Abstract
A total of 60 sheep were exposed to Anaplasma phagocytophilum infection on an enclosed area of Ixodes ricinus-infested pasture in North Wales, United Kingdom, and rapidly acquired acute A. phagocytophilum infections detectable by PCR and blood smear examination. Of the ticks that had engorged in the previous instar on infected sheep, 52% of adult ticks and 28% of nymphs were PCR positive; a significant, 10-fold increase in prevalence compared to that of ticks that engorged on sheep preinfection was observed (P = 0.015). The likelihood that ticks were PCR positive, after feeding on the sheep and molting to the next instar, increased marginally with increasing numbers of infected neutrophils per milliliter of blood of their sheep host (P = 0.068) and increased significantly when they were collected from sheep carrying higher numbers of adult female ticks (P = 0.017), but increasing numbers of feeding nymphs had a significant negative effect on transmission (P = 0.049). The numbers of circulating neutrophils and of infected neutrophils also varied significantly with the numbers of ticks feeding on the sheep when the blood was collected. Our study suggests that ruminants are efficient reservoirs of A. phagocytophilum during the acute and post-acute phases of infection. The risk of ruminant-derived infections may, however, be strongly affected by variations in tick densities, which may influence transmission from acutely infected animals via effects on the numbers of infected cells in the blood and possibly by within-skin modulation of infection.
Anaplasma phagocytophilum (formerly Ehrlichia phagocytophilum, Ehrlichia equi, and the agent of human granulocytic ehrlichiosis [8]) is an obligate intracellular bacterium that mostly targets granulocytes in its mammalian hosts (43). Although it has been recognized for some years as a pathogen of veterinary importance, the discovery of human granulocytic ehrlichiosis in the United States and Europe (6, 21) has generated increasing public health interest in this organism.
A. phagocytophilum is transmitted by ixodid ticks. In the United States the principal vectors are Ixodes scapularis and I. pacificus (35, 36), while in Europe the main exophilic tick vector is I. ricinus (22). A. phagocytophilum is transstadially transmitted by these vector ticks, and there is no evidence of transovarial transmission (22, 27, 29, 37). Most studies to date that have investigated the importance of mammalian hosts of A. phagocytophilum and its tick vectors have focused on rodents (e.g., see references 5, 20, and 37), but this organism has a wide mammalian host range, infecting domesticated cats, dogs, sheep, cows, and horses (4, 9, 13, 22). Ruminants such as deer and sheep are frequently very important hosts for vector ticks in North America and Europe (2, 26, 38) and are also potentially important reservoir hosts of A. phagocytophilum (2, 3, 22).
In the present study we have investigated the role of sheep in the acute and post-acute phases of infection (rather than that of “carrier” sheep [29]) as a source of A. phagocytophilum infection for I. ricinus ticks by exposing infection-naïve sheep to tick-borne infection under controlled conditions in the field. This design was necessary to investigate effects of natural rates and densities of tick attachment that are difficult (if not impossible) to reproduce in the laboratory. A number of factors that may affect the ability of ticks to acquire A. phagocytophilum from acutely infected sheep (and possibly other ruminant hosts) were considered. These included factors that may influence the delivery of A. phagocytophilum via blood to feeding I. ricinus ticks, such as (i) the numbers of circulating, potentially infected granulocytes and (ii) the level of bacteremia (using the number of infected cells per milliliter of blood as an index), as well as factors that may modulate the delivery of neutrophils to lesions caused by feeding ticks, hereafter referred to as feeding-tick lesions, such as (iii) resistance to ticks (1) and (iv) the numbers of ticks feeding per sheep. The last factor may provide an index to the local or systemic dose of immunomodulatory tick saliva that each sheep receives with potential consequences for transmission (14, 15).
MATERIALS AND METHODS
Study site and study animals.
A total of 60 18-month-old female Welsh Mountain sheep, reared mostly indoors on an I. ricinus-free farm, were used in the study. The sheep were sorted into two groups of equal size (A and B) by random turn, individually identified by ear tagging, and EDTA treated, and clotted-blood samples were collected from each. The sheep were then introduced onto a fenced enclosure of I. ricinus-infested upland pasture on a nearby farm during late summer and autumn of 2001. The enclosure for the study was created on pasture where A. phagocytophilum infection and tick dynamics had been monitored for some years and where A. phagocytophilum infection was naturally maintained in I. ricinus ticks by infections of carrier sheep (29). Sheep of group A were introduced to the enclosure on 17 August 2001, while group B sheep were introduced 4 weeks later to investigate potential confounding effects of temporal factors such as the onset of breeding (with potential effects on immune responses [30, 31]), potential (albeit unlikely) variations in preexisting A. phagocytophilum infection prevalence in nymphal I. ricinus ticks, potential differences in genotypes of infecting A. phagocytophilum (36), and variations in the emergence or activity of different tick cohorts (34). Following introduction to the pasture, the sheep were gathered weekly when the numbers of ticks, of each instar, feeding on the head of each animal were counted (as an index of the total numbers of ticks feeding per animal [28]). At these times, EDTA-treated blood samples (for hematological and A. phagocytophilum infection analyses) and clotted blood samples (for serological analysis) were collected from each sheep and any fully engorged nymphal or larval ticks were also collected. Ticks were considered fully engorged on the basis of size, shape, and color and when they could be easily dislodged with one fingertip. Any apparently engorged tick that required more effort to remove was considered not to have completed feeding and was not collected. Collection of samples continued until 20 October 2001, by which time the sheep of group A had been in the tick-infested enclosure for 9 weeks and those of group B had been in the enclosure for 5 weeks.
Analysis of sheep blood and serum samples.
Blood samples collected into EDTA-coated tubes were used for estimation of total leukocyte numbers, for extraction of DNA for PCR analysis, and for preparation of smears for differential counts of leukocytes and counts of the numbers of infected cells per milliliter of blood.
An ABC Vet automated blood cell counter (ABX Haematologie, Montpellier, France) was used to determine total leukocyte numbers. In Giema-stained smears, 200 neutrophils and all other leukocytes were examined for the presence of intracytoplasmic inclusions (morulae) typical of A. phagocytophilum infection (43). These data were then used as described previously (43) to estimate the actual number of detectably infected cells per milliliter of blood. Using Quiex II extraction columns (Qiagen, Crawley, West Sussex, United Kingdom), DNA was extracted from 200 μl of each sheep blood sample and subjected to a nested PCR specifically targeting the 16S DNA coding for rRNA (rDNA) of A. phagocytophilum as previously described (23). Throughout, a single water-only negative control was included per five test samples during DNA extraction and a further single water-only negative control was included per five test samples during PCR. Four PCR products (two from sheep blood samples and two from engorged nymphal ticks collected from the sheep) were cloned using the TOPO TA cloning kit (Invitrogen, Paisley, United Kingdom) and sequenced using an ABI 377 automated sequencer. Using the BLAST program on the National Center for Biotechnology website, the sequences were compared to previously published 16S rDNA sequences of A. phagocytophilum on GenBank.
Sera obtained from clotted blood samples collected from each sheep prior to and 3 and 4 weeks after introduction to the tick-infested enclosure were assayed with an immunofluorescent antibody test for the presence of immunoglobulin G (IgG) antibodies specific to A. phagocytophilum. Blood from an acutely infected sheep experimentally infected with an Old Sourhope strain of A. phagocytophilum was used to prepare antigen slides as described previously (11, 32). The secondary antibody was a rabbit anti-sheep IgG whole-molecule-fluorescein isothiocyanate conjugate (Sigma, Dorset, United Kingdom). The cutoff dilution of serum samples for nonspecific reactions was determined to be 1 in 50 (data not shown).
Analysis of engorged immature I. ricinus ticks.
Engorged immature (larval and nymphal) ticks collected from the sheep were weighed to provide an index of tick resistance in the sheep from which they were collected and then subjected to PCR, either immediately following weighing (in the engorged state) or after molting to the next instar in the laboratory, to detect A. phagocytophilum infection.
Engorged ticks were collected into tubes containing filter paper moistened with autoclaved, distilled, and deionized water and then maintained overnight in the laboratory at 80% humidity and at room temperature. The ticks, excluding any that died overnight, were then weighed using a microbalance (BP211D; Sartorius, Goettingen, Germany) and randomly allocated into one of two treatments: direct extraction of DNA for PCR analysis (following storage at −20°C) or incubation in the laboratory at room temperature and 80% humidity until the ticks molted, followed by DNA extraction and PCR analysis. In either case, DNA was extracted from the ticks by maceration followed by alkaline digestion as previously described (17, 29) and A. phagocytophilum infection was detected by nested PCR as described above for sheep blood samples.
Statistical analysis.
With the use of STATA (version 6) software for Windows, variations in the delivery of A. phagocytophilum-infected cells in sheep blood to feeding ticks during the course of infection were investigated in two regression models in which the outcome variables were (i) the numbers of circulating neutrophils per milliliter of sheep blood pre- and postinfection and (ii) the numbers of infected neutrophils per milliliter of blood postinfection. The time point of infection of each sheep was defined as the week in which blood from that sheep was PCR positive for the first time. For outcome i, the explanatory variables included as indicated the week postexposure to the enclosure and the proportions of infected neutrophils of sheep blood at the time blood was collected; for outcome ii, the explanatory variables included the week postinfection. In both models, the number of ticks feeding on the sheep in each developmental stage and the identity of the experimental sheep group (A or B) were also considered as explanatory variables.
Factors influencing transmission of infection from the sheep to the ticks were investigated in two multivariable generalized linear mixed-effects models. The two outcome variables were (i) the probability that an engorged immature tick collected from a study sheep was PCR positive and (ii) the probability that an immature tick collected from a study sheep was PCR positive after molting to the next instar. Explanatory variables in these models were the results of the sheep blood PCRs, the proportions (and number per milliliter of blood) of infected neutrophils in sheep blood at the time engorged ticks were collected, tick instar (nymph or larva), experimental sheep group (A or B), tick weight (as an index of sheep resistance to ticks), and the numbers of feeding ticks of each instar counted on the sheep at the time ticks were collected. When tick weight was investigated as an explanatory variable, account was made of sex differences in engorged nymph weights by including a binary variable for nymphal tick sex as previously described (30). Also, the number of weeks since the sheep first acquired infection was included as a factor in these models.
The development of sheep resistance to ticks was investigated in linear regression models with engorged nymph weight as the outcome variable and with the following explanatory variables: tick sex, week postexposure to ticks in the enclosure (as a factor), sheep group (A or B), the numbers of feeding ticks of each instar counted on the sheep, sheep blood PCR result, and the proportions and numbers of infected neutrophils per milliliter of sheep blood at the time the ticks were collected.
In all statistical models, sheep identity number was included as a random effect to account for repeated sampling of individual sheep and to adjust for the statistical dependence of ticks collected from the same sheep at the same time (7). In all models, the most parsimonious model was sought (by a process of forward and backward elimination) in which no variables or interactions could be removed without significantly altering model deviance. Standard model checking methods were used throughout, and the critical probability was P < 0.05.
RESULTS
Tick infestations.
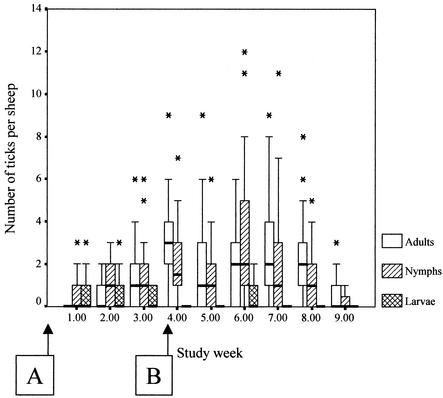
The study spanned an expected autumn period of I. ricinus tick activity. Larvae, nymphs, and adult females were found on sheep at all sampling periods. Nymphal and adult tick numbers appeared to be coincident, being low at the start of the study and highest during weeks 4 to 7 and declining thereafter. Larvae were found in their greatest numbers on sheep in the early weeks of the study (Fig. 1).
FIG. 1.
Box plots showing medians, quartiles, and outlier values of the numbers of I. ricinus ticks counted on sheep during the study. Letters in boxes refer to the groups of sheep in the study, and the arrows indicate the time points at which these groups of sheep were introduced to the I. ricinus-infested study site.
Sheep infections, serology, and hematology.
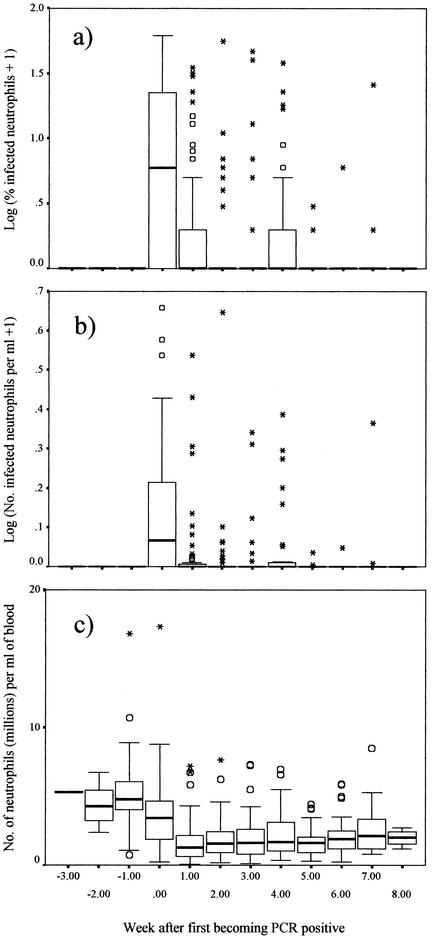
All sheep were PCR negative, smear negative, and seronegative prior to introduction to the tick-infested enclosure. All sheep became PCR positive, most by the first or second week of exposure to ticks (median, week 2 postexposure for both groups of sheep). One sheep of group A remained PCR negative until the third week of exposure to ticks. Most remained PCR positive until the end of the study although blood samples from 31 sheep were PCR negative during one or more intervening weeks. All sheep were seropositive after 4 weeks of exposure, most (44/60) were seropositive by 3 weeks postexposure. Blood samples obtained from all but four sheep were positive for A. phagocytophilum by light microscopy on at least one occasion, and no sheep blood sample that was positive by microscopy was PCR negative. Smears of blood samples became positive by microscopy at the same time as or 1 week after the first detection of A. phagocytophilum in blood by PCR. A total of 19 of the sheep (11 from group A and 8 from group B) had positive blood smears on two or three separate occasions, having had negative blood smears for 1 or 2 weeks between the weeks when the smears were positive. Of these sheep, 11 also became temporarily PCR negative during one of the weeks separating the peaks in the appearance of inclusions. The proportions of morula-containing cells ranged from 1 to 60% (mean, 13.5%) during the first peak of detectable inclusions in sheep blood samples and from 1 to 39% (mean, 8.7%) in the second or subsequent peaks (Fig. 2a). A total of 10 sheep were smear positive in two consecutive weeks during the early peak in detectable inclusions, but otherwise, individual peaks in individual sheep were only detected by microscopy in one week.
FIG. 2.
Proportion of A. phagocytophilum-infected circulatory neutrophils (log10 transformed) (A), numbers of infected cells per milliliter of blood (log10 transformed) (B), and numbers of neutrophils per milliliter of blood (C) in blood samples from the study sheep before and after the sheep acquired A. phagocytophilum infection (as detected by PCR of blood samples). Box plots show the medians, quartiles, and outlier (circles) and extreme (stars) values.
The total numbers of neutrophils per milliliter of the blood of the study sheep declined significantly after the sheep became PCR positive, from a mean of 5.01 × 106 per ml preinfection to a mean of 2.39 × 106 per ml subsequently (P < 0.001) (Table 1 and Fig. 2c), but were significantly greater the higher was the proportion of neutrophils that were detected as infected (P < 0.001). Accounting for these effects, the total numbers of neutrophils per milliliter were also significantly greater in sheep carrying higher numbers of nymphs at the time of sampling (P = 0.037) and significantly lower in sheep carrying higher numbers of adult ticks (P < 0.001; Table 1). The random effect of sheep identity number was highly significant (P < 0.001).
TABLE 1.
Significant determinants of the numbers of neutrophils and of A. phagocytophilum-infected neutrophils per milliliter in whole-blood samples of the study sheep in multivariable regression models
| Factor | Effect on no. of neutrophils/ml of blood
|
Effect on no. of infected neutrophils/ml of blood
|
||
|---|---|---|---|---|
| Coefficient (SE) | Wald Z (P)b | Coefficient (SE) | Wald Z (P)b | |
| Number of adult ticks per sheep | −0.215 (0.062) | −3.497 (<0.001) | −0.298 (0.124) | −2.400 (0.016) |
| Number of nymphal ticks per sheep | 0.109 (0.052) | 2.058 (0.037) | 0.113 (0.061) | 1.865 (0.062) |
| Proportion of neutrophils infected | 0.048 (0.011) | 4.424 (<0.001) | 1.448 (0.300) | 4.828 (<0.001) |
| Wk pre- vs wk postinfectiona | −2.737 (0.287) | −9.534 (<0.001) | ||
| Intercept | 7.886 (0.503) | 15.658 (<0.001) | −2.152 (0.272) | −7.902 (<0.001) |
| Random effect of sheep identity | 0.635 (0.166) | 3.826 (<0.001) | ||
The time point of infection was defined as the first occasion when blood from an individual sheep was found to be PCR positive.
Wald Z values represent parameter estimates divided by their asymptotic standard errors.
Regression models with Poisson errors were used as dictated by model-checking procedures to investigate variations in the numbers of peripheral blood neutrophils infected with A. phagocytophilum. In the most parsimonious multivariable regression model, the numbers of infected circulating neutrophils were significantly larger in the first week of infection (i.e., the first week the sheep's blood was PCR positive) than in subsequent weeks (P < 0.001) but there were no significant differences between the second and subsequent weeks of infection (likelihood ratio statistic [LRS] = 2.82, degrees of freedom (df) = 6, P > 0.1; Fig. 2b). This was accounted for by the fact that blood samples contained significantly lower numbers of infected cells per milliliter of blood in proportion to increases in the numbers of adult ticks feeding at the time of sampling (P = 0.016). Increases in numbers of nymphal ticks feeding on the sheep were associated with a marginally significant increase in the numbers of circulating infected cells (P = 0.062). The random effect of sheep identity was not significant (P > 0.1). There were no significant differences between the two groups of sheep in any of the models described in this report. Band (immature) neutrophils were more frequently found in sheep following infection, but these cells (and eosinophils) were nearly always found in low numbers (ranges, 0 to 2.32 for band neutrophils and 0 to 1.95 for eosinophils; median, 0 for both) and none of these cells were detected as being infected with morulae during this study.
Transmission of infection to immature ticks.
Tick infection data are summarized in Table 2. Over the whole study, 72% of engorged nymphs collected from the sheep were PCR positive while 47.7% of adult ticks that molted from engorged nymphs collected from the sheep were PCR positive. Of the engorged larvae collected from the sheep, 59.3% were PCR positive, and 18.5% of nymphs that molted from engorged larvae were PCR positive. Of six nymphs collected before their host sheep was PCR positive for the first time, three were PCR positive, while one engorged larva of three collected from these sheep was PCR positive. None of five nymphs that molted from larvae and 1 of 13 adult ticks that molted from nymphs that engorged on these sheep were PCR positive. Serendipitously, of the ticks collected from sheep preinfection, all bar three of the molted ticks were collected from group A animals while all bar one of the engorged ticks were collected from group B animals (Table 2).
TABLE 2.
The proportions of PCR-detected A. phagocytophilum infections in engorged and molted I. ricinus ticks collected from the two groups of sheep during the study
| Sheep group (engorged tick growth stage) | No. (%) of engorged ticks collected before sheep became PCR positive that were:
|
No. (%) of engorged ticks collected after sheep became PCR positive that were:
|
||
|---|---|---|---|---|
| PCR positive before molting | PCR positive after molting | PCR positive before molting | PCR positive after molting | |
| A | ||||
| Nymphs | 0/1 (0) | 0/10 (0) | 42/67 (62.7) | 52/96 (54.2) |
| Larvae | 0/1 (0) | 0/5 (0) | 25/40 (62.5) | 5/21 (23.8) |
| B | ||||
| Nymphs | 3/5 (60.0) | 1/3 (33.3) | 50/60 (83.3) | 9/22 (40.9) |
| Larvae | 1/2 (50.0) | 0/0 | 6/11 (54.5) | 0/0 |
| Total for both groups | ||||
| Nymphs | 3/6 (50) | 1/13 (8.7) | 92/127 (72.4) | 61/118 (51.7) |
| Larvae | 1/3 (33.3) | 0/5 (0) | 31/51 (60.8) | 5/21 (23.8) |
Engorged nymphs were significantly more likely to be PCR positive than were engorged larvae (P = 0.037). There were no significant variations in the likelihood that an engorged tick was PCR positive that correlated with the week of infection of the sheep, numbers of circulating infected neutrophils, engorged tick weights, or the numbers of ticks feeding on the sheep.
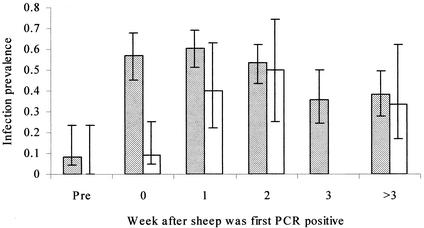
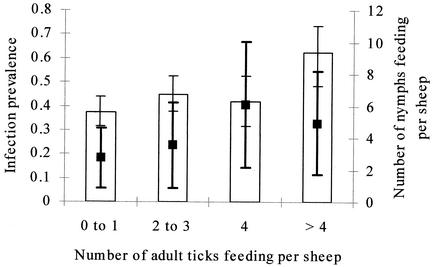
In the most parsimonious model, engorged immature ticks collected in any week after sheep became PCR positive were significantly more likely to be PCR positive after molting than those collected from sheep before they were detected by PCR as being infected (P = 0.015; Fig. 3). The likelihood that a molted tick was PCR positive declined in later stages of sheep infections (Fig. 3), but this was not significant. Adult ticks (molted from collected engorged nymphs) were more likely to be PCR positive than nymphal ticks (molted from collected engorged larvae) (P = 0.005). The likelihood that an immature tick was PCR positive following molting to the next instar increased with the numbers of infected neutrophils per milliliter in blood samples from the sheep from which the tick was collected, but this was marginally significant (P = 0.068). Variations in the numbers of infected cells per milliliter of blood were accounted for by the fact that ticks were significantly more likely to be PCR positive after molting when the sheep from which they were collected carried larger numbers of feeding adult female ticks (for sheep carrying four or more adult ticks compared to those carrying fewer ticks [odds ratio {OR} = 8.50, P = 0.017]; Fig. 4). There was a significant interaction between the numbers of nymphal ticks and the numbers of adult ticks carried by the sheep at the time engorged ticks were collected, such that increasing numbers of nymphs reduced the effect of adult tick numbers on the likelihood that a molted tick was PCR positive (for every nymph carried on sheep with four or more adults [OR = 0.70, P = 0.049]; Fig. 4). The random effect of sheep identity was not significant (P > 0.1). These data are summarized in Table 3. The effects of tick infestations on the tick infections that were detected and of the numbers of infected neutrophils did not conflict. Effects on the numbers of feeding ticks remained similarly significant in models that included week postinfection as a factor, although there was some confounding between week of infection and the numbers of circulating infected cells. There were no significant differences between the two groups of sheep or significant variations in transmission associated with variations in engorged weights of immature ticks, numbers of feeding larval ticks, or numbers of circulating eosinophils, monocytes, or lymphocytes in any of the models described in this report (P > 0.5 for all).
FIG. 3.
Prevalence of A. phagocytophilum infection in adult ticks that molted from engorged nymphs (stippled bars [± exact binomial SE]) and in nymphal ticks that molted from engorged larvae (open bars [± exact binomial SE]) from sheep preinfection and in the first and subsequent weeks of infection.
FIG. 4.
Prevalence of infection in adult ticks (bar graphs [± exact binomial SE adjusted for intrasheep correlation]) that molted from nymphs that had engorged on sheep carrying different numbers of adult female ticks. The mean numbers of nymphal ticks carried by these sheep (± SE) are indicated by black squares. Heavy black error bars refer to counts of nymphs.
TABLE 3.
Determinants of PCR-detected A. phagocytophilum infection prevalence in ticks molted from engorged immature I. ricinus collected from study sheep
| Factor | OR (95% CI)a | Wald Z (P)b |
|---|---|---|
| Tick collected from sheep pre- vs postinfection | 13.63 (1.65-112.17) | 2.427 (0.015) |
| Tick molted from engorged nymph vs larva | 5.75 (1.72-19.29) | 2.833 (0.005) |
| Sheep carrying 4 or more adult ticks vs sheep carrying 3 or fewer | 8.50 (1.46-49.90) | 2.377 (0.017) |
| No. of infected cells per ml of blood | 1.75 (0.96-3.19) | 1.827 (0.068) |
| Interaction of no. of feeding nymphs on sheep carrying 4 or more adult ticks | 0.70 (0.49-0.98) | −1.968 (0.049) |
| Intercept | 0.01 (0.01-0.10) | −3.022 (0.003) |
CI, confidence interval.
Wald Z values represent parameter estimates divided by their asymptotic standard errors.
Sheep resistance to ticks.
The engorged weights of 348 engorged nymphs were recorded. Accounting for differences in tick sex (female nymphs being heavier than males [coefficient = 1.166, standard error {SE} = 0.050, P < 0.001]), nymphs that engorged on sheep during the first 5 weeks of exposure were significantly heavier (means, 4.45 mg for females and 2.75 mg for males) than those that engorged in subsequent weeks (means, 4.24 mg for females and 2.70 mg for males [coefficient = 0.127, SE = 0.056, P = 0.024]). Sheep of group B did not, therefore, appear to acquire resistance to ticks during the study, although there was no significant difference between groups in the multivariable model (P > 0.5). There was no variation observed in weights of ticks collected during the first 5 weeks of exposure (LRS = 3.48, df = 4, P > 0.1) or in those collected during the subsequent weeks of exposure (LRS = 0.53, df = 2, P > 0.1). Engorged nymphs were significantly heavier when collected from sheep carrying higher numbers of nymphs (coefficient = 0.032, SE = 0.009, P < 0.001). No method of identification of sheep infections was associated with variations in engorged tick weights.
16S rDNA sequences.
The two sequences obtained from sheep blood (GenBank accession numbers AY149634 and AY149635) and one of those from an engorged I. ricinus nymph (GenBank accession number AY149636) were identical. The sequence obtained from the second engorged I. ricinus nymph (GenBank accession number AY149637) had a guanine rather than an adenine at both positions 77 and 84 (using the number designations of Chen et al. [6]). All the sequences had 99.9 or 100% similarity with published 16S rDNA sequences of other isolates of A. phagocytophilum. For example, the rather different sequence obtained from an engorged tick was 100% homologous with the sequence of an isolate from a red deer (Cervus elaphus) in Slovenia (GenBank accession number AF481853), while the other sequences were 100% homologous with that of a human isolate from California (AF093788).
DISCUSSION
The A. phagocytophilum-naïve sheep of this study, when naturally exposed to I. ricinus ticks, rapidly acquired acute A. phagocytophilum infections which were detectable by PCR. Nearly all of the sheep had detectable A. phagocytophilum morulae in circulatory neutrophils, usually in the first or second week of PCR-detectable infections. The period during which morulae were detectable in the blood of individual sheep (1 to 2 weeks) and the proportions of infected neutrophils (up to 60%) in the naturally infected study sheep were comparable to these parameters in experimentally infected sheep (43). Many of the sheep showed evidence of two or more episodes when morulae were detectable during the course of infection. Sequence data indicated that more than one genotype of A. phagocytophilum was present on the site, so reinfection of sheep might be one explanation (10, 39). This phenomenon was, however, observed in both groups of sheep, with similar time intervals of 1 to 2 weeks between the initial and second peaks in the appearance of morulae. This suggests, therefore, that sheep suffer cyclic rickettsemia following natural A. phagocytophilum infections, as do ruminants following infection with Anaplasma marginale (16). The main hematological consequence of infection was a decline in the numbers of circulating neutrophils, with a slight relative increase in the numbers of neutrophils during periods when morulae were detectable in blood smears; these are variations that have been observed in sheep experimentally infected with A. phagocytophilum (43). Following infection, the blood of most sheep remained persistently PCR positive for the duration of the study, with the group A sheep that were monitored for the longest period showing a small decline in the likelihood of being PCR positive towards the end of the study. Some sheep also became transiently PCR negative during the intervals between peaks of rickettsemia, suggesting that variations in the numbers of circulating infected cells were sometimes large.
Tests of immature ticks in the engorged state that fed on the sheep showed that a high proportion acquired PCR-detectable A. phagocytophilum infections. Some engorged immature ticks were PCR positive even though they were collected prior to the acquisition by the sheep host of infection that was detectable in peripheral blood by PCR. Some host-seeking nymphal ticks may have already been infected, but the previous estimate by Ogden et al. of nymphal tick infection prevalence from this site (on which sheep management has not changed for many years) was 1.5% (29), much lower than the prevalence observed in the engorged nymphs collected from sheep in the preinfection period. Some amplification of infection intensity in feeding ticks infected previously might have occurred to raise detected infection prevalence (12), but one PCR-positive engorged larva was collected from a PCR-negative sheep, so either transmission of infection between cofeeding ticks occurred (19) or, because neutrophils accumulate at feeding-tick lesions in sheep (1), perhaps PCR of engorged ticks more sensitively detected early A. phagocytophilum infections in sheep than did PCR of whole blood.
There was at least a 10-fold amplification of A. phagocytophilum infection prevalence from one tick instar to the next after ticks fed on infected sheep, but nymphs were more likely to acquire infections, and maintain them through molting, than were larvae. Transmission efficiency declined somewhat in the fourth and subsequent weeks of infection, but this was not significant, suggesting that transmission efficiency declines gradually from high levels in the acute and immediate post-acute phases of infection to the low levels seen in sheep that have been infected for much longer periods (29). The appearance of specific anti-A. phagocytophilum IgG antibodies in the sheep was not associated with any observed effect on transmission. In studies on other intracellular tick-borne pathogens, variation in detectable levels of circulatory infection has been considered the main determinant of host-to-tick transmission efficiency (24, 25, 33, 44). In our study, the likelihood that molted ticks were PCR positive did increase with increasing numbers of detectably infected circulating neutrophils in the sheep on which they engorged, but this was only marginally significant despite potentially wide fluctuations in levels of bacteremia between the first and subsequent weeks of infection. However, the likelihood that engorged larvae or nymphs acquired infections (that were maintained through the molt) varied significantly with the numbers of ticks feeding on the sheep at the time the ticks engorged. This suggested that feeding ticks somehow modulated A. phagocytophilum infections of the sheep, affecting sheep-to-tick transmission efficiency in a density-dependent way. The effect appeared relatively important: a 10-fold increase in the number of adult female ticks feeding per sheep had an effect on A. phagocytophilum transmission similar to that of a 5-fold increase in the numbers of detectably infected circulating neutrophils per milliliter of blood. Increases in the numbers of nymphal ticks feeding on the sheep had a simultaneous but conflicting effect on A. phagocytophilum transmission from sheep to the molted ticks.
The effect of feeding-tick population density on transmission may have been due in part to the effects of the numbers of feeding adult female and nymphal ticks on the numbers of circulating neutrophils and infected cells per milliliter of blood in infected sheep. Accounting for variations that occurred with the stage of infection, infected sheep that carried greater numbers of feeding adult female ticks had lower numbers of neutrophils, and lower numbers of infected neutrophils, circulating per milliliter of their blood. Infected sheep that carried larger numbers of feeding nymphs had larger numbers of circulating neutrophils but only marginally larger numbers of infected neutrophils. These findings are consistent with a net movement of neutrophils from the circulation to feeding-tick lesions (1) in response to increasing numbers of feeding adult female ticks and a net influx of neutrophils into the circulation from the spleen or hemopoietic tissues in response to the numbers of feeding nymphal ticks, with consequent effects on sheep-to-tick transmission efficiency. The different influences of nymphal and adult tick numbers may have reflected different qualities of the inflammatory response at nymphal- and adult-feeding-tick lesions (40). Different influences of nymphal and adult tick numbers may also be consistent, however, with different short-term effects (better indicated by counts of feeding nymphs) and long-term effects (better indicated by counts of adult females that feed for longer than nymphs) of changes in the numbers of feeding ticks of any stage on the dynamics of neutrophil populations and their infection. In an experimental study in which corticosteroids were used to stimulate neutrophilia in A. phagocytophilum-infected sheep, newly recruited neutrophils in the circulation of the sheep were less likely to be infected and so, in the short term, stimulation of neutrophilia did not increase the numbers of infected neutrophils in the circulation (42), a result similar to that obtained with increased numbers of feeding nymphs as observed in the present study.
The numbers of ticks feeding on the sheep may have had effects on sheep-to-tick transmission over and above effects on variations in the numbers of circulating infected neutrophils. A high proportion of immature ticks encountered infection while engorging on the sheep, but variations in the proportions of ticks that remained infected following molting were observed; these were associated with differences in the numbers of feeding ticks when variations in the numbers of infected cells circulating in sheep blood were taken into account. This suggests, first, that the observed differences in efficiency of transmission from sheep to molted ticks were due to variations in the numbers of bacteria acquired by engorging ticks and, second, that increasing numbers of feeding ticks had additional local, within-skin effects on multiplication of A. phagocytophilum that caused variations in the levels of the infective dose acquired by feeding ticks. Thus, increases in the numbers of feeding adult ticks increased the infective dose acquired by the ticks and increases in the numbers of feeding nymphs had an opposite effect. This result may be consistent with a longer-term effect, namely, that increases in the density of feeding-tick lesions (for which the counted numbers of adult ticks were an index) promoted further increases in the numbers of A. phagocytophilum-infected cells in the skin, an outcome which might have been due to effects of tick saliva on host immune responses (41). Studies on mice suggest that neutrophils migrate readily within skin between feeding-tick lesions (18), a property that must be essential for cofeeding transmission of A. phagocytophilum to occur (19). Dilution of the numbers of infected neutrophils per feeding-tick lesion may, therefore, have occurred in the shorter term (for which the counted numbers of nymphal ticks were an index) when the densities of feeding-tick lesions on the sheep increased. We have no evidence from this study that variations in sheep resistance to ticks had direct effects on transmission, although most sheep appeared to acquire resistance late or not at all, which is in contrast to the findings of other studies (1, 30). Potential inhibition of resistance when the numbers of feeding nymphs were high might, however, have been partly responsible for effects on the numbers of circulatory neutrophils (31).
In summary, this study has provided evidence that sheep are efficient reservoirs of A. phagocytophilum during the acute and post-acute phases of infection. Sheep-to-tick transmission efficiency varied with the number of circulating infected cells, which underwent cyclical variations during the post-acute phase of infection, but also with variations in the numbers of ticks feeding on the sheep at the time that ticks engorged. There was evidence that the latter effect may have been due to influences of feeding ticks on the numbers of circulating infected neutrophils encountered by feeding ticks and on local within-skin effects on transmission, possibly involving cofeeding transmission. Sheep-to-tick transmission efficiency may generally increase with increasing densities of feeding ticks, although a short-term effect of an increase in the numbers of feeding ticks (particularly nymphs) can be a transient reduction in transmission efficiency. If our findings are generally applicable among ruminant hosts of A. phagocytophilum in foci of infection where ruminants are important reservoirs, then there might be a very strong and direct relationship between tick density and the prevalence of host-seeking ticks that carry infections of ruminant origin. Further studies are required to test this hypothesis and to investigate more fully the mechanisms underlying the effects of feeding-tick population density on systemic infections and transmission of A. phagocytophilum.
Acknowledgments
This work was funded by the Biotechnology and Biological Sciences Research Council.
We gratefully acknowledge the kind cooperation of the land owner.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Abdul-Amir, I. M., and J. S. Gray. 1987. Resistance of sheep to laboratory infestations of the tick Ixodes ricinus. Res. Vet. Sci. 43:266-267. [PubMed] [Google Scholar]
- 2.Alberdi, M. P., A. R. Walker, and K. A. Urquhart. 2000. Field evidence that roe deer (Capreolus capreolus) are a natural host for Ehrlichia phagocytophila. Epidemiol. Infect. 124:315-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belongia, E. A., K. D. Reed, P. D. Mitchell, C. P. Kolbert, D. H. Persing, J. S. Gill, and J. J. Kazmierczak. 1997. Prevalence of granulocytic Ehrlichia infection among white-tailed deer in Wisconsin. J. Clin. Microbiol. 35:1465-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjoersdorff, A., L. Svendenius, J. H. Owens, and R. F. Massung. 1999. Feline granulocytic ehrlichiosis—a report of a new clinical entity and characterisation of the infectious agent. J. Small Anim. Pract. 40:20-24. [DOI] [PubMed] [Google Scholar]
- 5.Castro, M. B., W. L. Nicholson, V. L. Kramer, and J. E. Childs. 2001. Persistent infection in Neotoma fuscipes (Muridae: Sigmodontinae) with Ehrlichia phagocytophila sensu lato. Am. J. Trop. Med. Hyg. 65:261-267. [DOI] [PubMed] [Google Scholar]
- 6.Chen, S. M., J. S. Dumler, J. S. Bakken, and D. H. Walker. 1994. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J. Clin. Microbiol. 32:589-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diggle, P. J., K.-Y. Liang, and S. L. Zeger. 1994. Analysis of longitudinal data. Oxford Science Publications, Oxford, United Kingdom.
- 8.Dumler, J. S., A. F. Barbet, C. P. J. Bekker, G. A. Dasch, G. H. Palmer, S. C. Ray, Y. Rikihisa, and F. R. Rurangirwa. 2001. Reorganisation of the genera of the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new combinations and designations of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila. Int. J. Syst. E vol. Microbiol. 51:2145-2165. [DOI] [PubMed] [Google Scholar]
- 9.Engvall, E. O., B. Pettersson, M. Persson, K. Artursson, and K.-E. Johansson. 1996. A 16S rRNA-based assay for detection and identification of granulocytic Ehrlichia species in dogs, horses and cattle. J. Clin. Microbiol. 34:2170-2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foggie, A. 1951. Studies on the infectious agent of tick-borne fever in sheep. J. Pathol. Bacteriol. 63:1-15. [DOI] [PubMed] [Google Scholar]
- 11.Gokce, H. I. 1998. Studies on the effect of Ehrlichia (Cytoecetes) phagocytophila on some cellular immune responses in sheep. Ph.D. thesis. University of Liverpool, Liverpool, United Kingdom.
- 12.Hodzic, E., D. Fish, C. M. Maretzki, A. M. de Silva, S. L. Feng, and S. W. Barthold. 1998. Acquisition and transmission of the agent of human granulocytic ehrlichiosis by Ixodes scapularis ticks. J. Clin. Microbiol. 36:3574-3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hudson, J. R. 1950. The recognition of tick-borne fever as a disease of cattle. Br. Vet. J. 106:3-17. [Google Scholar]
- 14.Inokuma, H., R. L. Kerlin, D. H. Kemp, and P. Willadsen. 1994. Effects of cattle tick (Boophilus microplus) infestation on the bovine immune system. Vet. Parasitol. 53:293-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones, L. D., W. R. Kaufman, and P. A. Nuttall. 1992. Modification of the skin feeding site by tick saliva mediates virus transmission. Experientia 48:779-782. [DOI] [PubMed] [Google Scholar]
- 16.Kieser, S. T., I. S. Eriks, and G. H. Palmer. 1990. Cyclic rickettsemia during persistent Anaplasma marginale infection of cattle. Infect. Immun. 58:1117-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurtenbach, K., M. Peacey, S. G. T. Rijpkema, A. N. Hoodless, P. A. Nuttall, and S. E. Randolph. 1998. Differential transmission of the genospecies of Borrelia burgdorferi sensu lato by game birds and small rodents in England. Appl. Environ. Microbiol. 64:1169-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Labuda, M., J. M. Austyn, E. Zuffova, O. Kozuch, N. Fuchsberger, J. Lysy, and P. A. Nuttall. 1996. Importance of localized skin infection in tick-borne encephalitis virus transmission. Virology 219:357-366. [DOI] [PubMed] [Google Scholar]
- 19.Levin, M. L., and D. Fish. 2000. Immunity reduces reservoir host competence of Peromyscus leucopus for Ehrlichia phagocytophila. Infect. Immun. 68:1514-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liz, J. S., L. Anderes, J. W. Sumner, R. F. Massung, L. Gern, B. Rutti, and M. Brossard. 2001. PCR detection of granulocytic Ehrlichiae in Ixodes ricinus ticks and wild small mammals in western Switzerland. J. Clin. Microbiol. 38:1002-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lotric-Furlan, S., M. Petrovec, T. A. Zupanc, W. L. Nicholson, J. W. Sumner, J. E. Childs, and F. Strle. 1998. Human granulocytic ehrlichiosis in Europe: clinical and laboratory findings for four patients from Slovenia. Clin. Infect. Dis. 27:424-428. [DOI] [PubMed] [Google Scholar]
- 22.MacLeod, J., and W. S. Gordon. 1933. Studies on tick-borne fever in sheep. I. Transmission by the tick Ixodes ricinus and description of the disease produced. Parasitology 25:273-283. [Google Scholar]
- 23.Massung, R. F., K. Slater, J. H. Owens, W. L. Nicholson, T. N. Mather, V. B. Solberg, and J. G. Olson. 1998. Nested PCR assay for detection of granulocytic ehrlichiae. J. Clin. Microbiol. 36:1090-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Medley, G. F., B. D. Perry, and A. S. Young. 1993. Preliminary analysis of the transmission dynamics of Theileria parva in Eastern Africa. Parasitology 106:251-264. [DOI] [PubMed] [Google Scholar]
- 25.O'Callaghan, C. J., G. F. Medley, T. F. Peter, and B. D. Perry. 1998. Investigating the epidemiology of heartwater (Cowdria ruminantium infection) by means of a transmission dynamics model. Parasitology 117:49-61. [DOI] [PubMed] [Google Scholar]
- 26.Ogden, N. H., P. A. Nuttall, and S. E. Randolph. 1997. Natural Lyme disease cycles maintained via sheep by co-feeding ticks. Parasitology 115:591-599. [DOI] [PubMed] [Google Scholar]
- 27.Ogden, N. H., K. Bown, B. K. Horrocks, Z. Woldehiwet, and M. Bennett. 1998. Granulocytic Ehrlichia infection in Ixodid ticks and mammals in woodlands and uplands of the U.K. Med. Vet. Entomol. 12:423-429. [DOI] [PubMed] [Google Scholar]
- 28.Ogden, N. H., R. S. Hailes, and P. A. Nuttall. 1998. Interstadial variation in the attachment site of Ixodes ricinus ticks to sheep. Exp. App. Acarol. 22:227-232. [DOI] [PubMed] [Google Scholar]
- 29.Ogden, N. H., A. N. J. Casey, N. P. French, K. J. Bown, J. D. W. Adams, and Z. Woldehiwet. 2002. Natural Ehrlichia phagocytophila transmission coefficients from sheep ‘carriers’ to Ixodes ricinus ticks vary with the numbers of feeding ticks. Parasitology 124:127-136. [DOI] [PubMed] [Google Scholar]
- 30.Ogden, N. H., A. N. J. Casey, N. P. French, J. D. W. Adams, and Z. Woldehiwet. 2002. Field evidence for density-dependent facilitation amongst Ixodes ricinus ticks feeding on sheep. Parasitology 124:117-125. [DOI] [PubMed] [Google Scholar]
- 31.Ogden, N. H., A. N. J. Casey, C. Lawrie, N. P. French, Z. Woldehiwet, and S. D. Carter. 2002. IgG responses to salivary gland extract of Ixodes ricinus ticks vary inversely with resistance in naturally exposed sheep. Med. Vet. Entomol. 16:186-192. [DOI] [PubMed] [Google Scholar]
- 32.Paxton, E. A., and G. R. Scott. 1989. Detection of antibodies to the agent of tick-borne fever by indirect immuno-fluorescence. Vet. Microbiol. 21:133-138. [DOI] [PubMed] [Google Scholar]
- 33.Randolph, S. E. 1995. Quantifying parameters in the transmission of Babesia microti by the tick Ixodes trianguliceps amongst voles (Clethrionomys glareolus). Parasitology 110:287-295. [DOI] [PubMed] [Google Scholar]
- 34.Randolph, S. E., R. M. Green, A. N. Hoodless, and M. F. Peacey. 2002. An empirical quantitative framework for the seasonal population dynamics of the tick Ixodes ricinus. Int. J. Parasitol. 32:979-989. [DOI] [PubMed] [Google Scholar]
- 35.Richter, P. J., R. B. Kimsey, J. E. Madigan, J. E. Barlough, J. S. Dumler, and D. L. Brooks. 1996. Ixodes pacificus (Acari: Ixodidae) as a vector of Ehrlichia equi (Rickettsiales: Ehrlichieae). J. Med. Entomol. 33:1-5. [DOI] [PubMed] [Google Scholar]
- 36.Stuen, S., I. Van De Pol, K. Bergstrom, and L. M. Schouls. 2002. Identification of Anaplasma phagocytophila (formerly Ehrlichia phagocytophila) variants in blood from sheep in Norway. J. Clin. Microbiol. 40:3192-3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Telford, S. R., J. E. Dawson, P. Katavlos, C. K. Warner, C. P. Kolbert, and D. H. Persing. 1996. Perpetuation of the agent of human granulocytic ehrlichiosis in a deer tick-rodent cycle. Proc. Natl. Acad. Sci. USA 93:6209-6214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thompson, C., A. Spielman, and P. J. Krause. 2001. Coinfecting deer-associated zoonoses: Lyme disease, babesiosis, and ehrlichiosis. Clin. Infect. Dis. 33:676-685. [DOI] [PubMed] [Google Scholar]
- 39.Tuomi, J. 1967. Experimental studies on bovine tick-borne fever. (3) Immunological strain differences. Acta Pathol. Microbiol. Scand. 71:89-100. [PubMed] [Google Scholar]
- 40.Whelan, A. C., and S. K. Wikel. 1993. Acquired resistance of guinea pigs to Dermacentor andersoni mediated by humoral factors. J. Parasitol. 79:908-912. [PubMed] [Google Scholar]
- 41.Wikel, S. K. 1999. Tick modulation of host immunity: an important factor in pathogen transmission. Int. J. Parasitol. 29:851-859. [DOI] [PubMed] [Google Scholar]
- 42.Woldehiwet, Z., and G. R. Scott. 1982. Corticosteroid therapy of tick-borne fever. Vet. Rec. 110:151-152. [DOI] [PubMed] [Google Scholar]
- 43.Woldehiwet, Z., and G. R. Scott. 1993. Tick-borne (pasture) fever. In Z. Woldehiwet and M. Ristic (ed.), Rickettsial and chlamydial diseases of domestic animals. Pergamon Press, Oxford, United Kingdom.
- 44.Young, A. S., T. T. Dolan, S. P. Morzaria, F. N. Mwakima, R. A. I. Norval, J. Scott, A. Sherriff, and G. Gettinby. 1996. Factors influencing infections in Rhipicephalus appendiculatus ticks fed on cattle infected with Theileria parva. Parasitology 113:255-266. [DOI] [PubMed] [Google Scholar]