EGFP cDNA insert (GFP), plasmid vector with AgMuc1 cDNA insert (mucin), plasmid vector with Ag-Aper1 cDNA insert (PM1), and plasmid vector with all blood-fed mosquito midgut cDNA library inserts. Additionally, two separate vector and cDNA library groups were given a final immunization of midgut protein and are referred to as Vec+Prot and Lib+Prot, respectively. These protein immunizations were administered subcutaneously 10 days after the final DNA immunization, with 15 homogenized A. gambiae midguts obtained 24 h after feeding on human blood. Each midgut contains approximately 2 to 3 μg of total protein. The midguts were homogenized in phosphate-buffered saline and were mixed 1:1 with incomplete Freund's adjuvant (total volume, 100 μl).
Serum and splenocyte harvests.
Sera were collected from all mice by tail bleeding immediately prior to immunization. After mosquitoes fed on their blood, mice were sacrificed, total serum was collected, and spleens were harvested for antigen restimulation assays. All sera were obtained from blood that was separated in microtainers (Becton Dickenson) and were immediately frozen until later use.
Antibody analysis.
Western blots were performed with midgut antigens, and immune mouse serum was used as primary antibody. The blotted antigens in each lane consisted of one midgut (either fed on sugar or 24 h after feeding on blood) or recombinant PM1 or mucin (10 μg/lane). Blots were probed with group mice sera (pooled from the seven immunized mice per group) diluted 1:500 followed by goat anti-mouse immunoglobulin G (IgG)-horseradish peroxidase diluted 1:5,000 and were visualized with chemiluminescent substrate.
Serum titers of midgut antigen-specific total Ig, IgG1, and IgG2a were assayed by sandwich enzyme-linked immunosorbant assays (ELISA). Homogenized midguts of A. gambiae taken 24 h after feeding on blood were used as the antigen. Serially diluted serum was incubated on the antigen, and binding antibody was detected by using secondary antibody alkaline phosphatase (AKP) conjugates diluted 1:1,000 (AKP-anti-mouse IgG2a, AKP-anti-mouse IgG1, and AKP-anti-mouse Ig). Two optical density readings taken from the plotted linear range of the serum serial dilutions were normalized for dilution factor, averaged, and blanked against the readings obtained with preimmune sera. Final antibody concentration was calculated on the basis of a regression plot by using known standards. Due to the limited amount of sera obtained from mice tails, group sera (from seven mice) were pooled for all assays requiring antibodies.
Cytokine assays.
Antigen restimulation assays of splenocytes from immunized mice were used to measure mouse cytokine profiles as previously described (9). Briefly, macrophages were harvested from the peritoneal cavity of naive mice 4 days after injection with incomplete Freund's adjuvant. Macrophages (105) were added to each well prior to incubation with antigen, and 106 splenocytes/well were overlayed on the antigen-presenting macrophages. A. gambiae midguts (taken 24 h after feeding on blood) were dissected in RPMI-10 medium (RPMI, 10% heat-inactivated fetal bovine serum, 1× antibiotic/antimycotic [Sigma]), frozen, thawed, and homogenized, and the homogenate was incubated at 65°C for 1 h prior to incubation with macrophages. Splenocytes from each mouse were incubated with each macrophage-presented antigen (midguts of mosquitoes after feeding on blood, 5 guts/well; ovalbumin, −20 μg/well) or without presenting antigen (no antigen) in duplicate. Culture supernatant was collected on day 3 and was assayed for secreted cytokines by using OptEIA sandwich ELISA kits (BD Pharmingen). Duplicate well readings were averaged, and cytokine quantities were calculated on the basis of a regression plot by using known standards according to the manufacturer's instructions. Statistics were calculated on the basis of the seven replicate mice in each group. Intra- and intergroup statistical comparisons were made by the Student's t test.
Mosquito survival and fecundity assays.
A. gambiae larvae were reared on ground fish food tablets, and adults were fed 10% Karo syrup. All mosquitoes were reared in an insectary at 28°C, 75% humidity on a 14 h-10 h light-dark cycle. Adult females were transferred to clean pint-size containers (one for each mouse in the experiment) 2 to 6 days postpupation and were held without sugar overnight prior to feeding on blood. Seven days following the final boost for each group, mice were anesthetized with ketamine-xylazine (80:1) and were placed on the netting of a mosquito cage. Mosquitoes were allowed to feed to repletion. Unfed mosquitoes were removed from each cage and were discarded. A mean number of 70 mosquitoes were fed on each mouse in each group. Five fed mosquitoes were randomly removed and placed in individual oviposition tubes to lay eggs. All mosquitoes were given fresh sugar and water ad libitum until the end of the experiments. Dead mosquitoes in each pint container were counted and removed each day. Seven days following bloodfeeding the surviving mosquitoes were counted. The number of eggs laid in each oviposition tube was recorded 4 days after bloodfeeding. Life tables were constructed for each cage of mosquitoes, and survival curves were analyzed by using Kaplan-Meier log rank analysis. Mosquito survival was first analyzed within groups to identify outliers. Since no outliers were found, data were then pooled to construct group life tables of mosquito survival, and survival curves were analyzed between groups. The egg-laying data were pooled within groups, and data were analyzed between groups by log10 transformation followed by one-way analysis of variance and Student's t test.
For membrane feeding, serum harvested from groups was thawed, pooled, mixed 1:1 with washed human erythrocytes, and fed to A. gambiae mosquitoes via membrane feeders. Human erythrocytes were used to prevent possible binding of any anti-mouse erythrocyte antibodies that may potentially be in the protein-boosted mice sera (Vec+Prot and Lib+Prot). These in vitro serum group feedings were replicated at least twice by using separate cages of A. gambiae mosquitoes. Statistical analysis of mortality was performed as previously described.
RESULTS
Immune profiles of mice immunized with certain mosquito cDNAs are characterized by low yet specific antibody titers and elevated gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) cytokines, while mosquito protein boosts in cDNA-immunized mice shifted immunity toward a Th2-type response. To initially demonstrate that our immunization plasmid containing cDNA is expressed in mice, we immunized both mouse muscle and footpad with the pcDNA:GFP construct. Cryosections of the injection sites showed that muscle fibers expressing GFP were visible 48 h postinjection, and migrating amoeboid-like cells (presumably dendritic cells) expressing GFP were visible in the footpad 24 h postinjection (data not shown). All subsequent immunizations were administered only in the footpad.
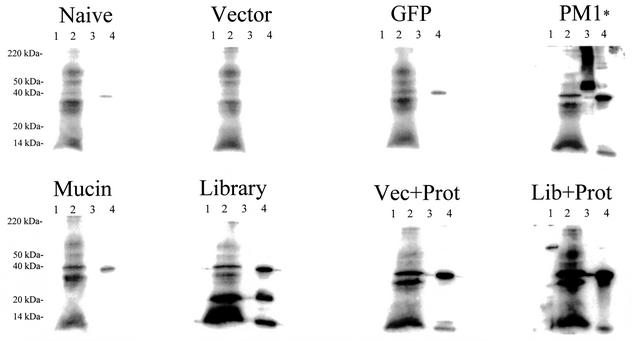
Western blots of various midgut antigens were probed by using mouse group serum as the primary antibody (Fig. 1). These figures demonstrate that immunized mice produced IgG against protein corresponding to the translated midgut cDNAs with which they were immunized. By comparison, the naive, vector, and GFP groups' serum IgG were nonreactive toward midgut proteins. Quantification of serum Ig by ELISA revealed that the titers of total Ig, IgG1, and IgG2a from mice immunized with DNA alone were negligible (Table 1). However, those groups boosted with midgut protein from blood-fed mosquitoes (Vec+Prot and Lib+Prot) generated high antibody titers against midgut antigen. In particular, total Ig concentrations from midgut protein-boosted mice were greater than those of mice immunized with DNA alone, and IgG1 titers were higher than IgG2a titers among the midgut protein-boosted mice.
FIG. 1.
Western blot assays showing mosquito midgut antigens probed with immune mice serum from each immunization group. Lanes 1, midgut; lanes 2, midgut 24 h after feeding on blood; lanes 3, recombinant PM1; lanes 4, recombinant mucin. The recombinant mucin contains a multihistidine tag which may contribute to the observed nonspecific cross-reactivity from several different control groups, and background reactivity is apparent on all blood-fed midgut lanes (lanes 2) due to nonspecific cross-reactivity of the secondary antibody with blood meal components. The PM1* group serum was taken at the time of sacrifice.
TABLE 1.
Blood-fed midgut-specific antibody concentration in immune seruma
| Group | Total Ig | IgG1 | IgG2a |
|---|---|---|---|
| Naive | 0 | 0 | 0 |
| Vector | 0 | 0 | 0 |
| GFP | 0.07 | 0 | 0 |
| PM1 | ND | ND | ND |
| Mucin | 0 | 0 | 0 |
| Library | 0 | 0 | 0 |
| Vec + Prot | 8.14 | 0.07 | 0 |
| Lib + Prot | 2.36 | 0.19 | 0.07 |
Sera were harvested immediately prior to mosquito feeding. The units are micrograms/milliliter; ND, not determined.
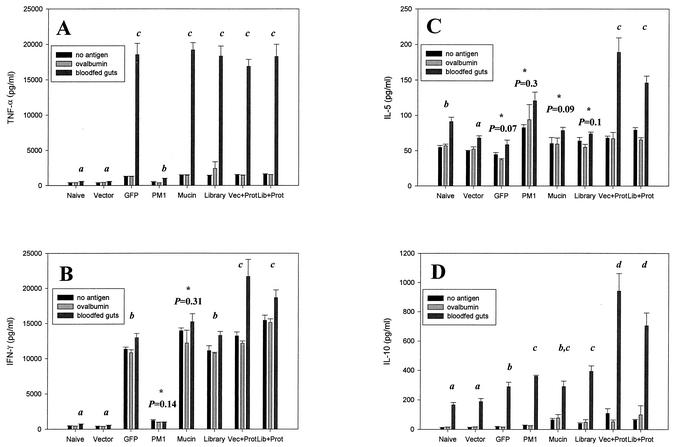
Cytokine profiles were examined by antigen restimulation assays with mouse splenocytes (Fig. 2). TNF-α and IFN-γ were examined as measures of proinflammatory and Th1 cytokines, respectively, and interleukin-5 (IL-5) and IL-10 were examined as measures of Th2 cytokines. The naive, vector, and PM1 groups had dramatically lower TNF-α titers than all other groups (Fig. 2A). IFN-γ levels from these same three groups were very low as well. Interestingly, nonspecific induction of IFN-γ was apparent in the other five groups, and IFN-γ levels were significantly elevated in the protein-boosted groups (Vec+Prot and Lib+Prot) compared to that of the GFP-, mucin-, and cDNA library-immunized groups (Fig. 2B). Th2 cytokine analysis revealed that these same protein-boosted groups generated significantly higher IL-5 and IL-10 levels than did the groups immunized with DNA alone (Fig. 2C and D).
FIG. 2.
The quantity of cytokines TNF-α (A), IFN-γ (B), IL-5 (C), and IL-10 (D) secreted from immunized mouse splenocytes after antigen restimulation. Error bars represent the standard errors of the means from the seven mice in each immunization group. Data was compared within groups and between groups by using the Student's t test. Asterisks denote that cytokine stimulation by blood-fed guts within a group is not significantly higher (P > 0.05) than cytokine stimulation within a group receiving ovalbumin or no antigen; the P value is included below the asterisk. These groups were not included in intergroup comparisons. Groups that did not significantly differ (P > 0.05) in their levels of blood-fed gut-specific cytokine secretion are marked with matching letters (a, b, c, and d).
Mosquito survival is reduced by direct feeding on specific mosquito cDNA-immunized mice.
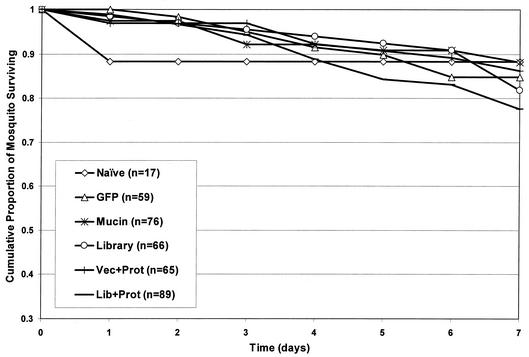
The A. gambiae mosquitoes that fed on immunized mice were monitored for 1 week after their blood meal for changes in mortality. We plotted the cumulative proportion of mosquitoes surviving for more than 7 days, calculated from the life tables of each cage of mosquitoes that fed on each mouse within each immunization group. Each set of survival curves was compared within groups by Kaplan-Meier log rank analysis to look for the presence of outliers. Graphically, the curves exhibit a consistent pattern within each group, and no statistical outliers were found (data not shown). Therefore, mortality data from each immunization group were pooled and a single survival curve was calculated for the entire group (Fig. 3). These data revealed that the mucin- and cDNA library-immunized groups generated significant mosquitocidal immunity compared to that generated by all other groups. The log rank analysis (and significance) of these pooled survival curves is presented in Table 2. cDNA library and/or mucin group immunizations and mosquito feedings were repeated in separate experiments (Table 3). Although not as dramatic, these data verify the induction of mosquitocidal immunity and show that we were able to replicate the results. Mosquitoes feeding on mice from the original experiment were also monitored for changes in egg laying (Table 4). Mosquitoes that fed on the Lib+Prot group exhibited reduced egg laying ability (only 46% of fed mosquitoes), but while the geometric mean of eggs laid per mosquito of the Lib+Prot group is significantly different from that of most other groups (P ≤ 0.01), it does not differ significantly from the number of eggs laid by vector group-fed mosquitoes (6 versus 11 eggs; P = 0.36). Finally, the serum taken at the time of mouse sacrifice was thawed, mixed with red blood cells, and fed to caged A. gambiae mosquitoes via membrane feeders. When the mortality of mosquitoes in these cages was examined, no differences were observed among all groups tested (Fig. 4).
FIG. 3.
The cumulative proportion of surviving mosquitoes each day following a blood meal, plotted from life tables. Mosquitoes fed on mice of the naive group (four mice) and the immunization groups vector, GFP, PM1, mucin, library, Vec+Prot, and Lib+Prot (seven mice each). n, the total number of mosquitoes fed per group. An average of 70 mosquitoes fed on each mouse within each group.
TABLE 2.
Kaplan-Meier log rank statistic and significance from life table comparisons between immunization groups
| Group | Group log rank statistic (significance)
|
||||||
|---|---|---|---|---|---|---|---|
| Naive | Vector | GFP | PM1 | Mucin | Library | Vec + Prot | |
| Vector | 0.15 (0.6975) | ||||||
| GFP | 38.34 (<0.0001) | 41.08 (<0.0001) | |||||
| PM1 | 3.33 (0.0681) | 2.36 (0.1246) | 19.16 (<0.0001) | ||||
| Mucin | 84.3 (<0.0001) | 95.91 (<0.0001) | 23.85 (<0.0001) | 57.55 (<0.0001) | |||
| Library | 62.15 (<0.0001) | 69.3 (<0.0001) | 8.59 (0.0034) | 38.76 (<0.0001) | 3.38 (0.066) | ||
| Vec + Prot | 7.83 (0.0051) | 6.89 (0.0087) | 19.91 (<0.0001) | 0.59 (0.4406) | 74.14 (<0.0001) | 46.86 (<0.0001) | |
| Lib + Prot | 0.13 (0.7221) | 0.84 (0.3599) | 73.93 (<0.0001) | 6.4 (0.0114) | 151.6 (<0.0001) | 113.48 (<0.0001) | 15.75 (<0.0001) |
TABLE 3.
Survival data for mosquitoes
| Group and replicate expta | Cumulative proportion of mosquitoes surviving on day:
|
Kaplan-Meier log rank statistic (P value) vs vector control | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||
| First expt | |||||||||
| Vector (n = 89) | 1 | 0.94 | 0.91 | 0.9 | 0.88 | 0.87 | 0.81 | 0.74 | |
| Mucin (n = 91) | 1 | 0.88 | 0.82 | 0.82 | 0.79 | 0.75 | 0.71 | 0.63 | 3.00 (0.0835) |
| Library (n = 85) | 1 | 0.88 | 0.82 | 0.8 | 0.8 | 0.73 | 0.71 | 0.64 | 2.62 (0.1055) |
| Second expt | |||||||||
| Vector (n = 116) | 1 | 0.96 | 0.93 | 0.9 | 0.85 | 0.85 | 0.83 | 0.8 | |
| Mucin (n = 115) | 1 | 0.96 | 0.91 | 0.9 | 0.83 | 0.78 | 0.75 | 0.67 | 4.55 (0.0329) |
Three mice/group; three injections/mouse.
TABLE 4.
Fecundity data from mosquitoes fed on immunized mouse groups
| Group | % Mosquitoes laying eggs | Mean no. of eggs per egg-laying mosquito (±SEM) | Geometric mean no. of eggs per mosquito |
|---|---|---|---|
| Naive | 70 | 125 ± 19 | 22 |
| Vector | 55 | 93 ± 9 | 11 |
| GFP | 69 | 110 ± 9 | 23 |
| PM1 | 69 | 139 ± 12 | 28 |
| Mucin | 77 | 108 ± 10 | 30 |
| Library | 71 | 140 ± 10 | 30 |
| Vec + Prot | 80 | 120 ± 9 | 40 |
| Lib + Prot | 46 | 86 ± 12 | 6 |
FIG. 4.
The cumulative proportion of surviving mosquitoes each day following blood meals on group sera through membrane feeders. Mouse serum was thawed, pooled within groups, and mixed 1:1 with washed human erythrocytes. The experiment was performed two separate times, and data was pooled between mosquito cages that fed on the same sera. n, the total number of mosquitoes fed per group. An average of 31 mosquitoes fed on each membrane feeder.
DISCUSSION
In this study we used cDNA immunization combined with detailed immune analysis to elucidate factors that contribute to immunization-induced mosquitocidal immunity. Our results demonstrate that host immunization with specific mosquito midgut cDNAs can induce an immune response that is lethal to bloodfeeding mosquitoes. Furthermore, the generation of a mosquitocidal immune response is correlated with the type of immunity generated by each antigen, whereas only high Th1-type and proinflammatory immunity coupled with low Th2-type immunity generated a mosquitocidal immune response; this immunity is not based solely on serum factors such as antibodies.
The individual midgut antigens mucin and PM1 were chosen for immunization for several reasons. Both antigens are potential targets for the development of transmission-blocking vaccines against malaria (14, 45, 46, 56). Additionally, they are both exposed in the lumen of the midgut and thus have the potential to be recognized by ingested immune components of the blood. Furthermore, these targets could possibly help identify influences of molecular location for immune attack on the midgut. PM1 is associated with the PM and thus is not directly attached to the epithelium, but mucin is attached via a GPI anchor. The cDNA library-immunized mouse immunization data represent only the first round of expression library immunization screening (4). However, we feel that these first-round expression library immunization data alone provide an insightful comparison with the other immunization groups that help to explain the results. Likewise, the mosquitocidal immunity achieved from whole cDNA library immunization is notable in itself as it, along with the mucin immunization groups, provides the first demonstration of this novel method to immunologically kill mosquitoes.
The Western blot data reveal that antibodies are indeed produced against the protein forms of the immunogens. This assay is more sensitive than the ELISA and is optimally used as a measure of the absence or presence of targeting antibodies. By comparison, the ELISA data are a measure of anti-midgut antibody quantity in the immune sera, which, in mice immunized with DNA alone, are low enough to be mostly indistinguishable from preimmune sera. However, the ELISA data reveal high titers of anti-midgut antibody in mice boosted with midgut protein and higher quantities of midgut-binding Th2 antibodies (IgG1) than Th1 antibodies (IgG2a) in these same mice.
The cytokine data from antigen restimulation assays provide further insight into the type of immunity generated and, together with the antibody data, can be correlated with the induction of a mosquitocidal immune response. Splenocytes from mouse groups exhibiting the greatest mosquitocidal effects, mucin- and cDNA library-immunized mice (and to a lesser extent GFP-immunized mice), secreted relatively large quantities of TNF-α and IFN-γ but small quantities of Th2 cytokines (IL-5 and IL-10). The vector and PM1 groups were immunized with cDNA alone, but their splenocytes secreted relatively small amounts of proinflammatory and Th1 cytokines and these groups exhibited no mosquitocidal immunity. Finally, splenocytes from the groups given a midgut protein boost secreted relatively large quantities of Th2 cytokines; correspondingly, they generated high midgut-specific antibody titers and exhibited no mosquitocidal immunity.
Our data are consistent with the general observation that protein immunization tends to shift immunity towards a Th2-type, antibody-driven immune response. The groups given protein boost immunizations following immunizations with the midgut library or empty vector were chosen to be included in the experiment a priori due to concerns that no detectable immune response would be found with DNA immunizations alone. Indeed, antibody titers in the DNA-only immunization groups were mostly undetectable. But ultimately these groups were key to demonstrating the immune profile shift to a Th2-type response and the corresponding loss of mosquitocidal immunity.
Many researchers have reported on antimosquito immunity from immunization with midgut protein (1, 2, 18, 28, 29, 36-41, 48, 49). While there is strong evidence that antibodies are the primary mediators of anti-vector transmission-blocking immune responses (28, 41, 48), the data are not so clear for mosquitocidal immunity, primarily because of the variability in existing reports. All animals used in these mosquitocidal immunity studies were immunized with a mix of crude or partially purified mosquito antigens. The methods for isolating antigen has varied from using whole ground mosquitoes (1, 49) to immunizing with midgut antigens that only bind wheat germ agglutinin (41); also, at least five different mosquito species have been studied. Likewise, the vaccinated host animals ranged from rabbits to guinea pigs to mice, and most studies reported that only one or a few of the animals in the experiment generated an immune response that affected mosquito physiology. Increases in mosquito mortality and decreases in mosquito fecundity were reported in some of these studies, yet these effects were most often mutually exclusive of each other (18, 38, 40, 49), and the sample size of affected mosquito groups was often too small for robust statistical analysis. Some of the studies report feeding mosquitoes directly on immunized animals while others fed immune sera to mosquitoes by using membrane feeders, but such differences in methods could change the types of immune effectors that could act to kill the insect. Finally, these studies have only examined antibody titers or antibody-binding profiles of immune serum and suggested that antimosquito effects were due to these antibodies. However, sera from all our groups that were fed to mosquitoes via membrane feeders failed to increase mosquito mortality over control levels. Thus, at least for DNA immunization, soluble serum factors alone are not responsible for the observed mosquitocidal effects.
When mosquitoes feed directly on immunized animals, a spectrum of immune factors (antibodies, lectins, complement, and immune effector cells, such as cytotoxic T lymphocytes, natural killer cells, eosinophils, and macrophages) is ingested, and all may act separately or synergistically to impair the insect. Antibodies by themselves can bind to their antigen targets and could sterically hinder enzyme activity or the function of protein ion channels or could simply block pores, as is thought to be case with antibodies against peritrophins of the blowfly Lucilia cuprina (52). By using purified monoclonal antibodies fed to mosquitoes in membrane feeders, Lal et al. (28) provided evidence that antibodies alone may mediate mosquito killing. It has also been shown that antibodies can cross into the hemocoel of mosquitoes and thereby have access to many other critical vector targets (7, 10, 51). Acting as a receptor for complement, antibodies can direct membrane attack complexes to the surface of cells or they can direct lymphocytes toward the cell surfaces to which they are binding. Importantly, it has been shown that both monocytes/macrophages and polymorphonuclear granulocytes are functional for a time in the mosquito midgut; they can phagocytize malaria gametocytes for up to several hours after ingestion by the mosquito (19, 32, 47).
Our results are likely dependent on the specific DNA with which we chose to immunize mice. The differences in mosquito killing between mucin and PM1 immunizations may be due to their molecular location in the mosquito, as mucin is attached to the cellular membrane via a GPI anchor (45) and PM1 is secreted (46). It is also notable that these two midgut cDNAs induced very different immune profiles, probably due to the presence or absence of certain immunostimulatory epitopes. The effect of GFP cDNA immunization on blood-feeding mosquitoes was surprising because more mosquitoes feeding on this group died than did controls. The immune profile of the GFP group mirrors that from the mucin and cDNA library groups, especially the induction of proinflammatory and Th1 cytokines. These results suggest that DNA immunization resulting from this type of immune profile may have some nonspecific mosquitocidal effects. It may be possible that the GFP-induced effects are due to GFP toxicity which potentially could affect mouse immunity and stimulate proinflammatory cytokines; GFP is known to be toxic to cells, causing apoptosis and affecting regulation of gene expression in cells (17, 33). It is important to note, however, that blood meals from the mucin and cDNA library groups still resulted in significantly higher mosquito mortality than blood meals from the GFP group (P ≤ 0.0034), and anti-GFP sera failed to recognize midgut proteins on Western blots.
We hypothesize that the significant mosquitocidal effects from mucin and midgut library cDNA immunization are due their ability to induce proinflammatory or Th1-type immunity in combination with antigen localization on the midgut surface. In this antibody-dependent cellular cytotoxicity scenario we envision that antibodies bind to mucin or to other lumenal surface midgut proteins that were coded by genes in the midgut library, and immune effector cells recognize the bound antibody and direct secretion of cytotoxic factors toward the midgut cells.
In light of these data, further studies that examine anti-mosquito immunity generated by DNA immunization are warranted. Our data show that it is possible to generate reproducible mosquitocidal immunity, the past lack of which has hampered progress in this field (8, 22, 24, 53). DNA immunization may also stimulate discovery of specific molecular targets within mosquitoes with the help of present mosquito genomic projects, iterative screening of DNA libraries (14), and high-throughput screening methods for mosquitoes (25). Identification of these targets and the development of immunological methods to inhibit their function could have the dual effect of stimulating the generation of antivector vaccines and furthering the understanding of basic vector biology.
Acknowledgments
We thank Marcelo Jacobs-Lorena for his generous gifts of midgut cDNAs and the midgut cDNA library.
This work was funded by NIH grants U19-AI45511 and D43-TW01142, and B. D. Foy was supported by the Louisiana Educational Scholarship Fund.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Alger, N. E., and E. J. Cabrera. 1972. An increase in death rate of Anopheles stephensi fed on rabbits immunized with mosquito antigen. J. Econ. Entomol. 65:165-168. [DOI] [PubMed] [Google Scholar]
- 2.Almeida, A. P., and P. F. Billingsley. 1998. Induced immunity against the mosquito Anopheles stephensi Liston (Diptera: Culicidae): effects on mosquito survival and fecundity. Int. J. Parasitol. 28:1721-1731. [DOI] [PubMed] [Google Scholar]
- 3.Aultman, K. 2000. Report of a meeting on medical entomology research priorities. National Institute of Allergy and Infectious Disease, Bethesda, Md. [Online.] http://mim.nih.gov/english/events/insecticide_resistance_conf/presentations/aultman.doc.
- 4.Barry, M. A., W. C. Lai, and S. A. Johnston. 1995. Protection against mycoplasma infection using expression-library immunization. Nature 377:632-635. [DOI] [PubMed] [Google Scholar]
- 5.Beier, J. C. 1996. Frequent blood-feeding and restrictive sugar-feeding behavior enhance the malaria vector potential of Anopheles gambiae s.l. and An. funestus (Diptera:Culicidae) in western Kenya. J. Med. Entomol. 33:613-618. [DOI] [PubMed] [Google Scholar]
- 6.Beier, J. C. 1998. Malaria parasite development in mosquitoes. Annu. Rev. Entomol. 43:519-543. [DOI] [PubMed] [Google Scholar]
- 7.Beier, J. C., C. N. Oster, J. K. Koros, F. K. Onyango, A. K. Githeko, E. Rowton, D. K. Koech, and C. R. Roberts. 1989. Effect of human circumsporozoite antibodies in Plasmodium-infected Anopheles (Diptera: Culicidae). J. Med. Entomol. 26:547-553. [DOI] [PubMed] [Google Scholar]
- 8.Billingsley, P. F. 1994. Approaches to vector control: new and trusted. 2. Molecular targets in the insect midgut. Trans. R Soc. Trop. Med. Hyg. 88:136-140. [DOI] [PubMed] [Google Scholar]
- 9.Bowman, C. C., and J. D. Clements. 2001. Differential biological and adjuvant activities of cholera toxin and Escherichia coli heat-labile enterotoxin hybrids. Infect. Immun. 69:1528-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brennan, J. D., M. Kent, R. Dhar, H. Fujioka, and N. Kumar. 2000. Anopheles gambiae salivary gland proteins as putative targets for blocking transmission of malaria parasites. Proc. Natl. Acad. Sci. USA 97:13859-13864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casida, J. E., and G. B. Quistad. 1998. Golden age of insecticide research: past, present, or future? Annu. Rev. Entomol. 43:1-16. [DOI] [PubMed] [Google Scholar]
- 12.Coluzzi, M. 1992. Malaria vector analysis and control. Parasitol. Today 8:113-118. [DOI] [PubMed] [Google Scholar]
- 13.Curtis, C. F. 1994. Should DDT continue to be recommended for malaria vector control? Med. Vet. Entomol. 8:107-112. [DOI] [PubMed] [Google Scholar]
- 14.Foy, B. D., G. F. Killeen, T. Magalhaes, and J. C. Beier. 2002. Immunological targeting of critical insect antigens. Am. Entomol. 48:150-163. [Google Scholar]
- 15.Greenwood, B. M. 1997. Malaria transmission and vector control. Parasitol. Today 13:90-92. [DOI] [PubMed] [Google Scholar]
- 16.Gurunathan, S., D. M. Klinman, and R. A. Seder. 2000. DNA vaccines: immunology, application, and optimization. Annu. Rev. Immunol. 18:927-974. [DOI] [PubMed] [Google Scholar]
- 17.Hanazono, Y., K. Terao, H. Shibata, T. Nagashima, N. Ageyama, T. Asano, Y. Ueda, I. Kato, A. Kume, M. Hasegawa, and K. Ozawa. 2002. Introduction of the green fluorescent protein gene into hematopoietic stem cells results in prolonged discrepancy of in vivo transduction levels between bone marrow progenitors and peripheral blood cells in nonhuman primates. J. Gene Med. 4:470-477. [DOI] [PubMed] [Google Scholar]
- 18.Hatfield, P. R. 1988. Anti-mosquito antibodies and their effects on feeding, fecundity and mortality of Aedes aegypti. Med. Vet. Entomol. 2:331-338. [DOI] [PubMed] [Google Scholar]
- 19.Healer, J., A. Graszynski, and E. Riley. 1999. Phagocytosis does not play a major role in naturally acquired transmission-blocking immunity to Plasmodium falciparum malaria. Infect. Immun. 67:2334-2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hemingway, J. 1992. Genetics of insecticide resistance in mosquito vectors of disease. Parasitol. Today 8:296-298. [DOI] [PubMed] [Google Scholar]
- 21.Holder, A. A. 1999. Malaria vaccines. Proc. Natl. Acad. Sci. USA 96:1167-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacobs-Lorena, M., and F. J. A. Lemos. 1995. Immunologic strategies for control of insect disease vectors: a critical assessment. Parasitol. Today 11:144-147. [DOI] [PubMed] [Google Scholar]
- 23.Kaslow, D. C., and S. Welburn. 1996. Insect-transmitted pathogens in the insect midgut, p. 442-462. In M. J. Lehane and P. F. Billingsley (ed.), Biology of the insect midgut. Chapman & Hall, London, United Kingdom.
- 24.Kay, B. H., and D. H. Kemp. 1994. Vaccines against arthropods. Am. J. Trop. Med. Hyg. 50:87-96. [DOI] [PubMed] [Google Scholar]
- 25.Killeen, G. F., B. D. Foy, M. Shahabuddin, W. Roake, A. Williams, T. J. Vaughan, and J. C. Beier. 2000. Tagging blood meals with phagemids allows feeding of multiple-sample arrays to single cages of mosquitoes (Diptera: Culicidae) and the recovery of single recombinant antibody fragment genes from individual insects. J. Med. Entomol. 37:528-533. [DOI] [PubMed] [Google Scholar]
- 26.Killeen, G. F., F. E. McKenzie, B. D. Foy, C. Schieffelin, P. F. Billingsley, and J. C. Beier. 2000. A simplified model for predicting malaria entomologic inoculation rates based on entomologic and parasitologic parameters relevant to control. Am. J. Trop. Med. Hyg. 62:535-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lai, W. C., and M. Bennett. 1998. DNA vaccines. Crit. Rev. Immunol. 18:449-484. [DOI] [PubMed] [Google Scholar]
- 28.Lal, A. A., P. S. Patterson, J. B. Sacci, J. A. Vaughan, C. Paul, W. E. Collins, R. A. Wirtz, and A. F. Azad. 2001. Anti-mosquito midgut antibodies block development of Plasmodium falciparum and Plasmodium vivax in multiple species of Anopheles mosquitoes and reduce vector fecundity and survivorship. Proc. Natl. Acad. Sci. USA 98:5228-5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lal, A. A., M. E. Schriefer, J. B. Sacci, I. F. Goldman, V. Louis-Wileman, W. E. Collins, and A. F. Azad. 1994. Inhibition of malaria parasite development in mosquitoes by anti-mosquito-midgut antibodies. Infect. Immun. 62:316-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lehane, M. J., and P. F. Billingsley (ed.). 1996. Biology of the insect midgut. Chapman & Hall, London, United Kingdom.
- 31.Lemos, F. J., A. J. Cornel, and M. Jacobs-Lorena. 1996. Trypsin and aminopeptidase gene expression is affected by age and food composition in Anopheles gambiae. Insect Biochem. Mol. Biol. 26:651-658. [DOI] [PubMed] [Google Scholar]
- 32.Lensen, A. H., M. Bolmer-Van de Vegte, G. J. van Gemert, W. M. Eling, and R. W. Sauerwein. 1997. Leukocytes in a Plasmodium falciparum-infected blood meal reduce transmission of malaria to Anopheles mosquitoes. Infect. Immun. 65:3834-3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu, H. S., M. S. Jan, C. K. Chou, P. H. Chen, and N. J. Ke. 1999. Is green fluorescent protein toxic to the living cells? Biochem. Biophys. Res. Commun. 260:712-717. [DOI] [PubMed] [Google Scholar]
- 34.Manoutcharian, K., L. I. Terrazas, G. Gevorkian, and T. Govezensky. 1998. Protection against murine cysticercosis using cDNA expression library immunization. Immunol. Lett. 62:131-136. [DOI] [PubMed] [Google Scholar]
- 35.Melby, P. C., G. B. Ogden, H. A. Flores, W. Zhao, C. Geldmacher, N. M. Biediger, S. K. Ahuja, J. Uranga, and M. Melendez. 2000. Identification of vaccine candidates for experimental visceral leishmaniasis by immunization with sequential fractions of a cDNA expression library. Infect. Immun. 68:5595-5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noden, B. H., J. A. Vaughan, M. S. Ibrahim, and J. C. Beier. 1995. An immunological factor that affects Anopheles gambiae survival. J. Am. Mosq. Control Assoc. 11:45-49. [PubMed] [Google Scholar]
- 37.Ramasamy, M. S., and R. Ramasamy. 1990. Effect of anti-mosquito antibodies on the infectivity of the rodent malaria parasite Plasmodium berghei to Anopheles farauti. Med. Vet. Entomol. 4:161-166. [DOI] [PubMed] [Google Scholar]
- 38.Ramasamy, M. S., R. Ramasamy, B. H. Kay, and C. Kidson. 1988. Anti-mosquito antibodies decrease the reproductive capacity of Aedes aegypti. Med. Vet. Entomol. 2:87-93. [DOI] [PubMed] [Google Scholar]
- 39.Ramasamy, M. S., M. Sands, B. H. Kay, I. D. Fanning, G. W. Lawrence, and R. Ramasamy. 1990. Anti-mosquito antibodies reduce the susceptibility of Aedes aegypti to arbovirus infection. Med. Vet. Entomol. 4:49-55. [DOI] [PubMed] [Google Scholar]
- 40.Ramasamy, M. S., K. A. Srikrishnaraj, S. Wijekoone, L. S. Jesuthasan, and R. Ramasamy. 1992. Host immunity to mosquitoes: effect of antimosquito antibodies on Anopheles tessellatus and Culex quinquefasciatus (Diptera: Culicidae). J. Med. Entomol. 29:934-938. [DOI] [PubMed] [Google Scholar]
- 41.Ramasamy, R., I. C. Wanniarachchi, K. A. Srikrishnaraj, and M. S. Ramasamy. 1997. Mosquito midgut glycoproteins and recognition sites for malaria parasites. Biochim. Biophys. Acta. 1361:114-122. [DOI] [PubMed] [Google Scholar]
- 42.Roberts, D. R., S. Manguin, and J. Mouchet. 2000. DDT house spraying and re-emerging malaria. Lancet 356:330-332. [DOI] [PubMed] [Google Scholar]
- 43.Robinson, H. L., H. S. Ginsberg, H. L. Davis, S. A. Johnston, and M. A. Liu. 1997. The scientific future of DNA for immunization. American Academy of Microbiology critical issues colloquia, American Academy of Microbiology, Washington, D.C.
- 44.Rogers, W. O., J. K. Baird, A. Kumar, J. A. Tine, W. Weiss, J. C. Aguiar, K. Gowda, R. Gwadz, S. Kumar, M. Gold, and S. L. Hoffman. 2001. Multistage multiantigen heterologous prime boost vaccine for Plasmodium knowlesi malaria provides partial protection in rhesus macaques. Infect. Immun. 69:5565-5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shen, Z., G. Dimopoulos, F. C. Kafatos, and M. Jacobs-Lorena. 1999. A cell surface mucin specifically expressed in the midgut of the malaria mosquito Anopheles gambiae. Proc. Natl. Acad. Sci. USA 96:5610-5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shen, Z., and M. Jacobs-Lorena. 1998. A type I peritrophic matrix protein from the malaria vector Anopheles gambiae binds to chitin. Cloning, expression, and characterization. J. Biol. Chem. 273:17665-17670. [DOI] [PubMed] [Google Scholar]
- 47.Sinden, R. E., and M. E. Smalley. 1976. Gametocytes of Plasmodium falciparum: phagocytosis by leucocytes in vivo and in vitro. Trans. R Soc. Trop. Med. Hyg. 70:344-345. [DOI] [PubMed] [Google Scholar]
- 48.Srikrishnaraj, K. A., R. Ramasamy, and M. S. Ramasamy. 1995. Antibodies to Anopheles midgut reduce vector competence for Plasmodium vivax malaria. Med. Vet. Entomol. 9:353-357. [DOI] [PubMed] [Google Scholar]
- 49.Sutherland, G. B., and A. B. Ewen. 1974. Fecundity decrease in mosquitoes ingesting blood from specifically sensitized mammals. J. Insect Physiol. 20:655-660. [DOI] [PubMed] [Google Scholar]
- 50.Trager, W. 1939. Acquired immunity to ticks. J. Parasitol. 25:137-139. [Google Scholar]
- 51.Vaughan, J. A., and A. F. Azad. 1988. Passage of host immunoglobulin G from blood meal into hemolymph of selected mosquito species (Diptera: Culicidae). J. Med. Entomol. 25:472-474. [DOI] [PubMed] [Google Scholar]
- 52.Wijffels, G., S. Hughes, J. Gough, J. Allen, A. Don, K. Marshall, B. Kay, and D. Kemp. 1999. Peritrophins of adult dipteran ectoparasites and their evaluation as vaccine antigens. Int. J. Parasitol. 29:1363-1377. [DOI] [PubMed] [Google Scholar]
- 53.Willadsen, P., and P. F. Billingsley. 1996. Immune intervention against blood-feeding insects, p. 323-340. In M. J. Lehane and P. F. Billingsley (ed.), Biology of the insect midgut. Chapman & Hall, London, United Kingdom.
- 54.Willadsen, P., P. Bird, G. S. Cobon, and J. Hungerford. 1995. Commercialisation of a recombinant vaccine against Boophilus microplus. Parasitology 110:S43-S50. [DOI] [PubMed]
- 55.Willadsen, P., G. A. Riding, R. V. McKenna, D. H. Kemp, R. L. Tellam, J. N. Nielsen, J. Lahnstein, G. S. Cobon, and J. M. Gough. 1989. Immunologic control of a parasitic arthropod. Identification of a protective antigen from Boophilus microplus. J. Immunol. 143:1346-1351. [PubMed] [Google Scholar]
- 56.Zieler, H., J. P. Nawrocki, and M. Shahabuddin. 1999. Plasmodium gallinaceum ookinetes adhere specifically to the midgut epithelium of Aedes aegypti by interaction with a carbohydrate ligand. J. Exp. Biol. 202(Part 5):485-495. [DOI] [PubMed] [Google Scholar]