Abstract
The cis-acting replication element (CRE) is a 61-nucleotide stem-loop RNA structure found within the coding sequence of poliovirus protein 2C. Although the CRE is required for viral RNA replication, its precise role(s) in negative- and positive-strand RNA synthesis has not been defined. Adenosine in the loop of the CRE RNA structure functions as the template for the uridylylation of the viral protein VPg. VPgpUpUOH, the predominant product of CRE-dependent VPg uridylylation, is a putative primer for the poliovirus RNA-dependent RNA polymerase. By examining the sequential synthesis of negative- and positive-strand RNAs within preinitiation RNA replication complexes, we found that mutations that disrupt the structure of the CRE prevent VPg uridylylation and positive-strand RNA synthesis. The CRE mutations that inhibited the synthesis of VPgpUpUOH, however, did not inhibit negative-strand RNA synthesis. A Y3F mutation in VPg inhibited both VPgpUpUOH synthesis and negative-strand RNA synthesis, confirming the critical role of the tyrosine hydroxyl of VPg in VPg uridylylation and negative-strand RNA synthesis. trans-replication experiments demonstrated that the CRE and VPgpUpUOH were not required in cis or in trans for poliovirus negative-strand RNA synthesis. Because these results are inconsistent with existing models of poliovirus RNA replication, we propose a new four-step model that explains the roles of VPg, the CRE, and VPgpUpUOH in the asymmetric replication of poliovirus RNA.
Poliovirus possesses a single-stranded positive-polarity RNA genome that is 7,441 nucleotides (nt) in length (39). VPg, a virally encoded 22-amino-acid protein, is covalently linked to the 5′ uridine of poliovirus RNA. When released into the cytoplasm of susceptible cells, VPg is removed from the 5′ terminus of poliovirus RNA by a host enzyme (1, 2). Following the removal of VPg, viral RNA functions sequentially as an mRNA for viral protein synthesis and then as a template for negative-strand RNA synthesis during viral RNA replication. Translation of the long open reading frame in viral mRNA yields a single polyprotein which undergoes both co- and posttranslational processing, culminating in the synthesis of viral capsid and replication proteins (21, 24, 32). The viral replication proteins interact with both the viral mRNA and components of the host cell to produce membrane-associated ribonucleoprotein complexes (9-11, 44, 45). cis-active RNA structures found within the 5′ and 3′ nontranslated regions (NTRs) of poliovirus RNA mediate the sequential translation and replication of viral RNA (4, 8, 20, 28, 38, 41). Poliovirus mRNA, like other eukaryotic mRNAs (43), may circularize within messenger ribonucleoprotein complexes. Likewise, because viral negative-strand RNA synthesis requires RNA structures from both the 5′ and 3′ NTRs, the template for viral negative-strand RNA synthesis may exist in a circularized conformation via RNA-protein-protein-RNA interactions (8, 20). Amplification of viral RNA within membranous replication complexes occurs asymmetrically. Viral RNA is first copied into a negative strand, which then becomes the template for the reiterative synthesis of multiple copies of positive-polarity RNA identical to the genome of the virus that initiated the infection.
Recent studies identifying cis-active structures within the open reading frames of picornaviruses seem to complicate models of translation and replication based exclusively on interactions between the 5′ and 3′ termini of viral RNA. In 1998, an RNA structure located within the open reading frame of human rhinovirus 14 was identified as being necessary for RNA replication (27). Mutations in this cis-acting replication element (CRE) prevented any detectable RNA replication in cells (27). Since 1998, CRE structures have been described within the open reading frames of the rhinoviruses (15, 27), enteroviruses (17, 35), and cardioviruses (25) and are predicted to exist within the open reading frames of the aphthovirus, hepatovirus, and teschovirus genera (48). Biochemical characterization of the CREs from poliovirus type 1 Mahoney (40) and human rhinovirus type 2 (16) indicate that the CRE functions as a template for the uridylylation of viral protein 3B (VPg). The CREs described thus far are all simple stem-loop structures in which the conserved sequence 5′AAAC-3′ can be found (Fig. 1A) (50). Reaction mixtures containing the CRE, VPg, UTP, and 3DPol were used to demonstrate that the uridylylation of VPg is based on the most 5′ A of the conserved 5′AAAC-3′ sequence (40). Viral protein 3CD dramatically stimulates VPg uridylylation in these reactions (35, 40). After addition a single uridine to VPg, the nascent VPgpUOH-polymerase-substrate complex is believed to slide back on the template sequence 5′AAAC-3′ to allow a second uridine residue to be incorporated to form VPgpUpUOH (40). Once VPgpUpUOH is formed, the polymerase-substrate complex is suspected to dissociate from the template, as VPgpUpUOH accumulates both in infected cells and in reconstituted reaction mixtures containing the CRE, VPg, and poliovirus 3DPol (13, 35, 40). Before the discovery of CRE structures within the open reading frames of picornaviruses, it was thought that the 3′-terminal poly(A) tails of viral RNA functioned as the templates for VPg uridylylation (36). Because the 5′ cloverleaf RNA structure of poliovirus is required in cis for both the initiation of negative-strand RNA synthesis and the uridylylation of VPg (26), a more complicated conformation of viral RNA than that previously proposed (8, 20) may be involved in RNA replication.
FIG. 1.
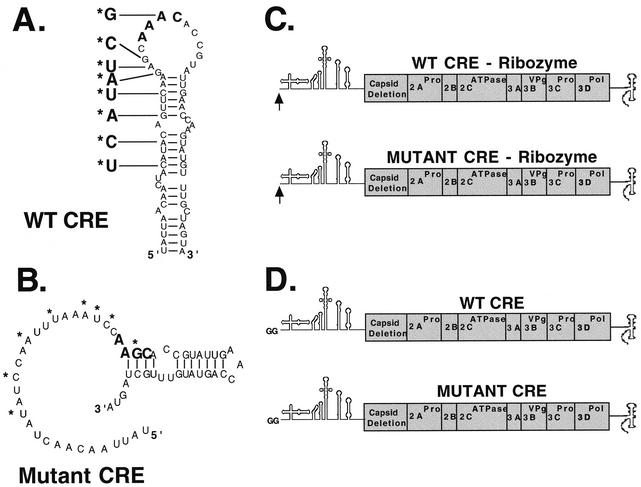
Poliovirus CRE and RNA replicons. (A) Wild-type (WT) CRE structure and mutations engineered into the CRE structure. The CRE presented is based on the enterhino CRE common motif proposed by Yang et al. (50) . (B) Mutant CRE RNA structure. (C and D) Diagrams of RNA replicons possessing either wild-type CRE or mutant CREs. (C) Replicons used to assay positive-strand RNA synthesis. Replicons with 5′-terminal hammerhead ribozymes were used to generate replicon RNAs with authentic 5′ termini necessary for positive-strand RNA synthesis. (D) Replicons used to assay negative-strand RNA synthesis. Replicons with two 5′-terminal guanosine residues (capable of inhibiting positive-strand RNA synthesis) were used to characterize negative-strand RNA synthesis that is independent of positive-strand RNA synthesis.
Current models for poliovirus RNA replication suggest that, once formed, VPgpUpUOH is translocated from the CRE RNA template (via an unknown mechanism) to the 3′ terminus of the viral RNA, where it serves to prime negative-strand RNA synthesis (35). VPgpUpUOH is also believed to prime positive-strand RNA synthesis. VPgpUpUOH may form Watson-Crick base pairs with viral RNA templates to facilitate the initiation of RNA synthesis (19). The 3′-terminal poly(A) tail of positive-strand RNA as well as the two 3′-terminal adenosine residues of negative-strand RNA may accommodate complementarity with VPgpUpUOH primers. Two 5′-terminal nonviral guanosine residues on T7 transcripts of poliovirus RNA prevent positive-strand RNA synthesis but do not prevent negative-strand RNA synthesis (19). It is likely that the two 5′-terminal guanosine residues on T7 transcripts of poliovirus positive-strand RNA are copied into 3′-terminal cytosine residues on negative-strand RNA. 3′-terminal cytosine residues on negative-strand RNA would preclude complementarity with VPgpUpUOH primers, consistent with the observed inhibition in positive-strand RNA synthesis (19). Therefore, it is believed that the interaction of VPgpUpUOH with the 3′ terminus of the negative strand would allow VPgpUpUOH to prime positive-strand RNA synthesis in a manner that would faithfully reproduce the viral genome (19).
The steps of poliovirus replication affected by lethal mutations in viral RNA are difficult to determine in experiments with transfected cells. In contrast, sequential translation and replication of poliovirus RNA in cell-free reactions along with synchronous RNA replication within preinitiation RNA replication complexes allows one to identify the precise steps of replication affected by lethal mutations in viral RNA. Reaction mixtures containing cytoplasmic extracts from uninfected HeLa cells faithfully support the sequential translation and replication of poliovirus RNA in vitro (5, 29). In the presence of 2 mM guanidine HCl, a reversible inhibitor of viral protein 2CATPase (6, 37), preinitiation RNA replication complexes containing poliovirus RNA templates accumulate in these reaction mixtures (5). Preinitiation RNA replication complexes are advantageous because they catalyze the sequential synthesis of radiolabeled negative- and positive-strand RNA upon their incubation in reaction mixtures containing 32P-nucleoside triphosphates (6). Preinitiation RNA replication complexes also catalyze the synthesis of VPgpUpUOH (26). In this study, we used preinitiation RNA replication complexes containing poliovirus RNA templates with either wild-type or mutant CREs to evaluate the role of the CRE in RNA replication. Our data confirm that the CRE is the predominate template for the uridylylation of VPg. Furthermore, contrary to previous predictions, our data indicate that CRE-dependent VPg uridylylation is not required for the initiation of negative-strand RNA synthesis but is required for the synthesis of positive-polarity RNAs. Our data demonstrate that, while VPgpUpUOH is not required for the initiation of negative-strand RNA synthesis, the tyrosine hydroxyl of VPg is absolutely required for negative-strand RNA synthesis. Based on these results, we propose a new four-step model that explains the mechanisms by which the CRE, VPg, and VPgpUpUOH mediate the asymmetric replication of poliovirus RNA.
MATERIALS AND METHODS
cDNA and cloning. (i) pRNA2.
pRNA2 contains the subgenomic wild-type poliovirus (Mahoney, type 1) RNA replicon, which possesses two 5′ nonviral guanosine residues (Fig. 1D). It was kindly provided by James B. Flanegan (University of Florida College of Medicine, Gainesville). This plasmid encodes RNA2, a subgenomic poliovirus replicon containing an in-frame deletion of poliovirus nt 1175 to 2956 within the capsid genes (12). T7 transcription of MluI-linearized RNA2 cDNA produces RNA2 replicon RNA. RNA2 has two nonviral guanosine residues at its 5′ terminus.
(ii) pDJB14.
pDJB14 is the subgenomic RNA template for negative-strand RNA synthesis (see Fig. 5A) and was kindly provided by James B. Flanegan, (University of Florida College of Medicine). T7 transcription of MluI-linearized pDJB14 yields DJB14 RNA. DJB14 RNA consists of the 5′-terminal 629 nt of poliovirus RNA, poliovirus nt 6012 to 6056, and the 3′-terminal 1,007 nt of poliovirus RNA with a poly(A) tail 83 bases in length.
FIG. 5.
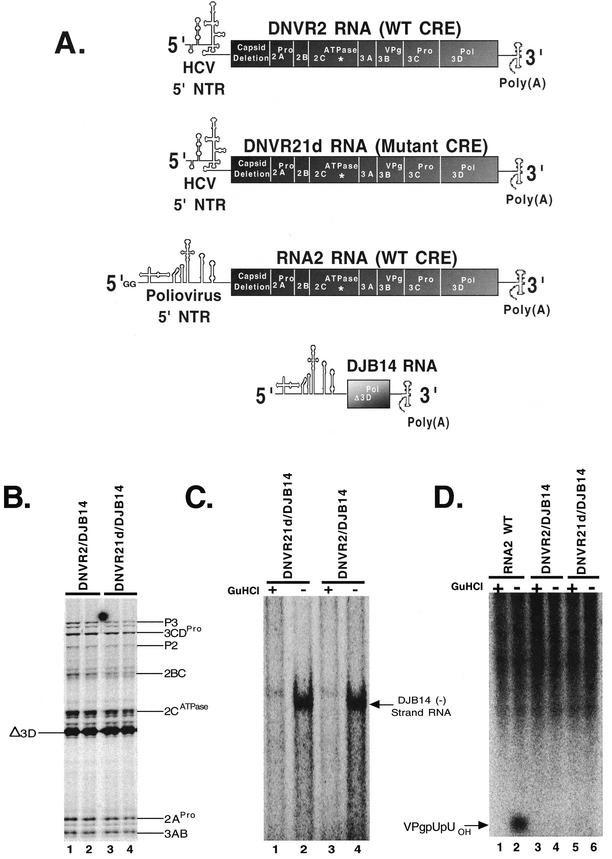
Poliovirus negative-strand RNA synthesis does not require the CRE in cis or in trans. Poliovirus protein synthesis, negative-strand RNA synthesis, and VPg uridylylation were assayed as described in Materials and Methods. Preinitiation RNA replication complexes containing DJB14 RNA were prepared by cotranslating DNVR2 or DNVR21d (Fig. 5A) with DJB14 RNA as previously described (30). (A) Diagrams of DNVR2, DNVR21d, wild-type (WT) RNA2, and DJB14 RNAs. The * in the 2C coding region of DNVR2, DNVR21d, and wild-type RNA2 indicates the approximate location of the CRE. The CRE is not present within DJB14 RNA. (B) SDS-PAGE. Poliovirus proteins were synthesized in reaction mixtures containing [35S]methionine, DJB14 RNA, and DNVR2 RNA (duplicate samples in lanes 3 and 4) or mutant CRE DNVR21d RNA (duplicate samples in lanes 1 and 2). Radiolabeled proteins were fractionated by SDS-PAGE and detected by phosphorimaging. (C) Negative-strand RNA synthesis. Preinitiation RNA replication complexes formed during cotranslation reactions were assayed for their ability to synthesize negative-strand RNA when the helper virus possessed either a wild-type (lanes 1 and 2) or a mutant (lanes 3 and 4) CRE as indicated in the figure. Reactions were performed in the presence (lanes 1 and 3) and absence (lanes 2 and 4) of 2 mM guanidine HCl (GuHCl). Products from the reactions were fractionated by electrophoresis in a denaturing methylmercury hydroxide agarose gel. Radiolabled negative-strand RNA in the gel was detected by phosphorimaging. (D) VPg uridylylation. Preinitiation complexes formed during cotranslation were evaluated for their ability to synthesize VPgpUpUOH when the helper virus contained either a wild-type CRE (lanes 3 and 4) or a mutant CRE (lanes 5 and 6). Wild-type RNA was used as a positive control (lanes 1 and 2). Reactions were performed in the presence (lanes 1, 3, and 5) or the absence (lanes 2, 4, and 6) of 2 mM guanidine HCl. Products from the reactions were fractionated by polyacrylamide-Tris-Tricine gel electrophoresis and detected by phosphorimaging.
(iii) pDNVR2.
pDNVR2 contains wild-type CRE helper RNA (see Fig. 5A) and, as previously described (26), is a cDNA encoding a chimeric viral RNA composed of the 5′ NTR of hepatitis C virus and the P2/P3 coding sequence, 3′ NTR, and poly(A) tail of poliovirus.
(iv) pDNVR21d.
A mutation was engineered into the CRE coding sequence of pDNVR2. This mutation was designed to disrupt the structure of the CRE (Fig. 1A and B and see Fig. 5A) without changing the coding sequence of the viral protein 2CATPase. pDNVR21d was made using a Stratagene (La Jolla, Calif.) Quickchange site-directed mutagenesis kit and the following two primers: 21A (5′CATACTATTAACAACTATATCCAATTTAAATCCAAGCACCGTATTGAACC3′) and 21B (5′GGTTCAATACGGTGCTTGGATTTAAATTGGATATAGTTGTTAATAGTATG3′).
(v) pDNVR26.
pDNVR26 (with a mutant CRE in an RNA2 background [Fig. 1D]). was created by cutting pRNA2 and pDNVR21d with PvuI and AvrII. Gel purification of fragments was followed by ligation (sticky-sticky), yielding pDNVR26.
(vi) pDNVR27.
pMO-3, a plasmid encoding wild-type poliovirus, was generously provided by Craig E. Cameron (Pennsylvania State University, University Park). pMO-3 encodes a 5′-terminal hammerhead ribozyme such that in vitro T7 transcription produces an RNA possessing an authentic poliovirus 5′ terminus. pDNVR27 (with a wild-type CRE in an RNA2 ribozyme construct [Fig. 1c]) was generated by cutting both pRNA2 and pMO-3 with AgeI and MluI. Following digestion, the cut fragments were gel purified and ligated.
(vii) pDNVR28.
As for pDNVR27, pDNVR28 (a mutant CRE-ribozyme construct [Fig. 1C]) was created by cutting pMO-3 and, in this case, pDNVR26 with AgeI and MluI. Appropriate fragments were gel purified and ligated.
(viii) pY3F.
A mutation was engineered into the 3B coding sequence of pRNA2. This mutation was designed to change the tyrosine residue in position 3 of viral protein 3B to a phenylalanine. pY3F was made with the Stratagene Quickchange site-directed mutagenesis kit and the following two primers: primer Y3F (forward) (5′GGACACCAGGGAGCATTCACTGGTTTACCAAAC3′) and primer Y3F (reverse) (5′GTTTGGTAAACCAGTGAATGCTCCCTGGTGTCC3′). The sequences of all cDNA constructs were confirmed by restriction analyses and DNA sequencing.
Viral RNA.
Poliovirus RNAs were generated by T7 transcription of MluI-linearized plasmids by using a commercially available kit (Epicentre, Madison Wis.). MluI linearizes all of the plasmids immediately downstream of the 3′-terminal poly(A) tail of the viral cDNAs. With the exception of RNAs denoted “-ribozyme,” all RNAs were produced with two 5′-terminal nonviral guanosine residues. RNAs were extracted with 5 M NH4+CH3CO2 followed by ethanol precipitation. Viral RNA was quantified by determining the optical density at 260 nm.
HeLa S10 translation-replication reactions.
HeLa cell S10 extracts (S10) and HeLa cell translation initiation factors were prepared as previously described (7). HeLa S10 translation-replication reaction mixtures contained 50% (by volume) S10, 20% (by volume) translation initiation factors, 10% (by volume) 10× nucleotide reaction mix (10 mM ATP, 2.5 mM GTP, 2.5 mM CTP, or 2.5 mM UTP; 600 mM KCH3CO2; 300 mM creatine phosphate; 4 mg of creatine kinase per ml; and 155 mM HEPES-KOH [pH 7.4]), 2 mM guanidine hydrochloride, and T7 transcripts of viral RNA at 45 μg/ml. Reaction mixtures were incubated at 34°C for 4 h.
Viral RNA translation.
Poliovirus mRNA translation was assayed by including [35S]methionine (1.2 mCi/ml; Amersham) in HeLa S10 translation-replication reaction mixtures. [35S]methionine-labeled proteins synthesized in HeLa S10 translation-replication reactions were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Samples (4 μl) of the HeLa S10 translation-replication reaction mixtures containing [35S]methionine were solubilized in SDS-PAGE sample buffer (2% SDS σ, 62.5 mM Tris-HCl [pH 6.8], 0.5% 2-mercaptoethanol, 0.1% bromophenol blue, 20% glycerol). The samples were heated at 100°C for 5 min and separated by gel electrophoresis in SDS-9 to 18% polyacrylamide gels as previously described (7). The gels were fixed by soaking them in 50% trichloroacetic acid. The gels were dried, and the [35S]methionine-labeled proteins were detected by phosphorimaging (Bio-Rad, Hercules, Calif.).
Negative-strand RNA synthesis.
Poliovirus negative-strand RNA synthesis was assayed by using preinitiation RNA replication complexes containing poliovirus RNA templates with two 5′-terminal nonviral guanosine residues as previously described (6). Following 4 h of incubation at 34°C, preinitiation RNA replication complexes were isolated from HeLa S10 translation-replication reaction mixtures by centrifugation at 13,000 × g. Pellets containing preinitiation RNA replication complexes were resuspended in 50-μl labeling reaction mixtures containing [32P]CTP and incubated at 37°C for 1 h. Under these conditions, the radiolabel is incorporated into nascent negative-strand RNA as it is synthesized by the viral RNA replication complexes. Two 5′-terminal nonviral guanosine residues on the T7 RNA transcripts prevent the initiation of positive-strand RNA synthesis within the preinitiation complexes. The products of the reactions were phenol-chloroform extracted, ethanol precipitated, denatured with 50 mM methylmercury hydroxide, and separated by electrophoresis in 1% agarose. 32P-labeled RNAs were detected and quantified by phosphorimaging (Bio-Rad).
Positive-strand RNA synthesis.
Poliovirus positive-strand RNA synthesis was assayed by using preinitiation RNA replication complexes containing poliovirus RNA templates with authentic 5′ termini as previously described (5, 6). Following 4 h of incubation at 34°C, preinitiation RNA replication complexes were isolated from HeLa S10 translation-replication reactions by centrifugation at 13,000 × g. Pellets containing preinitiation RNA replication complexes were resuspended in 50-μl labeling reaction mixtures containing [32P]CTP and incubated at 37°C for 1 h. Under these conditions, the radiolabel was first incorporated into nascent negative-strand RNA and then into positive-strand RNA. The products of the reactions were phenol-chloroform extracted and ethanol precipitated. Reaction products were resuspended in a nondenaturing formaldehyde-formamide gel loading buffer [10% 200 mM 3-(N-morpholino) propanesulfonic acid (MOPS), 17.5% formaldehyde, 50% formamide, 8% glycerol, and 0.1% bromophenol blue] and separated by electrophoresis in 1% agarose-MOPS-formaldehyde (200 mM MOPS, 2% formaldehyde). 32P-labeled RNAs were detected and quantified by phosphorimaging (Bio-Rad).
VPg uridylylation.
VPg uridylylation was assayed by using preinitiation RNA replication complexes containing the indicated poliovirus RNA templates as previously described (26). Preinitiation RNA replication complexes were isolated from HeLa S10 translation-replication reactions in the same manner as that used for negative-strand RNA. The preinitiation RNA replication complexes were resuspended in reaction mixtures containing [α-32P]UTP rather than [α-32P]CTP. The reaction mixtures were incubated for 60 min at 37°C. Following incubation, the reaction mixtures were centrifuged at 13,000 × g to pellet the viral RNA replication complexes. Radiolabeled VPgpUpUOH and viral RNA remained in the replication complexes and were not released into the soluble portion of the reaction mixtures. The supernatant containing unincorporated radiolabel was discarded unless otherwise indicated. The pellets containing VPgpUpUOH were solubilized in Tris-Tricine sample buffer (3% SDS σ, 62.5 mM Tris-hydrochloride [pH 6.8], 5% β-mercaptoethanol, 10% glycerol, and 0.1% bromophenol blue). The samples were fractionated by electrophoresis (75 mA of constant current for 15 min followed by 11 mA of constant current for 17 h) in a 0.75-mm-thick polyacrylamide (29:1 ratio of acrylamide to bis-acrylamide)-Tris-Tricine gel system (a 4% polyacrylamide stacking gel [4% polyacrylamide, 0.7 M Tris-HCl {pH 8.45}, and 0.1% SDS] and a 12% polyacrylamide separating gel [1 M Tris-HCl {pH 8.45} and 0.1% SDS]) by using a Tris-Tricine running buffer (0.1 M Tris-Tricine [pH 8.25], 0.3% SDS). Radiolabeled products in the gel were detected by phosphorimaging.
RESULTS
A CRE mutation completely inhibited VPg uridylylation and positive-strand RNA synthesis.
To study the role of the CRE in poliovirus replication, eight bases were mutated within poliovirus replicons to disrupt the structure of the CRE (Fig. 1). The 5′-terminal A in the conserved 5′AAAC-3′ sequence of the CRE, the template for VPg uridylylation, could not be mutated without changing the lysine residue encoded by the 2C gene. The eight bases mutated disrupted the structure of the CRE without altering the coding sequence of the 2C gene (as illustrated in Fig. 1A and B). A comparable mutation was shown by Goodfellow et al. to prevent poliovirus RNA replication in transfected cells (17); however, the precise defect in viral RNA replication was not defined. In order to evaluate more precisely the effects of the CRE mutations on negative- and positive-strand RNA synthesis independently, we engineered this CRE mutation into two slightly different wild-type poliovirus replicons (Fig. 1C and D). To assay the effect of the CRE mutation on positive-strand RNA synthesis, a replicon with a 5′-terminal hammerhead ribozyme was used to generate replicon RNAs with authentic 5′ termini (as illustrated in Fig. 1C). To assay the effect of the CRE mutation on negative-strand RNA synthesis, a replicon with two nonviral guanosine residues at the 5′ terminus was employed (as illustrated in Fig. 1D).
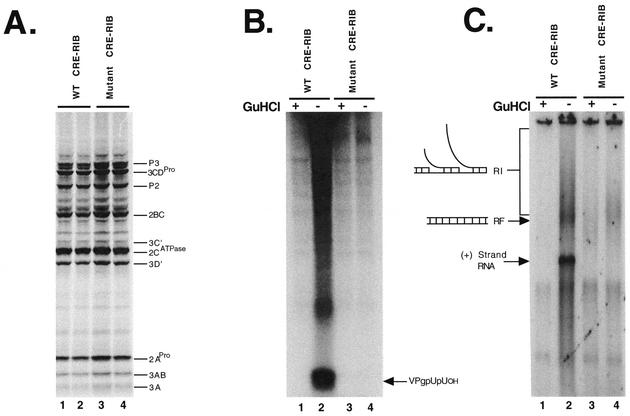
RNA replicons with authentic 5′ termini allowed us to evaluate the effect of the CRE mutation on positive-strand RNA synthesis (Fig. 2). Disruption of the CRE structure had no effect on viral protein synthesis or polyprotein processing (Fig. 2A, lanes 3 and 4 versus lanes 1 and 2). Preinitiation RNA replication complexes containing wild-type replicon RNA templates synthesized robust amounts of both VPgpUpUOH (Fig. 2B, lane 2) and viral RNA (Fig. 2C, lane 2). Radiolabeled VPgpUpUOH and viral RNA products were not released into the soluble portion of the reaction mixtures but remained associated with the repelleted membranous replication complexes (see “VPg uridylylation” in Materials and Methods) (26). As previously described (26), 2 mM guanidine HCl inhibited the uridylylation of VPg (Fig. 2B, lanes 1 and 3) and viral RNA synthesis (Fig. 2C, lanes 1 and 3). Replicative-intermediate, replicative-form (RF), and positive-strand RNAs were synthesized by using preinitiation RNA replication complexes containing wild-type RNA templates (Fig. 2C, lane 2). Quantifying the RF RNA and the positive-strand RNA (Fig. 2C) indicated that ∼20 positive strands were synthesized for each negative-strand RNA molecule. As predicted, VPg uridylylation was completely inhibited within preinitiation RNA replication complexes containing the replicon RNA with the mutant CRE (Fig. 2B, lane 4). Preinitiation RNA replication complexes containing mutant CRE RNA templates were able to synthesize the RF RNA (Fig. 2C, lane 4) but were unable to synthesize any detectable positive-strand RNA (Fig. 2C, lane 4). The CRE mutation led to diminished incorporation of radiolabel into RF RNA (Fig. 2C, lane 4 versus lane 2) because wild-type RNA replicons incorporated radiolabel into both the negative and positive strands of the RF RNA, while radiolabel was incorporated only into the negative strand of the mutant RF RNA. These data suggest that negative-strand RNA synthesis was supported by both wild-type and CRE-mutant templates, but only the wild-type RNA template supported VPg uridylylation and positive-strand RNA synthesis.
FIG. 2.
CRE-dependent VPg uridylylation is required for positive-strand RNA synthesis. Poliovirus protein synthesis, VPg uridylylation, and positive-strand RNA synthesis were assayed as described in Materials and Methods. Poliovirus RNA replicons with wild-type (WT) or mutant CREs in an RNA2 background and authentic 5′ termini were used as illustrated in Fig. 1C. (A) SDS-PAGE. Poliovirus proteins were synthesized in reaction mixtures containing [35S]methionine and the wild-type CRE replicon (duplicate samples in lanes 1 and 2) or mutant CRE replicon (duplicate samples in lanes 3 and 4). Radiolabeled proteins were fractionated by SDS-PAGE and detected by phosphorimaging. (B) VPg uridylylation. VPgpUpUOH was synthesized in reaction mixtures containing [α-32P]UTP by preinitiation RNA replication complexes containing wild-type poliovirus RNA templates (lanes 1 and 2) or mutant CRE RNA templates (lanes 3 and 4). Reactions were performed in the presence (lanes 1 and 3) and absence (lanes 2 and 4) of 2 mM guanidine HCl (GuHCl). Products from the reactions were fractionated by polyacrylamide-Tris-tricine gel electrophoresis and detected by phosphorimaging. (C) Positive-strand RNA synthesis. Poliovirus RNA was synthesized in reaction mixtures containing [α-32P]CTP by preinitiation RNA replication complexes containing wild-type poliovirus RNA templates (lanes 1 and 2) or mutant CRE RNA templates (lanes 3 and 4). Reactions were performed in the presence (lanes 1 and 3) and absence (lanes 2 and 4) of 2 mM guanidine HCl. Products from the reactions were fractionated by electrophoresis in a nondenaturing agarose gel. Radiolabeled RNA in the gel was detected by phosphorimaging. The mobility of positive-strand, RF, and replicative-intermediate (RI) RNA is indicated.
CRE-dependent uridylylation of VPg is not required for negative-strand RNA synthesis.
To more precisely analyze the role of CRE-dependent VPg uridylylation and VPgpUpUOH in the synthesis of negative-strand RNA, we used replicon RNAs with two nonviral guanosine residues at the 5′ terminus (as illustrated in Fig. 1D). The presence of the two nonviral guanosine residues at the 5′ terminus of the RNA prevents the initiation of positive-strand RNA synthesis without inhibiting negative-strand RNA synthesis (19), allowing us to specifically evaluate the effect of the CRE mutation on negative-strand RNA synthesis. In the absence of 2 mM guanidine HCl, preinitiation RNA replication complexes containing wild-type RNA were able to robustly synthesize VPgpUpUOH (Fig. 3A, lane 2). Additionally, the wild-type construct was found to be a competent template for negative-strand RNA synthesis (Fig. 3B, lane 2). The negative-strand RNA synthesized (Fig. 3B, lane 2) possessed two times more radiolabel than VPgpUpUOH (Fig. 3A, lane 2). The specific radioactivities of CTP and UTP used to radiolabel negative-strand RNA and VPgpUpUOH were identical. VPgpUpUOH possessed two uracil residues, while the negative-strand RNA synthesized in these experiments possessed 1,791 cytosine residues. Therefore, ∼500 VPgpUpUOH molecules were synthesized for each negative-strand RNA molecule (Fig. 3, lanes 2). Preinitiation RNA replication complexes containing mutant CRE RNA templates revealed no VPgpUpUOH synthesis (Fig. 3A, lane 4); however, the mutant CRE RNA was an efficient template for negative-strand RNA synthesis (Fig. 3B, lane 4). Quantitation of the viral RNA in Fig. 3B indicated that the CRE mutation had no adverse effect on negative-strand RNA synthesis. Thus, the CRE mutation completely abolished VPgpUpUOH synthesis without inhibiting negative-strand RNA synthesis.
FIG. 3.
CRE-dependent VPg uridylylation is not required for negative-strand RNA synthesis. Poliovirus VPg uridylylation and negative-strand RNA synthesis were assayed as described in Materials and Methods. Poliovirus RNA replicons with wild-type (WT) or mutant CREs and two nonviral guanosine residues at their 5′ termini were used as illustrated in Fig. 1D. (A) VPg uridylylation. VPgpUpUOH was synthesized in reaction mixtures containing [α-32P]UTP by preinitiation RNA replication complexes containing wild-type poliovirus RNA templates (lanes 1 and 2) or mutant CRE RNA templates (lanes 3 and 4). Reactions were performed in the presence (lanes 1 and 3) and absence (lanes 2 and 4) of 2 mM guanidine HCl (GuHCl). Products from the reactions were fractionated by polyacrylamide-Tris-Tricine gel electrophoresis and detected by phosphorimaging. (B) Negative-strand RNA synthesis. Poliovirus RNA was synthesized in reaction mixtures containing [α-32P]CTP by preinitiation RNA replication complexes containing wild-type poliovirus RNA templates (lanes 1 and 2) or mutant CRE RNA templates (lanes 3 and 4). Reactions were performed in the presence (lanes 1 and 3) and absence (lanes 2 and 4) of 2 mM guanidine HCl. Products from the reactions were fractionated by electrophoresis in a denaturing methylmercury hydroxide agarose gel. Radiolabeled negative-strand RNA in the gel was detected by phosphorimaging.
VPg is required for the synthesis of negative-strand RNA.
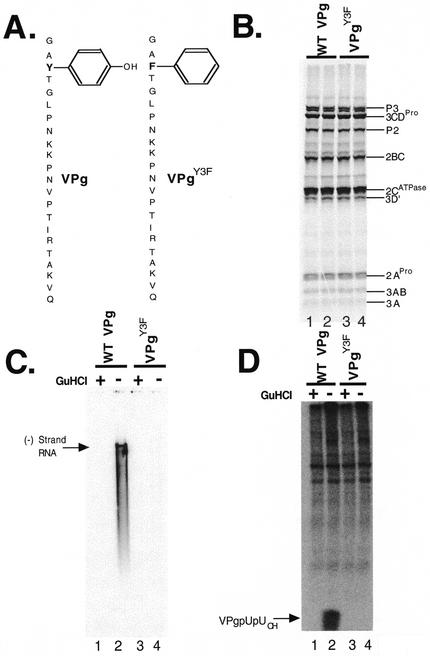
To assess the role of VPg in negative-strand RNA synthesis, we engineered a tyrosine-to-phenylalanine mutation in VPg (Fig. 4A). Phenylalanine is structurally similar to tyrosine; however, phenylalanine lacks the critical phenolic hydroxyl moiety necessary for the uridylylation of VPg by 3DPol (Fig. 4A) (15, 16, 22, 23, 42). Protein synthesis and polyprotein processing were unaffected by the Y3F mutation (Fig. 4B, lanes 3 and 4 versus lanes 1 and 2). As predicted, the Y3F mutation in VPg prevented negative-strand RNA synthesis (Fig. 4C, lane 4). Additionally, the Y3F mutation prevented the synthesis of VPgpUpUOH (Fig. 4D, lane 4). These results indicate that the tyrosine residue of VPg is required for VPg uridylylation and for negative-strand RNA synthesis.
FIG. 4.
VPg is required for negative-strand RNA synthesis. Poliovirus protein synthesis, negative-strand RNA synthesis, and VPg uridylylation were assayed as described in Materials and Methods. Poliovirus RNA2 replicons with two 5′-terminal nonviral guanosines encoding wild-type (WT) or Y3F mutant VPg (VPgY3F) were used. (A) Diagrams of wild-type VPg and VPgY3F. (B) SDS-PAGE. Poliovirus proteins were synthesized in reaction mixtures containing [35S]methionine and the wild-type (WT) RNA2 replicon (duplicate samples in lanes 1 and 2) or VPgY3F replicon (duplicate samples in lanes 3 and 4). Radiolabeled proteins were fractionated by SDS-PAGE and detected by phosphorimaging. (C) Negative-strand RNA synthesis. Poliovirus RNA was synthesized in reaction mixtures containing [α-32P]CTP by preinitiation RNA replication complexes containing wild-type poliovirus RNA2 templates (lanes 1 and 2) or VPgY3F RNA templates (lanes 3 and 4). Reactions were performed in the presence (lanes 1 and 3) and absence (lanes 2 and 4) of 2 mM guanidine HCl. Products from the reactions were fractionated by electrophoresis in a denaturing methylmercury hydroxide agarose gel. Radiolabled negative-strand RNA in the gel was detected by phosphorimaging. (D) VPg uridylylation. VPgpUpUOH was synthesized in reaction mixtures containing [α-32P]UTP by preinitiation RNA replication complexes containing wild-type poliovirus RNA templates (lanes 1 and 2) or mutant VPgY3F RNA templates (lanes 3 and 4). Reactions were performed in the presence (lanes 1 and 3) and absence (lanes 2 and 4) of 2 mM guanidine HCl. Products from the reactions were fractionated by polyacrylamide-Tris-Tricine gel electrophoresis and detected by phosphorimaging.
Poliovirus negative-strand RNA synthesis does not require the CRE in cis or in trans.
Previous trans-replication experiments established that the poliovirus CRE RNA structure was not required in cis for negative-strand RNA synthesis (8, 30). These experiments, however, did not rule out the possibility that the CRE RNA structure could function in trans. To further analyze the possibility of the CRE functioning in trans, we engineered the CRE mutation illustrated in Fig. 1 into DNVR2 RNA (thereby creating DNVR21d) (Fig. 5A). The absence of the 5′ cloverleaf of poliovirus on DNVR2 RNA prevents both VPg uridylylation and negative-strand RNA synthesis (26, 30). Therefore, DNVR2 and DNVR21d are replication-incompetent helper RNAs (26, 30). Both DNVR2 and DNVR21d RNAs contain the 5′ NTR of hepatitis C virus fused to the poliovirus P2/P3 open reading frame and the PV 3′ NTR (as illustrated in Fig. 5A). DJB14 RNA is a subgenomic poliovirus template for negative-strand RNA synthesis (as illustrated in Fig. 5A). DNVR2 RNA and CRE-mutant DNVR21d RNA expressed equal amounts of poliovirus replication proteins when they were cotranslated with DJB14 RNA (Fig. 5B, lanes 1 to 4). DJB14 RNA does not possess a CRE or encode any of the viral replication proteins (Fig. 5A). Nonetheless, as previously described (30), DJB14 RNA functioned perfectly well as a template for negative-strand RNA synthesis when it was cotranslated with DNVR2 helper RNA (Fig. 5C, lane 4). Mutating the CRE within the helper RNA had no effect on the ability of DJB14 to function as a template for negative-strand RNA synthesis (Fig. 5C, lane 4 versus lane 2). Furthermore, DJB14 negative-strand RNA synthesis occurred in reactions that did not synthesize VPgpUpUOH (Fig. 5C and D). These findings, like those illustrated in Fig. 3, demonstrate that the CRE and VPgpUpUOH are not required in cis or in trans for the synthesis of negative-strand RNA.
Poliovirus polyproteins and RNA synthesis.
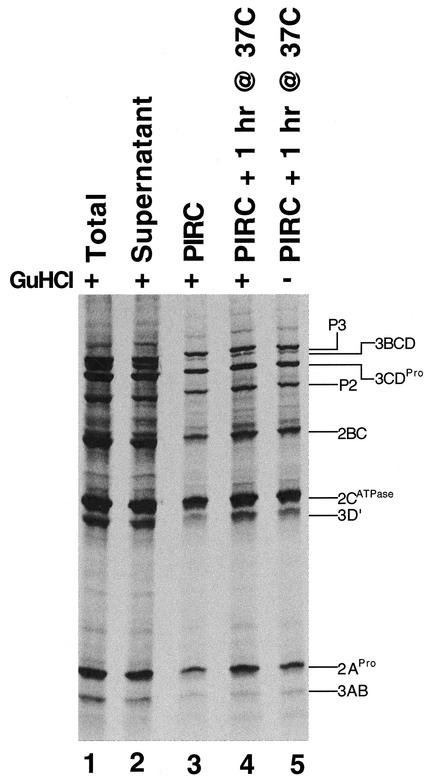
To examine the viral proteins associated with RNA replication complexes, we radiolabeled poliovirus proteins with [35S]methionine in HeLa S10 reactions and examined the viral proteins before and after fractionation (Fig. 6). Although most viral proteins were found in the supernatant fraction, a significant portion of viral proteins cofractionated with preinitiation RNA replication complexes (Fig. 6, compare lane 3 with lanes 1 and 2). Significant amounts of poliovirus proteins P3, P2, 3BCD, 3CD, 2BC, 3AB, 2C, and 2A cofractionated with preinitiation RNA replication complexes (Fig. 6, lane 3). Incubation of preinitiation RNA replication complexes for 1 h in the presence and absence of 2 mM guanidine HCl revealed very little additional processing of the viral polyproteins (Fig. 6, lanes 4 and 5). As shown previously (5), poliovirus RNA replication complexes have very little mature 3DPol (Fig. 6; 3DPol is not readily visible) and inhibition of RNA synthesis with 2 mM guanidine HCl has no effect on viral polyprotein processing (Fig. 6).
FIG. 6.
Poliovirus polyproteins associated with RNA replication complexes. Poliovirus proteins were radiolabeled in reaction mixtures containing [35S]methionine and wild-type (WT) RNA2 in the presence of 2 mM guanidine HCl (GuHCl) for 4 h at 34°C. Preinitiation RNA replication complexes (PIRC) were isolated by centrifugation as described in Materials and Methods. Samples of poliovirus proteins before (total, lane 1) and after (supernatant, lane 2; preinitiation RNA complex, lane 3) fractionation were examined by SDS-PAGE. Preinitiation RNA replication complexes were then incubated for 1 h at 37°C in the presence (lane 4) and absence (lane 5) of 2 mM guanidine HCl to allow for RNA replication and VPg uridylylation.
DISCUSSION
VPg and/or VPgpUpUOH is a potential primer for poliovirus 3DPol (36). In this study, we have shown that CRE-dependent VPg uridylylation is required for the synthesis of positive-strand RNA but that CRE-dependent VPg uridylylation is not required for negative-strand RNA synthesis. We also show that the tyrosine residue of VPg is required for negative-strand RNA synthesis and VPg uridylylation. These results indicate that negative-strand RNA synthesis and positive-strand RNA synthesis are differentially primed. We suggest that VPg primes negative-strand RNA synthesis and that VPgpUpUOH primes positive-strand RNA synthesis.
Poliovirus RNA replication.
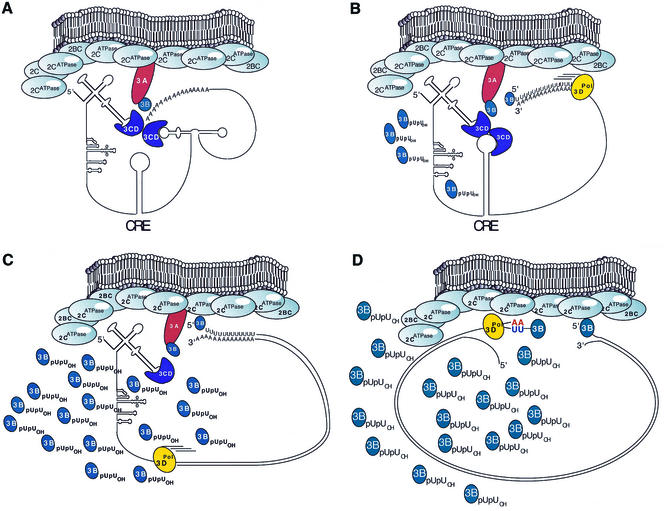
Not long ago, it was believed that the poly(A) tail of poliovirus RNA functioned as the template for VPg uridylylation (36). VPg primed the synthesis of VPgpUOH, VPgpUpUOH, and VPg-poly(U) in reaction mixtures containing poly(A) templates and poliovirus 3DPol (36). More recently, the CRE was found to be a more efficient template for VPg uridylylation (35, 40). In this study, we found that mutations in the CRE completely abolished VPgpUpUOH synthesis despite the presence of poly(A) tails on the viral RNA templates within preinitiation RNA replication complexes (Fig. 2 and 3). Because of this observation, we conclude that the poly(A) tail of poliovirus RNA is not used as a template for VPgpUpUOH synthesis within membranous RNA replication complexes. Other recent studies led to the suggestion that VPgpUpUOH, generated on the CRE RNA template, might prime both negative- and positive-strand RNA synthesis (19, 35). Our results do not support this possibility. We found that the CRE and VPgpUpUOH are not required for negative-strand RNA synthesis (Fig. 3 and 5). In other recent studies, we found that the 5′ cloverleaf of poliovirus RNA was required in cis for negative-strand RNA synthesis (8, 20, 26). These results suggested that the 5′ cloverleaf of poliovirus RNA is required coordinately with the 3′ NTR for the initiation of negative-strand RNA synthesis. The 5′ cloverleaf of poliovirus RNA was also found to be necessary for the uridylylation of VPg within preinitiation RNA replication complexes (26). These results suggest that the 5′ cloverleaf of poliovirus RNA may work coordinately with the CRE to uridylylate VPg. In light of these observations, we propose a new four-step model to explain the mechanisms for the asymmetric replication of poliovirus RNA (Fig. 7).
FIG. 7.
Four-step model for the asymmetric replication of poliovirus RNA. (A) Initiation of negative-strand RNA synthesis (first step); (B) initiation of VPg uridylylation(second step); (C) termination of ongoing VPg Uridylylation(third step); (D) initiation of positive-strand RNA synthesis (fourth step). See Discussion in the text for an explanation of the individual steps of replication.
Model of poliovirus RNA replication.
We propose that VPg primes the initiation of negative-strand RNA synthesis coordinately with ribonucleoproteins present at the 5′ and 3′ termini of poliovirus RNA (Fig. 7A). The CRE, illustrated as the simple stem-loop in the middle of poliovirus RNA in Fig. 7A, is not required for negative-strand RNA synthesis. Membranes and 2CATPase, as evidenced by the inhibitory action of guanidine, are also necessary for the initiation of negative-strand RNA synthesis. Viral protein 3AB is a likely precursor of VPg (46) at the site of negative-strand RNA synthesis initiation (Fig. 7A) (18, 49). Viral protein 3CD binds to both the 5′ cloverleaf RNA (3, 4, 14, 33) and the 3′ NTR of poliovirus RNA (18). 3CD protein-protein bridges between both termini of poliovirus RNA may coordinately cleave 3AB and 3CD, releasing VPg and 3DPol at the site of initiation along the poly(A) tail. The tyrosine hydroxyl of VPg appears to prime negative-strand RNA synthesis without the synthesis of stable VPgpUpUOH intermediates (Fig. 3 to 5). Other viral and cellular proteins, as proposed by others (20, 33, 47), may also be involved in the initiation of negative-strand RNA synthesis.
After the initiation of negative-strand RNA synthesis, we propose that the elongating polymerase destroys the protein-RNA interactions within the 3′-terminal ribonucleoprotein complex to prevent repeated initiation of negative-strand RNA synthesis (Fig. 7B). After the 3′-terminal ribonucleoprotein complex is disassembled by the elongating negative-strand polymerase, we propose that a reformed 5′-terminal ribonucleoprotein interacts with ribonucleoprotein complexes containing the CRE to initiate VPg uridylylation (Fig. 7B). The CRE, like the 3′ NTR, may form ribonucleoprotein complexes containing 3CD (18, 34). Analogous to the situation described above for the initiation of negative-strand RNA synthesis, protein-protein interactions between 3CD moieties bound to the 5′ cloverleaf and the CRE may coordinately cleave 3AB and 3CD, releasing VPg and 3DPol at the site of VPg uridylylation along the loop of the CRE (Fig. 7B). Our inability to detect significant 3CD and 3AB polyprotein processing coordinate with RNA synthesis in crudely purified replication complexes (Fig. 6) suggests that a population of preprocessed protein pools could be used during RNA synthesis. Alternatively, more rigorously purified replication complexes may reveal polyprotein processing coordinate with RNA synthesis.
During the time necessary for the elongation of one molecule of negative-strand RNA, we detected the synthesis of ∼500 molecules of VPgpUpUOH (data from Fig. 3). We suggest that VPg uridylylation involves repeated formation of ribonucleoproteins containing 3CD, the 5′ cloverleaf, and the CRE (Fig. 7B). The ability of guanidine HCl to inhibit VPg uridylylation suggests that protein 2CATPase activity modifies the ribonucleoprotein complexes involved in VPg uridylylation. We propose that ongoing VPg uridylylation is terminated by elongating 3DPol (Fig. 7C), analogous to the termination of negative-strand RNA synthesis initiation by elongating 3DPol (Fig. 7B). Presumably, elongating 3DPol would permanently alter the nature of the template RNA, preventing the continued formation of the 5′ cloverleaf-, the CRE-, and 3CD-containing ribonucleoprotein complexes.
Because CRE-dependent VPg uridylylation is required for positive-strand RNA synthesis, we propose that accumulated VPgpUpUOH primes the initiation of positive-strand RNA synthesis (Fig. 7D). We find that VPgpUpUOH is not released into the soluble portion of reaction mixtures but that it remains associated with membranous replication complexes (Fig. 2 to 4). Because proteolytic processing of new 3AB- and 3CD-containing ribonucleoprotein complexes into VPg and 3DPol may be involved in the synthesis of each VPgpUpUOH molecule, each VPgpUpUOH molecule may remain associated with the 3DPol involved in its formation (free VPgpUpUOH molecules were illustrated rather than VPgpUpUOH-3DPol complexes for simplicity in Fig. 7B to D). Accumulated VPgpUpUOH-3DPol molecules may prime positive-strand RNA synthesis via the complementarity of VPgpUpUOH with the 3′-terminal adenosine residues of negative-strand RNA templates. Because significant amounts of VPgpUpUOH accumulate in poliovirus-infected cells (13) as well as in cell-free reaction mixtures (Fig. 2 to 4), VPgpUpUOH priming of positive-strand RNA synthesis may be rather inefficient. The magnitude of asymmetry of poliovirus RNA replication in cells is approximately 20 to 50 positive-strand RNA molecules per negative-strand RNA (31). In this study, we found that preinitiation RNA replication complexes catalyzed the synthesis of ∼20 positive-strand RNA molecules per negative-strand RNA molecule (Fig. 2C, lane 2) while synthesizing ∼500 VPgpUpUOH molecules (Fig. 3). These results suggest that greater than 90% of VPgpUpUOH molecules were never used as primers for positive-strand RNA synthesis.
As predicted by this model, the magnitude of asymmetry of poliovirus RNA replication might be determined by the amount of VPgpUpUOH made during negative-strand RNA synthesis. There are approximately 3,000 bases between the 3′ poly(A) tail, where negative-strand RNA synthesis initiates, and the CRE, where VPgpUpUOH is synthesized. The distance between the 3′-terminal poly(A) template and the CRE RNA template and the elongation rate of the negative-strand polymerase may be the dominant factors affecting the magnitude of asymmetry. Intriguingly, the CREs of various picornaviruses are found at different positions within the viral open reading frame (48). For poliovirus and other enteroviruses, the CRE is found within the 2C gene. In rhinovirus type 2, the CRE is found within the 2A gene. The rhinovirus 14 CRE is found within VP1, while cardiovirus CREs are found within the VP2 gene. Experimentally, rhinovirus type 14 and poliovirus CREs can be moved to alternative locations within replicon RNAs (17, 27). The consequences of alternative CRE locations on the magnitude of the asymmetric replication of particular viruses are yet to be determined.
The new model of poliovirus replication proposed above presents a number of testable predictions not previously proposed in other models. This model suggests that (i) The CRE is not involved in or required for negative-strand RNA synthesis; (ii) CRE-dependent VPg uridylylation begins immediately after the initiation of negative-strand RNA synthesis; (iii) the only fundamental difference between the initiation of negative-strand RNA synthesis and the uridylylation of VPg is the nature and conformation of the viral RNA template [the 3′ NTR-poly(A) tail for the initiation of negative-strand RNA synthesis and the CRE for the uridylylation of VPg]; (iv) the poliovirus 5′ cloverleaf and associated ribonucleoprotein complex is required in cis coordinately with the 3′NTR-poly(A) tail and the CRE for the initiation of negative-strand RNA synthesis and VPg uridylylation, respectively; and (v) no cis-active viral RNA structure, including the 5′ cloverleaf as previously proposed (3), may be involved directly in the initiation of positive-strand RNA synthesis except as necessary for the synthesis of negative-strand RNA and VPgpUpUOH as illustrated in Fig. 7A and B.
Largely undefined, due to limited data, are the precise protein-protein interactions and RNA-protein-protein-RNA bridges between distal cis-active structures.
Acknowledgments
We thank James B. Flanegan, University of Florida, College of Medicine, Gainesville, Fla., for providing the poliovirus subgenomic replicon plasmids pRNA2 and pDJB14 and Craig E. Cameron, Pennsylvania State University, University Park, Pa., for providing the poliovirus plasmid pMO-3. We are grateful to Allan W. Roberts and Rebecca Hoogstraten for excellent technical assistance. We thank Naushad Ali and Aleem Siddiqui for helpful comments.
This work was supported by Public Health Service grant AI42189 from the National Institutes of Health.
REFERENCES
- 1.Ambros, V., and D. Baltimore. 1980. Purification and properties of a HeLa cell enzyme able to remove the 5′-terminal protein from poliovirus RNA. J. Biol. Chem. 255:6739-6744. [PubMed] [Google Scholar]
- 2.Ambros, V., R. F. Pettersson, and D. Baltimore. 1978. An enzymatic activity in uninfected cells that cleaves the linkage between poliovirion RNA and the 5′ terminal protein. Cell 15:1439-1446. [DOI] [PubMed] [Google Scholar]
- 3.Andino, R., G. E. Rieckhof, P. L. Achacoso, and D. Baltimore. 1993. Poliovirus RNA synthesis utilizes an RNP complex formed around the 5′-end of viral RNA. EMBO J. 12:3587-3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andino, R., G. E. Rieckhof, and D. Baltimore. 1990. A functional ribonucleoprotein complex forms around the 5′ end of poliovirus RNA. Cell 63:369-380. [DOI] [PubMed] [Google Scholar]
- 5.Barton, D. J., E. P. Black, and J. B. Flanegan. 1995. Complete replication of poliovirus in vitro: preinitiation RNA replication complexes require soluble cellular factors for the synthesis of VPg-linked RNA. J. Virol. 69:5516-5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barton, D. J., and J. B. Flanegan. 1997. Synchronous replication of poliovirus RNA: initiation of negative-strand RNA synthesis requires the guanidine-inhibited activity of protein 2C. J. Virol. 71:8482-8489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barton, D. J., B. J. Morasco, and J. B. Flanegan. 1996. Assays for poliovirus polymerase, 3DPol, and authentic RNA replication in HeLa S10 extracts. Methods Enzymol. 275:35-57. [DOI] [PubMed] [Google Scholar]
- 8.Barton, D. J., B. J. O'Donnell, and J. B. Flanegan. 2001. 5′ cloverleaf in poliovirus RNA is a cis-acting replication element required for negative-strand synthesis. EMBO J. 20:1439-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bienz, K., D. Egger, and L. Pasamontes. 1987. Association of polioviral proteins of the P2 genomic region with the viral replication complex and virus-induced membrane synthesis as visualized by electron microscopic immunocytochemistry and autoradiography. Virology 160:220-226. [DOI] [PubMed] [Google Scholar]
- 10.Bienz, K., D. Egger, and T. Pfister. 1994. Characteristics of the poliovirus replication complex. Arch. Virol. 9(Suppl.):147-157. [DOI] [PubMed] [Google Scholar]
- 11.Bienz, K., D. Egger, T. Pfister, and M. Troxler. 1992. Structural and functional characterization of the poliovirus replication complex. J. Virol. 66:2740-2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collis, P. S., B. J. O'Donnell, D. J. Barton, J. A. Rogers, and J. B. Flanegan. 1992. Replication of poliovirus RNA and subgenomic RNA transcripts in transfected cells. J. Virol. 66:6480-6488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crawford, N. M., and D. Baltimore. 1983. Genome-linked protein VPg of poliovirus is present as free VPg and VPg-pUpU in poliovirus-infected cells. Proc. Natl. Acad. Sci. USA 80:7452-7455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gamarnik, A. V., and R. Andino. 2000. Interactions of viral protein 3CD and poly(rC) binding protein with the 5′ untranslated region of the poliovirus genome. J. Virol. 74:2219-2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerber, K., E. Wimmer, and A. V. Paul. 2001. Biochemical and genetic studies of the initiation of human rhinovirus 2 RNA replication: identification of a cis-replicating element in the coding sequence of 2Apro. J. Virol. 75:10979-10990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerber, K., E. Wimmer, and A. V. Paul. 2001. Biochemical and genetic studies of the initiation of human rhinovirus 2 RNA replication: purification and enzymatic analysis of the RNA-dependent RNA polymerase 3Dpol. J. Virol. 75:10969-10978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodfellow, I., Y. Chaudhry, A. Richardson, J. Meredith, J. W. Almond, W. Barclay, and D. J. Evans. 2000. Identification of a cis-acting replication element within the poliovirus coding region. J. Virol. 74:4590-4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris, K. S., W. Xiang, L. Alexander, W. S. Lane, A. V. Paul, and E. Wimmer. 1994. Interaction of poliovirus polypeptide 3CDpro with the 5′ and 3′ termini of the poliovirus genome. Identification of viral and cellular cofactors needed for efficient binding. J. Biol. Chem. 269:27004-27014. [PubMed] [Google Scholar]
- 19.Herold, J., and R. Andino. 2000. Poliovirus requires a precise 5′ end for efficient positive-strand RNA synthesis. J. Virol. 74:6394-6400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herold, J., and R. Andino. 2001. Poliovirus RNA replication requires genome circularization through a protein-protein bridge. Mol. Cell 7:581-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krausslich, H. G., M. J. Nicklin, C. K. Lee, and E. Wimmer. 1988. Polyprotein processing in picornavirus replication. Biochimie 70:119-130. [DOI] [PubMed] [Google Scholar]
- 22.Kuhn, R. J., H. Tada, M. F. Ypma-Wong, J. J. Dunn, B. L. Semler, and E. Wimmer. 1988. Construction of a “mutagenesis cartridge” for poliovirus genome-linked viral protein: isolation and characterization of viable and nonviable mutants. Proc. Natl. Acad. Sci. USA 85:519-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuhn, R. J., H. Tada, M. F. Ypma-Wong, B. L. Semler, and E. Wimmer. 1988. Mutational analysis of the genome-linked protein VPg of poliovirus. J. Virol. 62:4207-4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lawson, M. A., and B. L. Semler. 1990. Picornavirus protein processing—enzymes, substrates, and genetic regulation. Curr. Top. Microbiol. Immunol. 161:49-87. [PubMed] [Google Scholar]
- 25.Lobert, P. E., N. Escriou, J. Ruelle, and T. Michiels. 1999. A coding RNA sequence acts as a replication signal in cardioviruses. Proc. Natl. Acad. Sci. USA 96:11560-11565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lyons, T., K. E. Murray, A. W. Roberts, and D. J. Barton. 2001. Poliovirus 5′-terminal cloverleaf RNA is required in cis for VPg uridylylation and the initiation of negative-strand RNA synthesis. J. Virol. 75:10696-10708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKnight, K. L., and S. M. Lemon. 1998. The rhinovirus type 14 genome contains an internally located RNA structure that is required for viral replication. RNA 4:1569-1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mirmomeni, M. H., P. J. Hughes, and G. Stanway. 1997. An RNA tertiary structure in the 3′ untranslated region of enteroviruses is necessary for efficient replication. J. Virol. 71:2363-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Molla, A., A. V. Paul, and E. Wimmer. 1991. Cell-free, de novo synthesis of poliovirus. Science 254:1647-1651. [DOI] [PubMed] [Google Scholar]
- 30.Murray, K. E., A. W. Roberts, and D. J. Barton. 2001. Poly(rC) binding proteins mediate poliovirus mRNA stability. RNA 7:1126-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Novak, J. E., and K. Kirkegaard. 1991. Improved method for detecting poliovirus negative strands used to demonstrate specificity of positive-strand encapsidation and the ratio of positive to negative strands in infected cells. J. Virol. 65:3384-3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pallansch, M. A., O. M. Kew, B. L. Semler, D. R. Omilianowski, C. W. Anderson, E. Wimmer, and R. R. Rueckert. 1984. Protein processing map of poliovirus. J. Virol. 49:873-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parsley, T. B., J. S. Towner, L. B. Blyn, E. Ehrenfeld, and B. L. Semler. 1997. Poly (rC) binding protein 2 forms a ternary complex with the 5′-terminal sequences of poliovirus RNA and the viral 3CD proteinase. RNA 3:1124-1134. [PMC free article] [PubMed] [Google Scholar]
- 34.Pathak, H. B., S. K. B. Ghosh, A. W. Roberts, S. D. Sharma, J. D. Yoder, J. J. Arnold, D. W. Gohara, D. J. Barton, A. V. Paul, and C. E. Cameron. 2002. Structure-function relationships of the RNA-dependent RNA polymerase from poliovirus 3DPol: a surface of the primary oligomerization domain functions in capsid precursor processing and VPg uridylylation. J. Biol. Chem. 277:31551-31562. [DOI] [PubMed] [Google Scholar]
- 35.Paul, A. V., E. Rieder, D. W. Kim, J. H. van Boom, and E. Wimmer. 2000. Identification of an RNA hairpin in poliovirus RNA that serves as the primary template in the in vitro uridylylation of VPg. J. Virol. 74:10359-10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paul, A. V., J. H. van Boom, D. Filippov, and E. Wimmer. 1998. Protein-primed RNA synthesis by purified poliovirus RNA polymerase. Nature 393:280-284. [DOI] [PubMed] [Google Scholar]
- 37.Pfister, T., and E. Wimmer. 1999. Characterization of the nucleoside triphosphatase activity of poliovirus protein 2C reveals a mechanism by which guanidine inhibits poliovirus replication. J. Biol. Chem. 274:6992-7001. [DOI] [PubMed] [Google Scholar]
- 38.Pilipenko, E. V., K. V. Poperechny, S. V. Maslova, W. J. Melchers, H. J. Slot, and V. I. Agol. 1996. Cis-element, oriR, involved in the initiation of (−) strand poliovirus RNA: a quasi-globular multi-domain RNA structure maintained by tertiary (′kissing') interactions. EMBO J. 15:5428-5436. [PMC free article] [PubMed] [Google Scholar]
- 39.Racaniello, V. R., and D. Baltimore. 1981. Molecular cloning of poliovirus cDNA and determination of the complete nucleotide sequence of the viral genome. Proc. Natl. Acad. Sci. USA 78:4887-4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rieder, E., A. V. Paul, D. W. Kim, J. H. van Boom, and E. Wimmer. 2000. Genetic and biochemical studies of poliovirus cis-acting replication element cre in relation to VPg uridylylation. J. Virol. 74:10371-10380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rohll, J. B., D. H. Moon, D. J. Evans, and J. W. Almond. 1995. The 3′ untranslated region of picornavirus RNA: features required for efficient genome replication. J. Virol. 69:7835-7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rothberg, P. G., T. J. Harris, A. Nomoto, and E. Wimmer. 1978. O4-(5′-uridylyl)tyrosine is the bond between the genome-linked protein and the RNA of poliovirus. Proc. Natl. Acad. Sci. USA 75:4868-4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sachs, A. B., P. Sarnow, and M. W. Hentze. 1997. Starting at the beginning, middle, and end: translation initiation in eukaryotes. Cell 89:831-838. [DOI] [PubMed] [Google Scholar]
- 44.Schlegel, A., T. H. Giddings, Jr., M. S. Ladinsky, and K. Kirkegaard. 1996. Cellular origin and ultrastructure of membranes induced during poliovirus infection. J. Virol. 70:6576-6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Teterina, N. L., D. Egger, K. Bienz, D. M. Brown, B. L. Semler, and E. Ehrenfeld. 2001. Requirements for assembly of poliovirus replication complexes and negative-strand RNA synthesis. J. Virol. 75:3841-3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Towner, J. S., M. M. Mazanet, and B. L. Semler. 1998. Rescue of defective poliovirus RNA replication by 3AB-containing precursor polyproteins. J. Virol. 72:7191-7200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Waggoner, S., and P. Sarnow. 1998. Viral ribonucleoprotein complex formation and nucleolar-cytoplasmic relocalization of nucleolin in poliovirus-infected cells. J. Virol. 72:6699-6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Witwer, C., S. Rauscher, I. L. Hofacker, and P. F. Stadler. 2001. Conserved RNA secondary structures in Picornaviridae genomes. Nucleic Acids Res. 29:5079-5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xiang, W., A. Cuconati, D. Hope, K. Kirkegaard, and E. Wimmer. 1998. Complete protein linkage map of poliovirus P3 proteins: interaction of polymerase 3Dpol with VPg and with genetic variants of 3AB. J. Virol. 72:6732-6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang, Y., R. Rijnbrand, K. L. McKnight, E. Wimmer, A. Paul, A. Martin, and S. M. Lemon. 2002. Sequence requirements for viral RNA replication and VPg uridylylation directed by the internal cis-acting replication element (cre) of human rhinovirus type 14. J. Virol. 76:7485-7494. [DOI] [PMC free article] [PubMed] [Google Scholar]