Abstract
A chimeric protein consisting of a divalent pertussis toxin (PT) S1 fragment linked to the cholera toxin (Ctx) A2B fragment was constructed. The chimera induced a mucosal immunoglobulin A (IgA) and a serum IgG immune response to PT and CtxB in BALB/c mice following intranasal immunization. The immune sera neutralized PT in vitro. In the mouse model of Bordetella pertussis respiratory infection, the chimera-immunized animals showed a significant reduction in bacterial lung counts (P = 0.01) from that of the sham control group. Thus, a divalent S1 fragment CtxA2B chimera is an immunogenic antigen and can elicit a protective immunity.
Pertussis is a severe respiratory disease caused by Bordetella pertussis. Among the virulence factors produced by B. pertussis, pertussis toxin (PT) is one of most important (18) and is the prominent component of all acellular pertussis vaccines, which are given parenterally. The A-protomer (S1 subunit) of PT is immunodominant (4). Antibodies against the S1 subunit have been shown to neutralize the toxin in vitro and protect mice from B. pertussis infection in aerosol and intracerebral challenges (7, 16, 17).
Cholera toxin (Ctx) is also an AB toxin in which the toxic A1 moiety (22 kDa) is linked to the pentameric B subunit (CtxB) (11 kDa) by an A2 fragment (5 kDa) which is derived from the proteolytic cleavage of the C terminus of the A subunit (5, 14). CtxB binds to the GM1 ganglioside and can serve as a mucosal adjuvant (6, 12). Hajishengalis et al. (6) previously have shown that the A1 moiety can be replaced by a saliva-binding region (SBR) of the Streptococcus mutans antigen I/II, and the chimera induced an excellent immune response to SBR when given orally to mice.
A mucosal pertussis vaccine offers a number of potential advantages over the conventional parenteral vaccines, such as the ease in administration and the generation of mucosal antibodies that can prevent colonization by B. pertussis. However, induction of a protective antibody immune response at mucosal surfaces is not readily achieved by parenteral or mucosal immunization with soluble antigens. Since CtxB is an excellent mucosal adjuvant, we exploited this property to deliver the PT S1 fragment to the mucosal immune system.
Construction of the MBPS1S1CtxA2B chimera.
Two copies of the DNA coding for the N-terminal 179-amino-acid sequence of the PT S1 subunit were cloned in tandem into the middle portion of spaP by ligating a 1.4-kb SmaI-KpnI fragment from pPTS1 into the NruI-KpnI sites of pRJMI (11). The DNA coding for the CtxA2 motif and CtxB was then cloned downstream to the SpaPS1S1 construct using the NruI-KpnI sites. The CtxA2B DNA was amplified from pRIT10814 (13) using Taq DNA polymerase and the primers SL155 (ATGATATCGCTTGGAGGGAAGAG; EcoRV site underlined) and SL156 (ACGGTACCATAATACGCACTAAGG; KpnI site underlined) under conditions described previously (8). The cloning placed the CtxA2 sequence in-frame to the S1 sequence, creating the DNA coding for the SpaPS1S1CtxA2 fusion as one gene and ctxB as a second gene with its authentic ribosomal binding site and leader sequence. The S1S1CtxA2B DNA was further subcloned into pMalp (New England Biolabs, Mississauga, Ontario, Canada), creating an in-frame fusion between the S1S1CtxA2 protein and the maltose binding protein. The S1S1CtxA2B fusion carried on a 1.9-kb HindIII-KpnI fragment was blunted with Klenow and subcloned into the blunted EcoRI site of pMalp. The resulting plasmid was named pMalpS1S1CtxA2B.
Purification and characterization of the MBPS1S1CtxA2B chimera.
Initial expression studies with Escherichia coli (pMalpS1S1CtxA2B) showed that large quantities of the MBPS1S1CtxA2 fusion protein and CtxB were produced as insoluble aggregates following induction with 0.3 mM isopropyl-β-d-thiogalactoside. Solubilization of the aggregates with 6 M urea followed by dialysis resulted in a very low yield of the active MBPS1S1CtxA2B chimera, as indicated by GM1-binding enzyme-linked immunosorbent assay (ELISA) using anti-PT as the detecting antibody. The highest yield of the active form of the chimera was from noninduced cultures at 37°C. Therefore, batches of noninduced cultures (2 liters) were used as the source (clarified sonicate) for the isolation of the chimera by affinity chromatography using an amylose column (20 ml; New England Biolabs) followed with a d-galactose column (15 ml; Pierce, Rockford, Ill.) according to the manufacturer's suggestions. The yield of the chimera was ca. 50 μg per liter of culture.
The protein composition was examined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on a 12% gel and Western immunoblotting. An unheated sample of the affinity-isolated chimera showed a single band with a mass of ca. 105 kDa on the SDS-PAGE gel, suggesting purity. The boiled sample showed the disappearance of the 105-kDa band and appearance of two bands of 76 and 11 kDa. Western immunoblots showed that the 105- and 76-kDa bands were recognized by the monoclonal anti-S1 antibody (7). The 105-kDa band and eight other bands from the unheated sample were recognized by the anti-CtxB antibody (1/2,000; List Biological Laboratories, Campbell, Calif.). The multiple banding pattern is likely due to the different aggregated forms of the chimera. In the heated sample, only the 11-kDa band was recognized by the anti-CtxB antibody.
The molecular mass of the native chimeric protein was estimated by gel permeation chromatography on a Sephacryl S-200HR column (2.5 by 96 cm). The eluted protein was assayed by GM1-binding ELISA. The single peak that was immunoreactive to both anti-PT and anti-CtxB antibodies has a molecular weight of 122,500 as estimated by comparisons to known protein standards (Sigma). The result suggests that the chimera is the expected MBPS1S1A2(CtxB)5. It is interesting that the MBPS1S1CtxA2B chimera is considerably larger than the holocholera toxin (84 kDa) and the SBRCtxA2B chimera (107.9 kDa [6]). Our results indicate that the A2 fragment can direct a polypeptide as large as 76 kDa for association with the CtxB pentamer.
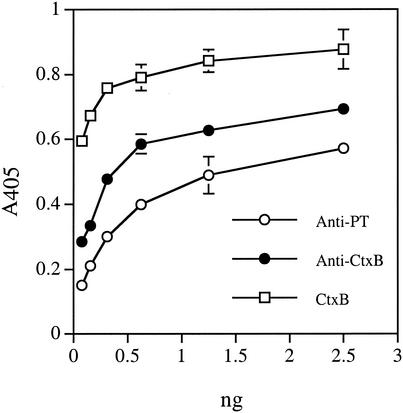
The functionality of the isolated chimeric protein was demonstrated by GM1-binding ELISA in a dose-dependent manner. In the assay, microtiter plates were coated with 230 ng of GM1 ganglioside (CalBiochem, San Diego, Calif.) and blocked with 1% gelatin in phosphate-buffered saline (PBS) with 0.1% Tween 20 and 5 mM MgCl2, and samples were added. The chimeric protein bound to GM1 was detected with a rabbit anti-PT (1/300; [11]) or a goat anti-CtxB antibody followed by the goat anti-rabbit immunoglobulin G (IgG) (1/30,000; Sigma) or rabbit anti-goat IgG (1/20,000; Sigma) alkaline phosphatase conjugates, respectively. As shown in Fig. 1, the titration curves in GM1-binding ELISA probed with anti-PT and anti-CtxB antibodies were very similar. The curves were also very similar to that of a commercial CtxB.
FIG. 1.
GM1-binding ELISA of MBPS1S1CtxA2B. The chimera was detected with a rabbit anti-PT antibody or a goat anti-CtxB antibody. Open squares represent a parallel ELISA with a commercial CtxB probed with the goat anti-CtxB antibody.
The chimera did not showed any cytotoxicity to Chinese hamster ovary (CHO) cells at concentrations of below 45 μg/ml in the CHO cell clustering assay (7). In comparison, the native PT was cytotoxic at 98 pg/ml.
Immunogenicity of the MBPS1S1CtxA2B chimera.
BALB/c mice (n = 5; female; 3 weeks old) were immunized intranasally with 25 μg of MBPS1S1CtxA2B at days 1, 11, 21, 41, 51, and 61 by pipetting the immunogen into the nostrils. A second cohort of mice (n = 5) was similarly immunized with 25 μg of pertussis toxoid (SmithKline Beecham, Rixensart, Belgium), and a third cohort (n = 5) was sham immunized with PBS. Saliva and blood were collected at days 0, 18, 28, 48, 57, and 67 as described previously (9, 10). Vaginal washes (VW) were collected at days 0 and 67 (9, 10). Bronchoalveolar lavage (BAL) fluids were obtained with 1 ml of PBS at day 67 (7, 9). Specific antibodies in samples were measured by ELISA in end-point dilutions as described previously (10). Titers of the specific antibodies were defined as the highest dilution that gave an A405 value at least 0.05 higher than that of a pool of pre- or nonimmune sample.
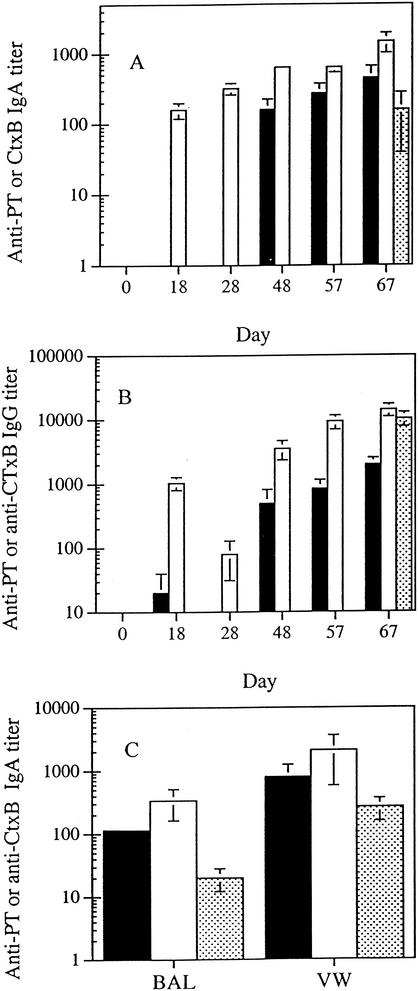
Following a series of four intranasal immunizations, the chimera elicited a salivary IgA response to PT (Fig. 2A). The titer of the anti-PT IgA antibodies was tripled following two additional immunizations. In contrast, the salivary IgA response to CtxB was much more rapid and greater in magnitude than the anti-PT response. IgA antibodies to PT and CtxB were also found in BAL and VW samples from animals immunized with the chimera (Fig. 2C). The animals that received the soluble pertussis toxoid (PTd) also demonstrated a mucosal IgA response, but the titers of antibody in saliva, BAL, and VW were three-, six-, and threefold lower (P = 0.05; Student's t test) than that from animals immunized with the chimera.
FIG. 2.
Immune responses following intranasal immunization with MBPS1S1CtxA2B. (A) Specific titers of IgA antibody in saliva. (B) Specific titers of IgG antibody in sera. (C) Specific titers of IgA antibody in BAL and VW. Symbols: ▪, anti-PT antibodies; □, anti-CtxB antibodies; , anti-PT antibodies in PTd-immunized samples. In panels A and B, for the PTd group, only samples from the last time point were assayed. Results are mean titers (± standard error; n = 5) for individual animal samples.
Intranasal immunization with the chimera also elicited a serum IgG response to PT and CtxB (Fig. 2B). Similar to the salivary IgA response, the anti-PT IgG antibodies appeared only after the fourth immunization, while the anti-CtxB IgG response was much more rapid and intense. Interestingly, the anti-PT IgG titer elicited by the pertussis toxoid was about five times higher than that elicited by the chimera. The serum antibodies raised with the chimera recognized the S1 subunit of PT in Western immunoblots, suggesting specificity (data not shown).
In vitro PT neutralization.
The ability of the antiserum to neutralize the cytotoxic effect of PT was assessed by the CHO cell clustering assay using the method described previously (7). The neutralization titers (reciprocal of dilution which showed complete neutralization) for the preimmune and chimera-immunized sera (day 67) were <2 and 256, respectively. The neutralization titer for the Ptd sera (day 67) was 1,024. In comparison, the serum from a human subject (CD95) who had pertussis showed a neutralization titer of >4,096. The PT neutralization titer for the chimera-immunized serum is comparable to that for serum obtained from parenteral immunization using diphtheria toxin subunit A-S1 fusion protein (1) or S1-tetanus toxic fragment C fusion protein (2).
Protection in the mouse respiratory model.
The ability of the chimera to confer protection in vivo was tested using the mouse respiratory model of B. pertussis infection (7). Briefly, cohorts of BALB/c mice were immunized as described above and were exposed to an aerosol of 5 × 108 CFU of virulent B. pertussis (Tohama)/ml at day 68. As shown in Fig. 3, the animals that were immunized with the chimera had a bacterial count in the lungs of 9.80 × 103 on day 7, which was significantly lower than the sham group's count of 1.86 × 105 bacteria (P = 0.01; Student's t test). The lung bacterial count for the pertussis toxoid group was 5.20 × 104 (P = 0.136 compared to the sham group and P = 0.135 compared to the chimera group). On day 15, the lung bacterial counts were 4.27 × 103, 7.87 × 103, and 1.48 × 103 for the chimera, sham, and pertussis toxoid groups, respectively.
FIG. 3.
Protection against B. pertussis respiratory infection in BALB/c mice. Each point represents mean (± standard error) for four or five animals. Day zero counts were determined for animals euthanatized within an hour after inoculation.
On day 7, the chimera-immunized animals had lung bacterial counts of ca. 2 log10 units lower (P = 0.01) than that for the sham control group, suggesting protection. The level of protection is comparable to that found in studies in which PTd and filamentous hemagglutinin were used together as soluble antigens (15) or encapsulated in biodegradable particles (3). Since our chimera contains only the S1 fragment, the observed protection is actually quite remarkable. Although it is not statistically significant, the PTd-immunized animals showed a lower lung count than the sham group but a higher count than the chimera group. The reason for a better protection conferred by the chimera than by the PTd is unclear, although a higher mucosal anti-PT IgA immune response was observed in the chimera group than in the PTd group.
In conclusion, a divalent pertussis toxin S1 fragment fused to CtxA2B is an immunogenic antigen and can elicit protective immunity against B. pertussis infection in a mouse model. A similar strategy may be employed to investigate the mucosal immunogenicity and protective potential of other pertussis vaccine antigens.
Acknowledgments
We thank Y.-J. Li for her excellent technical assistance and M. Russell for providing pRIT10814.
This study is supported by an MRC/CIHR Regional Partnership Program grant with the IWK Health Centre. D. Salloum was a recipient of an IWK Graduate Studentship Award.
Editor: J. T. Barbieri
REFERENCES
- 1.Barbieri, J. T., D. Armellini, J. Molkentin, and R. Rappuoli. 1992. Construction of a diphtheria toxin A fragment-C180 peptide fusion protein which elicits a neutralizing antibody response against diphtheria toxin and pertussis toxin. Infect. Immun. 60:5071-5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boucher, P., H. Sato, Y. Sato, and C. Locht. 1994. Neutralizing antibodies and immunoprotection against pertussis and tetanus obtained by use of a recombinant pertussis toxin-tetanus toxin fusion protein. Infect. Immun. 62:449-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conway, M. A., L. Madrigal-Estebas, S. McClean, D. J. Brayden, and K. H. Mills. 2001. Protection against Bordetella pertussis infection following parenteral or oral immunization with antigens entrapped in biodegradable particles: effects of formulation and route of immunization on induction of Th1 and Th2 cells. Vaccine 19:1940-1950. [DOI] [PubMed] [Google Scholar]
- 4.De Magistris, M. T., M. Romano, A. Bartoloni, R. Rappuoli, and A. Tagliabue. 1989. Human T cell clones define S1 subunit as the most immunogenic moiety of pertussis toxin and determine its epitope map. J. Exp. Med. 169:1519-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gill, D. M. 1976. The arrangement of subunits in cholera toxin. Biochemistry 12:3558-3563. [DOI] [PubMed] [Google Scholar]
- 6.Hajishengallis, G., S. K. Hollingshead, T. Koga, and M. W. Russell. 1995. Mucosal immunization with a bacterial protein antigen genetically coupled to cholera toxin A2/B subunits. J. Immun. 154:4322-4332. [PubMed] [Google Scholar]
- 7.Halperin, S. A., T. B. Issekutz, and A. Kasina. 1991. Modulation of Bordetella pertussis infection with monoclonal antibodies to pertussis toxin. J. Infect. Dis. 163:355-361. [DOI] [PubMed] [Google Scholar]
- 8.Homonylo-McGavin, M. K., and S. F. Lee. 1996. Role of the C terminus in antigen P1 surface localization in Streptococcus mutans and two related cocci. J. Bacteriol. 178:801-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee, S. F., S. A. Halperin, J. B. Knight, and A. Tait. 2002. Purification and immunogenicity of a recombinant Bordetella pertussis S1S3FHA fusion protein expressed by Streptococcus gordonii. Appl. Environ. Microbiol. 68:4253-4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee, S. F., S. A. Halperin, H. Wang, and A. MacArthur. 2002. Oral colonization and immune responses to Streptococcus gordonii expressing a pertussis toxin S1 fragment in mice. FEMS Microbiol. Lett. 208:175-178. [DOI] [PubMed] [Google Scholar]
- 11.Lee, S. F., R. March, S. A. Halperin, G. Faulkner, and L. Q. Gao. 1999. Surface expression of a protective recombinant pertussis toxin S1 subunit fragment in Streptococcus gordonii. Infect. Immun. 67:1511-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKenzie, S. J., and J. F. Halsey. 1984. Cholera toxin B subunit as a carrier protein to stimulate a mucosal immune response. J. Immunol. 133:1818-1824. [PubMed] [Google Scholar]
- 13.Mekalanos, J. J., D. J. Swartz, G. D. N. Pearson, N. Harford, F. Groyne, and M. de Wilde. 1983. Cholera toxin genes: nucleotide sequence, deletion analysis and vaccine development. Nature 306:551-557. [DOI] [PubMed] [Google Scholar]
- 14.Merritt, E. A., S. Sarfaty, F. van Den Akker, C. L'Hoir, J. A. Martial, and W. G. J. Hol. 1994. Crystal structure of cholera toxin B-pentamer bound to receptor GM1 pentasaccharide. Protein Sci. 3:166-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryan, E. J., E. McNeela, G. A. Murphy, H. Stewart, D. O'Hagan, M. Pizza, R. Rappuoli, and K. H. Mills. 1999. Mutants of Escherichia coli heat-labile toxin act as effective mucosal adjuvants for nasal delivery of an acellular pertussis vaccine: differential effects of the nontoxic AB complex and enzyme activity on Th1 and Th2 cells. Infect. Immun. 67:6270-6280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sato, H., A. Ito, J. Chiba, and Y. Sato. 1984. Monoclonal antibody against pertussis toxin: effect on toxin activity and pertussis infections. Infect. Immun. 46:422-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sato, H., Y. Sato, and I. Ohishi. 1991. Comparison of pertussis toxin (PT)-neutralizing activities and mouse-protective activities of anti-PT mouse monoclonal antibodies. Infect. Immun. 59:3832-3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weiss, A. A., and E. L. Hewlett. 1986. Virulence factors of Bordetella pertussis. Annu. Rev. Microbiol. 40:661-686. [DOI] [PubMed] [Google Scholar]