Abstract
Chronic viral infections often result in ineffective CD8 T-cell responses due to functional exhaustion or physical deletion of virus-specific T cells. However, how persisting virus impacts various CD8 T-cell effector functions and influences other aspects of CD8 T-cell dynamics, such as immunodominance and tissue distribution, remains largely unknown. Using different strains of lymphocytic choriomeningitis virus (LCMV), we compared responses to the same CD8 T-cell epitopes during acute or chronic infection. Persistent infection led to a disruption of the normal immunodominance hierarchy of CD8 T-cell responses seen following acute infection and dramatically altered the tissue distribution of LCMV-specific CD8 T cells in lymphoid and nonlymphoid tissues. Most importantly, CD8 T-cell functional impairment occurred in a hierarchical fashion in chronically infected mice. Production of interleukin 2 and the ability to lyse target cells in vitro were the first functions compromised, followed by the ability to make tumor necrosis factor alpha, while gamma interferon production was most resistant to functional exhaustion. Antigen appeared to be the driving force for this loss of function, since a strong correlation existed between the viral load and the level of exhaustion. Further, epitopes presented at higher levels in vivo resulted in physical deletion, while those presented at lower levels induced functional exhaustion. A model is proposed in which antigen levels drive the hierarchical loss of different CD8 T-cell effector functions during chronic infection, leading to distinct stages of functional impairment and eventually to physical deletion of virus-specific T cells. These results have implications for the study of human chronic infections, where similar T-cell deletion and functional dysregulation has been observed.
Considerable evidence suggests that an effective CD8 T-cell response can control or eradicate viral infections. CD8 T cells appear to be particularly important in the immune response to chronic infections and in the long-term control of latent and reactivating viruses (2, 26). Indeed, potent human immunodeficiency virus (HIV)-specific CD8 T-cell responses correlate with acute viral control and long-term nonprogression (16, 42, 55, 56, 61, 66). Further, rhesus macaques infected with simian immunodeficiency virus (SIV) show significantly higher viral loads if CD8 T cells are eliminated by antibody-mediated depletion (38). Evidence also suggests that CD8 T cells are important for the acute phase as well as long-term control of other persistent viruses, such as Epstein-Barr virus (EBV), cytomegalovirus, hepatitis B virus (HBV), and hepatitis C virus (HCV) (18, 24, 63, 83). However, in many patients CD8 T cells fail to contain viral replication, particularly during HIV, HBV, and HCV infections. Recent studies suggest that functional impairment (exhaustion) and/or physical deletion of CD8 T cells can accompany ineffective viral control (44, 85). However, the impact of chronic viral infection on the induction and maintenance of epitope-specific CD8 T-cell responses and the impact of persisting antigen on distinct T-cell effector functions are not well understood.
Infection of mice with lymphocytic choriomeningitis virus (LCMV)4 offers an excellent model with which to investigate the effect of chronic infection on CD8 T-cell function. CD8 T cells are critical for the control of LCMV infection, and the adoptive transfer of memory CD8 T cells into persistently infected mice can lead to viral elimination (37). In addition, different LCMV strains result in either an acute or a chronic infection, allowing detailed comparison between effective responses when virus is eliminated and ineffective responses when virus persists (4). The Armstrong strain of LCMV is cleared by 8 days postinfection (p.i.), while LCMV Cl-13 infection results in viremia that can last up to 3 months, with virus persisting in some tissues indefinitely. Importantly, LCMV Armstrong and LCMV Cl-13 differ by only two amino acids, preserving all known T-cell epitopes, allowing a direct comparison between T-cell responses to dominant and subdominant epitopes (49, 50).
It has long been known that the CD8 T-cell response, as assessed by in vitro 51Cr release assays, is suppressed during persistent LCMV infections (3, 4, 15, 40, 50). It has recently been demonstrated that CD8 T cells specific for the NP396 epitope, which is dominant following acute LCMV infection, are physically eliminated during chronic infection (58, 89, 90). In contrast, CD8 T cells specific for the GP33 epitope, another dominant epitope, remain detectable by major histocompatibility complex (MHC) tetramer staining throughout chronic infection, but these cells lose the ability to make gamma interferon (IFN-γ) and have been termed functionally exhausted (89). However, the reason one response is physically eliminated while another is present but nonfunctional and whether these phenotypes are common features of chronic infections remain unknown. Further, the extent to which other effector functions are impaired and their relative sensitivity to inactivation remain to be determined.
In the present study we have examined the impact of chronic infection on CD8 T-cell responses by comparing responses to the same epitopes following acute infection or during chronic infection. Our results revealed several important aspects of CD8 T-cell responses during chronic infection. First, a dramatically altered immunodominance hierarchy, including the deletion of several dominant specificities and a heightened frequency of some subdominant populations, occurred during chronic LCMV infection. Second, chronic infection resulted in substantial redistribution of virus-specific CD8 T cells to the bone marrow and nonlymphoid tissues, such as the liver. Nearly 30% of the CD8 T cells isolated from the liver (or ∼4 × 105 cells) were specific for one LCMV epitope, compared to ∼3.5% (or ∼2 × 105 cells) from the spleen. Third, LCMV-specific CD8 T-cell effector functions were impaired during chronic infection in a hierarchical manner. An evaluation of multiple functional properties demonstrated a clear hierarchy in this loss of function, since in vitro lytic capacity and interleukin 2 (IL-2) production were lost early during infection, tumor necrosis factor alpha (TNF-α) synthesis was lost soon thereafter, and IFN-γ production was most resistant to exhaustion. Last, antigen load was a critical factor in both the loss of effector function and epitope-specific deletion. In fact, a high level of persisting epitope drove deletion while lower levels led to functional exhaustion. The hierarchical loss of function suggests a model of progressive loss of different effector functions with increasing viral load and/or time of infection.
MATERIALS AND METHODS
Mice and virus.
Four- to six-week-old female C57Bl/6 mice were purchased from Jackson Laboratory (Bar Harbor, Maine). Mice received 2 × 105 PFU of Armstrong intraperitoneally to initiate acute infection or 2 × 106 PFU of Cl-13 intravenously to initiate chronic infection (89). It should be noted that there is no evidence for either the Armstrong or Cl-13 strains of LCMV infecting CD8 T cells directly (1, 40). Titers of virus were determined by plaque assay on Vero cells as previously described (4).
Flow cytometry and tetramer staining.
MHC class I tetramers of H-2Db or H-2Kb complexed with LCMV NP396-404, GP33-41, GP34-42, or GP276-286 were produced as previously described (53). Biotinylated complexes were tetramerized using allophycocyanin-conjugated streptavidin (Molecular Probes, Eugene, Ore.). All antibodies were purchased from BD/Pharmingen (San Diego, Calif.). Splenocytes from LCMV-infected mice were stained as previously described (53) and acquired using a FACSCalibur flow cytometer (Becton Dickinson). Data were analyzed using CELLQuest software (Becton Dickinson).
Intracellular cytokine staining.
For intracellular cytokine analysis, 106 splenocytes were cultured in the absence or presence of the indicated peptide (0.2 μg/ml) and brefeldin A for 5 to 6 h at 37°C. Following staining for surface antigens as described above, cells were stained for intracellular cytokines using the Cytofix/Cytoperm kit (BD/Pharmingen) according to the manufacturer's instructions. Antibodies used for intracellular cytokine detection were anti-IFN-γ (clone XMG1.2), anti-IL-2 (clone JES6-5H4), and anti-TNF-α (clone MP6-XT22) and an isotype control (clone A95-1) and were purchased from Pharmingen.
Isolation of lymphocytes from nonlymphoid tissues.
To isolate intrahepatic lymphocytes, livers were first perfused by cutting the hepatic vein and injecting 5 ml of ice-cold phosphate-buffered saline injected directly into the hepatic artery. The gall bladder was removed, and the entire liver was excised and homogenized. Following incubation in 0.25 mg of collagenase B (Boehringer Mannheim)/ml and 1 U of DNase (Sigma, St. Louis, Mo.)/ml at 37°C for 45 min, the digested liver was centrifuged and the pellet was resuspended in 5 to 10 ml of 44% Percoll (Sigma). This suspension was underlaid with 56% Percoll and spun at 850 × g for 20 min at 20°C, the intrahepatic lymphocyte population was harvested from the interface, and red blood cells were lysed using 0.83% ammonium chloride. Lymphocytes were isolated from the lungs similarly. This procedure was found to have little impact on the expression of most cell surface molecules (data not shown). In addition, splenocytes isolated in the same manner as liver lymphocytes showed functional properties similar to those of splenocytes isolated by normal procedures (data not shown). Lymphocytes were isolated from the brain essentially as described previously (13). Briefly, brains were removed, rinsed once in ice-cold RPMI, and dissociated using an ice-cold Tenbroeck homogenizer. Homogenate was adjusted to 30% Percoll and underlaid with 1 to 2 ml 70% Percoll. The Percoll gradient was centrifuged at 800 × g for 20 min at 20°C. Mononuclear cells were recovered from the interface and washed once in RPMI, red blood cells were lysed using 0.83% ammonium chloride, and the cells were washed and counted. The number of total bone marrow cells was determined as previously described by multiplying the number of cells recovered from two femurs by 7.9 (70).
51Chromium release assay.
51Chromium release assays were performed using LCMV-infected or peptide-pulsed MC57 fibroblasts as targets as previously described (53) with the following modifications. Peptide-pulsed targets were labeled with 0.2 μg of the indicated peptide/ml for 60 to 75 min during 51Cr labeling. Effector populations were stained with MHC tetramer to determine the frequency of epitope-specific CD8 T cells. Based on this frequency, the starting effector-to-target cell ratio was adjusted to obtain identical ratios of tetramer-positive CD8 T cells to target cells for all effector populations. In addition, the total number of cells/well was kept constant by the addition of naïve C57Bl/6 splenocytes.
Analysis of T-cell proliferation by CFSE.
Splenocyte populations from LCMV Cl-13-infected mice to be used as antigen-presenting cells (APC) were depleted of CD8 T cells using magnetic-activated cell sorter (MACS) beads and magnetic column purification (Miltenyi Biotech, Gladbach, Germany). Responder CD8 T-cell populations from LCMV Armstrong-immune mice (>30 days p.i.) were purified using MACS columns and labeled with carboxyl fluorescein succinimidyl ester (CFSE) (Molecular Probes) as described previously (54). APC and responders were mixed at a 1:1 ratio and incubated for 60 h, and cultures were stained using MHC class I tetramers and anti-CD8 as described above. Proliferation was determined by dilution of the CFSE dye.
RESULTS
Dynamics of viral control during acute or chronic LCMV infection.
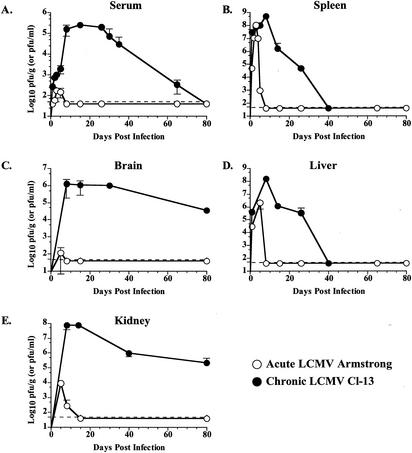
LCMV causes either an acute infection or a chronic infection depending on the strain of virus; LCMV Armstrong is cleared rapidly, while its variant Cl-13 causes a chronic infection (4). Figure 1 summarizes the major differences in viral load following infection with LCMV Armstrong or Cl-13. Initial viral loads in the spleens of Armstrong-infected mice and Cl-13-infected mice were similar during the first 3 to 4 days (Fig. 1B). However, while Armstrong was rapidly cleared from all tissues by day 8 p.i., Cl-13 persisted (Fig. 1B to E). Following Cl-13 infection, virus was observed in the spleen and liver for more than 1 month (Fig. 1B and D), viremia persisted for somewhat longer as serum virus was detectable for 75 to 90 days (Fig. 1A), and high levels of virus remained in the brain and kidneys for more than 3 months (Fig. 1C and E). Since Armstrong and Cl-13 share all known T-cell epitopes (49, 50), these two strains of LCMV were used to investigate the impact of chronic infection on the dynamics and function of virus-specific CD8 T cells.
FIG. 1.
Viral kinetics during acute and chronic LCMV infection. Panels A to E show the titer of infectious virus in the indicated tissues at various times p.i. with either LCMV Armstrong (open symbols) or LCMV Cl-13 (filled symbols). (A) Titers in serum, expressed in PFU per milliliter. (B and C) Titers in the spleen, brain, liver, and kidney, respectively, expressed as PFU per gram of tissue. Data are the average for two to eight mice/time point.
Chronic infection skews immunodominance.
An advantage of studying T-cell responses to LCMV is that multiple dominant and subdominant CD8 T-cell responses have been identified following LCMV infection (53, 79, 80). Specifically, the H-2Db-restricted NP396-404 (Db/NP396), H-2Db-restricted GP33-41 (Db/GP33), and H-2Kb-restricted GP34-43 (Kb/GP34) epitopes are dominant, while the response to H-2Db-restricted GP276-286 (Db/GP276) is subdominant (53, 79, 80). We used MHC-peptide tetramers to track CD8 T-cell responses to these four epitopes over time following acute LCMV infection or during chronic LCMV infection. Infection with either strain of LCMV results in the generation of potent responses to all four epitopes (Fig. 2A). Following resolution of acute infection, the hierarchy of the epitope-specific memory CD8 T-cell populations is maintained in the following order (from most to least dominant): Db/NP396, Db/GP33, Kb/GP34, and Db/GP276 (Fig. 2B). This immunodominance hierarchy is a reproducible characteristic of LCMV Armstrong-immune mice (53, 79, 80). In contrast to the stable hierarchy maintained following acute infection, chronic LCMV infection resulted in an altered immunodominance profile. Although responses to all epitopes were initially detected, the normally dominant Db/NP396 and Kb/GP34 responses were somewhat diminished as early as day 8 p.i. and became essentially undetectable above background levels by day 25 (Fig. 2A and B). This deletion constituted an ∼80 to 90% reduction in cell numbers from those for LCMV Armstrong-immune mice. In contrast, the Db/GP33 and Db/GP276 responses remained detectable, and in fact the normally subdominant Db/GP276 response became the dominant population in chronically infected mice (Fig. 2B). Thus, chronic infection can severely perturb the immunodominance pattern of virus-specific CD8 T-cell responses. Responses to some epitopes, Db/GP276, were dramatically increased, while responses to others, Db/NP396 and Kb/GP34, were deleted.
FIG. 2.
Chronic LCMV infection alters CD8 T-cell immunodominance. (A) Responses to four LCMV T-cell epitopes were determined using MHC tetramer staining. The number of CD8 T cells/spleen specific for the Db/NP396, Db/GP33, Kb/GP34, or Db/GP276 epitope is plotted over time following infection with LCMV Armstrong (open symbols) or LCMV Cl-13 (closed symbols). Data are the average for two to eight mice/time point. The dotted line indicates the level of detection based on tetramer staining of splenocytes from naive mice. (B) The percent of CD8 T cells staining positive for each of the four MHC class I tetramers is shown for a representative LCMV Armstrong-immune (around day 400 p.i.) mouse (top) and a representative LCMV Cl-13-infected (day 60 p.i.) mouse (bottom). Fluorescence-activated cell sorter plots are gated on CD8+ T cells, and the number indicates the percent of CD8 T cells staining with the indicated tetramer. It should be noted that the Db/GP33 and Kb/GP34 tetramers stain distinct nonoverlapping CD8 T-cell populations (data not shown).
Localization of LCMV-specific T cells in lymphoid and nonlymphoid tissues during chronic infection.
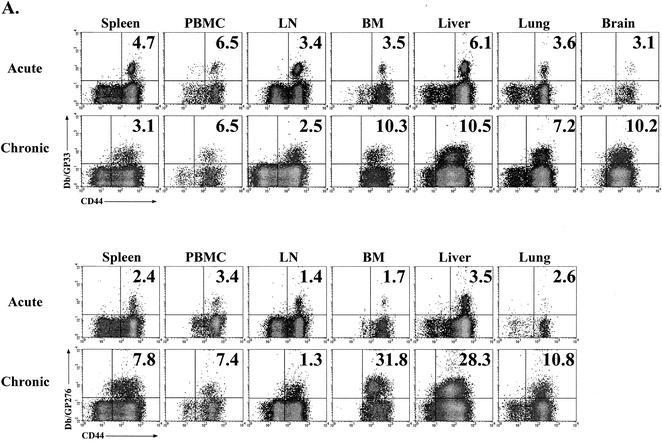
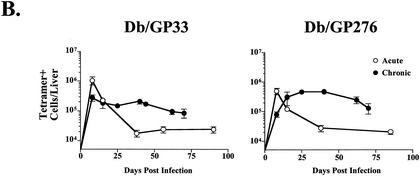
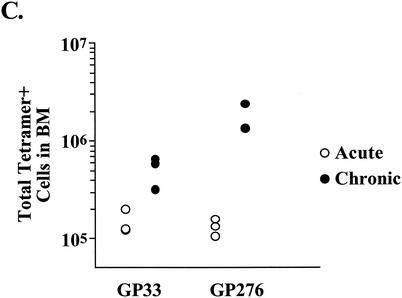
Several recent reports have demonstrated the presence of antigen-specific memory T cells in nonlymphoid tissues (46, 47, 62). To determine whether chronic LCMV infection influenced the localization of virus-specific CD8 T cells, lymphocytes were isolated from four lymphoid compartments and three nonlymphoid locations 25 days after infection with LCMV Cl-13 and analyzed by MHC tetramer staining (Fig. 3A). Consistent with what was observed in spleen tissue, Db/NP396- and Kb/GP34-specific CD8 T cells were not detected in other sites (data not shown). The frequencies of Db/GP33-specific CD8 T cells in the spleen, peripheral blood mononuclear cells (PBMC), and lymph nodes (LN) of LCMV Cl-13-infected mice were similar to those observed with Armstrong-immune mice, while the frequency of Db/GP276-specific cells was slightly increased in these locations (Fig. 3A), consistent with the immunodominance data presented in Fig. 2. Interestingly, the frequency of both Db/GP33- and Db/GP276-specific CD8 T cells was dramatically elevated in the bone marrow as well as nonlymphoid sites including the liver, lungs, and brain during Cl-13 infection (Fig. 3A). Up to 30% of the CD8 T cells in the bone marrow or liver of a chronically infected animal were specific for Db/GP276, compared to ∼1 to 4% in these locations for LCMV Armstrong-immune mice. To determine whether this increased frequency also corresponded to a larger absolute number of cells at nonlymphoid sites, the liver was chosen for more detailed longitudinal examination. Following acute infection with LCMV, the number of Db/GP33- or Db/GP276-specific cells in the liver stabilized at ∼1 × 104 to 3 × 104 cells/liver, approximately 1/10 the number found in the spleen by day 30 p.i. (Fig. 3B; compare to Fig. 2A). In striking contrast, during chronic infection the number of Db/GP33- or Db/GP276-specific CD8 T cells in the liver reached 1 × 105 to 4.5 × 105 cells/organ, approximately 10 to 20 times the number found in immune mice at similar time points (Fig. 3B). Larger numbers of Db/GP33- or Db/GP276-specific CD8 T cells were often found in the liver than in the spleens of chronically infected mice, particularly at ∼1 month p.i. (∼2 × 105 versus 1 × 105 for Db/GP33 and ∼4.5 × 105 versus 2 × 105 for Db/GP276 for liver versus spleen, respectively) (Fig. 3B and 2A). To determine whether LCMV-specific CD8 T-cell numbers were also increased in the bone marrow, another site of unexpectedly high tetramer frequencies, absolute numbers of Db/GP33- and Db/GP276-specific CD8 T cells were determined at 2 months p.i. with LCMV Armstrong or Cl-13. Following Armstrong infection, the number of Db/GP33- or Db/GP276-specific CD8 T cells in the bone marrow stabilized at ∼1.2 × 105 to 1.6 × 105 cells (Fig. 3C). During Cl-13 infection, roughly 4.7 × 105 Db/GP33-specific and 1.3 × 106 Db/GP276-specific CD8 T cells were isolated from the bone marrow, an increase of ∼3-fold and nearly 10-fold, respectively, compared to results with the LCMV Armstrong-immune mice (Fig. 3C). Thus, during chronic LCMV infection the tissue distribution of virus-specific CD8 T cells became significantly altered from that observed for a normal memory T-cell population. Two points should be noted. First, the frequency of antigen-specific cells in the blood did not reflect the dramatic skewing of specific CD8 T cells to locations such as the bone marrow, liver, lungs, and brain. Second, while it is likely that antigen plays a role in this redistribution of LCMV-specific CD8 T cells, other factors may also be involved, since the kinetics of viral control for the spleen and that for the liver were similar, but the frequencies and numbers of Db/GP33- and Db/GP276-specific cells in these locations were dramatically different.
FIG. 3.
Chronic LCMV infection substantially alters the tissue distribution of virus-specific CD8 T cells. (A) Lymphocytes were isolated from four lymphoid tissues (spleen, PBMC, LN, and bone marrow [BM]) and three nonlymphoid tissues (liver, lung, and brain) of LCMV Armstrong-immune mice (around day 400 p.i.; similar results were observed at numerous time points between days 30 and 400; data not shown) or LCMV Cl-13-chronically infected mice (day 25 p.i.; similar results were observed at days 30, 60 and 70 p.i.; data not shown) and stained with MHC tetramers of Db/GP33 (upper two rows) and Db/GP276 (lower two rows). All plots are gated on CD8+ cells, and the numbers indicate the percentages of CD8 T cells staining with the indicated tetramers. (B) The number of Db/GP33-positive (left panel) or Db/GP276-positive (right panel) CD8 T cells in the liver was determined over time by MHC tetramer staining following acute LCMV Armstrong infection (open symbols) or during chronic LCMV Cl-13 infection (filled symbols). (C) The number of Db/GP33- and Db/GP276-specific CD8 T cells in the bone marrow was determined at day 60 p.i. for either LCMV Armstrong or Cl-13. The data indicate the number of cells in the total bone marrow calculated as described in Materials and Methods. A similar trend was observed at day 30 p.i. (D) CD44 expression on Db/GP33 (left panel) or Db/GP276 (right panel) tetramer-positive cells from LCMV Armstrong-immune (around day 400 p.i.) mice (gray histogram) or LCMV Cl-13-infected (day 25 p.i.) mice (open histogram). Histograms are gated on CD8+/tetramer-positive cells from the spleen. Similar results were observed for all tissues examined (data not shown and see panel A).
Interestingly, LCMV-specific CD8 T cells found in all locations during chronic infection displayed markedly lower levels of CD44 expression (Fig. 3D). This is illustrated for the spleen in Fig. 3D, but similar two- to threefold decreases in CD44 mean fluorescence intensity were observed in all tissues (Fig. 3A and data not shown).
A spectrum of T-cell functional impairment during chronic infection.
In addition to producing IFN-γ upon encountering an antigen, virus-specific CD8 T cells can also produce TNF-α and IL-2. Following acute infection, all CD8 T cells specific for NP396, GP33, or GP276 were capable of producing IFN-γ at days 8, 15 and 30 p.i. (Fig. 4A). At day 8, 30 to 70% of these epitope-specific populations produced TNF-α and ∼5% of the effectors made IL-2 (Fig. 4A). Once the infection had been resolved and these populations had differentiated into memory cells, between 75 and 95% of the tetramer-positive populations synthesized TNF-α and 10 to 25% made IL-2, consistent with previous results showing greater TNF-α production by memory T cells (72). We wanted to evaluate how chronic infection impacted the ability of virus-specific CD8 T cells to produce these three cytokines. Initially, at day 8 p.i., most tetramer-positive CD8 T cells from chronically infected mice made IFN-γ upon peptide stimulation (Fig. 4A). However, this capacity was progressively lost, and by day 15 for NP396 and day 30 for GP33 and GP276, ∼50% of the tetramer-positive cells were unable to produce IFN-γ upon stimulation. In contrast to this delayed loss of IFN-γ production, the ability to produce TNF-α was ablated early, observed only for a minority of the GP33 and GP276 cells at day 8, and nearly undetectable by day 15. Similarly, the ability to make IL-2 was strikingly suppressed, essentially absent even at early time points. This progressive loss of different effector functions falls into three patterns of functional exhaustion, illustrated in Fig. 4B. An example of functional cytokine responses by memory CD8 T cells is shown in the top row for comparison. For this Armstrong-immune mouse, 2.3% of the CD8 T cells were specific for Db/GP33 by tetramer staining. One hundred percent of these produced IFN-γ upon peptide stimulation. Ninety-four percent of the tetramer-positive cells also made TNF-α, while ∼1/4 produced IL-2. The first stage of exhaustion during chronic infection, “partial exhaustion I,” is characterized by tetramer-positive cells that were capable of producing IFN-γ upon peptide stimulation (Fig. 4B, row 2). However, the level of production of this cytokine appeared to be diminished, since the mean fluorescence intensity of IFN-γ staining was substantially reduced (551, top row, versus 95, second row). Also, less than 10% of this population produced TNF-α, and virtually none could make IL-2. This can occur as early as day 8 during LCMV Cl-13 infection. The second state of dysfunction, “partial exhaustion II,” is characterized by IFN-γ production by only a subset of tetramer-positive CD8 T cells (Fig. 4B, row 3). Only 45% of the Db/GP33 tetramer-positive cells were IFN-γ+ following stimulation, and essentially none could make TNF-α or IL-2. Last, in some cases a complete loss of all cytokine production or “full exhaustion” was observed (Fig. 4B, bottom row). In this example, only 6% of the tetramer-positive population could make IFN-γ, and both TNF-α- and IL-2-positive cells were undetectable. This complete loss of function is most often observed in the absence of CD4 T-cell help (89) but also can arise during Cl-13 infection of normal mice when viral load is high (see data below). Together, these results are consistent with a hierarchical loss of the ability to produce different cytokines during chronic infections. Production of TNF-α and IL-2 appeared to be silenced early during infection even when IFN-γ production was relatively intact. Eventually, however, the ability to synthesize IFN-γ was also compromised.
FIG. 4.
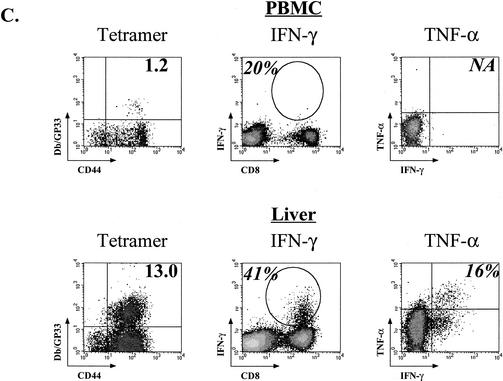
Progressive loss of cytokine production during chronic LCMV Cl-13 infection. (A) The fraction of tetramer-positive cells able to produce IFN-γ (top row) was determined for the NP396-, GP33-, and GP276-specific populations at the indicated times p.i. with LCMV Cl-13 (Chronic) or Armstrong (Acute). The percentage of the tetramer-positive population able to produce TNF-α (middle row) or IL-2 (bottom row) was determined using three-color intracellular cytokine staining. Asterisks indicate too few cells available for analysis as a result of deletion. Open bars indicate LCMV Armstrong infection, while the black bars indicate LCMV Cl-13 infection. The data represent the average and standard deviation for three to six mice/time point. (B) An example of fully functional GP33-specific memory CD8 T cells from an LCMV Armstrong-immune mouse (top row) and three examples of different states of functional exhaustion of GP33-specific T cells during LCMV Cl-13 infection are shown. The percentage in the left column indicates the percentage of CD8 T cells that is Db/GP33 tetramer-positive. The percentage in the second, third, or fourth column indicates the percentage of tetramer-positive cells that is IFN-γ+, TNF-α+, or IL-2+, respectively. Plots for tetramer and IFN-γ staining are gated on all lymphocytes. Plots for TNF-α and IL-2 are gated on CD8 T cells. Similar results were observed for the GP276 response (data not shown). (C) Lymphocytes from the PBMC (upper panels) or intrahepatic lymphocytes (lower panels) of LCMV Cl-13-infected mice (chronic; day 35 p.i. for PBMC, day 60 p.i. for liver) were examined for functional exhaustion. The left columns show the percent Db/GP33 tetramer-positive cells (numbers indicate percentages of CD8 T cells). Production of IFN-γ (gated on all lymphocytes) and TNF-α (gated on CD8 T cells) is shown in the middle and right columns, respectively. Numbers indicate the percentage of tetramer-positive cells making cytokine. No IL-2-producing cells were detected from CD8 T cells from chronically infected mice (data not shown). Similar results were observed at other time points and for GP276-specific responses (data not shown). Note that the GP33 peptide used for intracellular cytokine staining stimulates both the Db/GP33 and Kb/GP34 response. Therefore, the percent functional of tetramer-positive cells shown in panels A, B, and C was determined using both DbGP33 and KbGP34 tetramers. As shown in Fig. 2, the Kb/GP34 becomes negligible by 2 to 3 weeks p.i. Therefore, only the Db/GP33 tetramer stain is shown. No specific staining was observed when using isotype control antibodies.
The above functional analysis was performed using cells from the spleen. It was possible that LCMV-specific T cells found in distinct anatomical locations, such as those thought to contain effector memory T cells, differ functionally during chronic infection. We analyzed the functionality of virus-specific CD8 T cells from a nonlymphoid site by intracellular cytokine staining using cells isolated from the liver as well as the PBMC ∼40 days after infection with Cl-13 (Fig. 4C). Similar to what was observed for the spleen, the majority of cells that stained with Db/GP33 tetramers were unable to produce IFN-γ or TNF-α (or IL-2; data not shown) following peptide stimulation (Fig. 4C). Similar results were observed for GP276 (data not shown). Epitope-specific populations isolated from PBMC and liver of LCMV Armstrong-immune mice were fully capable of IFN-γ production, and ∼70% and ∼80% made TNF-α, respectively (data not shown). Thus, even antigen-specific CD8 T cells present in an effector-like environment, such as the liver, are functionally impaired during chronic infection with LCMV, suggesting that T cells in nonlymphoid tissues experience functional exhaustion in a manner similar to that for those found in the spleen.
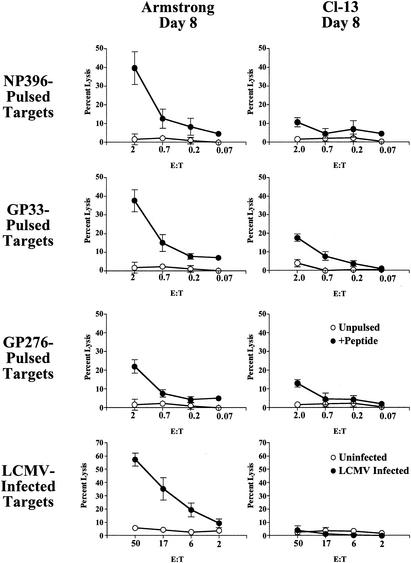
In addition to cytokine production, CD8 T cells are able to control viral infections through cell-mediated cytotoxicity. Previous studies have demonstrated that ex vivo cytotoxic T lymphocyte (CTL) activity was comparable for LCMV Armstrong and Cl-13 infections at day 5 (15), but this in vitro target cell lysis becomes substantially reduced by day 8 of Cl-13, but not Armstrong, infection (3, 4, 40, 50). In earlier work, however, it was unclear whether this loss of in vitro lytic activity was a result of decreased numbers of LCMV-specific CD8 T cells or qualitative differences in the cytotoxic potential of epitope-specific T-cell populations. To examine the impact of chronic infection on the cytotoxic activity of individual epitope-specific populations, ex vivo 51Cr release assays were performed using spleen populations from LCMV Armstrong-infected or LCMV Cl-13-infected mice that had been normalized to contain the same number of tetramer-positive cells. In this approach the lytic activities of individual NP396-, GP33-, and GP276-specific CD8 T-cell populations from LCMV Cl-13-infected mice were compared on a per-cell basis to those for the same tetramer-positive populations from LCMV Armstrong-infected mice. Eight days after either acute or chronic LCMV infection, splenocytes were isolated and tested for the ability to lyse either peptide-pulsed or virally infected targets. Effector CD8 T cells isolated 8 days after acute infection displayed strong in vitro cytolytic activity, detected using both sets of targets (Fig. 5). In contrast, little or no epitope- or virus-specific cytotoxicity was observed in vitro from splenocytes isolated 8 days after LCMV Cl-13 infection. In vitro cytotoxicity was undetectable from chronically infected mice at time points after day 8 (data not shown)(89). These results suggested that the normal antigen-specific cytotoxic activity of LCMV-specific CD8 T cells, at least as assessed by 51Cr release assays, was impaired as early as 8 days p.i., suggesting that cytotoxic function is highly sensitive to exhaustion.
FIG. 5.
In vitro cytotoxicity is impaired during chronic infection. Splenocytes isolated from LCMV Armstrong- or LCMV Cl-13-infected mice at day 8 p.i. were tested for cytotoxicity in a 5-h 51Cr release assay ex vivo. To accurately compare cytotoxicity from LCMV Armstrong- and LCMV Cl-13-infected mice, the effector/target cell (E:T) ratios were adjusted for the individual Db/NP396, Db/GP33 plus Kb/GP34, and Db/GP276 tetramer-positive populations in the first, second, and third rows, respectively. Since the GP33 peptide stimulates both the Db/GP33 and Kb/GP34 populations, the numbers of Db/GP33 and Kb/GP34 tetramer-positive cells were combined to obtain an accurate E:T ratio for GP33-pulsed targets. Targets were peptide-pulsed (top three rows) or LCMV-infected (bottom row) MC57 fibroblasts. E:T ratios for LCMV-infected targets indicate total splenocytes to targets (bottom row). Filled symbols represent peptide-coated or LCMV-infected targets, while open symbols are unpulsed, uninfected targets.
The influence of viral load on functional impairment.
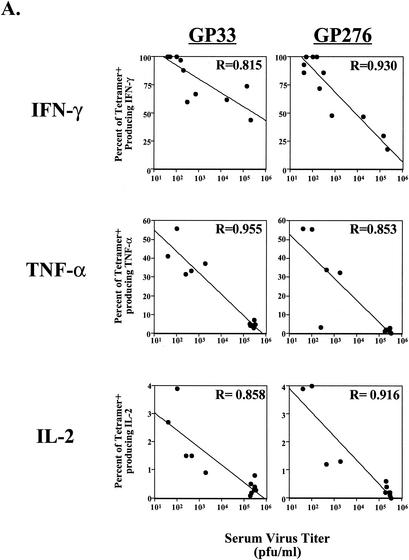
We next investigated the influence of antigen load on loss of CD8 T-cell effector function during chronic infection. As shown in Fig. 6A, there was a strong correlation between viral load and effector function. In the case of IFN-γ, loss of function became apparent when titers of virus in serum were above 103 PFU/ml. The loss of TNF-α and IL-2 also correlated strongly with viral load. Substantial functional impairment for these two cytokines was observed at lower viral loads (<103 PFU/ml) than for IFN-γ (Fig. 6A; note the difference in scale for cytokine-positive cells). As suggested by the progressive loss of different functions, the correlation between viral load and loss of TNF-α and IL-2 is readily apparent at day 15, but the correlation for loss of IFN-γ only becomes significant at later time points (results for days 30 to 60 are shown in Fig. 6A, top row). Since titers in serum generally reflect viral load in many tissues (48) (Fig. 1), these data imply that virus-specific T cells that continuously encounter antigen for weeks to months in vivo are those that are likely to become the most functionally impaired. Together with the data in Fig. 4 and 5 on the timing of loss of effector function, these data suggest that persistent T-cell stimulation by antigen was a driving force in the hierarchical loss of distinct CD8 T-cell functional properties.
FIG. 6.
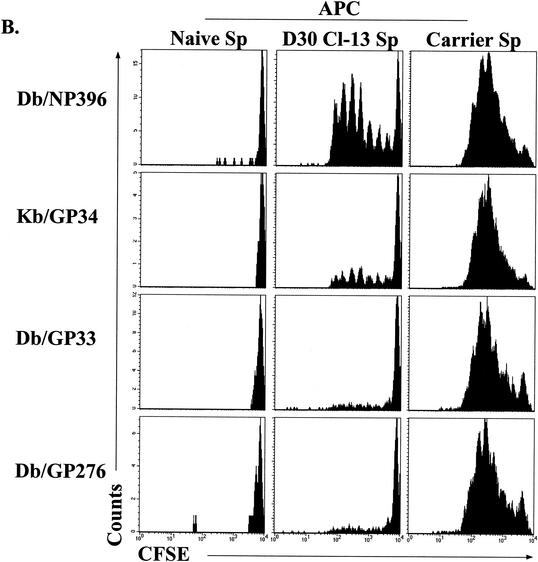
Correlation of antigen load to functional impairment of virus-specific CD8 T cells. (A) Virus in serum was quantitated by plaque assay, and the level of virus was plotted against cytokine responses. Top row, the percentage of tetramer-positive (tetramer+) cells producing IFN-γ in chronically infected mice (1 to 2 months p.i.) is plotted against viral load. Middle and bottom rows, the percentage of tetramer-positive cells that can synthesize TNF-α or IL-2 at day 15 after Cl-13 infection is plotted against the viral load. Lines indicate the linear regression best fit. (B) Splenocytes from uninfected mice (Naive Sp; left column), LCMV Cl-13-infected mice (day 30 p.i.; D30 Cl-13 Sp, middle column), or LCMV carrier mice (Carrier Sp, right column) were depleted of CD8 T cells and used as APC to stimulate CFSE-labeled, purified memory CD8 T cells from LCMV Armstrong-immune mice (>30 days p.i.). Proliferation was assessed after 60 h of coincubation by determining loss of CFSE fluorescence in the four tetramer-positive populations indicated on the left. Similar results were observed when APC were not depleted of CD8 T cells (data not shown).
Analysis of in vivo epitope presentation.
An important unresolved question is why some epitope-specific populations, such as the Db/NP396- and Kb/GP34-specific populations, undergo physical deletion while others become functionally exhausted during chronic infection. Since the overall viral load strongly influenced functional exhaustion, we hypothesized that antigen load at the level of specific epitopes may also influence the differential fate of distinct CD8 T-cell populations during chronic infection. Therefore, we developed an assay to examine the presentation of individual peptides during chronic LCMV infection. Splenocytes from LCMV Cl-13-infected mice (∼30 days p.i.) were used as APC. CFSE-labeled memory CD8 T cells from LCMV Armstrong-immune mice were used as a readout to probe for the presentation of different epitopes by the splenocytes from chronically infected mice by coculturing the CFSE-labeled T cells and APC. After 60 h of stimulation, cell division was assessed by staining cultures with MHC-peptide tetramers of Db/NP396, Kb/GP34, Db/GP33, or Db/GP276 and determining the dilution of CFSE fluorescence. Importantly, substantial division of Db/NP396+ and some division of Kb/GP34+ CD8 T cells was observed, while the division of the Db/GP33+ and Db/GP276+ CD8 T cells was minimal (Fig. 6B), indicating that the Db/NP396 and Kb/GP34 epitopes continue to be presented at higher levels in vivo than Db/GP33 and Db/GP276. Stimulation of responder CD8 T cells with splenocytes from uninfected mice resulted in no proliferation of any tetramer-positive population. In contrast, the addition of peptide caused substantial proliferation of the appropriate specificity, and peptide-induced proliferation was similar for all four epitope-specific populations (data not shown). As an additional control, splenocytes from LCMV carrier mice were used as APC. Infection of mice neonatally or in utero results in a carrier state in which mice are rendered tolerant to LCMV and high levels of systemic virus (>104 PFU/ml in serum) persist for life. Substantial, but similar, proliferation was observed for all four epitope-specific populations using LCMV carrier splenocytes as APC. This result demonstrates two points. First, all four CD8 T-cell populations are capable of undergoing similar levels of proliferation to peptide even when present in the same culture. Second, all four epitopes can be efficiently presented by splenocytes in vivo. This indicated that the substantially greater proliferation of Db/NP396- and Kb/GP34-specific CD8 T cells than that of Db/GP33- and Db/GP276-specific populations induced by Cl-13 spleen-derived APC was not a result of a general inability of splenocytes to present the Db/GP33 and Db/GP276 epitopes in vivo. These results indicate that during chronic LCMV Cl-13 infection, the level of stimulation available for Db/NP396- and Kb/GP34-specific populations was significantly greater than that for Db/GP33 and Db/GP276 populations. Careful examination reveals that proliferation of Db/GP33- and Db/GP276-specific cells using Cl-13 day 30 splenocytes as APC was detectable above background levels (naive APC). These results indicate that the level of individual peptides presented in vivo was an important determinant of both functional exhaustion and physical deletion and suggested that a high level of persistent epitope presentation may lead to deletion while lower-level stimulation may result in functional impairment.
DISCUSSION
To examine how chronic viral infection impacts CD8 T-cell responses, we have studied the dynamics of epitope-specific CD8 T-cell responses following acute LCMV infection or during chronic LCMV infection. This study demonstrated that several aspects of the normal CD8 T-cell response following acute infection become altered during chronic infection: (i) immunodominance was dramatically modified, (ii) tissue distribution was skewed, since substantially more LCMV-specific CD8 T cells were found in the bone marrow and nonlymphoid organs, such as the liver, (iii) functional responses were impaired in a hierarchical fashion, and (iv) antigen load was the driving force behind functional exhaustion and physical deletion, since higher viral loads correlated with increased exhaustion and high in vivo epitope presentation was associated with physical deletion.
Functional exhaustion during chronic infections has been reported for antigen-specific CD8 T cells during human infections with HIV (31, 36, 41, 44, 69), HBV (65), HCV (34), and human T-cell lymphotrophic virus (33), during malignant melanoma (43), in primate models of SIV infection (82, 86), and in other models of murine chronic infections (21, 30, 45) or persisting antigen (74). However, precisely what functions are impaired and the degree of impairment differ considerably in these reports. In addition, while highly valuable, the data from many human or monkey studies are often limited by the availability of PBMC for analysis and the inability to compare directly epitope-specific CD8 T-cell responses from a chronic infection to effective responses to the same epitopes generated following an acute infection.
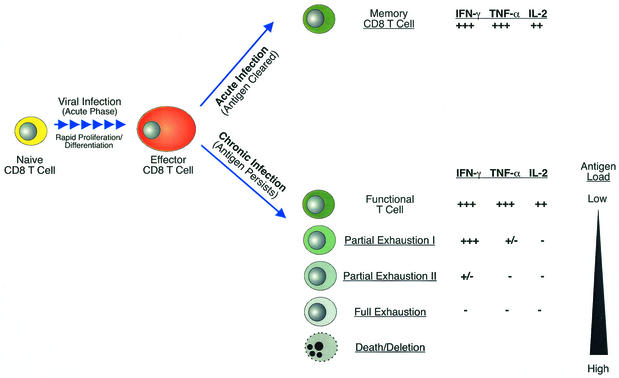
Our results suggest that CD8 T-cell functional exhaustion follows a pattern of progressive loss of function during chronic infection. Figure 7 depicts a model illustrating how multiple stages of CD8 T-cell exhaustion may arise during chronic infections. Virus-specific memory cells that persist in the absence of antigen following resolution of an acute infection (Fig. 7, top) are maintained as fully functional populations capable of immediate synthesis of IFN-γ, TNF-α, and IL-2 upon reencountering an antigen. As effectors during acute infection, these CD8 T cells are highly cytolytic. As memory T cells they can secrete cytokines, begin to proliferate, and become cytolytic effectors more rapidly than naive cells upon reexposure to antigen (9). In contrast, when virus persists, CD8 T cells with various levels of function may arise (Fig. 7, bottom). First, functional T-cell populations can coexist with persisting virus if the level of viral antigen expression is low or antigen encounter by T cells is infrequent, as may be the case during some latent infections or when antigen is anatomically separated from the immune system. Partial exhaustion I represents the stage in which a subset of effector functions is lost. The results presented here indicate that IL-2 and to a slightly lesser extent TNF-α were highly sensitive to negative regulation during chronic infection. These two cytokines failed to be produced even when IFN-γ production was only mildly affected. Cell-mediated cytotoxicity also appeared to be impaired at this stage, since day 8 effectors from LCMV Cl-13-infected mice were weakly cytolytic in in vitro 51Cr release assays compared to their counterparts during acute LCMV infection. Partial exhaustion II exists when functions more resistant to loss, such as IFN-γ production, begin to be blunted. At this point, some IFN-γ-producing T cells were detected but many cells were functionally inert, unable to synthesize IL-2, TNF-α, or IFN-γ. Next, full exhaustion is the complete loss of all effector functions. These cells were incapable of in vitro cytotoxicity or IL-2 and TNF-α production and have now also completely lost the ability to synthesize IFN-γ in response to peptide stimulation. In the LCMV system, exhaustion of essentially all effector functions is magnified if CD4 T-cell help is unavailable (89). Cells in this state of exhaustion are likely to encounter antigen nearly continuously in vivo, since they express early activation markers suggesting recent T-cell-receptor stimulation (43, 89). Finally, if the antigen load in the form of peptide-MHC complexes presented in vivo is high, epitope-specific CD8 T cells can be physically deleted. During chronic LCMV infection, this was the case for the Db/NP396- and Kb/GP34-specific population, since these cells were essentially undetectable by tetramer staining ∼3 weeks postinfection. Careful examination of the Db/NP396-specific cells at early time points p.i. revealed that functional exhaustion precedes deletion (data not shown) (89). We propose that antigen-specific CD8 T cells responding to a chronic infection may be fully functional, partially exhausted, fully exhausted, or physically deleted depending on the viral, or more precisely epitope, load and duration of infection. It should be noted that a given T-cell population may represent a mixture of these phenotypes. This may be especially true for PBMC where some cells may have recently encountered antigen (e.g., in the liver during HBV or HCV) whereas others may have transited from a relatively antigen-free environment. Two reports should be mentioned in the context of this model of hierarchical loss of effector functions. First, during SIV infection, the loss of the ability of virus-specific CD8 T cells to produce TNF-α and IL-2 correlated better with the severity of disease than did the loss of IFN-γ (51). A second study examining the function of HIV-specific CD8 T cells demonstrated that perforin expression, cytotoxicity, and TNF-α production were all impaired in cells that maintained the ability to produce IFN-γ (7). Both of these reports are consistent with the model of hierarchical loss of function proposed above, since in vitro cytotoxicity, IL-2, and TNF-α production were impaired while IFN-γ synthesis, the function most resistant to inactivation, remained detectable. In addition, it has recently been demonstrated that molecules involved in target cell lysis, such as granzyme B and perforin, are differentially expressed by human virus-specific CD8 T cells during distinct persisting infections (6), suggesting that cytotoxic activity may vary depending on the infection or antigen load. It will be interesting in the future to determine how the expression of these cytolytic molecules corresponds with the different states of functional exhaustion during chronic LCMV infection and to examine lytic activity in vivo.
FIG. 7.
Model for hierarchical loss of T-cell function during chronic viral infection. Memory T cells that persist in the absence of antigen following an acute infection are fully functional and capable of immediate synthesis of IFN-γ, TNF-α, and IL-2 upon antigen reencounter (top). Persisting virus results in CD8 T cells with various levels of function (bottom). Functional T cells can coexist with virus if antigen encounters are infrequent. Partial Exhaustion I represents the stage in which IL-2 and TNF-α are impaired even while CD8 T cells maintain the ability to produce IFN-γ. Partial Exhaustion II represents a stage where IFN-γ production also becomes impaired. At this point some functional cells can be detected by IFN-γ production, but many cells are exhausted. Full Exhaustion is the complete loss of all effector functions including IL-2, TNF-α, and IFN-γ production. Finally, deletion of epitope-specific CD8 T cells can result if epitope presentation to T cells is high and/or sustained. This hierarchical loss of function is dramatically influenced by antigen such that in the presence of low levels of virus, T cells maintain greater functional capacity, but as viral load increases, effector functions are progressively lost. Ultimately, a high antigen load leads to the physical deletion of specific T cells.
One of the most important factors regulating T-cell function is antigen. However, the impact of persisting antigen on T-cell function during chronic human infections remains controversial. While some studies examining the function of HIV- or SIV-specific CD8 T cells suggest that high viral loads can negatively impact cytokine production and/or cytotoxicity (27, 60), other reports have found no apparent correlation between plasma HIV viral load and T-cell function (32, 52). Using the LCMV model, our studies show a strong correlation between virus levels and loss of CD8 T-cell function during chronic infection. Further, this functional impairment is progressive; loss of the ability to produce IFN-γ was more severe at day 30 of LCMV Cl-13 infection than at day 15 even though viremia had changed only modestly. Importantly, persisting virus did not impact all T-cell populations equally during chronic LCMV infection. Epitopes that are displayed or persist at higher levels during Cl-13 infection, such as NP396 and GP34, appeared to drive clonal deletion, while those with lesser stimulatory capacity, including GP33 and GP276, resulted in functional exhaustion. How can this data be reconciled with the diverse reports from the human and primate systems? Several points are worth highlighting in this regard. The first is whether antigen persistence during a chronic infection is determined virologically or immunologically. This concept is illustrated by the disparity between persisting viremia and substantially different epitope-specific stimulation of CD8 T-cell populations during Cl-13 infection. Thus, while the level of viral persistence can be an indicator of antigen levels, the level of viral peptide perceived by an individual T-cell population is likely the most relevant parameter in causing immune dysfunction. Some epitope-specific populations may be rapidly driven to exhaustion and deletion, while others show little functional impairment in the presence of similar levels of virus. The second point is the location of viral replication in vivo. One reason LCMV is an excellent model for these studies is that it replicates efficiently in numerous organs throughout the mouse (3, 4, 49). Titers of virus in serum in this model are a reflection of viral infection in many tissues where viral peptides are available for T-cell stimulation. During other infections, the proportional relationship between serum virus levels and the number of epitope-bearing APC in vivo may differ dramatically. This may be influenced not only by the tropism of the virus (e.g., CD4 T cells, macrophages, or hepatocytes) and the anatomical location of viral replication but also by the amount of progeny virus produced/infected cell. Virus may coexist with functional CD8 T cells if the two are effectively ignorant of each other (e.g., viral epitopes are not available to CD8 T cells at high levels). This has been suggested as a potential mechanism for persistent HIV replication in LN in patients with HIV-specific CD8 T cells in the PBMC that lack LN homing molecules (22, 59). A third and important consideration, particularly with respect to infection with error-prone RNA viruses, is epitope mutation. Numerous reports have demonstrated that HIV, SIV, and HCV can escape immune control by mutating individual or multiple CTL epitopes (28, 84, 87). However, even a mixed quasispecies containing both wild-type and mutated epitopes could reduce T-cell stimulation, leading perhaps to a less severe loss of function. A last consideration is the diverse replication patterns of persisting viruses. This ranges from latent infections that undergo periodic reactivation (e.g., herpes simplex virus, varicella-zoster virus, and EBV) to “smoldering” chronic infections, such as cytomegalovirus, and finally to chronic infections with high viremia (e.g., HCV, HBV, HIV/AIDS, etc.). The first situation is likely to be essentially ignored by the immune system due to a lack of antigen synthesis and/or inefficient antigen presentation during latency. At the opposite end of the spectrum, continuous persistence of very high viral loads is likely to result in immune dysfunction. However, some latent viruses will provide effective T-cell boosts during periods of reactivation and maintain a large population of functional virus-specific T cells, as seen for EBV (18). This situation might also arise during highly active antiretroviral therapy or structured treatment interruptions of highly active antiretroviral therapy for HIV patients, since occasional viremic episodes can be associated with increased HIV-specific CD8 T-cell frequencies (57). The benefit of low-level viral persistence or periodic viral reactivation for maintaining elevated T-cell numbers, however, is likely to be determined by the level of epitope presentation in vivo and/or the frequency of T-cell encounter with antigen. Higher-level or more frequent T-cell stimulation can lead to exhaustion or deletion, while lower levels may result in occasional, useful boosts of virus-specific T-cell responses. However, even when the level of persistent viral replication is low, epitope escape mutants can arise and result in a loss of immune control (11).
CD4 help has been implicated as being critical for the maintenance of CD8 T-cell functions during chronic infections. In fact, during chronic LCMV infection, functional exhaustion is more extreme in the absence of CD4 T cells (48, 89). Loss of CD4 T cells often precedes or is associated with CD8 T-cell dysfunction and AIDS progression during HIV infection (5, 27), correlates with CD8 T-cell exhaustion during EBV-related non-Hodgkin's lymphoma (77), and leads to impaired long-term control of murine gamma herpesvirus infection (21). We would speculate that CD4 T-cell help may increase the threshold antigen level at which CD8 T-cell function is lost. This may occur via the release of cytokines, such as IL-2 (73), and/or the conditioning of APC (12, 64, 67). Conversely, virus-specific CD4 T cells could have antiviral effects, either directly or via help for antibody production, and in their absence, viral load may increase, leading to impaired CD8 T-cell responses.
One interesting observation during these studies was the striking change in immunodominance during chronic infection. Similar alterations have been reported during persistent infection of the central nervous system with mouse hepatitis virus (13) and in macaques infected with SIV (27). Chronic LCMV infection of BALB/c mice resulted in a relative increase in subdominant responses and a decrease in the dominant CTL population (81), but the reason for this change in hierarchy was not clear. Our results suggest that one contributing factor to this altered immunodominance is the deletion of some specificities experiencing the strongest stimulus. The known LCMV CD8 T-cell epitopes in B6 mice can be divided into those derived from nucleoprotein (NP) (NP396, NP205) and those derived from glycoprotein (GP) (GP33, GP34, GP276, GP118) (79). The epitopes derived from NP (NP396 [this study] and NP205 [data not shown]) decrease or are deleted during chronic infection, while three of the four GP-specific responses remain stable or are substantially increased (GP33 and GP276 [this study] and GP118 [data not shown]). Interestingly, NP is present at higher levels in infected cells than is GP (19). Also, recent studies have demonstrated that the generation of an LCMV NP-derived H-2Ld-restricted CD8 T-cell epitope is dependent on neosynthesis of protein (39), suggesting that defective ribosomal products (88) are the major source of this epitope. A high level of NP translation compared to that of GP is one parameter that could lead to the more frequent deletion of NP-specific responses. The binding capacities of NP396 and GP34 for MHC class I are high, with 50% inhibitory concentrations (IC50s) of 4.4 and 22 nM, respectively (61a, 79). The higher IC50s (less binding) for GP33 (775 nM), GP276 (52 nM), and GP118 (371 nM) (79) suggest that binding affinity for MHC class I might also be a critical factor that influences functional exhaustion versus deletion. However, the IC50 for NP205 is 170 nM, indicating that the correlation between MHC class I binding affinity and functional exhaustion and deletion is also not absolute. Since neither the protein source nor the MHC class I binding capacity correlates perfectly with deletion versus functional exhaustion, it is likely that changes in immunodominance during chronic infection are multifactorial, similar to what has been reported following acute influenza infection (23). The binding affinity of peptides for MHC class I molecules, as well as their dissociation rate, could contribute significantly to the overall level of T-cell stimulation and immunodominance (78). Perhaps differential antigen processing or APC usage could also alter the dominance of different epitopes, since both of these factors, particularly the presence of professional APC (15), may change during a chronic infection. Interestingly, both partial proteasome inhibition and APC type have been shown to differentially impact GP276 presentation in vitro (20, 68). Future experiments will be necessary to dissect these issues and elucidate the mechanisms that lead to altered immunodominance during chronic viral infections.
Several recent studies have demonstrated that memory T cells can be found in peripheral tissues (46, 47, 62). It was therefore not surprising to find LCMV-specific memory CD8 T cells in several tissues examined following acute infection. During chronic LCMV infection, however, virus-specific CD8 T cells massively accumulated in nonlymphoid organs as well as the bone marrow. In fact, LCMV-specific CD8 T-cell numbers in the livers of chronically infected mice often exceeded the numbers detected in the spleens. Importantly, the functional impairment of LCMV-specific CD8 T cells isolated from different locations was similar to that observed for the spleens of the same mice. This skewed localization of LCMV-specific CD8 T cells is consistent with the results of He et al., who reported that HCV-specific CD8 T cells could be isolated from liver biopsies of chronic HCV patients at ∼20-fold higher frequencies than from the PBMC of the same individuals (35). Antigen is likely to play a role in this altered localization, but because the kinetics of LCMV clearance was similar for the spleen and liver during Cl-13 infection, other signals may also contribute to nonlymphoid localization. Analysis of LCMV-specific CD8 T cells from multiple tissues suggests that sampling of one compartment during chronic infections may not predict the number of antigen-specific T cells present in other locations.
LCMV-specific CD8 T cells isolated from all locations during chronic infection exhibited a significantly lower level of CD44 expression than cells from LCMV Armstrong-immune mice. A similar decrease in CD44 expression on antigen-specific CD8 T cells was also recently reported following postexposure vaccination during murine gamma herpesvirus infection (45). Expression of CD44 is upregulated upon antigen encounter and remains high on memory T cells. CD44 interacts with extracellular matrix components including hyaluronate and may play a role in lymphocyte migration (14). Further, CD44 can bind soluble factors, such as osteopontin (8, 14), and since its intracellular domains can associate with signaling molecules, it may transduce signals about the extracellular environment or act as a costimulatory molecule (14). It will be interesting to determine whether the lower level of CD44 expression on CD8 T cells from chronically infected mice has functional consequences.
Why do functional responses disappear during chronic viral infections? LCMV is a noncytolytic virus, and tissue damage is a result of immunopathology (17, 19). Therefore, functional inactivation in the presence of a high antigen load may represent a mechanism of peripheral tolerance. The hierarchical pattern of exhaustion during LCMV infection suggests that the functions silenced first, including cytolysis and TNF-α production, are most dangerous to the host. Unregulated cell-mediated cytotoxicity in the presence of excess antigen might quickly lead to severe tissue damage. TNF-α overproduction can also have drastic consequences if not controlled. TNF-α contributes to septic shock, fever, and cachexia, can induce apoptosis, and can influence vasoregulation and vascular integrity (25, 29). In contrast, the immunopathological consequences of prolonged or overproduction of IFN-γ may be less severe. Interestingly, it has recently been shown that while IFN-γ production continued for up to 24 h during continuous contact with antigen, TNF-α synthesis was shut off after only 4 h (10, 71), consistent with the dangers of overproduction of this cytokine. The high sensitivity of CD8 T-cell-produced IL-2 to exhaustion is intriguing. IL-2 may play a role in driving the expansion of antigen-specific CD8 T-cell responses (75), and its silencing may prevent an overexpansion of virus-specific CD8 T cells. Alternatively, IL-2 has been shown to sustain CD8 IFN-γ production (73). Interestingly, culture of CD8 T cells from SIV-infected macaques in IL-2 alone restored IFN-γ functional responsiveness (86). Thus, the autonomous production of IL-2 by virus-specific CD8 T cells may facilitate the prolonged ability to perform effector functions. However, the presence of IL-2 during peptide stimulation or pretreating cells with IL-2 for 24 h did not alter the IFN-γ or TNF-α production by LCMV-specific CD8 T cells (data not shown). Overall, the differential loss of effector functions may reflect a balance between minimizing immunopathology and maintaining some level of antiviral capability. If taken to the extreme, as with CD4-deficient, chronically LCMV-infected mice where viremia is persistently high, a state of permanent tolerance may result. From a mechanistic point of view, disruption of signal transduction pathways and/or transcriptional-translational regulation may play a role in preventing CD8 T-cell function in the presence of viral antigens. Evidence suggests that proximal signaling events may be impaired in nonfunctional HIV-specific CD8 T cells, since CD3ζ is downregulated (76). Events further downstream may also be compromised or silenced in exhausted T cells, since phorbol myristate acetate-ionomycin stimulation of functionally impaired LCMV-specific (89) or melanoma-specific (43) CD8 T cells does not fully restore cytokine production.
T-cell exhaustion is likely to be a common feature of the immune response during chronic infections. The molecular basis of functional inactivation remains unknown but is likely to provide important insights into both the normal regulation of T-cell function and potential targets for immunotherapy. A key question in this regard is whether T cells that are present in an inactivated state can recover functional properties. If so, functionally exhausted T cells may provide useful targets for specific intervention to augment antiviral responses. Future studies are under way to examine the molecular nature of T-cell exhaustion and the ability to reverse this phenotype.
Acknowledgments
We thank D. Barber, S. Crotty, S. Kaech, and D. Masopust for helpful discussions and for critically reading the manuscript and P. Yeiser, K. Madhavi-Krishna, and S. Jenkins for their technical assistance.
This work was supported by National Institutes of Health grant AI30048 (to R.A.) and The Cancer Research Institute postdoctoral fellowship (to E.J.W.).
REFERENCES
- 1.Ahmed, R., C. C. King, and M. B. Oldstone. 1987. Virus-lymphocyte interaction: T cells of the helper subset are infected with lymphocytic choriomeningitis virus during persistent infection in vivo. J. Virol. 61:1571-1576. [DOI] [PMC free article] [PubMed]
- 2.Ahmed, R., L. A. Morrison, and D. M. Knipe. 1996. Viral persistence, p. 181-206. In N. Nathanson (ed.), Viral pathogenesis. Lippincontt-Raven, Philadelphia, Pa.
- 3.Ahmed, R., and M. B. Oldstone. 1988. Organ-specific selection of viral variants during chronic infection. J. Exp. Med. 167:1719-1724. (Erratum, 168:457.) [DOI] [PMC free article] [PubMed]
- 4.Ahmed, R., A. Salmi, L. D. Butler, J. M. Chiller, and M. B. Oldstone. 1984. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J. Exp. Med. 160:521-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Altfeld, M., and E. S. Rosenberg. 2000. The role of CD4(+) T helper cells in the cytotoxic T lymphocyte response to HIV-1. Curr. Opin. Immunol. 12:375-380. [DOI] [PubMed] [Google Scholar]
- 6.Appay, V., P. R. Dunbar, M. Callan, P. Klenerman, G. M. Gillespie, L. Papagno, G. S. Ogg, A. King, F. Lechner, C. A. Spina, S. Little, D. V. Havlir, D. D. Richman, N. Gruener, G. Pape, A. Waters, P. Easterbrook, M. Salio, V. Cerundolo, A. J. McMichael, and S. L. Rowland-Jones. 2002. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat. Med. 8:379-385. [DOI] [PubMed] [Google Scholar]
- 7.Appay, V., D. F. Nixon, S. M. Donahoe, G. M. Gillespie, T. Dong, A. King, G. S. Ogg, H. M. Spiegel, C. Conlon, C. A. Spina, D. V. Havlir, D. D. Richman, A. Waters, P. Easterbrook, A. J. McMichael, and S. L. Rowland-Jones. 2000. HIV-specific CD8(+) T cells produce antiviral cytokines but are impaired in cytolytic function. J. Exp. Med. 192:63-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ashkar, S., G. F. Weber, V. Panoutsakopoulou, M. E. Sanchirico, M. Jansson, S. Zawaideh, S. R. Rittling, D. T. Denhardt, M. J. Glimcher, and H. Cantor. 2000. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science 287:860-864. [DOI] [PubMed] [Google Scholar]
- 9.Bachmann, M. F., M. Barner, A. Viola, and M. Kopf. 1999. Distinct kinetics of cytokine production and cytolysis in effector and memory T cells after viral infection. Eur. J. Immunol. 29:291-299. [DOI] [PubMed] [Google Scholar]
- 10.Badovinac, V. P., G. A. Corbin, and J. T. Harty. 2000. Cutting edge: OFF cycling of TNF production by antigen-specific CD8+ T cells is antigen independent. J. Immunol. 165:5387-5391. [DOI] [PubMed] [Google Scholar]
- 11.Barouch, D. H., J. Kunstman, M. J. Kuroda, J. E. Schmitz, S. Santra, F. W. Peyerl, G. R. Krivulka, K. Beaudry, M. A. Lifton, D. A. Gorgone, D. C. Montefiori, M. G. Lewis, S. M. Wolinsky, and N. L. Letvin. 2002. Eventual AIDS vaccine failure in a rhesus monkey by viral escape from cytotoxic T lymphocytes. Nature 415:335-339. [DOI] [PubMed] [Google Scholar]
- 12.Bennett, S. R. M., F. R. Carbone, F. Karamalis, R. A. Flavell, J. F. A. P. Miller, and W. R. Heath. 1998. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature 393:478-480. [DOI] [PubMed] [Google Scholar]
- 13.Bergmann, C. C., J. D. Altman, D. Hinton, and S. A. Stohlman. 1999. Inverted immunodominance and impaired cytolytic function of CD8+ T cells during viral persistence in the central nervous system. J. Immunol. 163:3379-3387. [PubMed] [Google Scholar]
- 14.Borland, G., J. A. Ross, and K. Guy. 1998. Forms and functions of CD44. Immunology 93:139-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borrow, P., C. F. Evans, and M. B. Oldstone. 1995. Virus-induced immunosuppression: immune system-mediated destruction of virus-infected dendritic cells results in generalized immune suppression. J. Virol. 69:1059-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borrow, P., H. Lewicki, B. H. Hahn, G. M. Shaw, and M. B. Oldstone. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 68:6103-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borrow, P., and M. B. A. Oldstone. 1997. Lymphocytic choriomeningitis virus, p. 593-628. In N. Nathanson (ed.), Viral pathogenesis. Lippincott-Raven, Philadelphia, Pa.
- 18.Borysiewicz, L. K., and J. G. Sissons. 1994. Cytotoxic T cells and human herpes virus infections. Curr. Top. Microbiol. Immunol. 189:123-150. [DOI] [PubMed] [Google Scholar]
- 19.Buchmeier, M. J., and A. J. Zajac. 1999. Lymphocytic choriomeningitis virus., p. 575-605. In R. Ahmed and I. S. Y. Chen (ed.), Persistent viral infections. John Wiley & Sons, Chichester, United Kingdom.
- 20.Butz, E., and M. Bevan. 1998. Differential presentation of the same MHC class I epitopes by fibroblasts and dendritic cells. J. Immunol. 160:2139-2144. [PubMed] [Google Scholar]
- 21.Cardin, R. D., J. W. Brooks, S. R. Sarawar, and P. C. Doherty. 1996. Progressive loss of CD8+ T cell-mediated control of a gamma-herpesvirus in the absence of CD4+ T cells. J. Exp. Med. 184:863-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen, G., P. Shankar, C. Lange, H. Valdez, P. R. Skolnik, L. Wu, N. Manjunath, and J. Lieberman. 2001. CD8 T cells specific for human immunodeficiency virus, Epstein-Barr virus, and cytomegalovirus lack molecules for homing to lymphoid sites of infection. Blood 98:156-164. [DOI] [PubMed] [Google Scholar]
- 23.Chen, W., L. C. Anton, J. R. Bennink, and J. W. Yewdell. 2000. Dissecting the multifactorial causes of immunodominance in class I-restricted T cell responses to viruses. Immunity 12:83-93. [DOI] [PubMed] [Google Scholar]
- 24.Chisari, F. V., and C. Ferrari. 1995. Hepatitis B virus immunopathology. Springer Semin. Immunopathol. 17:261-281. [DOI] [PubMed] [Google Scholar]
- 25.Clauss, M., C. Sunderkotter, B. Sveinbjornsson, S. Hippenstiel, A. Willuweit, M. Marino, E. Haas, R. Seljelid, P. Scheurich, N. Suttorp, M. Grell, and W. Risau. 2001. A permissive role for tumor necrosis factor in vascular endothelial growth factor-induced vascular permeability. Blood 97:1321-1329. [DOI] [PubMed] [Google Scholar]
- 26.Doherty, P. C., and R. Ahmed. 1996. Immune responses to viral infection, p. 143-162. In N. Nathanson (ed.), Viral pathogenesis. Lippincott-Raven, Philadelphia, Pa.
- 27.Edwards, B. H., A. Bansal, S. Sabbaj, J. Bakari, M. J. Mulligan, and P. A. Goepfert. 2002. Magnitude of functional CD8+ T-cell responses to the gag protein of human immunodeficiency virus type 1 correlates inversely with viral load in plasma. J. Virol. 76:2298-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erickson, A. L., Y. Kimura, S. Igarashi, J. Eichelberger, M. Houghton, J. Sidney, D. McKinney, A. Sette, A. L. Hughes, and C. M. Walker. 2001. The outcome of hepatitis C virus infection is predicted by escape mutations in epitopes targeted by cytotoxic T lymphocytes. Immunity 15:883-895. [DOI] [PubMed] [Google Scholar]
- 29.Fiers, W. 1991. Tumor necrosis factor. Characterization at the molecular, cellular and in vivo level. FEBS Lett. 285:199-212. [DOI] [PubMed] [Google Scholar]
- 30.Gallimore, A., A. Glithero, A. Godkin, A. Tissot, A. Pluckthun, T. Elliott, H. Hengartner, and R. Zinkernagel. 1998. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J. Exp. Med. 187:1383-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goepfert, P. A., A. Bansal, B. H. Edwards, G. D. Ritter, Jr., I. Tellez, S. A. McPherson, S. Sabbaj, and M. J. Mulligan. 2000. A significant number of human immunodeficiency virus epitope-specific cytotoxic T lymphocytes detected by tetramer binding do not produce gamma interferon. J. Virol. 74:10249-10255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goulder, P. J., Y. Tang, C. Brander, M. R. Betts, M. Altfeld, K. Annamalai, A. Trocha, S. He, E. S. Rosenberg, G. Ogg, C. A. O'Callaghan, S. A. Kalams, R. E. McKinney, Jr., K. Mayer, R. A. Koup, S. I. Pelton, S. K. Burchett, K. McIntosh, and B. D. Walker. 2000. Functionally inert HIV-specific cytotoxic T lymphocytes do not play a major role in chronically infected adults and children. J. Exp. Med. 192:1819-1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greten, T. F., J. E. Slansky, R. Kubota, S. S. Soldan, E. M. Jaffee, T. P. Leist, D. M. Pardoll, S. Jacobson, and J. P. Schneck. 1998. Direct visualization of antigen-specific T cells: HTLV-1 Tax11-19-specific CD8(+) T cells are activated in peripheral blood and accumulate in cerebrospinal fluid from HAM/TSP patients. Proc. Natl. Acad. Sci. USA 95:7568-7573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gruener, N. H., F. Lechner, M. C. Jung, H. Diepolder, T. Gerlach, G. Lauer, B. Walker, J. Sullivan, R. Phillips, G. R. Pape, and P. Klenerman. 2001. Sustained dysfunction of antiviral CD8+ T lymphocytes after infection with hepatitis C virus. J. Virol. 75:5550-5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He, X. S., B. Rehermann, F. X. Lopez-Labrador, J. Boisvert, R. Cheung, J. Mumm, H. Wedemeyer, M. Berenguer, T. L. Wright, M. M. Davis, and H. B. Greenberg. 1999. Quantitative analysis of hepatitis C virus-specific CD8(+) T cells in peripheral blood and liver using peptide-MHC tetramers. Proc. Natl. Acad. Sci. USA 96:5692-5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Islam, S. A., C. M. Hay, K. E. Hartman, S. He, A. K. Shea, A. K. Trocha, M. J. Dynan, N. Reshamwala, S. P. Buchbinder, N. O. Basgoz, and S. A. Kalams. 2001. Persistence of human immunodeficiency virus type 1-specific cytotoxic T-lymphocyte clones in a subject with rapid disease progression. J. Virol. 75:4907-4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jamieson, B. D., L. D. Butler, and R. Ahmed. 1987. Effective clearance of a persistent viral infection requires cooperation between virus-specific Lyt2+ T cells and nonspecific bone marrow-derived cells. J. Virol. 61:3930-3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jin, X., D. E. Bauer, S. E. Tuttleton, S. Lewin, A. Gettie, J. Blanchard, C. E. Irwin, J. T. Safrit, J. Mittler, L. Weinberger, L. G. Kostrikis, L. Zhang, A. S. Perelson, and D. D. Ho. 1999. Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 189:991-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khan, S., R. de Giuli, G. Schmidtke, M. Bruns, M. Buchmeier, M. van den Broek, and M. Groettrup. 2001. Cutting edge: neosynthesis is required for the presentation of a T cell epitope from a long-lived viral protein. J. Immunol. 167:4801-4804. [DOI] [PubMed] [Google Scholar]
- 40.King, C. C., R. de Fries, S. R. Kolhekar, and R. Ahmed. 1990. In vivo selection of lymphocyte-tropic and macrophage-tropic variants of lymphocytic choriomeningitis virus during persistent infection. J. Virol. 64:5611-5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kostense, S., G. S. Ogg, E. H. Manting, G. Gillespie, J. Joling, K. Vandenberghe, E. Z. Veenhof, D. van Baarle, S. Jurriaans, M. R. Klein, and F. Miedema. 2001. High viral burden in the presence of major HIV-specific CD8(+) T cell expansions: evidence for impaired CTL effector function. Eur. J. Immunol. 31:677-686. [DOI] [PubMed] [Google Scholar]
- 42.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68:4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee, P. P., C. Yee, P. A. Savage, L. Fong, D. Brockstedt, J. S. Weber, D. Johnson, S. Swetter, J. Thompson, P. D. Greenberg, M. Roederer, and M. M. Davis. 1999. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat. Med. 5:677-685. [DOI] [PubMed] [Google Scholar]
- 44.Lieberman, J., P. Shankar, N. Manjunath, and J. Andersson. 2001. Dressed to kill? A review of why antiviral CD8 T lymphocytes fail to prevent progressive immunodeficiency in HIV-1 infection. Blood 98:1667-1677. [DOI] [PubMed] [Google Scholar]
- 45.Liu, H., S. Andreansky, G. Diaz, T. Hogg, and P. C. Doherty. 2002. Reduced functional capacity of CD8+ T cells expanded by post-exposure vaccination of gamma-herpesvirus-infected CD4-deficient mice. J. Immunol. 168:3477-3483. [DOI] [PubMed] [Google Scholar]
- 46.Marshall, D. R., S. J. Turner, G. T. Belz, S. Wingo, S. Andreansky, M. Y. Sangster, J. M. Riberdy, T. Liu, M. Tan, and P. C. Doherty. 2001. Measuring the diaspora for virus-specific CD8+ T cells. Proc. Natl. Acad. Sci. USA 98:6313-6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Masopust, D., V. Vezys, A. L. Marzo, and L. Lefrancois. 2001. Preferential localization of effector memory cells in nonlymphoid tissue. Science 291:2413-2417. [DOI] [PubMed] [Google Scholar]
- 48.Matloubian, M., R. J. Concepcion, and R. Ahmed. 1994. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J. Virol. 68:8056-8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matloubian, M., S. R. Kolhekar, T. Somasundaram, and R. Ahmed. 1993. Molecular determinants of macrophage tropism and viral persistence: importance of single amino acid changes in the polymerase and glycoprotein of lymphocytic choriomeningitis virus. J. Virol. 67:7340-7349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matloubian, M., T. Somasundaram, S. R. Kolhekar, R. Selvakumar, and R. Ahmed. 1990. Genetic basis of viral persistence: single amino acid change in the viral glycoprotein affects ability of lymphocytic choriomeningitis virus to persist in adult mice. J. Exp. Med. 172:1043-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McKay, P. F., J. E. Schmitz, D. H. Barouch, M. J. Kuroda, M. A. Lifton, C. E. Nickerson, D. A. Gorgone, and N. L. Letvin. 2002. Vaccine protection against functional CTL abnormalities in simian human immunodeficiency virus-infected rhesus monkeys. J. Immunol. 168:332-337. [DOI] [PubMed] [Google Scholar]
- 52.Mueller, Y. M., S. C. De Rosa, J. A. Hutton, J. Witek, M. Roederer, J. D. Altman, and P. D. Katsikis. 2001. Increased CD95/Fas-induced apoptosis of HIV-specific CD8(+) T cells. Immunity 15:871-882. [DOI] [PubMed] [Google Scholar]
- 53.Murali-Krishna, K., J. D. Altman, M. Suresh, D. J. Sourdive, A. J. Zajac, J. D. Miller, J. Slansky, and R. Ahmed. 1998. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity 8:177-187. [DOI] [PubMed] [Google Scholar]
- 54.Murali-Krishna, K., L. L. Lau, S. Sambhara, F. Lemonnier, J. Altman, and R. Ahmed. 1999. Persistence of memory CD8 T cells in MHC class I-deficient mice. Science 286:1377-1381. [DOI] [PubMed] [Google Scholar]
- 55.Musey, L., J. Hughes, T. Schacker, T. Shea, L. Corey, and M. J. McElrath. 1997. Cytotoxic-T-cell responses, viral load, and disease progression in early human immunodeficiency virus type 1 infection. N. Engl. J. Med. 337:1267-1274. [DOI] [PubMed] [Google Scholar]
- 56.Ogg, G. S., X. Jin, S. Bonhoeffer, P. R. Dunbar, M. A. Nowak, S. Monard, J. P. Segal, Y. Cao, S. L. Rowland-Jones, V. Cerundolo, A. Hurley, M. Markowitz, D. D. Ho, D. F. Nixon, and A. J. McMichael. 1998. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science 279:2103-2106. [DOI] [PubMed] [Google Scholar]
- 57.Ortiz, G. M., J. Hu, J. A. Goldwitz, R. Chandwani, M. Larsson, N. Bhardwaj, S. Bonhoeffer, B. Ramratnam, L. Zhang, M. M. Markowitz, and D. F. Nixon. 2002. Residual viral replication during antiretroviral therapy boosts human immunodeficiency virus type 1-specific CD8+ T-cell responses in subjects treated early after infection. J. Virol. 76:411-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ou, R., S. Zhou, L. Huang, and D. Moskophidis. 2001. Critical role for alpha/beta and gamma interferons in persistence of lymphocytic choriomeningitis virus by clonal exhaustion of cytotoxic T cells. J. Virol. 75:8407-8423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pantaleo, G., H. Soudeyns, J. F. Demarest, M. Vaccarezza, C. Graziosi, S. Paolucci, M. B. Daucher, O. J. Cohen, F. Denis, W. E. Biddison, R. P. Sekaly, and A. S. Fauci. 1997. Accumulation of human immunodeficiency virus-specific cytotoxic T lymphocytes away from the predominant site of virus replication during primary infection. Eur. J. Immunol. 27:3166-3173. [DOI] [PubMed] [Google Scholar]
- 60.Pontesilli, O., M. R. Klein, S. R. Kerkhof-Garde, N. G. Pakker, F. de Wolf, H. Schuitemaker, and F. Miedema. 1997. Kinetics of immune functions and virus replication during HIV-1 infection. Immunol. Lett. 57:125-130. [DOI] [PubMed] [Google Scholar]
- 61.Pontesilli, O., M. R. Klein, S. R. Kerkhof-Garde, N. G. Pakker, F. de Wolf, H. Schuitemaker, and F. Miedema. 1998. Longitudinal analysis of human immunodeficiency virus type 1-specific cytotoxic T lymphocyte responses: a predominant gag-specific response is associated with nonprogressive infection. J. Infect. Dis. 178:1008-1018. [DOI] [PubMed] [Google Scholar]
- 61a.Puglielli, M. T., A. J. Zajac, R. G. van der Most, J. L. Dzuris, A. Sette, J. D. Altman, and R. Ahmed. 2001. In vivo selection of a lymphocytic choriomeningitis virus variant that affects recognition of the GP33-43 epitope by H-2Db but not H-2Kb. J. Virol. 75:5099-5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reinhardt, R. L., A. Khoruts, R. Merica, T. Zell, and M. K. Jenkins. 2001. Visualizing the generation of memory CD4 T cells in the whole body. Nature 410:101-105. [DOI] [PubMed] [Google Scholar]
- 63.Rickinson, A. B., and D. J. Moss. 1997. Human cytotoxic T lymphocyte responses to Epstein-Barr virus infection. Annu. Rev. Immunol. 15:405-431. [DOI] [PubMed] [Google Scholar]
- 64.Ridge, J. P., F. Di Rosa, and P. Matzinger. 1998. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature 393:474-478. [DOI] [PubMed] [Google Scholar]
- 65.Schlaak, J. F., G. Tully, H. F. Lohr, G. Gerken, and K. H. Meyer zum Buschenfelde. 1999. The presence of high amounts of HBV-DNA in serum is associated with suppressed costimulatory effects of interleukin 12 on HBV-induced immune response. J. Hepatol. 30:353-358. [DOI] [PubMed] [Google Scholar]
- 66.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 67.Schoenberger, S. P., R. E. Toes, E. I. van der Voort, R. Offringa, and C. J. Melief. 1998. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature 393:480-483. [DOI] [PubMed] [Google Scholar]
- 68.Schwarz, K., R. de Giuli, G. Schmidtke, S. Kostka, M. van den Broek, K. B. Kim, C. M. Crews, R. Kraft, and M. Groettrup. 2000. The selective proteasome inhibitors lactacystin and epoxomicin can be used to either up- or down-regulate antigen presentation at nontoxic doses. J. Immunol. 164:6147-6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shankar, P., M. Russo, B. Harnisch, M. Patterson, P. Skolnik, and J. Lieberman. 2000. Impaired function of circulating HIV-specific CD8(+) T cells in chronic human immunodeficiency virus infection. Blood 96:3094-3101. [PubMed] [Google Scholar]
- 70.Slifka, M. K., M. Matloubian, and R. Ahmed. 1995. Bone marrow is a major site of long-term antibody production after acute viral infection. J. Virol. 69:1895-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Slifka, M. K., F. Rodriguez, and J. L. Whitton. 1999. Rapid on/off cycling of cytokine production by virus-specific CD8+ T cells. Nature 401:76-79. [DOI] [PubMed] [Google Scholar]
- 72.Slifka, M. K., and J. L. Whitton. 2000. Activated and memory CD8+ T cells can be distinguished by their cytokine profiles and phenotypic markers. J. Immunol. 164:208-216. [DOI] [PubMed] [Google Scholar]
- 73.Su, H. C., L. P. Cousens, L. D. Fast, M. K. Slifka, R. D. Bungiro, R. Ahmed, and C. A. Biron. 1998. CD4+ and CD8+ T cell interactions in IFN-gamma and IL-4 responses to viral infections: requirements for IL-2. J. Immunol. 160:5007-5017. [PubMed] [Google Scholar]
- 74.Tanchot, C., S. Guillaume, J. Delon, C. Bourgeois, A. Franzke, A. Sarukhan, A. Trautmann, and B. Rocha. 1998. Modifications of CD8+ T cell function during in vivo memory or tolerance induction. Immunity 8:581-590. [DOI] [PubMed] [Google Scholar]
- 75.Tham, E. L., P. Shrikant, and M. F. Mescher. 2002. Activation-induced nonresponsiveness: a Th-dependent regulatory checkpoint in the CTL response. J. Immunol. 168:1190-1197. [DOI] [PubMed] [Google Scholar]
- 76.Trimble, L. A., and J. Lieberman. 1998. Circulating CD8 T lymphocytes in human immunodeficiency virus-infected individuals have impaired function and downmodulate CD3 zeta, the signaling chain of the T-cell receptor complex. Blood 91:585-594. [PubMed] [Google Scholar]
- 77.van Baarle, D., E. Hovenkamp, M. F. Callan, K. C. Wolthers, S. Kostense, L. C. Tan, H. G. Niesters, A. D. Osterhaus, A. J. McMichael, M. H. van Oers, and F. Miedema. 2001. Dysfunctional Epstein-Barr virus (EBV)-specific CD8(+) T lymphocytes and increased EBV load in HIV-1 infected individuals progressing to AIDS-related non-Hodgkin lymphoma. Blood 98:146-155. [DOI] [PubMed] [Google Scholar]
- 78.van der Burg, S. H., M. J. W. Visseren, R. M. P. Brandt, W. M. Kast, and C. J. M. Melief. 1996. Immunogenicity of peptides bound to MHC class I molecules depends on the MHC-peptide complex stability. J. Immunol. 156:3308-3314. [PubMed] [Google Scholar]
- 79.van der Most, R., K. Murali-Krishna, L. Whitton, C. Oseroff, J. Alexander, S. Southwood, J. Sidney, R. Chesnut, A. Sette, and R. Ahmed. 1998. Identification of Db and Kb-restricted subdominant cytotoxic T-cell responses in lymphocytic choriomeningitis virus-infected mice. Virology 240:158-167. [DOI] [PubMed] [Google Scholar]
- 80.van der Most, R. G., R. J. Concepcion, C. Oseroff, J. Alexander, S. Southwood, J. Sidney, R. W. Chesnut, R. Ahmed, and A. Sette. 1997. Uncovering subdominant cytotoxic T-lymphocyte responses in lymphocytic choriomeningitis virus-infected BALB/c mice. J. Virol. 71:5110-5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.van der Most, R. G., A. Sette, C. Oseroff, J. Alexander, K. Murali-Krishna, L. L. Lau, S. Southwood, J. Sidney, R. W. Chesnut, M. Matloubian, and R. Ahmed. 1996. Analysis of cytotoxic T cell responses to dominant and subdominant epitopes during acute and chronic lymphocytic choriomeningitis virus infection. J. Immunol. 157:5543-5554. [PubMed] [Google Scholar]
- 82.Vogel, T. U., T. M. Allen, J. D. Altman, and D. I. Watkins. 2001. Functional impairment of simian immunodeficiency virus-specific CD8+ T cells during the chronic phase of infection. J. Virol. 75:2458-2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Walker, C. M. 1999. Hepatitis C virus, p. 94-115. In R. Ahmed and I. S. Y. Chen (ed.), Persistent viral infections. John Wiley & Sons Ltd., New York, N.Y.
- 84.Weiner, A., A. L. Erickson, J. Kansopon, K. Crawford, E. Muchmore, A. L. Hughes, M. Houghton, and C. M. Walker. 1995. Persistent hepatitis C virus infection in a chimpanzee is associated with emergence of a cytotoxic T lymphocyte escape variant. Proc. Natl. Acad. Sci. USA 92:2755-2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Welsh, R. M. 2001. Assessing CD8 T cell number and dysfunction in the presence of antigen. J. Exp. Med. 193:F19-F22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xiong, Y., M. A. Luscher, J. D. Altman, M. Hulsey, H. L. Robinson, M. Ostrowski, B. H. Barber, and K. S. MacDonald. 2001. Simian immunodeficiency virus (SIV) infection of a rhesus macaque induces SIV-specific CD8(+) T cells with a defect in effector function that is reversible on extended interleukin-2 incubation. J. Virol. 75:3028-3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xu, X. N., G. R. Screaton, and A. J. McMichael. 2001. Virus infections: escape, resistance, and counterattack. Immunity 15:867-870. [DOI] [PubMed] [Google Scholar]
- 88.Yewdell, J., L. Anton, and J. Bennink. 1996. Defective ribosomal products (DRiPs): a major source of antigenic peptides for MHC class I molecules? J. Immunol. 157:1823-1826. [PubMed] [Google Scholar]
- 89.Zajac, A. J., J. N. Blattman, K. Murali-Krishna, D. J. Sourdive, M. Suresh, J. D. Altman, and R. Ahmed. 1998. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188:2205-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhou, S., R. Ou, L. Huang, and D. Moskophidis. 2002. Critical role for perforin-, Fas/FasL-, and TNFR1-mediated cytotoxic pathways in down-regulation of antigen-specific T cells during persistent viral infection. J. Virol. 76:829-840. [DOI] [PMC free article] [PubMed] [Google Scholar]