Abstract
The persistence of Bordetella pertussis and B. parapertussis within vaccinated populations and the reemergence of associated disease highlight the need to better understand protective immunity. The present study examined host immunity to bordetellae and addressed potential concerns about the mouse model by using a comparative approach including the closely related mouse pathogen B. bronchiseptica. As previously observed with B. pertussis, all three organisms persisted throughout the respiratory tracts of B-cell-deficient mice, indicating that B cells are required for bacterial clearance. However, adoptively transferred antibodies rapidly cleared B. bronchiseptica but not human pathogens. These results obtained with the mouse model are consistent with human clinical observations, including the lack of correlation between antibody titers and protection, as well as the limited efficacy of intravenous immunoglobulin treatments against human disease. Together, this evidence suggests that the mouse model accurately reflects substantial differences between immunities to these organisms. Although both B. pertussis and B. parapertussis are more closely related to B. bronchiseptica than they are to each other, they share the ability to resist rapid clearance from the lower respiratory tract by adoptively transferred antibodies, an adaptation that correlates with their emergence as human pathogens that circulate within vaccinated populations.
Bordetella bronchiseptica, B. pertussis, and B. parapertussis are closely related gram-negative respiratory pathogens that have recently been reclassified as subspecies (12, 16). B. pertussis and B. parapertussis appear to have diverged independently from a B. bronchiseptica-like progenitor and are highly infectious pathogens that primarily infect humans, causing the acute and severe disease pertussis or whooping cough (5, 6). In contrast, B. bronchiseptica infects a wide range of mammals (4), typically asymptomatically, and persists in the upper respiratory tract indefinitely (4). The basis for the interspecies differences in host range and severity of disease is not known, but these differences may be related to differences between bacterial subspecies or host differences in physiology or immune response to Bordetella infection.
Little is known definitively about the normal human immune response to Bordetella infection because it has generally been studied in individuals who were previously vaccinated (10). In the murine model, B cells are necessary to eliminate B. pertussis, suggesting that antibodies have a critical role in clearance (9). Although the importance of antibodies in immunity to other bacterial respiratory pathogens, such as Haemophilus influenzae and Pasteurella multocida, are well documented (10) and Bordetella-specific antibodies are generated in response to vaccination or infection (15), anti-Bordetella titers do not correlate well with protection in large clinical trials (3). In contrast to natural immunity following an infection, vaccination provides little, if any, protection against subclinical infection (10) and does not protect from cross infection with other Bordetella subspecies despite generating a strong antibody response (15, 17). Understanding natural immunity to bordetellae may allow the design of better vaccines that not only reduce the severity of the disease but also prevent infection and provide cross protection against other bordetellae.
In order to investigate the comparative biology of these closely related organisms, we have examined the basis for protective immunity to each in the mouse model. Experiments with SCID and Rag-1−/− mice indicated that adaptive immunity is required to clear all three organisms from the lower respiratory tract (4). B-cell-deficient mice fail to clear B. pertussis suggesting that antibodies may have a role in clearance of B. pertussis (9), but the role of antibodies in immunity to B. bronchiseptica and B. parapertussis is not known. Here we demonstrate that serum antibodies completely clear B. bronchiseptica from the lower respiratory tracts of wild-type and B-cell-deficient mice within 3 days but have no effect on the human-adapted pathogens in this time frame. This interspecies difference could not be attributed to antibody titers or differences in serum isotypes. We discuss the possibility that the human pathogens acquired resistance to serum antibodies during their apparently independent evolution from B. bronchiseptica-like animal pathogens in order to persist in immune populations.
MATERIALS AND METHODS
Bacteria.
Bacteria were maintained on Bordet-Gengou agar (Difco), inoculated into Stainer-Scholte broth at optical densities of 0.1 or lower, and grown to mid-log phase at 37°C on a roller drum. Wild-type strains of B. bronchiseptica (RB50), B. parapertussis (12822), and B. pertussis (BP536) have been described previously (4, 5).
Animal experiments.
C57BL/6 and MuMT mice were obtained from The Jackson Laboratory. Mice lightly sedated with isoflurane (Abbott Laboratories) were inoculated by pipetting 50 μl of phosphate-buffered saline (PBS) containing 5 × 105 bacteria onto the tip of the external nares. For time course experiments, groups of four animals were sacrificed on days 0, 3, 7, 14, 28, 49, 70, and 105 postinoculation. Colonization of various organs was quantified by homogenization of each tissue in PBS, plating onto Bordet-Gengou blood agar containing 20 μg of streptomycin per ml, and colony counting. For passive-transfer experiments, wild-type mice were inoculated with 5 × 105 CFU of B. bronchiseptica, B. parapertussis, or B. pertussis by the intranasal route as described above and serum was collected on day 28 postinoculation. Two hundred microliters of convalescent-phase or naive serum was injected intraperitoneally into mice before inoculation. Animals were sacrificed on days 0, 1, 3, 5, and 7 postinoculation or as indicated in each experiment. Colonization of various organs was quantified as described above. All animal experiments were carried out in accordance with institutional guidelines.
Antibodies.
Titers of anti-Bordetella antibody in convalescent-phase sera were determined by enzyme-linked immunosorbent assay with polyvalent anti-mouse secondary antibodies as described previously (1). Specific classes and isotypes of antibodies were determined by using appropriate secondary antibodies (Southern Biotechnology Associates and Pharmingen).
RESULTS
B cells are necessary for the clearance of bordetellae from the respiratory tracts of mice.
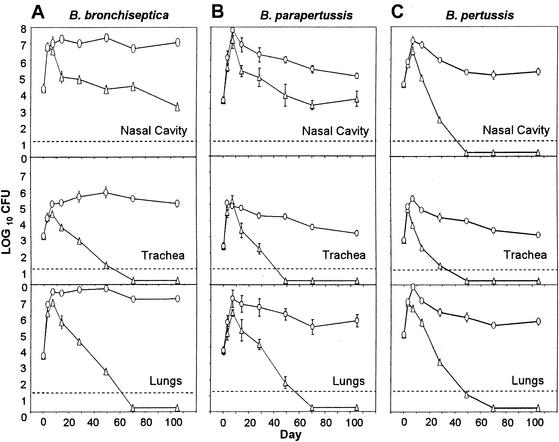
The role of B cells in immunity against bordetellae was investigated by using B-cell-deficient MuMT mice (7). Wild-type and MuMT mice were inoculated intranasally with 5 × 105 CFU of various Bordetella subspecies in 50 μl of PBS. This inoculation regimen consistently delivers approximately 105 CFU to the nasal cavity and lungs and 103 CFU to the trachea (4). Bacterial numbers were determined in the nasal cavity, trachea, and lungs at various time points. In wild-type mice, bacterial numbers began to decrease after day 7 and B. bronchiseptica and B. parapertussis were cleared from the lower respiratory tract (trachea and lungs) by day 70 while B. pertussis was cleared by day 49 postinoculation (Fig. 1). In contrast, MuMT mice failed to clear the three Bordetella subspecies from the lower respiratory tract even on day 105 postinoculation, and bacterial numbers were comparable to those recovered on day 7 (Fig. 1). These results agree with data published earlier showing that B. pertussis persists in MuMT mice (9) and indicate that B cells are required for the decrease in bacterial numbers observed after day 7 in wild-type mice.
FIG. 1.
MuMT mice are defective in clearing B. bronchiseptica, B. parapertussis, and B. pertussis from the respiratory tract. Groups of four 4- to 6-week-old C57BL/6 (▵) and MuMT (○) mice were inoculated with 5 × 105 CFU of B. bronchiseptica (A), B. parapertussis (B), or B. pertussis (C) delivered in a 50-μl volume of PBS into the nares. The number of bacteria recovered from the nasal cavity, trachea, or lungs at each indicated time postinoculation is expressed as the log10 mean ± the standard error.
MuMT mice were also defective in controlling bordetellae in the nasal cavity. Although B. bronchiseptica and B. parapertussis normally persist in the nasal cavities of wild-type mice beyond 105 days postinoculation, bacterial numbers were significantly (102- to 104-fold) higher in this site in MuMT mice than in wild-type mice (Fig. 1A and B). B. pertussis was cleared from wild-type mice by day 49 but was recovered from the nasal cavities of MuMT mice until at least day 105 in numbers similar to those of B. bronchiseptica and B. parapertussis (Fig. 1). These data indicate that B cells are required for the >100-fold reduction in the numbers of B. bronchiseptica and B. parapertussis and for the elimination of B. pertussis from the nasal cavities of wild-type mice.
Serum antibodies are sufficient to clear B. bronchiseptica, but not B. parapertussis or B. pertussis, from the lower respiratory tracts of mice.
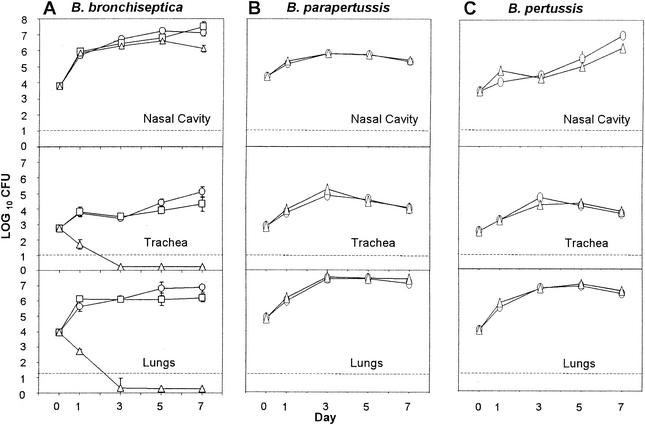
Although B cells appear to be required for the clearance of all three Bordetella subspecies, previous studies have shown that antibodies have little effect on B. pertussis numbers in vivo, leading to the conclusion that B cells are required for some function other than antibody production (8). To examine the specific role of antibodies in the control and clearance of bordetellae, serum from naive or convalescent animals previously infected with each of the Bordetella subspecies was adoptively transferred into naive animals immediately prior to a challenge with the respective subspecies. B. bronchiseptica-induced convalescent-phase serum (serum collected from mice 28 days postinoculation with B. bronchiseptica) rapidly cleared B. bronchiseptica from the tracheas and lungs of mice by day 3 postinoculation, whereas naive serum had no significant effect (Fig. 2A). In striking contrast, both human pathogens were unaffected by adoptive transfer of convalescent-phase serum (Fig. 2B and C). Interestingly, adoptive transfer of convalescent-phase serum had no significant effect on the number of bacteria recovered from the nasal cavity. These data reveal both tissue-specific and bacterium-specific effects of antibodies and, together with the data presented in Fig. 1, indicate that some factor that is missing from B-cell-deficient mice and is not replaced by transfer of serum antibodies is required for clearance of the human pathogens from the lower respiratory tract and for a greater-than-100-fold reduction in the numbers of all three bacterial subspecies in the nasal cavity.
FIG. 2.
Adoptive transfer of serum antibodies clears B. bronchiseptica but not B. parapertussis or B. pertussis from the lower respiratory tract. Groups of four 4- to 6-week-old C57BL/6 mice were inoculated with 5 × 105 CFU of B. bronchiseptica (A), B. parapertussis (B), or B. pertussis (C) delivered in a 50-μl volume of PBS into the nares. Two hundred microliters of PBS (□), naive serum (○), or convalescent-phase serum (▵) was given by intraperitoneal injection prior to inoculation. The number of bacteria recovered from the nasal cavity, trachea, or lungs at each indicated time postinoculation is expressed as the log10 mean ± the standard error.
To further investigate the possibility that B cells are required for some function other than antibody production, naive or convalescent-phase serum was transferred into B-cell-deficient MuMT mice challenged with B. bronchiseptica. As observed in wild-type animals, naive serum had no effect on bacterial numbers but convalescent-phase serum rapidly (by day 3) eliminated B. bronchiseptica from the lower respiratory tracts of these B-cell-deficient mice (Fig. 3). These results suggest that no additional B-cell functions are required for efficient serum antibody-mediated clearance of B. bronchiseptica from the lower respiratory tract. Additionally, serum from MuMT mice infected with B. bronchiseptica and collected on day 28 postinoculation was adoptively transferred into mice infected with B. bronchiseptica. Unlike wild-type serum, serum from MuMT mice had no effect on B. bronchiseptica numbers in the lower respiratory tracts of mice (data not shown). These data suggest that antibodies are responsible for the rapid clearance of B. bronchiseptica by convalescent-phase serum.
FIG. 3.
Adoptive transfer of serum antibodies clears B. bronchiseptica from the respiratory tracts of MuMT mice. Groups of four 4- to 6-week-old MuMT mice were inoculated with 5 × 105 CFU of B. bronchiseptica delivered in a 50-μl volume of PBS into the nares. Two hundred microliters of PBS (○), naive serum (▵), or convalescent-phase serum (□) was given by intraperitoneal injection prior to inoculation. The number of bacteria recovered from the nasal cavity, trachea, or lungs at each indicated time postinoculation is expressed as the log10 mean ± the standard error.
Antibody titers and clearance.
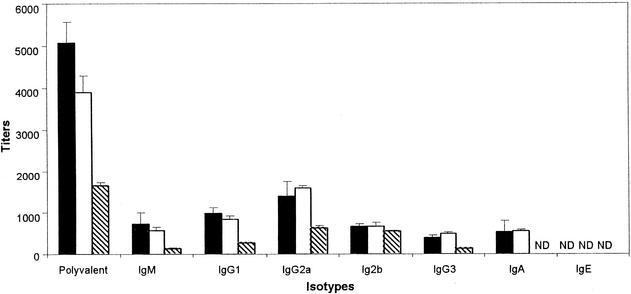
We hypothesized that the differences in the effectiveness of serum antibodies in clearing the three Bordetella subspecies from the lower respiratory tracts of mice could be due to quantitative and qualitative differences in the antibodies present in the respective convalescent-phase sera. We therefore determined the anti-Bordetella antibody titers of various classes and isotypes of antibodies in these sera. There was no significant difference in the overall titers or individual isotypes of anti-Bordetella antibodies generated by B. bronchiseptica or B. parapertussis, but both induced significantly higher antibody titers than did B. pertussis (Fig. 4). To investigate whether the lower antibody titer of B. pertussis-induced serum could be involved in the lack of antibody-mediated clearance in vivo, mice were administered three times the volume of B. pertussis-induced serum. Even when antibody titers were compensated for in this way, B. pertussis-induced serum had no effect on bacterial numbers within the respiratory tract (Fig. 5). To investigate the possibility that some qualitative characteristic of the antibodies was involved in their differential activity, B. bronchiseptica-induced serum was transferred into animals infected with B. pertussis. Although this serum recognized and bound B. pertussis in vitro as well as it did B. bronchiseptica, it did not affect B. pertussis numbers in the respiratory tracts of mice (data not shown). These data suggest that the difference in the susceptibility of human-adapted bordetellae to serum antibodies is not due to quantitative differences in the antibodies. To investigate the possibility of the presence of some inhibitors in B. pertussis-induced serum interfering with the action of antibodies, we adoptively transferred both B. pertussis- and B. bronchiseptica-induced sera into mice infected with B. bronchiseptica. These mice efficiently cleared B. bronchiseptica from the trachea and lungs within 3 days posttreatment (data not shown). These data suggest that B. pertussis-induced serum does not have inhibitors that interfere with the effects of antibodies.
FIG. 4.
Characterization of convalescent-phase sera. Anti-Bordetella antibody titers were compared in serum samples collected 28 days after intranasal inoculation of mice with a large dose (50 μl of PBS containing 5 × 105 CFU) of B. bronchiseptica (▪), B. parapertussis (□), or B. pertussis (▧). Whole cells of the indicated Bordetella subspecies were used as the antigen in each enzyme-linked immunosorbent assay. Bound anti-Bordetella antibodies were detected by secondary antibodies specific for total and indicated isotypes of immunoglobulin (Ig). Bars represent the means ± the standard errors of the immunoglobulin titers detected. ND, not detectable.
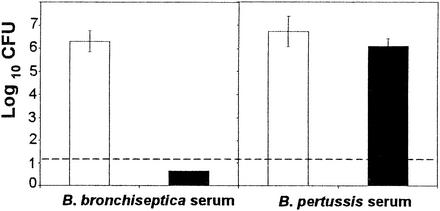
FIG. 5.
Increasing the volume of serum does not affect B. pertussis numbers in lungs. Groups of four 4- to 6-week-old C57BL/6 mice were inoculated with 5 × 105 B. pertussis bacteria delivered in a 50-μl volume of PBS into the nares. Six hundred microliters of convalescent-phase or naive serum was injected by the intraperitoneal route prior to inoculation. The number of bacteria recovered from the lungs on day 3 postinoculation is expressed as the log10 mean ± the standard error.
Coinfection and antibody-mediated clearance.
Previous experiments have shown that B. bronchiseptica induces an innate immune response that is substantially greater than that of B. pertussis (4), and we hypothesized that these differences may contribute to antibody-mediated bacterial clearance by increasing the access of antibodies, complement, and FcR-bearing phagocytes to the bacterial microcolonies in the respiratory tract. If B. bronchiseptica induces inflammation that contributes to antibody-mediated bacterial clearance, then inoculation of B. bronchiseptica along with B. pertussis would be predicted to allow B. pertussis-induced sera to eliminate B. pertussis. To test this hypothesis, B. bronchiseptica and B. pertussis were coinoculated into wild-type mice to which either B. bronchiseptica- or B. pertussis-induced serum was adoptively transferred. B. bronchiseptica-induced serum did not affect B. pertussis numbers but rapidly cleared B. bronchiseptica from the lower respiratory tract by day 3 postinoculation, again suggesting that B. pertussis does not inhibit some antibody function. However, B. pertussis-induced serum did not affect the numbers of either B. pertussis or B. bronchiseptica bacteria in the respiratory tract (Fig. 6), indicating that the inflammatory response elicited by B. bronchiseptica did not facilitate serum antibody-mediated clearance of B. pertussis. The failure of B. pertussis-induced serum to affect B. bronchiseptica is discussed below.
FIG. 6.
Coinoculation of B. bronchiseptica with B. pertussis does not affect B. pertussis CFU counts in the lungs. Groups of four 4- to 6-week-old C57BL/6 mice were inoculated with 5 × 105 B. bronchiseptica and B. pertussis bacteria delivered in 50 μl of PBS. Six hundred microliters of either B. bronchiseptica- or B. pertussis-induced serum was injected by the intraperitoneal route prior to inoculation. The number of B. bronchiseptica (▪) or B. pertussis (□) bacteria recovered from the lungs on day 3 postinoculation is expressed as the log10 mean ± the standard error.
DISCUSSION
To better understand immunity to bordetellae, we examined the two common human pathogens B. pertussis and B. parapertussis alongside B. bronchiseptica, which naturally infects mice. Our results show that B cells are required to clear all three Bordetella subspecies from the respiratory tracts of mice, in agreement with previous results obtained with B. pertussis (8, 9). Adoptive transfer of convalescent-phase serum was sufficient to rapidly clear the lower respiratory tract of the broad-host-range pathogen B. bronchiseptica but not the human-specific pathogens B. pertussis and B. parapertussis. These results obtained with the mouse model are consistent with human clinical trials, in which serum antibody titers could not be correlated with protection against B. pertussis and intravenous immunoglobulin therapy had modest effects, thus supporting the validity of the mouse model as accurately reflecting the roles of individual host immune functions (3, 11). Since B. pertussis and B. parapertussis appear to have emerged independently as human pathogens and each is more closely related to a B. bronchiseptica-like progenitor than each is to the other (16), these results raise the possibility that resistance to serum antibodies may relate to their adaptation to humans. Understanding the mechanism(s) involved in avoiding or delaying antibody-mediated clearance may contribute to the development of improved vaccines or treatments for human respiratory infections.
It is well established that B. bronchiseptica induces higher serum antibody titers than B. pertussis in the mouse model (4), and we hypothesized that qualitative and/or quantitative differences in the antibodies could explain their different in vivo effects. However, B. parapertussis and B. bronchiseptica induced similar antibody titers but these antibodies completely cleared only the latter without affecting the former. Additionally, when we compensated for the lower antibody titers of B. pertussis-induced serum by increasing the volume delivered, there was still no effect on B. pertussis, suggesting that the difference in their antibacterial function is not due to differences in overall antibody titers. It is possible that the antibodies differed qualitatively; however, the distribution of the various antibody isotypes was not significantly different among the three immune sera. Furthermore, antibodies raised against B. bronchiseptica also recognized B. pertussis and vice versa (5). In addition, each immune serum killed all three organisms in serum killing assays, indicating that they recognized, bound, and activated complement on the surface of each bacterium in vitro (data not shown). Together, these data suggest that differential antibody effects cannot be attributed to quantitative differences in the antibodies but may be due to qualitative differences or in vivo susceptibilities of the organisms.
We have shown that B. bronchiseptica induces substantially more inflammation than B. pertussis (4) and have recently observed that this inflammation is important in antibody-mediated bacterial clearance (P. B. Mann and E. T. Harvill, unpublished data). We therefore hypothesized that the greater inflammation induced by B. bronchiseptica might allow anti-B. pertussis antibodies to be more effective. Alternatively, B. pertussis could inhibit antibody access to, or function within, the respiratory tract. However, coinfection with these two organisms neither increased antibody-mediated clearance of B. pertussis nor inhibited clearance of B. bronchiseptica. Together, these results suggest that the human-specific bordetellae have a mechanism to resist antibody-mediated clearance that B. bronchiseptica does not. Interestingly, B. pertussis-induced serum did not affect B. bronchiseptica in coinfection experiments, although it did bind and kill B. bronchiseptica in serum killing assays in vitro. Differences in antibody activities have been shown to depend on the isotype, the cognate antigen, and even the epitope on that antigen (13). These parameters may determine whether antibodies are effective or whether they actually interfere with, or block, more effective antibodies (13). We are currently investigating these possibilities.
It is possible that differences in susceptibility to antibodies relate to the different epidemiologies of these organisms. B. bronchiseptica can persist for years within the nasal cavity of its host, where serum antibodies have no effect. Individual humans usually eliminate infections by bordetellae, yet B. pertussis persists within relatively dense and mobile human populations, including those in which vaccine coverage is very high. This environment would be expected to provide selection for organisms with the ability to infect hosts that were previously exposed via vaccination or prior infection. Therefore, the ability to resist antibody-mediated clearance, possibly acquired by the two human pathogens during their adaptation to a new host, may relate to their epidemiology. It is worth noting that B. bronchiseptica occasionally infects humans but does not appear to spread efficiently and is usually associated with immunocompromised individuals (19), perhaps reflecting its susceptibility to antibody-mediated clearance. In the light of these observations, it is possible that acquisition of resistance to antibody-mediated clearance represents an important step in the independent emergence of B. pertussis and B. parapertussis as human pathogens.
The effects of antibodies on B. pertussis in vitro have been well studied, and a number of observations may relate to the ability of this organism to resist antibody-mediated clearance in vivo. Stefanelli et al. have shown that phagocytosis of B. pertussis by a human macrophage-like cell line cannot be enhanced by opsonizing the bacteria with human immune serum in an in vitro assay (14). A similar observation with human neutrophils was made by Weiss et al., who also showed that B. pertussis expresses a protein, BrkA, that confers a level of resistance to serum complement in vitro (2, 18). However, our data show that convalescent-phase serum that binds and rapidly kills B. pertussis in vitro has no effect when adoptively transferred in vivo, consistent with previous observations with vaccine-induced serum. Therefore, it is difficult to assess the relevance of these, and other, in vitro observations to the effects of antibodies in vivo. Since B. bronchiseptica is both closely related to and the apparent progenitor of both human pathogens, it may be that understanding the mechanism(s) involved in antibody-mediated clearance of B. bronchiseptica will reveal the pathways that are blocked or avoided by B. pertussis and B. parapertussis.
Acknowledgments
This work was funded by a grant from Neose Corp., Pennsylvania Department of Agriculture grant ME440678 and USDA grant 2002-35204-11684 to E.T.H., and The U.S. Army's MSC LTHET (P.B.M.).
We thank E. Jane Pishko and other laboratory members for useful discussions and manuscript review. We thank Sheila J. Plock for technical help during the course of these studies.
Editor: D. L. Burns
REFERENCES
- 1.Allen, A. G., T. Isobe, and D. J. Maskell. 1998. Identification and cloning of waaF (rfaF) from Bordetella pertussis and use to generate mutants of Bordetella spp. with deep rough lipopolysaccharide. J. Bacteriol. 180:35-40. [DOI] [PMC free article] [PubMed]
- 2.Barnes, M. G., and A. A. Weiss. 2001. BrkA protein of Bordetella pertussis inhibits the classical pathway of complement after C1 deposition. Infect. Immun. 69:3067-3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giuliano, M., P. Mastrantonio, A. Giammanco, A. Piscitelli, S. Salmaso, and S. G. Wassilak. 1998. Antibody responses and persistence in the two years after immunization with two acellular vaccines and one whole-cell vaccine against pertussis. J. Pediatr. 132:983-988. [DOI] [PubMed] [Google Scholar]
- 4.Harvill, E. T., P. A. Cotter, and J. F. Miller. 1999. Pregenomic comparative analysis between Bordetella bronchiseptica RB50 and Bordetella pertussis Tohama I in murine models of respiratory tract infection. Infect. Immun. 67:6109-6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heininger, U., P. A. Cotter, H. W. Fescemyer, G. Martinez de Tejada, M. H. Yuk, J. F. Miller, and E. T. Harvill. 2002. Comparative phenotypic analysis of the Bordetella parapertussis isolate chosen for genomic sequencing. Infect. Immun. 70:3777-3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heininger, U., K. Stehr, S. Schmitt-Grohe, C. Lorenz, R. Rost, P. D. Christenson, M. Uberall, and J. D. Cherry. 1994. Clinical characteristics of illness caused by Bordetella parapertussis compared with illness caused by Bordetella pertussis. Pediatr. Infect. Dis. J. 13:306-309. [DOI] [PubMed] [Google Scholar]
- 7.Kitamura, D., J. Roes, R. Kuhn, and K. Rajewsky. 1991. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature 350:423-426. [DOI] [PubMed] [Google Scholar]
- 8.Leef, M., K. L. Elkins, J. Barbic, and R. D. Shahin. 2000. Protective immunity to Bordetella pertussis requires both B cells and CD4+ T cells for key functions other than specific antibody production. J. Exp. Med. 191:1841-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahon, B. P., B. J. Sheahan, F. Griffin, G. Murphy, and K. H. Mills. 1997. Atypical disease after Bordetella pertussis respiratory infection of mice with targeted disruptions of interferon-gamma receptor or immunoglobulin mu chain genes. J. Exp. Med. 186:1843-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mills, K. H. 2001. Immunity to Bordetella pertussis. Microbes Infect. 3:655-677. [DOI] [PubMed] [Google Scholar]
- 11.Morris, D., and J. C. McDonald. 1957. Failure of hyperimmune gammaglobulin to prevent whooping cough. Arch. Dis. Child. 32:236-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Musser, J. M., E. L. Hewlett, M. S. Peppler, and R. K. Selander. 1986. Genetic diversity and relationships in populations of Bordetella spp. J. Bacteriol. 166:230-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Netski, D., and T. R. Kozel. 2002. Fc-dependent and Fc-independent opsonization of Cryptococcus neoformans by anticapsular monoclonal antibodies: importance of epitope specificity. Infect. Immun. 70:2812-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stefanelli, P., R. Ippoliti, C. Fazio, and P. Mastrantonio. 2002. Role of immune sera in the in-vitro phagocytosis of Bordetella pertussis strains. Microb. Pathog. 32:135-141. [DOI] [PubMed] [Google Scholar]
- 15.Thomas, M. G., K. Redhead, and H. P. Lambert. 1989. Human serum antibody responses to Bordetella pertussis infection and pertussis vaccination. J. Infect. Dis. 159:211-218. [DOI] [PubMed] [Google Scholar]
- 16.van der Zee, A., F. Mooi, J. Van Embden, and J. Musser. 1997. Molecular evolution and host adaptation of Bordetella spp.: phylogenetic analysis using multilocus enzyme electrophoresis and typing with three insertion sequences. J. Bacteriol. 179:6609-6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watanabe, M., and M. Nagai. 2001. Reciprocal protective immunity against Bordetella pertussis and Bordetella parapertussis in a murine model of respiratory infection. Infect. Immun. 69:6981-6986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weingart, C. L., and A. A. Weiss. 2000. Bordetella pertussis virulence factors affect phagocytosis by human neutrophils. Infect. Immun. 68:1735-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woolfrey, B. F., and J. A. Moody. 1991. Human infections associated with Bordetella bronchiseptica. Clin. Microbiol. Rev. 4:243-255. [DOI] [PMC free article] [PubMed] [Google Scholar]