Abstract
RTX cytotoxins play an important role in virulence of numerous gram-negative pathogens. Unexpectedly, however, we show here that the RTX proteins of Neisseria meningitidis are dispensable for virulence in the infant rat model of infection.
The ability of Neisseria meningitidis to cause fulminant septicemic infections and large epidemics justifies the fear inspired by meningococcal disease. Moreover, with more than 500,000 cases each year, accounting for 50,000 deaths and significant morbidity, meningococcal infections remain one of the most serious medical emergencies worldwide (13). Paradoxically, N. meningitidis colonizes the nasopharynx of a substantial proportion of the world population, approximately 10%, without causing any symptoms (14). Occasionally, however, meningococci can cross the nasopharyngeal mucosa and gain access to the bloodstream, where they thrive by circumventing the host immune defenses and by acquiring iron from scavenger proteins such as hemoglobin, transferrin, and lactoferrin (6). Meningococcemia may further lead to crossing of the blood-brain barrier by the bacterium and development of life-threatening meningitis.
When bacterial pathogens are grown in iron-depleted conditions, similar to those encountered in the blood and mucosal secretions, where the levels of free iron are extremely low, the expression of a number of virulence factors is increased (6). Similarly, N. meningitidis expresses increased amounts of several proteins under iron-depleted conditions (2), most of which are involved in iron acquisition (6). Interestingly, in N. meningitidis FAM20, two of these Fe-regulated proteins (Frp), initially called FrpA and FrpC, were shown to be related to the RTX family of bacterial cytotoxins (10, 12). Like other members of this protein family, of which Escherichia coli alpha-hemolysin is the most studied prototype, these proteins contain, near their carboxy termini, copies repeated in tandem of a nonapeptide rich in glycine and aspartate (10, 12), which are usually implicated in binding of calcium and target cells. A further demonstration of their relationship to RTX toxins came from the observation that these proteins, which are found in the meningococcal outer membrane and the supernatant, can be secreted by the heterologous E. coli alpha-hemolysin secretory apparatus (11), which recognizes carboxy-terminal secretion signals. Finaly, in a recent epidemiological study it was demonstrated that frpC alleles of various lengths were present in all invasive and most carrier strains of N. meningitidis (5). Moreover, the detection of FrpC-specific antibodies in the sera of patients recovering from invasive meningococcal disease demonstrated that FrpC-like protein is produced in vivo during infection (5).
Although their biological activity remains unknown, all the observations above pointed towards the involvement of FrpC-like proteins in meningococcal virulence. We therefore examined the capacity of an frp mutant to cause systemic infection in infant rats, the model of infection most widely used to study N. meningitidis genes required for disseminated infection (7, 8).
Construction of an MC58 frpC double mutant.
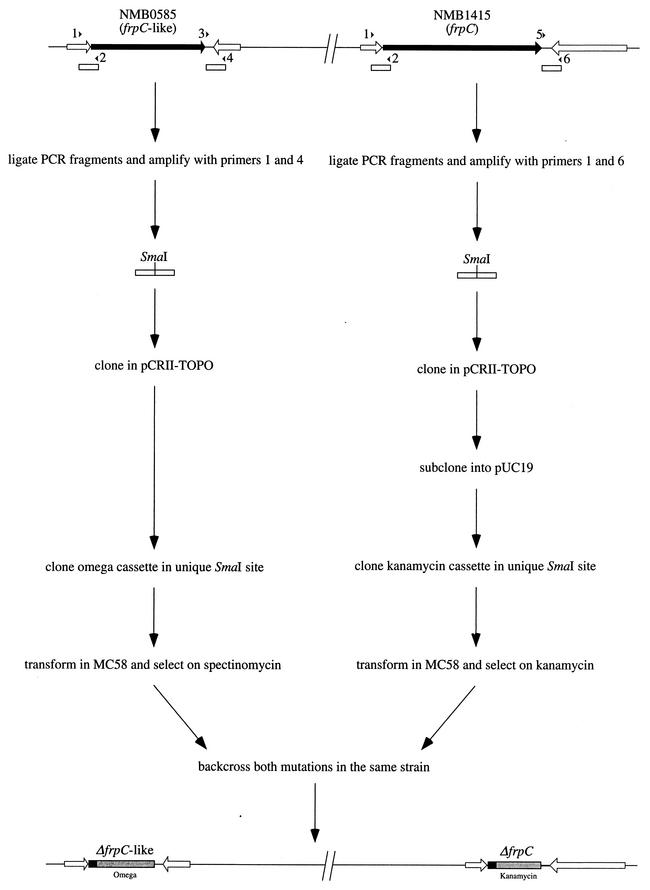
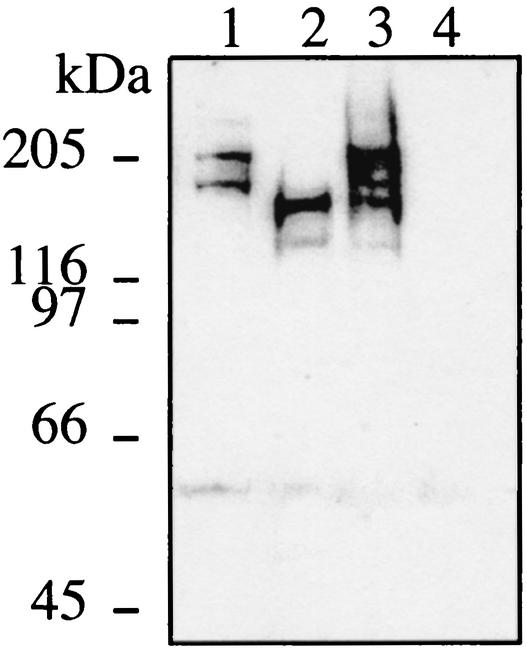
The availability of the genomic sequence of N. meningitidis MC58 (9) made this serogroup B strain the obvious choice for our study. In this strain, six open reading frames (ORF)s, scattered around the genome, present homologies to frpC and constitute the paralogous FrpC family: NMB0365, NMB0585, NMB1403, NMB1405, NMB1409, and NMB1415. However, only two of the corresponding proteins contained the classical nonapeptide repeats near their carboxy termini and could therefore be considered bona fide RTX proteins. They were encoded by NMB0585 (3,906 nucleotides) and NMB1415 (5,487 nucleotides). In contrast with what was observed in FAM20 (10, 12), the two ORFs were almost identical throughout their entire sequence, except for three internal fragments missing in NMB0585, and therefore clearly represent two different frpC alleles. NMB1415 encodes the prototype FrpC protein, whereas NMB0585 encodes an FrpC-like protein. Therefore, in order to test the involvement of RTX proteins in meningococcal virulence, it was crucial to construct a double mutant where both frpC alleles would be disrupted. Due to the lengths of the target genes, it was preferable to construct mutants where the two genes would be precisely deleted in order to avoid any residual activity. To perform this, we used a PCR-ligation-PCR mutagenesis method (1) (Fig. 1). First, a 681-bp region upstream of each gene was amplified by using primer 1 (5′-GGTTTGGATAGCGTGGACGATA-3′) and primer 2 (5′-TACCCGGGTGCAACCCGATCAAATTTCTC-3′), which contained an underlined SmaI site overhang. It should be noted that the amplified regions were identical and comprised part of an ORF of unknown function, potentially cotranscribed with the frp genes. Next, we amplified the differing regions downstream from the two frp genes (Fig. 1). The 689-bp region downstream of the frpC-like allele was amplified by using primers 3 (5′-GGAGCCTAATTACATTCATGGC-3′) and 4 (5′-CGCTTGGGCAACTACCAAATG-3′), whereas the 684-bp region downstream of the frpC allele was amplified by using primers 5 (5′-GGAGCCTAATCACATTCATGGC-3′) and 6 (5′-GCCGCCGCAGCGACACCCTGCAAC-3′). The amplified fragments, upstream and downstream, for frpC and frpC-like, respectively, were ligated and subsequently cloned into the pCRII-TOPO vector (Invitrogen) (Fig. 1). A kanamycin resistance cassette was inserted between the two fragments amplified for the frpC allele, whereas the Ω fragment encoding resistance to streptomycin was inserted between the two fragments amplified for the frpC-like allele (Fig. 1). This allowed the construction of individual knockout mutants of either of the frp alleles by allelic exchange in MC58. Finally the double mutant was constructed by backcrossing the frpC-like mutation in the frpC mutant and selecting on plates containing both kanamycin and spectinomycin (Fig. 1). PCR and Southern blot analyses confirmed precise deletions of both NMB0585 and NMB1415 genes (data not shown). In addition, the strains were analyzed by Western blotting with an FrpC-specific rabbit serum. This demonstrated that the double mutant does not produce any RTX protein (Fig. 2).
FIG.1.
Scheme of the construction of an MC58 frpC double mutant by PCR-ligation-PCR mutagenesis (1). The unique SmaI site was present within primer 2. The omega cassette was amplified from pT1Ω1E (4), whereas the kanamycin casette was obtained by amplifying the aphA-3 gene. The subcloning step, for the construction of the frpC mutant, was necessary because the pCRII-TOPO vector contains a kanamycin resistance gene.
FIG. 2.
Immunodetection of FrpC and FrpC-like proteins in MC58 wild-type and frp mutant strains. Strains were ΔNMB0585 (lane 1), ΔNMB1415 (lane 2), wild-type MC58 (lane 3), and frp double mutant (lane 4). The intermediate band (150 kDa) probably results from proteolytic processing, since meningococcal RTX proteins are known to be highly susceptible to proteolysis (12). A loopful of bacteria, grown overnight on GCB plates, was resuspended in 500 μl of RPMI medium, and 100 μl of this suspension was inoculated in 2 ml of RPMI medium. After 150 min of static culture at 37°C in an atmosphere containing 5% CO2, 200-μl aliquots were inoculated in 2 ml of RPMI medium, to which the iron chelator desferrioxamine (desferal) was added at a 200 μM final concentration in order to induce the expression of the frp genes. After 2 h, the bacteria were harvested by centrifugation, resuspended in loading buffer containing 8 M urea, boiled for 5 min, and loaded on a sodium dodecyl sulfate-7.5% polyacrylamide gel. After electrotransfer to nitrocellulose membrane, the Frp-derived proteins were detected by using an Frp-specific rabbit serum raised against purified recombinant FrpC (5) and were revealed by enhanced chemiluminescence detection with an anti-rabbit immunoglobulin G conjugated to horseradish peroxidase (Amersham Biosciences).
In vitro characterization of the double mutant.
As expected, the double mutant was not affected for growth in liquid GC base medium, confirming that the frp genes were dispensable for growth (data not shown). In addition, simple in vitro assays showed that the Frp proteins were neither involved in meningococcal serum resistance nor in adhesion to human endothelial cells, two phenotypes that are essential for virulence (14). The resistance of the double mutant to the bactericidal activity of the complement was tested as follows, by using a human serum obtained from a single individual: bacteria, grown overnight on GCB agar plates, were inoculated in 5 ml of RPMI medium and grown for 2 h at 37°C. Serum, RPMI medium, and bacteria were mixed in four-well NUNC plates (POLYLABO) so that the final 400-μl suspension contained 2 × 105 CFU in 25% serum (vol/vol). The reactions were incubated at 37°C in an atmosphere containing 5% CO2. Samples were taken from the reaction mixtures at different time points, and serial dilutions were plated on GCB. Colony counts were performed after overnight incubation of the plates. We first confirmed that an unencapsulated mutant, known to be serum sensitive, was killed within minutes (data not shown). In contrast, the double frpC mutant resisted in human serum as well as the wild-type MC58 strain (data not shown). Adhesion of meningococci to human umbilical vein endothelial cells was measured as described previously (3). This showed that, after a 5-hcontact with a monolayer of human umbilical vein endothelial cells, approximately 1.5 × 108 CFU ml−1 could be recovered for both the double frpC mutant and the MC58 parental strain, confirming that the frpC alleles are not important for adhesion to human endothelial cells.
In vivo analysis of the double mutant virulence.
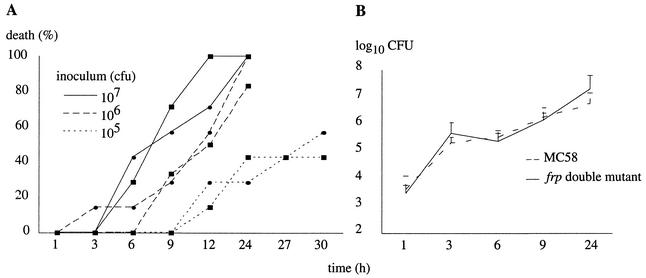
To determine whether the FrpC and FrpC-like RTX proteins were required for meningococcal virulence, the capacity of the double mutant to cause disseminated disease was assessed in the infant rat model of infection and compared to that of the parental MC58 strain. Lewis female rats with their litters of six or seven 4-day-old infants were obtained from Elevage JANVIER (France). Bacteria grown overnight on GCB agar plates were resuspended in 0.9% NaCl at different concentrations and were injected intraperitoneally, in 100-μl samples, to a total of six or seven infant animals coming from two different litters. The mortality rate, which was monitored over a 24- to 30-h course, was repeatedly found to be comparable in rats inoculated by the parental strain and the double frpC mutant (Fig. 3A). In parallel, the kinetics of meningococcemia caused by each strain was determined by counting the number of bacteria recovered from the blood of the infected animals after 3, 6, 9, and 24 h of infection. Five-microliter blood samples were taken from the tail of the surviving animals at the different time points and resuspended in 95 μl of phosphate-buffered saline, and 25 μl of serial dilutions was plated on GCB. Colony counts were performed after overnight incubation of the plate contents. As shown in Fig. 3B, no obvious difference in the course of bacteremia was seen between the wild-type strain and the double frpC mutant.
FIG. 3.
Time course of death (A) and meningococcemia (B) in 4-day-old infant rats inoculated with different doses of the MC58 parental strain (▪) and the isogenic frp double mutant (•). Mortality experiments, performed on a total of six or seven infant animals, were repeated twice, and representative results are shown. For the meningococcemia quantification experiment, six animals were injected with inocula of 7.7 × 105 and 6 × 105 CFU for the wild-type and the frp double mutant, respectively. Bars indicate standard errors of the means.
Despite numerous clues that point to a role in pathogenesis for the FrpC and FrpC-like proteins, we show here that an N. meningitidis MC58 isogenic double mutant, which does not produce these RTX-like proteins, is as virulent as the wild type in an infant rat model of infection. This suggests that these proteins do not appear to possess a toxin activity affecting the complement and/or phagocyte activities of the host, which would be necessary for systemic infection, because such activities would have been most likely detected in the used animal model. However, we cannot exclude that FrpC proteins might play a role in other steps essential for meningococcal virulence, such as colonization and dissemination in the nasopharynx or escape from the endocytic vacuoles, which could not be unraveled in the infant rat model of septicemia. Hence, it remains possible that in a more sensitive or adequate animal model, which is still sorely missing, a subtler contribution of the FrpC proteins to meningococcal pathogenesis could be detected. Therefore, if new animal models of infection for N. meningiti-dis become available in the future, the role of the RTX proteins in meningococcal virulence should be assessed again.
Acknowledgments
This work was supported by grants of the Grant Agency of the Czech Republic (310/02/1448) and of the Ministry of Education, Youth, and Sports of the Czech Republic (ME167), by French-Czech Research Collaboration awards DRI/637 MAE PECO and Barrande 2002-011-1, and by grants of the INSERM and the University of Paris 5.
Editor: D. L. Burns
REFERENCES
- 1.Ali, S. A., and A. Steinkasserer. 1995. PCR-ligation-PCR mutagenesis: a protocol for creating gene fusions and mutations. BioTechniques 18:746-750. [PubMed] [Google Scholar]
- 2.Black, J. R., D. W. Dyer, M. K. Thompson, and P. F. Sparling. 1986. Human immune response to iron-repressible outer membrane proteins of Neisseria meningitidis. Infect. Immun. 54:710-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eugène, E., I. Hoffmann, C. Pujol, P. O. Couraud, S. Bourdoulous, and X. Nassif. 2002. Microvilli-like structures are associated with the internalization of virulent capsulated Neisseria meningitidis into vascular endothelial cells. J. Cell Sci. 115:1231-1241. [DOI] [PubMed] [Google Scholar]
- 4.Klee, S. R., X. Nassif, B. Kusecek, P. Merker, J. L. Beretti, M. Achtman, and C. R. Tinsley. 2000. Molecular and biological analysis of eight genetic islands that distinguish Neisseria meningitidis from the closely related pathogen Neisseria gonorrhoeae. Infect. Immun. 68:2082-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Osicka, R., J. Kalmusova, P. Krizova, and P. Sebo. 2001. Neisseria meningitidis RTX protein FrpC induces high levels of serum antibodies during invasive disease: polymorphism of frpC alleles and purification of recombinant FrpC. Infect. Immun. 69:5509-5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schryvers, A. B., and I. Stojiljkovic. 1999. Iron acquisition systems in the pathogenic Neisseria. Mol. Microbiol. 32:1117-1123. [DOI] [PubMed] [Google Scholar]
- 7.Stojiljkovic, I., V. Hwa, J. Larson, L. Lin, M. So, and X. Nassif. 1997. Cloning and characterization of the Neisseria meningitidis rfaC gene encoding α-1,5 heptosyltransferase I. FEMS Microbiol. Lett. 151:41-49. [DOI] [PubMed] [Google Scholar]
- 8.Sun, Y. H., S. Bakshi, R. Chalmers, and C. M. Tang. 2000. Functional genomics of Neisseria meningitidis pathogenesis. Nat. Med. 6:1269-1273. [DOI] [PubMed] [Google Scholar]
- 9.Tettelin, H., N. J. Saunders, J. Heidelberg, A. C. Jeffries, K. E. Nelson, J. A. Eisen, K. A. Ketchum, D. W. Hood, J. F. Peden, R. J. Dodson, W. C. Nelson, M. L. Gwinn, R. DeBoy, J. D. Peterson, E. K. Hickey, D. H. Haft, S. L. Salzberg, O. White, R. D. Fleischmann, B. A. Dougherty, T. Mason, A. Ciecko, D. S. Parksey, E. Blair, H. Cittone, E. B. Clark, M. D. Cotton, T. R. Utterback, H. Khouri, H. Qin, J. Vamathevan, J. Gill, V. Scarlato, V. Masignani, M. Pizza, G. Grandi, L. Sun, H. O. Smith, C. M. Fraser, E. R. Moxon, R. Rappuoli, and J. C. Venter. 2000. Complete genome sequence of Neisseria meningitidis serogroup B strain MC58. Science 287:1809-1815. [DOI] [PubMed] [Google Scholar]
- 10.Thompson, S., L. L. Wang, and P. F. Sparling. 1993. Cloning and nucleotide sequence of frpC, a second gene from Neisseria meningitidis encoding a protein similar to RTX cytotoxins. Mol. Microbiol. 9:85-96. [DOI] [PubMed] [Google Scholar]
- 11.Thompson, S. A., and P. F. Sparling. 1993. The RTX cytotoxin-related FrpA protein of Neisseria meningitidis is secreted extracellularly by meningococci and by HlyBD+ Escherichia coli. Infect. Immun. 61:2906-2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thompson, S. A., L. L. Wang, A. West, and P. F. Sparling. 1993. Neisseria meningitidis produces iron-regulated proteins related to the RTX family of exoproteins. J. Bacteriol. 175:811-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tikhomirov, E., M. Santamaria, and K. Esteves. 1997. Meningococcal disease: public health burden and control. World Health Stat. Q. 50:170-177. [PubMed] [Google Scholar]
- 14.Tzeng, Y. K., and D. S. Stephens. 2000. Epidemiology and pathogenesis of Neisseria meningitidis. Microbes Infect. 2:687-700. [DOI] [PubMed] [Google Scholar]