Abstract
Staphylococcus aureus, an important pathogen of humans and other warm-blooded animals, is also capable of killing the nematode Caenorhabditis elegans. Here, we show that C. elegans organisms that are fed S. aureus die over the course of several days in a process that is correlated with the accumulation of bacteria within the nematode digestive tract. Several S. aureus virulence determinants known or speculated to be important in mammalian pathogenesis, including the quorum-sensing global virulence regulatory system agr and the global virulence regulator sarA, the alternative sigma factor σB, alpha-hemolysin, and V8 serine protease, are required for full pathogenicity in nematodes. In addition, several defined C. elegans mutants were examined for susceptibility to S. aureus infection. Enhanced susceptibility to S. aureus killing was observed with loss-of-function mutations in the C. elegans genes esp-2/sek-1 and esp-8/nsy-1, which encode components of a conserved p38 MAP kinase signaling pathway involved in nematode defense against multiple pathogens. These results suggest that key aspects of S. aureus pathogenesis have been conserved, irrespective of the host, and that specific C. elegans host factors can alter susceptibility to this gram-positive human pathogen.
The gram-positive bacterium Staphylococcus aureus is one of the leading causes of both community-acquired and hospital-acquired infections worldwide (30, 82) and is also an economically important cause of bovine and ovine mastitis (3, 80). S. aureus is a remarkably versatile pathogen in humans, capable of causing diseases as diverse as superficial skin infections and soft tissue abscesses and life-threatening infections, such as sepsis, endocarditis, pneumonia, and toxic shock syndrome (52). Treatment of S. aureus infections has become complicated by the emergence of widespread antimicrobial resistance. Isolates resistant to methicillin have steadily increased over the last 3 decades and now cause one-half of all nosocomial S. aureus infections in the United States (4, 30). Most methicillin-resistant S. aureus isolates are resistant to multiple antibiotics (73), and clinical S. aureus isolates with reduced susceptibility (39, 78) and full resistance (5) to vancomycin have been reported.
The ability of S. aureus to cause a wide spectrum of disease has been attributed to its ability to produce a broad array of pathogenicity factors (6). These factors can be subdivided into three general groups: cell-associated products, secreted exoproteins, and regulatory loci. Cell-associated products, including adhesins of the MSCRAMM (microbial surface components recognizing adhesive matrix molecules) family and capsular polysaccharide, facilitate binding to host tissue and help resist host immune responses. Secreted exoproteins, such as cytolysins (e.g., alpha-hemolysin) and extracellular proteases (e.g., V8 protease), are thought to combat host defenses and facilitate tissue invasion and nutrient acquisition (62). Expression of these pathogenicity factors is intricately regulated by a large number of interacting regulatory genes (agr, sarA, sarH1 [sarS], saeR-saeS, rot, srrA-srrB, and arlS-arlR) in response to a variety of environmental conditions (bacterial population density, osmolarity, catabolite concentration, oxygen tension, and pH) (62). The most extensively studied regulators are the global virulence regulatory loci agr and sarA.
The agr (for accessory gene regulator) locus—composed of two divergent transcripts, RNAII (encoding agrA, agrB, agrC, and agrD) and RNAIII—acts to suppress production of cell-associated products while enhancing secreted exoprotein production in response to high bacterial population density in vitro. AgrA and AgrC constitute a two-component histidine kinase-response regulator pair that responds to the extracellular accumulation of a thiolactone-modified octapeptide pheromone, generated by AgrD and AgrB. This autoinducing quorum-sensing circuit induces the expression of RNAIII, a regulatory RNA molecule that acts as the effector molecule of the agr system. agr mutants are attenuated in several animal models of S. aureus disease, including endocarditis, osteomyelitis, septic arthritis, renal and soft tissue abscesses, and endophthalmitis, confirming the importance of coordinate virulence gene regulation in vivo (2, 12, 18, 21, 27).
The sarA (for staphylococcal accessory regulator) locus encodes a 14.5-kDa transcriptional regulator (SarA) that is also involved in cell-associated and secreted protein production. SarA binds to agr promoter elements and is required for full activation of the agr locus (20, 25, 71). In addition, SarA can act independently of RNAIII to directly activate or repress virulence gene expression (9, 10, 17, 51, 84). For example, RNAIII induces exoprotease production, whereas SarA paradoxically represses exoprotease production (9, 17, 22). Thus, SarA has both agr-dependent and agr-independent effects on virulence gene expression. Like agr, the sarA locus has been shown to be important in a number of animal models of S. aureus pathogenesis, including endocarditis, septic arthritis, and endophthalmitis (13, 21, 61). Compared to single-locus disruptions, inactivation of both sarA and agr results in more marked attenuation in vivo (13, 21, 45).
Previously, our laboratories reported the development of a novel host-pathogen model system for gram-positive human pathogens using the nematode Caenorhabditis elegans as a model genetic host. We have shown that Enterococcus faecalis kills adult nematodes over the course of several days in a process that has the characteristics of a persistent infection. Several E. faecalis virulence-related factors, including cytolysin, the extracellular proteases gelatinase and serine protease, and the two-component quorum-sensing system fsr, are important for pathogenicity in both C. elegans and mammalian models of enterococcal infection (36, 59, 69, 76, 77). A p38-like mitogen-activated protein (MAP) kinase signaling pathway in C. elegans that is important in defense against E. faecalis killing has also been recently identified. Two strains with mutations of this pathway, esp-2 and esp-8 (esp for enhanced susceptibility to pathogen), were identified in a genetic screen as being hypersusceptible to killing by the gram-negative human pathogen Pseudomonas aeruginosa (46).
In a previous study of the C. elegans-E. faecalis model, it was briefly noted that a clinical isolate of S. aureus was capable of killing C. elegans (36). In the present study, we examined in detail the antagonistic interaction between S. aureus and C. elegans. C. elegans was killed by a variety of S. aureus strains, and this killing was associated with the accumulation of live bacteria within the nematode alimentary tract. Several S. aureus virulence determinants, known or speculated to be important in mammalian pathogenesis, were required for full virulence in nematodes. In turn, C. elegans esp-2 and esp-8 mutants were also more susceptible to S. aureus killing. Taken together, these results demonstrate that C. elegans can be used as a model host to explore S. aureus pathogenic mechanisms and the interaction with host innate immune defenses.
(This work was presented, in part, at the 102nd General Meeting of the American Society for Microbiology, Salt Lake City, Utah, in May 2002.)
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains used in this study are listed in Table 1. All strains were maintained at −70°C in tryptic soy (TS), brain-heart infusion (BHI), or Luria-Bertani medium (Difco Laboratories, Detroit, Mich.) containing 15% glycerol. S. aureus strains were grown with aeration at 37°C in TS broth that was supplemented with the following antibiotics when appropriate: nalidixic acid, 5 μg/ml; erythromycin, 5 μg/ml; and tetracycline, 10 μg/ml. Enterococcus faecium E007, a clinical strain that has relatively little nematocidal activity (36), was grown in BHI broth (Difco). Bacillus subtilis PY79, a laboratory strain that also has minimal nematocidal activity (36), was grown in TS broth. Escherichia coli strains were grown in Luria-Bertani broth supplemented when appropriate with ampicillin (50 μg/ml). Solid medium was prepared by the addition of 15 g of Bacto Agar (Difco) per liter.
TABLE 1.
Strains and plasmids used
| Strain or plasmid | Relevant genotype and/or phenotypea | Reference and/or source |
|---|---|---|
| S. aureus | ||
| A002 | Clinical S. aureus isolate | MGHb; this study |
| A003 | Clinical S. aureus isolate | 36 |
| A091 | Clinical S. aureus isolate | MGH; this study |
| NCTC 8325 | Wild-type strain; rsbU mutant | 41; The Staphylococcal Genetic Stock Center; J. J. Iandolo |
| NCTC 8325-4 | Prophage-cured derivative of NCTC 8325; rsbU mutant | 63 S. J. Foster |
| RN6390 | Prophage-cured derivative of NCTC 8325 | 68; A. L. Cheung |
| RN6390B | RN6390 reisolated in the Novick laboratory with stable alpha-hemolysin production | 64; M. J. McGavin |
| COL | Wild-type Mcr strain; mec | 75; G. B. Pier |
| Newman | ATCC 25904; high level of clumping factor; σB+ | 32; M. Bischoff |
| Reynolds | Wild-type strain; type 5 capsular polysaccharide | 49; J. C. Lee |
| RN6911 | RN6390 Δagr::tetM agr sarA+ Tcr | 47; A. L. Cheung |
| ALC488 | RN6390 sarA::ermC agr+sarA Emr | 19; A. L. Cheung |
| ALC837 | RN6390 hla::ermC Hla− Emr | 45; A. L. Cheung |
| ALC842 | RN6390 Δagr::tetM sarA::ermC agr sarA Tcr Emr | 19; A. L. Cheung |
| SP6391 | RN6390B sspA::ermAB SspA− Emr | 72; M. J. McGavin |
| IK194 | Newman ΔrsbUVW sigB::ermB σB− Emr | 8; M. Bischoff |
| ALC1435 | RN6390(pALC1420) sarA P1 promoter::gfpuv | 23; A. L. Cheung |
| SH1000 | NCTC 8325-4 rsbU+ | 40; S. J. Foster |
| E. coli | ||
| OP50 | Uracil auxotrophy | 15; laboratory collection |
| DH5α(pSMC2) | DH5α containing a stable plasmid which constitutively expresses a bright mutant of A. victoria GFP; Apr | 11 |
| E. faecium | ||
| E007 | Clinical E. faecium isolate | 36 |
| B. subtilis | ||
| PY79 | Laboratory strain | 85 |
| C. elegans | ||
| Bristol N2 | Wild-type strain | Laboratory strain |
| AU1 | esp-2(ag1) | 46; D. H. Kim |
| AU3 | esp-8(ag3) | 46; D. H. Kim |
| BA1 | fer-1(hc1) | Laboratory strain |
Abbreviations: Mc, methicillin; Tc, tetracycline; Em, erythromycin; Ap, ampicillin; Hla, alpha-hemolysin; SspA, V8 protease.
MGH, Massachusetts General Hospital.
C. elegans strains are listed in Table 1. The nematodes were maintained at 15°C on nematode growth medium plates spread with Escherichia coli strain OP50 as a food source (15, 50). The nematodes were manipulated using established techniques (50).
Nematode-killing assay.
Unless otherwise indicated, S. aureus, E. faecium, and B. subtilis assay plates were prepared as follows. S. aureus strains were grown overnight at 37°C in TS broth supplemented with selective antibiotics as needed. A 1:10 dilution of the saturated culture was made in TS broth, and 10 μl of the diluted culture was spread on 3.5-cm-diameter plates containing TS agar supplemented with 5 μg of nalidixic acid/ml. B. subtilis PY79 was grown overnight at 37°C in TS broth, and 10-μl aliquots of the saturated culture were spread on 3.5-cm-diameter plates containing TS agar. E. faecium E007 was grown overnight at 37°C in BHI broth, and 10-μl aliquots of the saturated culture were spread on 3.5-cm-diameter plates containing TS agar supplemented with 5 μg of nalidixic acid/ml. The plates were incubated at 37°C for 4 to 6 h and then allowed to equilibrate to room temperature for 30 to 60 min before being seeded with worms.
In each assay, 30 to 40 L4-stage nematodes were added per plate, and each assay was carried out in triplicate. The plates were incubated at 25°C and scored for live and dead worms at least every 24 h. A worm was considered dead when it failed to respond to plate tapping or gentle touch with a platinum wire. Worms that died as a result of getting stuck to the wall of the plate were censored from the analysis.
C. elegans pulse-chase experiments.
Sixty to 70 L4-stage nematodes were placed on S. aureus strain NCTC 8325 (hereafter referred to as 8325) lawns grown on TS agar plates, as described above and allowed to feed. After 14, 18, or 24 h, one-half of the total number (between 30 and 35 per plate) were transferred to TS agar plates containing E. faecium E007; the remainder were transferred to TS agar plates spread with 8325. E007 was used as an innocuous food source, because it specifically kills eggs and young hatchlings while allowing normal adult longevity, thus making it easier to keep track of the original adult nematodes through the course of the experiment.
ALC1435 contains the plasmid pALC1420, a recombinant vector derivative of the E. coli-S. aureus shuttle plasmid pSK236 containing a sarA P1 promoter::gfpuv transcriptional fusion (23). The worms were allowed to feed on ALC1435 on TS agar for 24 h and then were transferred to TS agar plates spread with lawns of 8325. After feeding on the 8325 lawn for defined periods, the worms were examined by confocal fluorescence microscopy.
Microscopy.
The nematodes were examined by differential interference contrast microscopy with Nomarski optics using a Zeiss Axioplan2 microscope and by confocal fluorescence microscopy using a Leica TCS NT confocal microscope with spectrophotometric detection by established methodologies (79).
Statistical analysis.
For each killing assay, nematode survival was calculated by the Kaplan-Meier method, and survival differences were tested for significance by use of the log rank test (GraphPad Prism, version 3.0; GraphPad Software, Inc., San Diego, Calif., 1999).
RESULTS
S. aureus kills C. elegans.
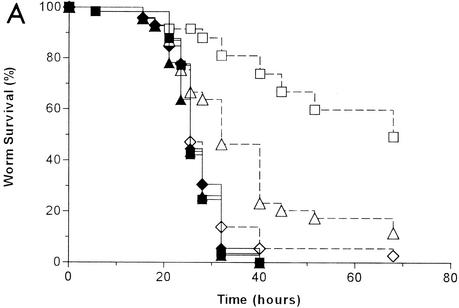
It was previously shown that the S. aureus clinical isolate A003 killed C. elegans over the course of several days (36). To determine whether the ability to kill C. elegans is a common property of S. aureus strains, we tested the abilities of 23 independent clinical S. aureus isolates to kill nematodes. A majority (70%) killed >70% of the nematodes during the course of a standard experiment (5 days). Representative data for three clinical isolates, two that demonstrated significant killing activity (isolates A003 and A091) and one that had marginal killing activity (isolate A002), are shown in Fig. 1A.
FIG. 1.
C. elegans killing by S. aureus. (A) Kaplan-Meier survival plots of worms fed S. aureus clinical isolates A002 (squares) (n = 78), A003 (circles) (n = 88), and A091 (triangles) (n = 91) and B. subtilis strain PY79 (diamonds) (n = 59; negative control). (B) Kaplan-Meier survival plots of worms fed S. aureus strains RN6390 (circles) (n = 85), Newman (squares) (n = 87), COL (triangles) (n = 92), Reynolds (solid diamonds) (n = 101) and B. subtilis strain PY79 (open diamonds) (n = 59; negative control).
We also tested the abilities of several well-characterized S. aureus laboratory strains to kill C. elegans. As shown in Fig. 1B, all of the laboratory strains tested exhibited a high level of nematocidal activity. 8325 is a well-studied strain used by Pattee and colleagues to generate a physical and genetic map of S. aureus (41). COL is a prototypical methicillin-resistant S. aureus strain (75). 8325 and COL are being fully sequenced at the University of Oklahoma Genome Center (http://www.genome.ou.edu/staph.html) and The Institute for Genomic Research (http://www.tigr.org), respectively, and both strains have been used in numerous animal models of S. aureus infection. RN6390 is a derivative of 8325 that has been cured of prophages and that exhibits stable alpha-hemolysin production (68). Newman (ATCC 25904) is a clinical isolate that produces a high level of clumping factor (32). Reynolds is a wild-type isolate with type 5 capsular polysaccharide used in experimental vaccine studies (49).
Nematodes progress through four larval stages (called L1 to L4) before maturing into egg-laying adults. All of these developmental stages were killed by S. aureus. Most S. aureus strains, including those clinical isolates that killed late larval (L4-stage) and adult worms poorly, efficiently killed the early larval stages so that no nematode population growth occurred under standard assay conditions. As has been reported for P. aeruginosa PA14 (55, 81), both the medium on which the bacterial lawn was grown and the developmental stage of the worms played important roles in determining the rate of killing. In the case of S. aureus killing, adult worms were moderately more susceptible than L4-stage worms, and the nematodes survived slightly longer on BHI agar than on TS agar (data not shown). No killing was observed when the nematodes were fed either heat- or antibiotic-killed S. aureus (data not shown), suggesting that killing requires the presence of live bacteria.
Killing is correlated with colonization of the nematode intestine by S. aureus.
When the worms were feeding on S. aureus, nematode locomotion, pharyngeal pumping, and foraging appeared normal for the first 16 to 20 h. Over the next 24 to 48 h, all of these activities progressively decreased until the worms became immobile and died. Many dead nematodes lost all apparent cellular architecture and appeared as “ghosts” in the bacterial lawn. We have observed this same phenotype after nematode feeding on E. faecalis, and O'Quinn and colleagues reported that nematodes that died while feeding on Burkholderia pseudomallei also appear as ghosts in the bacterial lawn (65). Moreover, we occasionally observed the so-called “bag of worms” phenotype, in which the eggs of a gravid hermaphrodite hatched internally and the resulting larvae consumed the parent (58). Bagging has been observed when nematodes feed on other pathogens and is particularly prominent with E. faecalis (36). Although it is not known why bagging is prevalent when nematodes feed on bacterial pathogens, one possibility is that infected worms may become too weak to lay eggs normally. Matricide is not the sole mechanism of killing, however, since males and the sterile mutant fer-1 were also killed by S. aureus (data not shown).
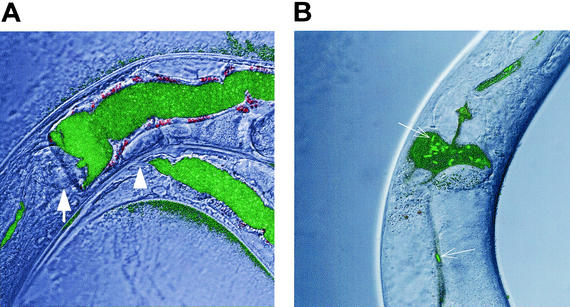
Worms fed S. aureus accumulated bacteria within their digestive tracts. Examination of the worms by Nomarski differential interference contrast microscopy showed significant distention of the intestinal lumen with large numbers of intact bacteria. In contrast, worms fed E. coli or B. subtilis had slender intestinal lumina with no visible intact bacteria (data not shown). To confirm that distention is due to the accumulation and/or proliferation of live S. aureus, we examined worms fed on lawns of strain ALC1435, a RN6390 transformant containing a shuttle vector with a sarA P1 promoter::gfpuv transcriptional fusion, which expresses Aequorea victoria green fluorescent protein (GFP) constitutively. After 24 h of nematode feeding on ALC1435, confocal fluorescence microscopy revealed innumerable fluorescent cocci throughout the distended lumens of the worm intestines (Fig. 2A). In contrast, nematodes fed GFP-expressing E. coli DH5α had only a small number of intact fluorescent bacilli in the proximal intestine (Fig. 2B).
FIG. 2.
Distention of the C. elegans digestive tract with S. aureus but not E. coli. Shown are confocal fluorescence photomicrographs of the terminal bulb and anterior intestinal tracts of nematodes after feeding for 24 h on lawns of S. aureus RN6390/gfp (ALC1435) (A) or E. coli DH5α/gfp (B). (A) The nematode intestinal lumen, from the terminal bulb (arrow) to the anus (arrowhead) was distended with GFP-expressing cocci after the worm was fed RN6390/gfp. (B) In contrast, only a few GFP-expressing bacilli (arrows) were observed in the nondistended intestinal tracts of worms feeding on DH5α/gfp. Magnification, ×63.
To further investigate the mechanisms of S. aureus killing of C. elegans, we tested the ability of worms to be rescued from S. aureus exposure. L4-stage worms were allowed to feed on lawns of 8325 for various lengths of time and then were transferred to either another plate of 8325 or a plate with the nonpathogenic bacterium E. faecium E007. To limit the ability of inadvertently transferred 8325 to grow on the E007 plate, we added gentamicin (25 μg/ml) to the media, which selectively kills 8325. As shown in Fig. 3A, transfer to the E. faecium plate rescued worms from S. aureus exposure until that exposure was 18 h or longer. Worms transferred at 8 h of exposure or earlier had normal life spans (data not shown). Interestingly, as described above, most worms appeared to feed and behave normally for the first 16 to 20 h of feeding. Similar results were obtained when worms were transferred from lawns of 8325 to E007 plates grown without gentamicin, suggesting that rescue was not simply a result of antibiotic treatment of S. aureus within the nematode intestinal tract. These data suggest that worms are unable to recover from the deadly effect of exposure to S. aureus once they have been in contact with the bacteria for a sufficient length of time (>8 h for strain 8325). These data also suggest that S. aureus can be cleared from the nematode gut once the worms are transferred to a new food source. In contrast, E. faecalis persistently colonizes and proliferates in the nematode gut, even after transfer to an innocuous food source (36).
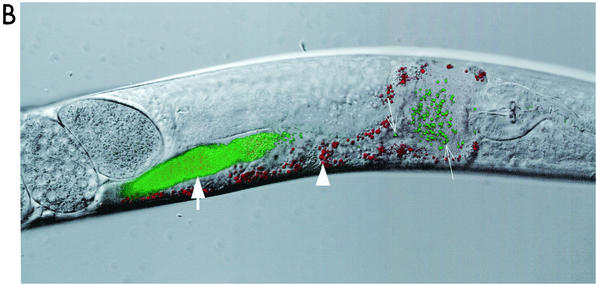
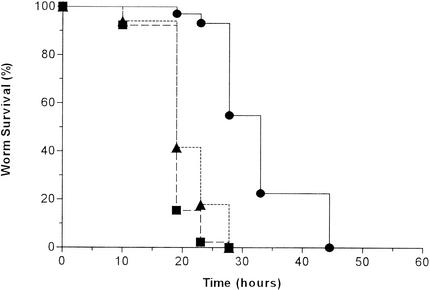
FIG. 3.
S. aureus does not persistently colonize the digestive tract of C. elegans. (A) C. elegans pulse-chase experiment (see Materials and Methods) showing nematode survival after transfer from S. aureus strain 8325 to either 8325 (solid symbols) or E. faecium E007 (open symbols): the worms were transferred after feeding for 14 (squares), 18 (triangles), and 24 (diamonds) h. (B) Confocal fluorescence photomicrograph of a nematode shifted to 8325 after feeding for 24 h on S. aureus RN6390/gfp. The image was obtained 45 min after transfer of the worm. Densely packed GFP-expressing cocci are visible in the midportion of the intestinal tract of the worm (thick arrow), whereas a few dozen GFP-expressing cocci are identifiable more proximally (thin arrows) in the distended anterior intestinal lumen, distal to the terminal bulb. Weak autofluorescence (red channel) of nematode intestinal cells is also visible (arrowhead). Magnification, ×63.
To explore the question of persistent colonization further, we examined worms that were first allowed to feed on ALC1435 for 24 h and were then transferred to plates of nonfluorescent RN6390. After the worms fed on RN6390, the bolus of fluorescent S. aureus moved down the intestinal lumen (Fig. 3B). After 2 h, no fluorescence could be detected in the nematode alimentary tract (data not shown).
Virulence determinants important for S. aureus pathogenesis in mammals are also involved in killing C. elegans.
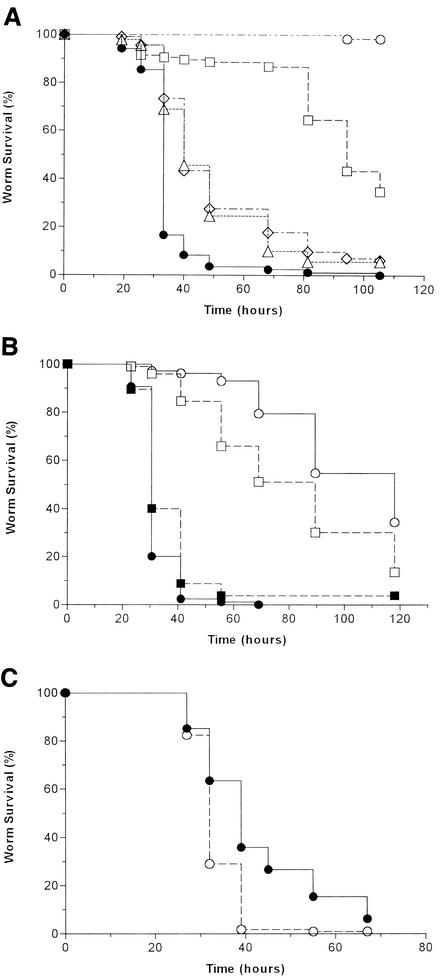
To test the hypothesis that S. aureus utilizes the same virulence strategies to infect both C. elegans and mammalian hosts, we evaluated in the nematode model system sets of isogenic S. aureus strains carrying mutations in virulence determinants important for mammalian infection. First, we tested the roles of the S. aureus global virulence regulators agr and sarA in nematocidal activity. Compared to the parental strain RN6390, C. elegans killing was highly attenuated while feeding on lawns of the agr mutant RN6911 and was moderately attenuated on lawns of the sarA mutant ALC488 and the agr-sarA double mutant ALC842 (Fig. 4A). There was no significant difference in killing between ALC488 and ALC842.
FIG. 4.
Mammalian virulence factors enhance S. aureus killing of C. elegans. (A) Kaplan-Meier survival plots of worms fed S. aureus strains RN6390 (wild type; n = 103) (solid circles), RN6911 (agr mutant; n = 105) (squares), ALC488 (sarA mutant; n = 92) (triangles), ALC842 (agr sarA double mutant; n = 113) (diamonds), and B. subtilis strain PY79 (negative control; n = 61) (open circles). Pairwise comparisons (log rank test) by strain were as follows: RN6390 versus RN6911, P < 0.0001; RN6390 versus ALC488, P < 0.0001; RN6390 versus ALC842, P < 0.0001; ALC488 versus ALC842, P = 0.6. (B) Kaplan-Meier survival plots of worms fed S. aureus strains RN6390 (wild type; n = 85) (solid circles), ALC837 (RN6390 hla mutant; n = 103) (open circles), RN6390B (wild type; n = 95) (solid squares), and SP6391 (RN6390B sspA mutant; n = 98) (open squares). Pairwise comparisons (log rank test) by strain were as follows: RN6390 versus ALC837, P < 0.0001; RN6390B versus SP6391, P < 0.0001. (C) Kaplan-Meier survival plots of worms fed S. aureus strains NCTC 8325-4 (wild type; n = 101) (solid circles) and SH1000 (NCTC 8325-4 rsbU+; n = 114) (open circles). Pairwise comparison (log-rank test) by strain: NCTC 8325-4 versus SH1000, P < 0.0001.
Next, we examined the role in C. elegans killing of S. aureus alpha-hemolysin, the production of which is positively regulated by both agr and sarA (dependent on and independent of agr) (17, 21). Alpha-hemolysin, encoded by hla, is a potent cytolytic pore-forming toxin that has been shown to be an important virulence factor in a number of mammalian models of S. aureus infection (14, 16, 45, 57, 67). We compared the survival of nematodes feeding on the hla mutant ALC837 to those feeding on the parental strain RN6390. As shown in Fig. 4B, ALC837 was significantly attenuated in worm killing compared to RN6390, demonstrating that alpha-hemolysin is important for S. aureus virulence in C. elegans as well as vertebrate hosts.
Considering that virulence gene regulation by sarA acts, in part, through agr, we were interested in further investigating the difference in phenotype between agr (highly attenuated) and sarA (moderately attenuated) mutants in C. elegans. We speculated that the difference in the agr and sarA mutant strains may be reflective of agr-independent sarA virulence gene regulation. To test this hypothesis, we evaluated the role of V8 protease, encoded by sspA, in C. elegans killing, since its production is contrarily regulated by the agr (a protease activator) and sarA (a protease repressor) loci (9, 17, 43). Recently, the importance of V8 protease to in vivo survival and virulence in three different animal models of infection was demonstrated by signature-tagged mutagenesis (27). The nematocidal activity of strain SP6391, which carries a nonpolar mutation in the V8 protease gene sspA, was examined. SP6391 was significantly attenuated in C. elegans killing compared to the parental strain RN6390B (Fig. 4B). Thus, increased production of certain virulence-related products, like V8 protease, in the sarA mutant may partially counterbalance the reduced production of other virulence factors, like alpha-hemolysin, thereby explaining the moderately attenuated phenotype of the sarA mutant.
Since the agr system acts to induce exoprotein production (including alpha-hemolysin and V8 protease) at high cell density, post-exponential-phase cells would be predicted to be more virulent than exponential-phase cells in nematode killing. To test this hypothesis, we compared C. elegans survival on lawns of S. aureus grown for 24 h (thick-lawn assay) with those grown for 4 h (standard assay). After 24 h of feeding, nematode survival was moderately shorter in the thick-lawn assay than in the standard assay for most strains tested, in agreement with this hypothesis. However, final nematode mortality was greater in the standard assay than in the thick-lawn assay for many strains (data not shown).
The role of the alternative sigma factor σB in the response of S. aureus to stress and its potential role in virulence has been the focus of several recent studies (37, 40, 48, 60). Although σB has been shown to positively regulate sarA expression, heat tolerance, hydrogen peroxide resistance, and biofilm formation in response to environmental stress (7, 29, 38, 70), a direct role for σB in virulence has not been demonstrated to date in vivo (60). Importantly, S. aureus strain 8325, used in most of the C. elegans-killing assays described above, is a natural σB mutant, by virtue of an 11-bp deletion in the sigB activator, rsbU, suggesting that σB may not play a significant role in C. elegans killing. To further investigate the role, if any, of σB in C. elegans killing, we tested two different sigB deletion mutants paired with their parental strains, as well as SH1000, a rsbU+ derivative of strain NCTC 8325-4. In all cases, the strains expressing σB were modestly (but significantly) more virulent than the isogenic σB-deficient strains. An example is shown in Fig. 4C, comparing SH1000 with its parent, NCTC 8325-4. To our knowledge, this is the first in vivo demonstration of a role for σB in virulence.
Mutants with mutations of the nematode innate immune system are hypersusceptible to S. aureus killing.
Two C. elegans mutants that exhibit enhanced susceptibility to pathogens, esp-2 and esp-8, have recently been characterized (46). These mutants have normal life spans when fed E. coli, the normal nematode food source, but are markedly more susceptible to P. aeruginosa-mediated killing than wild-type worms. esp-2 corresponds to the C. elegans sek-1 gene, which encodes a MAP kinase kinase, and esp-8 corresponds to the C. elegans nsy-1 gene, which encodes an upstream MAP kinase kinase kinase. SEK-1 is homologous to the MKK3/6 and MKK4 family of mammalian MAPKKs, while NSY-1 is a homologue of the mammalian ASK-1 MAPKKK. SEK-1/ESP-2 and NSY-1/ESP-8 function in a MAP kinase signaling pathway that activates the C. elegans p38 MAP kinase ortholog, PMK-1, suggesting that this pathway may be an ancient conserved component of the nematode immune response to pathogen attack (46). As shown in Fig. 5, esp-2 and esp-8 mutants of C. elegans were also more susceptible to killing by the wild-type S. aureus strain 8325.
FIG. 5.
C. elegans mutants with enhanced susceptibility to S. aureus killing. Shown are Kaplan-Meier survival plots of wild-type N2 C. elegans (n = 102) (circles), esp-2(ag1) mutant worms (n = 91) (squares), and esp-8(ag3) mutant worms (n = 84) (triangles) fed S. aureus strain 8325. Pairwise comparisons (log rank test) by worm strain were as follows: N2 versus esp-2(ag1), P < 0.0001; N2 versus esp-8(ag3), P < 0.0001.
DISCUSSION
There is growing interest in using nonvertebrate, genetically tractable organisms as model hosts to investigate virulence mechanisms of and defense responses against human pathogens (33, 34, 54, 83). In this study, we report the development of an S. aureus-C. elegans pathogenicity model system that demonstrates significant parallels to S. aureus infections in vertebrates at the molecular level.
A broad spectrum of clinical and laboratory S. aureus strains kill nematodes when substituted for E. coli as nutriment. Interestingly, different S. aureus strains killed nematodes with various efficiencies. Strain-specific differences in C. elegans killing have been observed as well for P. aeruginosa (28, 55, 81), E. faecalis (36), Salmonella enterica (1), and B. pseudomallei (35, 65), suggesting that different strains encode or express different complements of virulence-related factors (26). Worm death occurred after feeding on S. aureus for 48 to 72 h for most strains tested, which is faster than killing observed with E. faecalis but not as fast as that with Streptococcus pneumoniae (36). Like E. faecalis, P. aeruginosa, and S. enterica, killing by S. aureus was associated with the accumulation of live bacteria within the nematode alimentary tract. Moving nematodes to a benign food source cleared intestinal colonization with S. aureus. P. aeruginosa also accumulates but does not persist in the nematode intestine, whereas E. faecalis and S. enterica accumulate, persist, and proliferate in the nematode intestine (1, 36, 81). How some bacteria persist in the nematode digestive tract is not known.
Recently, Jansen et al. reported that killing of C. elegans by Streptococcus pyogenes and probably S. pneumoniae is mediated by hydrogen peroxide (42). Killing by these organisms occurs within a matter of hours and is not associated with the accumulation of bacteria within the nematode alimentary tract. In addition, the authors reported that S. aureus did not kill C. elegans in their assays, in contrast to our finding that most strains have potent nematocidal activity. Perhaps the strain used in their assays, SAI, H Mi1, has little intrinsic virulence toward C. elegans, as we have found with a subset of clinical isolates. Alternatively, the lack of killing may be a reflection of the different assay conditions used. For example, S. aureus was cultured in Todd-Hewitt medium supplemented with 0.5% yeast extract (THY medium) in the assays carried out by Jansen et al., whereas our assays were all performed on TS agar. We found that S. aureus grown on TS agar was more pathogenic than that grown on BHI agar. Similarly, C. elegans killing by P. aeruginosa is dependent on the media used (55, 81). The virulence to C. elegans of the S. aureus strains evaluated in this report, if grown in THY medium, is not known.
The S. aureus global virulence regulators agr and, to a lesser extent, sarA were required for full nematocidal activity; interestingly, an agr-sarA double mutant was no more attenuated than a sarA mutant itself. A homologue of the agr locus, the E. faecalis fsr virulence regulator, controls the production of two extracellular proteases, gelatinase and serine protease, which are also required for full virulence in both C. elegans and mice (69, 76). Production of extracellular proteases in S. aureus is similarly activated by the agr locus but is repressed by the sarA locus (9, 17, 43). Like E. faecalis serine protease, we found that S. aureus V8 protease was important in C. elegans killing. In addition to V8 protease, both agr and sarA regulate the production of other secreted products, such as alpha-hemolysin (17, 24, 47), and we found that alpha-hemolysin was also required for C. elegans killing. Differences in C. elegans killing between agr and sarA mutants, therefore, may be due, at least in part, to differences in regulation of virulence gene expression in these strains (31). The fact that the agr-sarA double mutant was no more attenuated in C. elegans killing than the sarA single mutant (and less attenuated that the agr single mutant) may reflect the complex interplay of virulence gene expression directed by RNAIII and SarA in S. aureus.
The alternative sigma factor σB controls the general stress response and influences the production of many virulence-associated products in S. aureus. Inactivation of σB had a small but demonstrable negative effect on virulence in C. elegans but not in four previously reported animal models (40, 60). The effect of σB on virulence in C. elegans but not in rodents may reflect a fundamental difference between nematodes and higher-order hosts. Alternatively, the ability to assay the survival of hundreds of worms in each experiment may allow the C. elegans model to detect small differences in S. aureus in vivo fitness not easily observed in vertebrate hosts. Interestingly, σB-positive strains have decreased production of V8 serine protease and alpha-hemolysin, possibly due to reduced levels of RNAIII (8, 38, 40, 66). Why σB-positive strains were measurably more virulent than isogenic σB-deficient strains in nematodes, despite having reduced levels of SspA and Hla, is not known. The most straightforward explanation is that altered levels of other σB-dependent gene products compensate for the reduced production of these extracellular proteins important for nematode infection.
C. elegans esp-2 and esp-8 mutants exhibited increased susceptibility to S. aureus infection, demonstrating that a conserved p38 MAP kinase signaling pathway is important in innate immunity against S. aureus, as previously shown for P. aeruginosa. In human neutrophils, phagocytosis of S. aureus activates p38 MAP kinase and induces apoptosis (53, 56). Inhibition of p38 MAP kinase along with p44/42 MAP kinase partially inhibits neutrophil destruction of S. aureus in vitro (74). While p38 MAP kinase appears to be important in nematode defense, the effectors of the innate immune response to S. aureus infection are not known. Recently, Kato and colleagues have identified a C. elegans antimicrobial peptide, called ABF-2, which is primarily produced in the worm pharynx and appears to be secreted into the pharyngeal lumen. Recombinant ABF-2 was shown to have broad antimicrobial activities against gram-positive bacteria, gram-negative bacteria, and yeast. Of the organisms tested, ABF-2 was most active against S. aureus, with a 50% microbicidal concentration of 0.005 μM (44). The role of ABF-2 in nematode defense against S. aureus in vivo is under investigation.
The experiments presented here demonstrate that S. aureus infects C. elegans, ultimately leading to worm death, and that key aspects of S. aureus pathogenesis and interaction with the innate immune system have been mechanistically conserved from nematodes through vertebrates. Based on our prior experience with similar pathogen-nematode systems, we predict that C. elegans will be a useful model for the identification of novel staphylococcal genes important in mammalian pathogenesis and for continued exploration of host innate immune defense systems.
Acknowledgments
C.D.S. and J.B. contributed equally to this work.
We thank M. Bischoff, A. L. Cheung, S. J. Foster, J. J. Iandolo, J. C. Lee, M. J. McGavin, and G. B. Pier for generously providing bacterial strains and A. L. Cheung, S. J. Foster, and M. J. McGavin for helpful discussions. Some C. elegans strains were originally obtained from the Caenorhabditis Genetics Center, which is supported by the National Institutes of Health National Center of Research Resources.
This work was supported by a Postdoctoral Research Fellowship for Physicians from the Howard Hughes Medical Institute to C.D.S. and by a research grant from Aventis Pharmaceuticals to F.M.A. and S.B.C.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Aballay, A., P. Yorgey, and F. M. Ausubel. 2000. Salmonella typhimurium proliferates and establishes a persistent infection in the intestine of Caenorhabditis elegans. Curr. Biol. 10:1539-1542. [DOI] [PubMed] [Google Scholar]
- 2.Abdelnour, A., S. Arvidson, T. Bremell, C. Ryden, and A. Tarkowski. 1993. The accessory gene regulator (agr) controls Staphylococcus aureus virulence in a murine arthritis model. Infect. Immun. 61:3879-3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson, D. C. 1983. Veterinary aspects of staphylococci, p. 193-241. In C. S. F. Easmon and C. Adlam (ed.), Staphylococci and staphylococcal disease, vol. 1. Academic Press, Inc., London, United Kingdom.
- 4.Anonymous. 2001. National Nosocomial Infections Surveillance (NNIS) System report, data summary from January 1992- June 2001, issued August 2001. Am. J. Infect. Control 29:404-421. [DOI] [PubMed] [Google Scholar]
- 5.Anonymous. 2002. Staphylococcus aureus resistant to vancomycin—United States, 2002. Morb. Mortal. Wkly. Rep. 51:565-567. [PubMed] [Google Scholar]
- 6.Archer, G. L. 1998. Staphylococcus aureus: a well-armed pathogen. Clin. Infect. Dis. 26:1179-1181. [DOI] [PubMed] [Google Scholar]
- 7.Bateman, B. T., N. P. Donegan, T. M. Jarry, M. Palma, and A. L. Cheung. 2001. Evaluation of a tetracycline-inducible promoter in Staphylococcus aureus in vitro and in vivo and its application in demonstrating the role of sigB in microcolony formation. Infect. Immun. 69:7851-7857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bischoff, M., J. M. Entenza, and P. Giachino. 2001. Influence of a functional sigB operon on the global regulators sar and agr in Staphylococcus aureus. J. Bacteriol. 183:5171-5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blevins, J. S., K. E. Beenken, M. O. Elasri, B. K. Hurlburt, and M. S. Smeltzer. 2002. Strain-dependent differences in the regulatory roles of sarA and agr in Staphylococcus aureus. Infect. Immun. 70:470-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blevins, J. S., A. F. Gillaspy, T. M. Rechtin, B. K. Hurlburt, and M. S. Smeltzer. 1999. The Staphylococcal accessory regulator (sar) represses transcription of the Staphylococcus aureus collagen adhesin gene (cna) in an agr-independent manner. Mol. Microbiol. 33:317-326. [DOI] [PubMed] [Google Scholar]
- 11.Bloemberg, G. V., G. A. O'Toole, B. J. Lugtenberg, and R. Kolter. 1997. Green fluorescent protein as a marker for Pseudomonas spp. Appl. Environ. Microbiol. 63:4543-4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Booth, M. C., R. V. Atkuri, S. K. Nanda, J. J. Iandolo, and M. S. Gilmore. 1995. Accessory gene regulator controls Staphylococcus aureus virulence in endophthalmitis. Investig. Ophthalmol. Vis. Sci. 36:1828-1836. [PubMed] [Google Scholar]
- 13.Booth, M. C., A. L. Cheung, K. L. Hatter, B. D. Jett, M. C. Callegan, and M. S. Gilmore. 1997. Staphylococcal accessory regulator (sar) in conjunction with agr contributes to Staphylococcus aureus virulence in endophthalmitis. Infect. Immun. 65:1550-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bramley, A. J., A. H. Patel, M. O'Reilly, R. Foster, and T. J. Foster. 1989. Roles of alpha-toxin and beta-toxin in virulence of Staphylococcus aureus for the mouse mammary gland. Infect. Immun. 57:2489-2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brenner, S. 1974. The genetics of Caenorhabditis elegans. Genetics 77:71-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Callegan, M. C., L. S. Engel, J. M. Hill, and R. J. O'Callaghan. 1994. Corneal virulence of Staphylococcus aureus: roles of alpha-toxin and protein A in pathogenesis. Infect. Immun. 62:2478-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan, P. F., and S. J. Foster. 1998. Role of SarA in virulence determinant production and environmental signal transduction in Staphylococcus aureus. J. Bacteriol. 180:6232-6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan, P. F., S. J. Foster, E. Ingham, and M. O. Clements. 1998. The Staphylococcus aureus alternative sigma factor σB controls the environmental stress response but not starvation survival or pathogenicity in a mouse abscess model. J. Bacteriol. 180:6082-6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheung, A., K. Eberhardt, and J. Heinrichs. 1997. Regulation of protein A synthesis by the sar and agr loci of Staphylococcus aureus. Infect. Immun. 65:2243-2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheung, A. L., M. G. Bayer, and J. H. Heinrichs. 1997. sar genetic determinants necessary for transcription of RNAII and RNAIII in the agr locus of Staphylococcus aureus. J. Bacteriol. 179:3963-3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheung, A. L., K. J. Eberhardt, E. Chung, M. R. Yeaman, P. M. Sullam, M. Ramos, and A. S. Bayer. 1994. Diminished virulence of a sar−/agr− mutant of Staphylococcus aureus in the rabbit model of endocarditis. J. Clin. Investig. 94:1815-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheung, A. L., J. M. Koomey, C. A. Butler, S. J. Projan, and V. A. Fischetti. 1992. Regulation of exoprotein expression in Staphylococcus aureus by a locus (sar) distinct from agr. Proc. Natl. Acad. Sci. USA 89:6462-6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheung, A. L., C. C. Nast, and A. S. Bayer. 1998. Selective activation of sar promoters with the use of green fluorescent protein transcriptional fusions as the detection system in the rabbit endocarditis model. Infect. Immun. 66:5988-5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheung, A. L., and P. Ying. 1994. Regulation of alpha- and beta-hemolysins by the sar locus of Staphylococcus aureus. J. Bacteriol. 176:580-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chien, Y., and A. L. Cheung. 1998. Molecular interactions between two global regulators, sar and agr, in Staphylococcus aureus. J. Biol. Chem. 273:2645-2652. [DOI] [PubMed] [Google Scholar]
- 26.Choi, J. Y., C. D. Sifri, B. C. Goumnerov, L. G. Rahme, F. M. Ausubel, and S. B. Calderwood. 2002. Identification of virulence genes in a pathogenic strain of Pseudomonas aeruginosa by representational difference analysis. J. Bacteriol. 184:952-961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coulter, S. N., W. R. Schwan, E. Y. Ng, M. H. Langhorne, H. D. Ritchie, S. Westbrock-Wadman, W. O. Hufnagle, K. R. Folger, A. S. Bayer, and C. K. Stover. 1998. Staphylococcus aureus genetic loci impacting growth and survival in multiple infection environments. Mol. Microbiol. 30:393-404. [DOI] [PubMed] [Google Scholar]
- 28.Darby, C., C. L. Cosma, J. H. Thomas, and C. Manoil. 1999. Lethal paralysis of Caenorhabditis elegans by Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 96:15202-15207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deora, R., T. Tseng, and T. K. Misra. 1997. Alternative transcription factor σSB of Staphylococcus aureus: characterization and role in transcription of the global regulatory locus sar. J. Bacteriol. 179:6355-6359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diekema, D. J., M. A. Pfaller, F. J. Schmitz, J. Smayevsky, J. Bell, R. N. Jones, and M. Beach. 2001. Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997-1999. Clin. Infect. Dis. 32(Suppl. 2):S114-S132. [DOI] [PubMed] [Google Scholar]
- 31.Dunman, P. M., E. Murphy, S. Haney, D. Palacios, G. Tucker-Kellogg, S. Wu, E. L. Brown, R. J. Zagursky, D. Shlaes, and S. J. Projan. 2001. Transcription profiling-based identification of Staphylococcus aureus genes regulated by the agr and/or sarA loci. J. Bacteriol. 183:7341-7353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duthie, E. S., and L. L. Lorenz. 1952. Staphylococcal coagulase: mode of action and antigenicity. J. Gen. Microbiol. 6:95-107. [DOI] [PubMed] [Google Scholar]
- 33.Ewbank, J. J. 2002. Tackling both sides of the host-pathogen equation with Caenorhabditis elegans. Microbes Infect. 4:247-256. [DOI] [PubMed] [Google Scholar]
- 34.Finlay, B. B. 1999. Bacterial disease in diverse hosts. Cell 96:315-318. [DOI] [PubMed] [Google Scholar]
- 35.Gan, Y. H., K. L. Chua, H. H. Chua, B. Liu, C. S. Hii, H. L. Chong, and P. Tan. 2002. Characterization of Burkholderia pseudomallei infection and identification of novel virulence factors using a Caenorhabditis elegans host system. Mol. Microbiol. 44:1185-1197. [DOI] [PubMed] [Google Scholar]
- 36.Garsin, D. A., C. D. Sifri, E. Mylonakis, X. Qin, K. V. Singh, B. E. Murray, S. B. Calderwood, and F. M. Ausubel. 2001. A simple model host for identifying Gram-positive virulence factors. Proc. Natl. Acad. Sci. USA 98:10892-10897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gertz, S., S. Engelmann, R. Schmid, A. K. Ziebandt, K. Tischer, C. Scharf, J. Hacker, and M. Hecker. 2000. Characterization of the σB regulon in Staphylococcus aureus. J. Bacteriol. 182:6983-6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giachino, P., S. Engelmann, and M. Bischoff. 2001. σB activity depends on RsbU in Staphylococcus aureus. J. Bacteriol. 183:1843-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hiramatsu, K., H. Hanaki, T. Ino, K. Yabuta, T. Oguri, and F. C. Tenover. 1997. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J. Antimicrob. Chemother. 40:135-136. [DOI] [PubMed] [Google Scholar]
- 40.Horsburgh, M. J., J. L. Aish, I. J. White, L. Shaw, J. K. Lithgow, and S. J. Foster. 2002. σB modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from Staphylococcus aureus 8325-4. J. Bacteriol. 184:5457-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iandolo, J. J. 2000. Genetic and physical map of the chromosome of Staphylococcus aureus 8325, p. 317-325. In V. A. Fischetti, R. P. Novick, J. J. Ferretti, D. A. Portnoy, and J. A. Rood (ed.), Gram-positive pathogens. ASM Press, Washington, D.C.
- 42.Jansen, W. T., M. Bolm, R. Balling, G. S. Chhatwal, and R. Schnabel. 2002. Hydrogen peroxide-mediated killing of Caenorhabditis elegans by Streptococcus pyogenes. Infect. Immun. 70:5202-5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karlsson, A., and S. Arvidson. 2002. Variation in extracellular protease production among clinical isolates of Staphylococcus aureus due to different levels of expression of the protease repressor sarA. Infect. Immun. 70:4239-4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kato, Y., T. Aizawa, H. Hoshino, K. Kawano, K. Nitta, and H. Zhang. 2002. abf-1 and abf-2, ASABF-type antimicrobial peptide genes in Caenorhabditis elegans. Biochem. J. 361:221-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kielian, T., A. Cheung, and W. F. Hickey. 2001. Diminished virulence of an alpha-toxin mutant of Staphylococcus aureus in experimental brain abscesses. Infect. Immun. 69:6902-6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim, D. H., R. Feinbaum, G. Alloing, F. E. Emerson, D. A. Garsin, H. Inoue, M. Tanaka-Hino, N. Hisamoto, K. Matsumoto, M.-W. Tan, and F. M. Ausubel. 2002. A conserved p38 MAP kinase pathway in Caenorhabditis elegans innate immunity. Science 297:623-626. [DOI] [PubMed] [Google Scholar]
- 47.Kornblum, J., B. N. Kreiswirth, S. J. Projan, H. Ross, and R. P. Novick. 1990. agr: a polycistronic locus regulating exoprotein synthesis in Staphylococcus aureus, p. 373-402. In R. P. Novick (ed.), Molecular biology of the staphylococci. VCH Publishers, Inc., New York, N.Y.
- 48.Kullik, I., P. Giachino, and T. Fuchs. 1998. Deletion of the alternative sigma factor σB in Staphylococcus aureus reveals its function as a global regulator of virulence genes. J. Bacteriol. 180:4814-4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee, J. C., M. J. Betley, C. A. Hopkins, N. E. Perez, and G. B. Pier. 1987. Virulence studies, in mice, of transposon-induced mutants of Staphylococcus aureus differing in capsule size. J. Infect. Dis. 156:741-750. [DOI] [PubMed] [Google Scholar]
- 50.Lewis, J. A., and J. T. Fleming. 1995. Basic culture methods, p. 3-29. In H. F. Epstein, and D. C. Shakes (ed.), Caenorhabditis elegans: modern biological analysis of an organism. Academic Press, San Diego, Calif.
- 51.Lindsay, J. A., and S. J. Foster. 1999. Interactive regulatory pathways control virulence determinant production and stability in response to environmental conditions in Staphylococcus aureus. Mol. Gen. Genet. 262:323-331. [DOI] [PubMed] [Google Scholar]
- 52.Lowy, F. D. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520-532. [DOI] [PubMed] [Google Scholar]
- 53.Lundqvist-Gustafsson, H., S. Norrman, J. Nilsson, and A. Wilsson. 2001. Involvement of p38-mitogen-activated protein kinase in Staphylococcus aureus-induced neutrophil apoptosis. J. Leukoc. Biol. 70:642-648. [PubMed] [Google Scholar]
- 54.Mahajan-Miklos, S., L. G. Rahme, and F. M. Ausubel. 2000. Elucidating the molecular mechanisms of bacterial virulence using non-mammalian hosts. Mol. Microbiol. 37:981-988. [DOI] [PubMed] [Google Scholar]
- 55.Mahajan-Miklos, S., M. W. Tan, L. G. Rahme, and F. M. Ausubel. 1999. Molecular mechanisms of bacterial virulence elucidated using a Pseudomonas aeruginosa-Caenorhabditis elegans pathogenesis model. Cell 96:47-56. [DOI] [PubMed] [Google Scholar]
- 56.McLeish, K. R., J. B. Klein, P. Y. Coxon, K. Z. Head, and R. A. Ward. 1998. Bacterial phagocytosis activates extracellular signal-regulated kinase and p38 mitogen-activated protein kinase cascades in human neutrophils. J. Leukoc. Biol. 64:835-844. [PubMed] [Google Scholar]
- 57.Menzies, B. E., and D. S. Kernodle. 1994. Site-directed mutagenesis of the alpha-toxin gene of Staphylococcus aureus: role of histidines in toxin activity in vitro and in a murine model. Infect. Immun. 62:1843-1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moerman, D. G., and A. Fire. 1997. Muscle: structure, function, and development, p. 417-470. In D. L. Riddle, T. Blumenthal, B. J. Meyer, and J. R. Priess (ed.), C. elegans II. Cold Spring Harbor Laboratory Press, Plainview, N.Y. [PubMed]
- 59.Mylonakis, E., M. Engelbert, X. Qin, C. D. Sifri, B. E. Murray, F. M. Ausubel, M. S. Gilmore, and S. B. Calderwood. 2002. The Enterococcus faecalis fsrB gene, a key component of the fsr quorum-sensing system, is associated with virulence in the rabbit endophthalmitis model. Infect. Immun. 70:4678-4681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nicholas, R. O., T. Li, D. McDevitt, A. Marra, S. Sucoloski, P. L. Demarsh, and D. R. Gentry. 1999. Isolation and characterization of a sigB deletion mutant of Staphylococcus aureus. Infect. Immun. 67:3667-3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nilsson, I. M., T. Bremell, C. Ryden, A. L. Cheung, and A. Tarkowski. 1996. Role of the staphylococcal accessory gene regulator (sar) in septic arthritis. Infect. Immun. 64:4438-4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Novick, R. P. 2000. Pathogenicity factors and their regulation, p. 392-407. In V. A. Fischetti, R. P. Novick, J. J. Ferretti, D. A. Portnoy, and J. A. Rood (ed.), Gram-positive pathogens. ASM Press, Washington, D.C.
- 63.Novick, R. P. 1990. The Staphylococcus as a molecular genetic system, p. 1-37. In R. P. Novick (ed.), Molecular biology of the staphylococci. VCH Publishers, Inc., New York, N.Y.
- 64.Novick, R. P., H. F. Ross, S. J. Projan, J. Kornblum, B. Kreiswirth, and S. Moghazeh. 1993. Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J. 12:3967-3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.O'Quinn, A. L., E. M. Wiegand, and J. A. Jeddeloh. 2001. Burkholderia pseudomallei kills the nematode Caenorhabditis elegans using an endotoxin-mediated paralysis. Cell. Microbiol. 3:381-393. [DOI] [PubMed] [Google Scholar]
- 66.Palma, M., and A. L. Cheung. 2001. σB activity in Staphylococcus aureus is controlled by RsbU and an additional factor(s) during bacterial growth. Infect. Immun. 69:7858-7865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Patel, A. H., P. Nowlan, E. D. Weavers, and T. Foster. 1987. Virulence of protein A-deficient and alpha-toxin-deficient mutants of Staphylococcus aureus isolated by allele replacement. Infect. Immun. 55:3103-3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peng, H. L., R. P. Novick, B. Kreiswirth, J. Kornblum, and P. Schlievert. 1988. Cloning, characterization, and sequencing of an accessory gene regulator (agr) in Staphylococcus aureus. J. Bacteriol. 170:4365-4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qin, X., K. V. Singh, G. M. Weinstock, and B. E. Murray. 2000. Effects of Enterococcus faecalis fsr genes on production of gelatinase and a serine protease and virulence. Infect. Immun. 68:2579-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rachid, S., K. Ohlsen, U. Wallner, J. Hacker, M. Hecker, and W. Ziebuhr. 2000. Alternative transcription factor σB is involved in regulation of biofilm expression in a Staphylococcus aureus mucosal isolate. J. Bacteriol. 182:6824-6826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rechtin, T. M., A. F. Gillaspy, M. A. Schumacher, R. G. Brennan, M. S. Smeltzer, and B. K. Hurlburt. 1999. Characterization of the SarA virulence gene regulator of Staphylococcus aureus. Mol. Microbiol. 33:307-316. [DOI] [PubMed] [Google Scholar]
- 72.Rice, K., R. Peralta, D. Bast, J. de Azavedo, and M. J. McGavin. 2001. Description of staphylococcus serine protease (ssp) operon in Staphylococcus aureus and nonpolar inactivation of sspA-encoded serine protease. Infect. Immun. 69:159-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Santos Sanches, I., R. Mato, H. de Lencastre, and A. Tomasz. 2000. Patterns of multidrug resistance among methicillin-resistant hospital isolates of coagulase-positive and coagulase-negative staphylococci collected in the international multicenter study RESIST in 1997 and 1998. Microb. Drug Resist. 6:199-211. [DOI] [PubMed] [Google Scholar]
- 74.Schnyder, B., P. C. Meunier, and B. D. Car. 1998. Inhibition of kinases impairs neutrophil activation and killing of Staphylococcus aureus. Biochem. J. 331:489-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shafer, W. M., and J. J. Iandolo. 1979. Genetics of staphylococcal enterotoxin B in methicillin-resistant isolates of Staphylococcus aureus. Infect. Immun. 25:902-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sifri, C. D., E. Mylonakis, K. V. Singh, X. Qin, D. A. Garsin, B. E. Murray, F. M. Ausubel, and S. B. Calderwood. 2002. Virulence effect of Enterococcus faecalis protease genes and the quorum-sensing locus fsr in Caenorhabditis elegans and mice. Infect. Immun. 70:5647-5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Singh, K. V., X. Qin, G. M. Weinstock, and B. E. Murray. 1998. Generation and testing of mutants of Enterococcus faecalis in a mouse peritonitis model. J. Infect. Dis. 178:1416-1420. [DOI] [PubMed] [Google Scholar]
- 78.Smith, T. L., M. L. Pearson, K. R. Wilcox, C. Cruz, M. V. Lancaster, B. Robinson-Dunn, F. C. Tenover, M. J. Zervos, J. D. Band, E. White, W. R. Jarvis, et al. 1999. Emergence of vancomycin resistance in Staphylococcus aureus. N. Engl. J. Med. 340:493-501. [DOI] [PubMed] [Google Scholar]
- 79.Sulston, J., and J. Hodgkin. 1988. Methods, p. 587-606. In W. B. Wood (ed.), The nematode Caenorhabditis elegans. Cold Spring Harbor Laboratory Press, Plainview, N.Y.
- 80.Sutra, L., and B. Poutrel. 1994. Virulence factors involved in the pathogenesis of bovine intramammary infections due to Staphylococcus aureus. J. Med. Microbiol. 40:79-89. [DOI] [PubMed] [Google Scholar]
- 81.Tan, M. W., S. Mahajan-Miklos, and F. M. Ausubel. 1999. Killing of Caenorhabditis elegans by Pseudomonas aeruginosa used to model mammalian bacterial pathogenesis. Proc. Natl. Acad. Sci. USA 96:715-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tenover, F. C., and R. P. Gaynes. 2000. The epidemiology of Staphylococcus infections, p. 414-421. In V. A. Fischetti, R. P. Novick, J. J. Ferretti, D. A. Portnoy, and J. A. Rood (ed.), Gram-positive pathogens. ASM Press, Washington, D.C.
- 83.Tzou, P., E. De Gregorio, and B. Lemaitre. 2002. How Drosophila combats microbial infection: a model to study innate immunity and host-pathogen interactions. Curr. Opin. Microbiol. 5:102-110. [DOI] [PubMed] [Google Scholar]
- 84.Wolz, C., P. Pohlmann-Dietze, A. Steinhuber, Y. T. Chien, A. Manna, W. van Wamel, and A. Cheung. 2000. Agr-independent regulation of fibronectin-binding protein(s) by the regulatory locus sar in Staphylococcus aureus. Mol. Microbiol. 36:230-243. [DOI] [PubMed] [Google Scholar]
- 85.Youngman, P., J. B. Perkins, and R. Losick. 1984. A novel method for the rapid cloning in Escherichia coli of Bacillus subtilis chromosomal DNA adjacent to Tn917 insertions. Mol. Gen. Genet. 195:424-433. [DOI] [PubMed] [Google Scholar]