Abstract
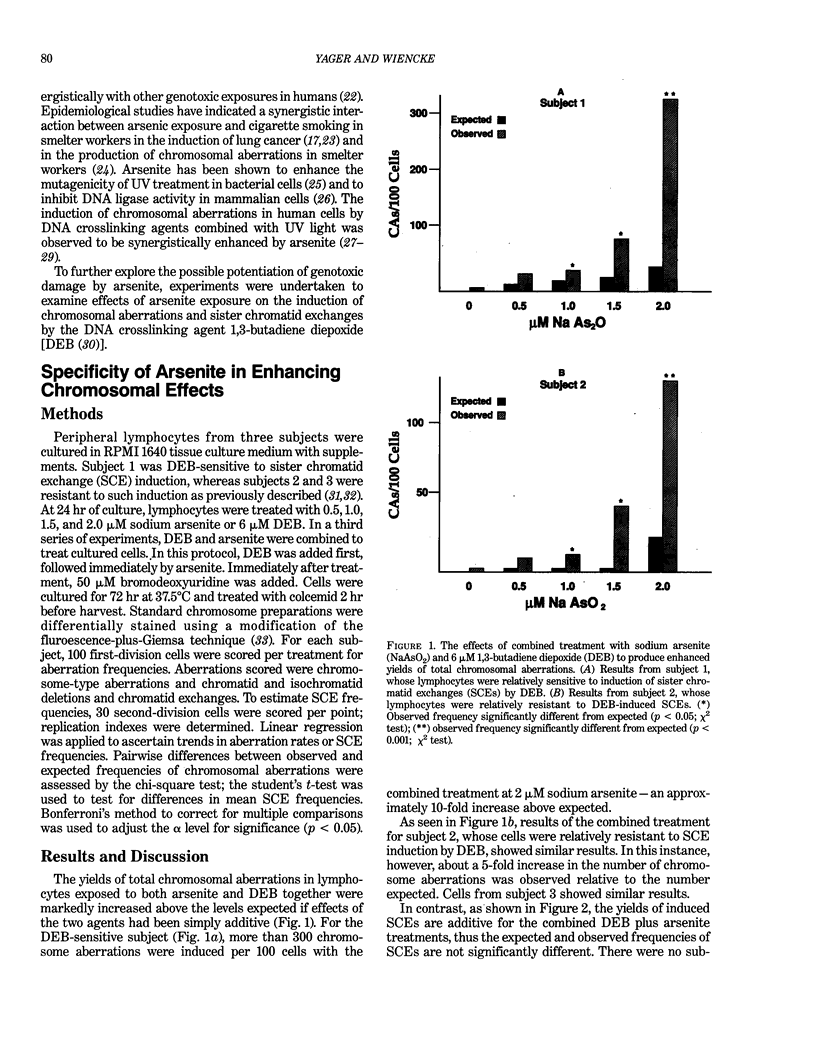
Arsenic is a naturally occurring metalloid that has been associated with increased incidence of human cancer in certain highly exposed populations. Arsenic is released to the environment by natural means such as solubilization from geologic formations into water supplies. It is also released to occupational and community environments by such activities as nonferrous ore smelting and combustion of fuels containing arsenic. Several lines of evidence indicate that arsenic acts indirectly with other agents to ultimately enhance specific genotoxic effects that may lead to carcinogenesis. Work described here indicates that arsenite specifically potentiates chromosomal aberrations induced by a DNA crosslinking agent, 1,3-butadiene diepoxide, but does not effect the induction of sister chromatid exchanges under the same treatment conditions. It is proposed that the specific co-clastogenic effects of arsenite seen here may be mediated by its interference with DNA repair activities. Further understanding of the mechanism by which arsenic interacts with other environmental agents will result in more accurate estimates of risk from exposure to arsenic.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelson O., Dahlgren E., Jansson C. D., Rehnlund S. O. Arsenic exposure and mortality: a case-referent study from a Swedish copper smelter. Br J Ind Med. 1978 Feb;35(1):8–15. doi: 10.1136/oem.35.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J. C., Lamb P. W., Wang T. C., Lee T. C. Mechanisms of arsenic-induced cell transformation. Biol Trace Elem Res. 1989 Jul-Sep;21:421–429. doi: 10.1007/BF02917284. [DOI] [PubMed] [Google Scholar]
- Beckman L., Nordenson I. Interaction between some common genotoxic agents. Hum Hered. 1986;36(6):397–401. doi: 10.1159/000153664. [DOI] [PubMed] [Google Scholar]
- Berg J. M. Zinc finger domains: hypotheses and current knowledge. Annu Rev Biophys Biophys Chem. 1990;19:405–421. doi: 10.1146/annurev.bb.19.060190.002201. [DOI] [PubMed] [Google Scholar]
- Chen C. J., Chuang Y. C., Lin T. M., Wu H. Y. Malignant neoplasms among residents of a blackfoot disease-endemic area in Taiwan: high-arsenic artesian well water and cancers. Cancer Res. 1985 Nov;45(11 Pt 2):5895–5899. [PubMed] [Google Scholar]
- Chen C. J., Wang C. J. Ecological correlation between arsenic level in well water and age-adjusted mortality from malignant neoplasms. Cancer Res. 1990 Sep 1;50(17):5470–5474. [PubMed] [Google Scholar]
- Enterline P. E., Marsh G. M. Cancer among workers exposed to arsenic and other substances in a copper smelter. Am J Epidemiol. 1982 Dec;116(6):895–911. doi: 10.1093/oxfordjournals.aje.a113492. [DOI] [PubMed] [Google Scholar]
- Enterline P. E., Marsh G. M. Mortality studies of smelter workers. Am J Ind Med. 1980;1(3-4):251–259. doi: 10.1002/ajim.4700010303. [DOI] [PubMed] [Google Scholar]
- Joshi S., Hughes J. B. Inhibition of coupling factor B activity by cadmium ion, arsenite-2,3-dimercaptopropanol, and phenylarsine oxide, and preferential reactivation by dithiols. J Biol Chem. 1981 Nov 10;256(21):11112–11116. [PubMed] [Google Scholar]
- Kelsey K. T., Christiani D. C., Wiencke J. K. Bimodal distribution of sensitivity to SCE induction by diepoxybutane in human lymphocytes. II. Relationship to baseline SCE frequency. Mutat Res. 1991 May;248(1):27–33. doi: 10.1016/0027-5107(91)90084-2. [DOI] [PubMed] [Google Scholar]
- Lee A. M., Fraumeni J. F., Jr Arsenic and respiratory cancer in man: an occupational study. J Natl Cancer Inst. 1969 Jun;42(6):1045–1052. [PubMed] [Google Scholar]
- Lee T. C., Lee K. C., Tzeng Y. J., Huang R. Y., Jan K. Y. Sodium arsenite potentiates the clastogenicity and mutagenicity of DNA crosslinking agents. Environ Mutagen. 1986;8(1):119–128. doi: 10.1002/em.2860080111. [DOI] [PubMed] [Google Scholar]
- Lee T. C., Oshimura M., Barrett J. C. Comparison of arsenic-induced cell transformation, cytotoxicity, mutation and cytogenetic effects in Syrian hamster embryo cells in culture. Carcinogenesis. 1985 Oct;6(10):1421–1426. doi: 10.1093/carcin/6.10.1421. [DOI] [PubMed] [Google Scholar]
- Lee T. C., Tanaka N., Lamb P. W., Gilmer T. M., Barrett J. C. Induction of gene amplification by arsenic. Science. 1988 Jul 1;241(4861):79–81. doi: 10.1126/science.3388020. [DOI] [PubMed] [Google Scholar]
- Lee T. C., Tzeng S. F., Chang W. J., Lin Y. C., Jan K. Y. Post-treatments with sodium arsenite during G2 enhance the frequency of chromosomal aberrations induced by S-dependent clastogens. Mutat Res. 1986 Dec;163(3):263–269. doi: 10.1016/0027-5107(86)90024-2. [DOI] [PubMed] [Google Scholar]
- Li J. H., Rossman T. G. Inhibition of DNA ligase activity by arsenite: a possible mechanism of its comutagenesis. Mol Toxicol. 1989 Winter;2(1):1–9. [PubMed] [Google Scholar]
- Nordberg G. F., Andersen O. Metal interactions in carcinogenesis: enhancement, inhibition. Environ Health Perspect. 1981 Aug;40:65–81. doi: 10.1289/ehp.814065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordenson I., Beckman G., Beckman L., Nordström S. Occupational and environmental risks in and around a smelter in northern Sweden. II. Chromosomal aberrations in workers exposed to arsenic. Hereditas. 1978;88(1):47–50. doi: 10.1111/j.1601-5223.1978.tb01601.x. [DOI] [PubMed] [Google Scholar]
- PINTO S. S., BENNETT B. M. EFFECT OF ARSENIC TRIOXIDE EXPOSURE ON MORTALITY. Arch Environ Health. 1963 Nov;7:583–591. doi: 10.1080/00039896.1963.10663587. [DOI] [PubMed] [Google Scholar]
- Perry P., Wolff S. New Giemsa method for the differential staining of sister chromatids. Nature. 1974 Sep 13;251(5471):156–158. doi: 10.1038/251156a0. [DOI] [PubMed] [Google Scholar]
- Pershagen G. The carcinogenicity of arsenic. Environ Health Perspect. 1981 Aug;40:93–100. doi: 10.1289/ehp.814093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman T. G. Enhancement of UV-mutagenesis by low concentrations of arsenite in E. coli. Mutat Res. 1981 May;91(3):207–211. doi: 10.1016/0165-7992(81)90032-4. [DOI] [PubMed] [Google Scholar]
- Tseng W. P., Chu H. M., How S. W., Fong J. M., Lin C. S., Yeh S. Prevalence of skin cancer in an endemic area of chronic arsenicism in Taiwan. J Natl Cancer Inst. 1968 Mar;40(3):453–463. [PubMed] [Google Scholar]
- Vahter M., Marafante E. Effects of low dietary intake of methionine, choline or proteins on the biotransformation of arsenite in the rabbit. Toxicol Lett. 1987 Jun;37(1):41–46. doi: 10.1016/0378-4274(87)90165-2. [DOI] [PubMed] [Google Scholar]
- Welch K., Higgins I., Oh M., Burchfiel C. Arsenic exposure, smoking, and respiratory cancer in copper smelter workers. Arch Environ Health. 1982 Nov-Dec;37(6):325–335. doi: 10.1080/00039896.1982.10667586. [DOI] [PubMed] [Google Scholar]
- Wiencke J. K., Wrensch M. R., Miike R., Petrakis N. L. Individual susceptibility to induced chromosome damage and its implications for detecting genotoxic exposures in human populations. Cancer Res. 1991 Oct 1;51(19):5266–5269. [PubMed] [Google Scholar]
- Wiencke J. K., Yager J. W. Specificity of arsenite in potentiating cytogenetic damage induced by the DNA crosslinking agent diepoxybutane. Environ Mol Mutagen. 1992;19(3):195–200. doi: 10.1002/em.2850190303. [DOI] [PubMed] [Google Scholar]


