Abstract
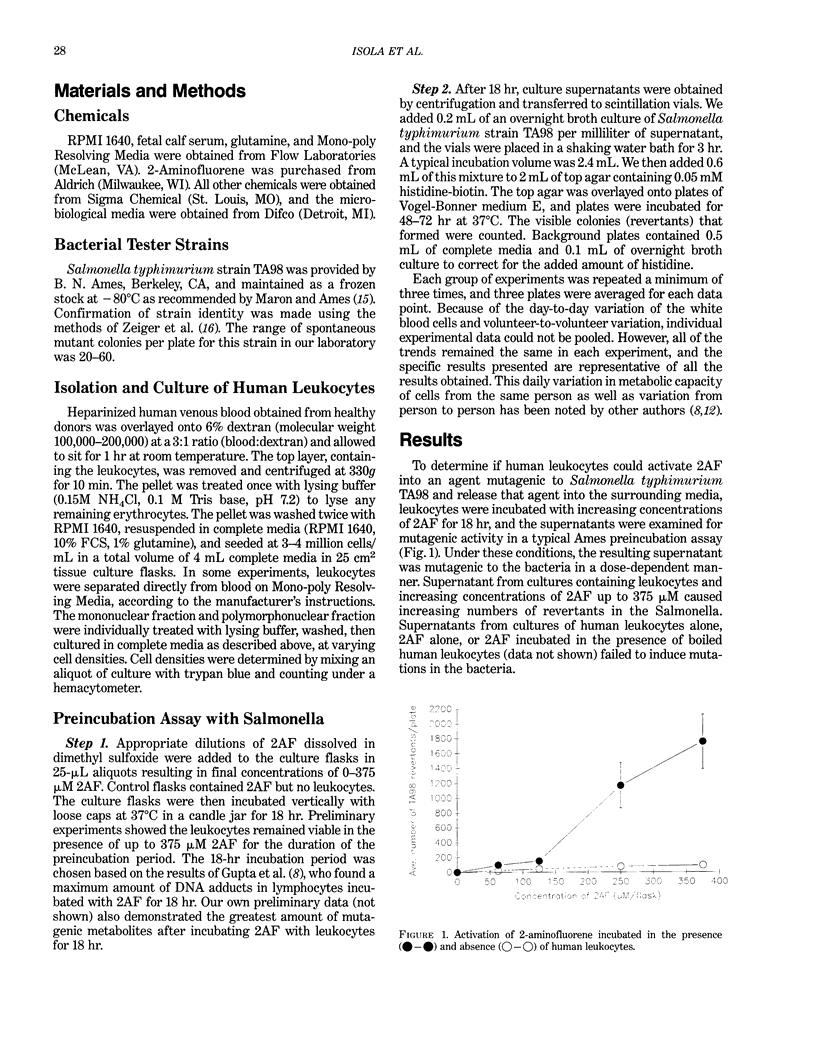
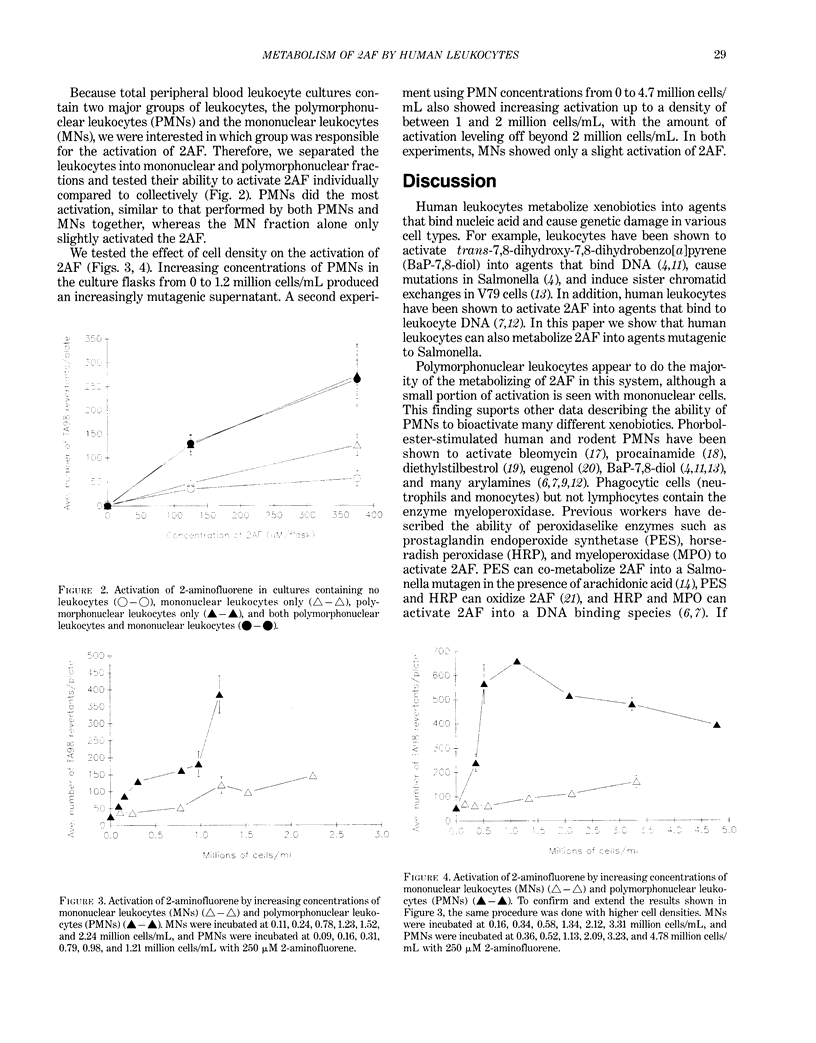
Recent investigations have demonstrated the ability of leukocytes to metabolize promutagens or procarcinogens into their genotoxic forms. As a possible explanation for the association between inflammation and cancer, we and others have hypothesized that local accumulations of leukocytes could take up nearby promutagens, metabolize them, and release genotoxic agents that may cause damage in the surrounding tissue. Using a modified, two-step preincubation protocol with Salmonella, we have tested this hypothesis. We have shown that total human peripheral blood leukocytes, cultured in the presence of 2-aminofluorene for 18 hr, can metabolize 2-aminofluorene into agents mutagenic to Salmonella typhimurium strain TA98. Furthermore, experiments in which polymorphonuclear leukocytes were separated from mononuclear leukocytes demonstrated that the PMNs metabolized 2-aminofluorene to a much greater extent than the MNs.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyd J. A., Eling T. E. Evidence for a one-electron mechanism of 2-aminofluorene oxidation by prostaglandin H synthase and horseradish peroxidase. J Biol Chem. 1984 Nov 25;259(22):13885–13896. [PubMed] [Google Scholar]
- Corbett M. D., Corbett B. R., Hannothiaux M. H., Quintana S. J. Metabolic activation and nucleic acid binding of acetaminophen and related arylamine substrates by the respiratory burst of human granulocytes. Chem Res Toxicol. 1989 Jul-Aug;2(4):260–266. doi: 10.1021/tx00010a008. [DOI] [PubMed] [Google Scholar]
- Corbett M. D., Corbett B. R. Nucleic acid binding of arylamines during the respiratory burst of human granulocytes. Chem Res Toxicol. 1988 Nov-Dec;1(6):356–363. doi: 10.1021/tx00006a006. [DOI] [PubMed] [Google Scholar]
- Corbett M. D., Hannothiaux M. H., Corbett B. R., Quintana S. J. A comparison of the HL-60 cell line and human granulocytes to effect the bioactivation of arylamines and related xenobiotics. The binding of 2-aminofluorene to nucleic acids as the result of the respiratory burst. Chem Biol Interact. 1991;78(1):33–54. doi: 10.1016/0009-2797(91)90101-c. [DOI] [PubMed] [Google Scholar]
- Eastmond D. A., French R. C., Ross D., Smith M. T. Metabolic activation of diethylstilbestrol by stimulated human leukocytes. Cancer Lett. 1987 Apr;35(1):79–86. doi: 10.1016/0304-3835(87)90059-0. [DOI] [PubMed] [Google Scholar]
- Gentile J. M., DeRuiter E. Promutagen activation in parasite-infected organisms: preliminary observations with Fasciola hepatica-infected mice and aflatoxin B1. Toxicol Lett. 1981 Jun-Jul;8(4-5):273–282. doi: 10.1016/0378-4274(81)90113-2. [DOI] [PubMed] [Google Scholar]
- Gentile J. M. Schistosome related cancers: a possible role for genotoxins. Environ Mutagen. 1985;7(5):775–785. doi: 10.1002/em.2860070514. [DOI] [PubMed] [Google Scholar]
- Gupta R. C., Earley K., Sharma S. Use of human peripheral blood lymphocytes to measure DNA binding capacity of chemical carcinogens. Proc Natl Acad Sci U S A. 1988 May;85(10):3513–3517. doi: 10.1073/pnas.85.10.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramers P. G., Gentile J. M., Gryseels B. J., Jordan P., Katz N., Mott K. E., Mulvihill J. J., Seed J. L., Frohberg H. International Commission for Protection Against Environmental Mutagens and Carcinogens. ICPEMC publication No. 18. Review of the genotoxicity and carcinogenicity of antischistosomal drugs; is there a case for a study of mutation epidemiology? Report of a task group on mutagenic antischistosomals. Mutat Res. 1991 Jan;257(1):49–89. doi: 10.1016/0165-1110(91)90019-r. [DOI] [PubMed] [Google Scholar]
- Krauss R. S., Angerman-Stewart J., Eling T. E., Dooley K. L., Kadlubar F. F. The formation of 2-aminofluorene-DNA adducts in vivo: evidence for peroxidase-mediated activation. J Biochem Toxicol. 1989 Summer;4(2):111–117. doi: 10.1002/jbt.2570040207. [DOI] [PubMed] [Google Scholar]
- Maron D. M., Ames B. N. Revised methods for the Salmonella mutagenicity test. Mutat Res. 1983 May;113(3-4):173–215. doi: 10.1016/0165-1161(83)90010-9. [DOI] [PubMed] [Google Scholar]
- Robertson I. G., Sivarajah K., Eling T. E., Zeiger E. Activation of some aromatic amines to mutagenic products by prostaglandin endoperoxide synthetase. Cancer Res. 1983 Feb;43(2):476–480. [PubMed] [Google Scholar]
- Seed J. L., Kensler T. W., Elia M., Trush M. A. Induction of sister-chromatid exchanges by polycyclic aromatic hydrocarbons following metabolic activation with phorbol ester-stimulated human polymorphonuclear leukocytes. Res Commun Chem Pathol Pharmacol. 1990 Mar;67(3):349–360. [PubMed] [Google Scholar]
- Shen J. H., Wegenke M., Wolff T. Capability of human blood cells to form the DNA adduct, C8-(N2-aminofluorenyl)-deoxyguanosine-3'-5'-diphosphate from 2-aminofluorene. Carcinogenesis. 1990 Aug;11(8):1441–1444. doi: 10.1093/carcin/11.8.1441. [DOI] [PubMed] [Google Scholar]
- Thompson D., Constantin-Teodosiu D., Norbeck K., Svensson B., Moldéus P. Metabolic activation of eugenol by myeloperoxidase and polymorphonuclear leukocytes. Chem Res Toxicol. 1989 May-Jun;2(3):186–192. doi: 10.1021/tx00009a011. [DOI] [PubMed] [Google Scholar]
- Trush M. A. Activation of bleomycin A2 to a DNA-damaging intermediate by phorbol ester-stimulated human polymorphonuclear leukocytes. Toxicol Lett. 1984 Mar;20(3):297–302. doi: 10.1016/0378-4274(84)90163-2. [DOI] [PubMed] [Google Scholar]
- Trush M. A., Seed J. L., Kensler T. W. Oxidant-dependent metabolic activation of polycyclic aromatic hydrocarbons by phorbol ester-stimulated human polymorphonuclear leukocytes: possible link between inflammation and cancer. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5194–5198. doi: 10.1073/pnas.82.15.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trush M. A., Twerdok L. E., Esterline R. L. Comparison of oxidant activities and the activation of benzo(a)pyrene-7,8-dihydrodiol by polymorphonuclear leucocytes from human, rat and mouse. Xenobiotica. 1990 Sep;20(9):925–932. doi: 10.3109/00498259009046908. [DOI] [PubMed] [Google Scholar]
- Tsuruta Y., Subrahmanyam V. V., Marshall W., O'Brien P. J. Peroxidase-mediated irreversible binding of arylamine carcinogens to DNA in intact polymorphonuclear leukocytes activated by a tumor promoter. Chem Biol Interact. 1985 Feb-Apr;53(1-2):25–35. doi: 10.1016/s0009-2797(85)80081-8. [DOI] [PubMed] [Google Scholar]
- Twerdok L. E., Trush M. A. Neutrophil-derived oxidants as mediators of chemical activation in bone marrow. Chem Biol Interact. 1988;65(3):261–273. doi: 10.1016/0009-2797(88)90111-1. [DOI] [PubMed] [Google Scholar]
- Uetrecht J., Zahid N., Rubin R. Metabolism of procainamide to a hydroxylamine by human neutrophils and mononuclear leukocytes. Chem Res Toxicol. 1988 Jan-Feb;1(1):74–78. doi: 10.1021/tx00001a013. [DOI] [PubMed] [Google Scholar]
- Zeiger E., Pagano D. A., Robertson I. G. A rapid and simple scheme for confirmation of Salmonella tester strain phenotype. Environ Mutagen. 1981;3(3):205–209. doi: 10.1002/em.2860030303. [DOI] [PubMed] [Google Scholar]


