Abstract
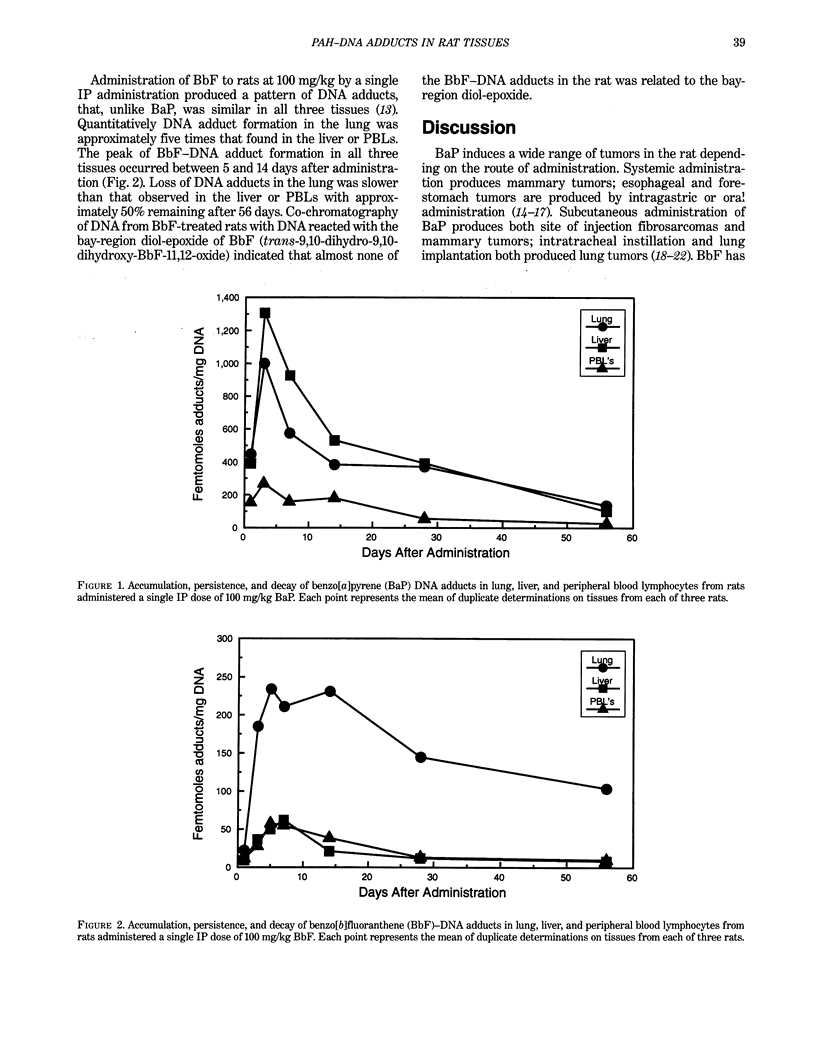
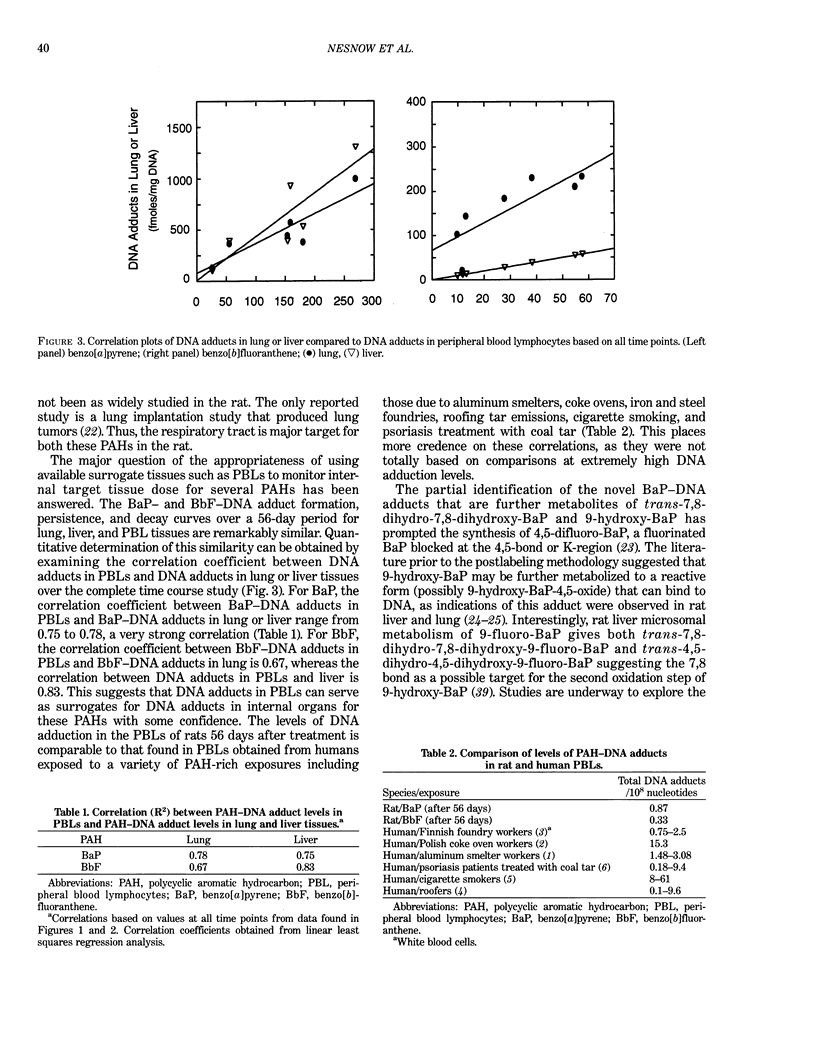
DNA-carcinogen adducts offer a potential dosimeter for environmental genotoxicants reaching the exposed individual. Because the target tissues for many chemical carcinogens are not readily accessible for monitoring adducts in humans, peripheral blood lymphocytes (PBLs) have served as surrogate sources of exposed DNA. Both benzo[a]pyrene (BaP) and benzo[b]fluoranthene (BbF) are widely distributed in the environment as components of complex mixtures, such as automobile exhaust, cigarette smoke, foods, water, and urban air. Thus, human exposure to these chemicals is widespread, and they probably contribute to overall human lung cancer risk. The interpretation of the results of such studies would be enhanced by an understanding of the pharmacokinetics of specific DNA adduct formation and persistence in both target and surrogate tissues. Polycyclic aromatic hydrocarbons (PAHs) were administered to male Sprague-Dawley rats IP at 100 mg PAH/kg body weight. Lung, liver, and PBL tissues were harvested 1, 3, 7, 14, 28, and 56 days after treatment. DNA was extracted from each tissue and 32P-postlabeling analysis of DNA adducts with nuclease P1 enhancement was conducted. In all three tissues, BaP-DNA adducts exhibit a similar pattern, reaching a maximum at 3-4 days, followed by a decrease to 56 days. For BbF, the maximum DNA adduct levels in each tissue were between 5 and 14 days after injection. By 56 days after administration, the total adducts remaining in all tissues were measurable. Correlation analyses of the amount of DNA adducts in lung or liver compared to those found in the PBL of the same animals suggest a range of correlations (R2 = 0.67-0.83).(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal S. C., Lambert G., Padgett W., Nesnow S. Synthesis of a novel fluorinated benzo[a]pyrene: 4,5-difluorobenzo[a]pyrene. Carcinogenesis. 1991 Sep;12(9):1647–1650. doi: 10.1093/carcin/12.9.1647. [DOI] [PubMed] [Google Scholar]
- Brune H., Deutsch-Wenzel R. P., Habs M., Ivankovic S., Schmähl D. Investigation of the tumorigenic response to benzo(a)pyrene in aqueous caffeine solution applied orally to Sprague-Dawley rats. J Cancer Res Clin Oncol. 1981;102(2):153–157. doi: 10.1007/BF00410666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buening M. K., Levin W., Wood A. W., Chang R. L., Agranat I., Rabinovitz M., Buhler D. R., Mah H. D., Hernandez O., Simpson R. B. Fluorine substitution as a probe for the role of the 6-position of benzo[a]pyrene in carcinogenesis. J Natl Cancer Inst. 1983 Aug;71(2):309–315. [PubMed] [Google Scholar]
- Buhler D. R., Unlu F., Thakker D. R., Slaga T. J., Newman M. S., Levin W., Conney A. H., Jerina D. M. Metabolism and tumorigenicity of 7-, 8-, 9-, and 10-fluorobenzo(a)pyrenes. Cancer Res. 1982 Nov;42(11):4779–4783. [PubMed] [Google Scholar]
- Deutsch-Wenzel R. P., Brune H., Grimmer G., Dettbarn G., Misfeld J. Experimental studies in rat lungs on the carcinogenicity and dose-response relationships of eight frequently occurring environmental polycyclic aromatic hydrocarbons. J Natl Cancer Inst. 1983 Sep;71(3):539–544. [PubMed] [Google Scholar]
- EVERETT N. B., CAFFREY R. W., RIEKE W. O. RECIRCULATION OF LYMPHOCYTES. Ann N Y Acad Sci. 1964 Feb 28;113:887–897. doi: 10.1111/j.1749-6632.1964.tb40710.x. [DOI] [PubMed] [Google Scholar]
- GIBEL W. EXPERIMENTELLER BEITRAG ZUR SYNKARZINOGENESE BEIM SPEISEROEHRENKARZINOM. Krebsarzt. 1964;19:268–272. [PubMed] [Google Scholar]
- Geddie J. E., Amin S., Huie K., Hecht S. S. Formation and tumorigenicity of benzo[b]fluoranthene metabolites in mouse epidermis. Carcinogenesis. 1987 Nov;8(11):1579–1584. doi: 10.1093/carcin/8.11.1579. [DOI] [PubMed] [Google Scholar]
- Gupta R. C. Enhanced sensitivity of 32P-postlabeling analysis of aromatic carcinogen:DNA adducts. Cancer Res. 1985 Nov;45(11 Pt 2):5656–5662. [PubMed] [Google Scholar]
- Hemminki K., Randerath K., Reddy M. V., Putman K. L., Santella R. M., Perera F. P., Young T. L., Phillips D. H., Hewer A., Savela K. Postlabeling and immunoassay analysis of polycyclic aromatic hydrocarbons--adducts of deoxyribonucleic acid in white blood cells of foundry workers. Scand J Work Environ Health. 1990 Jun;16(3):158–162. doi: 10.5271/sjweh.1798. [DOI] [PubMed] [Google Scholar]
- Herbert R., Marcus M., Wolff M. S., Perera F. P., Andrews L., Godbold J. H., Rivera M., Stefanidis M., Lu X. Q., Landrigan P. J. Detection of adducts of deoxyribonucleic acid in white blood cells of roofers by 32P-postlabeling. Relationship of adduct levels to measures of exposure to polycyclic aromatic hydrocarbons. Scand J Work Environ Health. 1990 Apr;16(2):135–143. doi: 10.5271/sjweh.1806. [DOI] [PubMed] [Google Scholar]
- Jernström B., Orrenius S., Undeman O., Gräslund A., Ehrenberg A. Fluorescence study of DNA-binding metabolites of benzo(a)pyrene formed in hepatocytes isolated from 3-methylcholanthrene-treated rats. Cancer Res. 1978 Aug;38(8):2600–2607. [PubMed] [Google Scholar]
- Kligerman A. D., Halperin E. C., Erexson G. L., Honoré G. The persistence of lymphocytes with dicentric chromosomes following whole-body X irradiation of mice. Radiat Res. 1990 Oct;124(1):22–27. [PubMed] [Google Scholar]
- McCormick D. L., Burns F. J., Albert R. E. Inhibition of benz[a]pyrene-induced mammary carcinogenesis by retinyl acetate. J Natl Cancer Inst. 1981 Mar;66(3):559–564. [PubMed] [Google Scholar]
- NORMAN A., SASAKI M. S., OTTOMAN R. E., FINGERHUT A. G. LYMPHOCYTE LIFETIME IN WOMEN. Science. 1965 Feb 12;147(3659):745–745. doi: 10.1126/science.147.3659.745. [DOI] [PubMed] [Google Scholar]
- ROBINSON S. H., BRECHER G., LOURIE I. S., HALEY J. E. LEUKOCYTE LABELING IN RATS DURING AND AFTER CONTINUOUS INFUSION OF TRITIATED THYMIDINE: IMPLICATIONS FOR LYMPHOCYTE LONGEVITY AND DNA REUTILIZATION. Blood. 1965 Sep;26:281–295. [PubMed] [Google Scholar]
- Reddy M. V., Randerath K. Nuclease P1-mediated enhancement of sensitivity of 32P-postlabeling test for structurally diverse DNA adducts. Carcinogenesis. 1986 Sep;7(9):1543–1551. doi: 10.1093/carcin/7.9.1543. [DOI] [PubMed] [Google Scholar]
- Rees E. D., Shuck A. E., Lowry J. Q., Smith T. M., Lipscomb H. Comparison of the clastogenic and carcinogenic effects of intravenous beta-propiolactone and benzo(a)pyrene in rats. J Environ Pathol Toxicol. 1979 Jul-Aug;2(6):1475–1485. [PubMed] [Google Scholar]
- Ross J. A., Nelson G. B., Holden K. L. DNA isolation from small tissue samples using salt and spermine. Nucleic Acids Res. 1991 Nov 11;19(21):6053–6053. doi: 10.1093/nar/19.21.6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J., Nelson G., Erexson G., Kligerman A., Earley K., Gupta R. C., Nesnow S. DNA adducts in rat lung, liver and peripheral blood lymphocytes produced by i.p. administration of benzo[a]pyrene metabolites and derivatives. Carcinogenesis. 1991 Oct;12(10):1953–1955. doi: 10.1093/carcin/12.10.1953. [DOI] [PubMed] [Google Scholar]
- Ross J., Nelson G., Kligerman A., Erexson G., Bryant M., Earley K., Gupta R., Nesnow S. Formation and persistence of novel benzo(a)pyrene adducts in rat lung, liver, and peripheral blood lymphocyte DNA. Cancer Res. 1990 Aug 15;50(16):5088–5094. [PubMed] [Google Scholar]
- Savela K., Hemminki K. DNA adducts in lymphocytes and granulocytes of smokers and nonsmokers detected by the 32P-postlabelling assay. Carcinogenesis. 1991 Mar;12(3):503–508. doi: 10.1093/carcin/12.3.503. [DOI] [PubMed] [Google Scholar]
- Schoket B., Phillips D. H., Hewer A., Vincze I. 32P-postlabelling detection of aromatic DNA adducts in peripheral blood lymphocytes from aluminium production plant workers. Mutat Res. 1991 May;260(1):89–98. doi: 10.1016/0165-1218(91)90084-y. [DOI] [PubMed] [Google Scholar]
- Sydnor K. L., Bergo C. H., Flesher J. W. Effect of various substituents in the 6-position on the relative carcinogenic activity of a series of benzo[a]pyrene derivatives. Chem Biol Interact. 1980 Feb;29(2):159–167. doi: 10.1016/0009-2797(80)90030-7. [DOI] [PubMed] [Google Scholar]
- Vigny P., Ginot Y. M., Kindts M., Cooper C. S., Grover P. L., Sims P. Fluorescence spectral evidence that benzo[a]pyrene is activated by metabolism in mouse skin to a diol-epoxide and a phenol-epoxide. Carcinogenesis. 1980;1(11):945–950. doi: 10.1093/carcin/1.11.945. [DOI] [PubMed] [Google Scholar]
- Zhang Y. J., Li Y., DeLeo V. A., Santella R. M. Detection of DNA adducts in skin biopsies of coal tar-treated psoriasis patients: immunofluorescence and 32P postlabeling. Skin Pharmacol. 1990;3(3):171–179. doi: 10.1159/000210867. [DOI] [PubMed] [Google Scholar]



