Abstract
Vesicular stomatitis virus (VSV) is a potent inducer of apoptosis in host cells. Recently, it has been shown that two VSV products are involved in the induction of apoptosis, the matrix (M) protein, and another viral product that has yet to be identified (S. A. Kopecky et. al., J. Virol. 75:12169-12181, 2001). Comparison of recombinant viruses containing wild-type (wt) or mutant M proteins showed that wt M protein accelerates VSV-induced apoptosis in HeLa cells, while wt M protein delays apoptosis in VSV-infected BHK cells. Our hypothesis to explain these results is that both effects of M protein are due to the ability of M protein to inhibit host gene expression. This hypothesis was tested by infecting cells with an M protein mutant virus defective in the inhibition of host gene expression (rM51R-M virus) in the presence or absence of actinomycin D, another inhibitor of host gene expression. Actinomycin D accelerated induction of apoptosis of HeLa cells infected with rM51R-M virus and delayed apoptosis in BHK cells infected with rM51R-M virus, similar to the effects of wt M protein. The idea that the induction of apoptosis by M protein in HeLa cells is due to its ability to inhibit host gene expression was further tested by comparing the activation of upstream caspase pathways by M protein versus that by actinomycin D or 5,6-dichlorobenzimidazole riboside (DRB). Expression of M protein activated both caspase-8 and caspase-9-like enzymes, as did treatment with actinomycin D or DRB. Induction of apoptosis by M protein, actinomycin D, and DRB was inhibited in stably transfected HeLa cell lines that overexpress Bcl-2, an antiapoptotic protein that inhibits the caspase-9 pathway. A synthetic inhibitor of caspase-8, Z-IETD-FMK, did not inhibit induction of apoptosis by M protein, actinomycin D, or DRB. Taken together, our data support the hypothesis that the induction of apoptosis by M protein is caused by the inhibition of host gene expression and that the caspase-9 pathway is more important than the caspase-8 pathway for the induction of apoptosis by M protein and other inhibitors of host gene expression.
Infection with many viruses causes death of host cells by inducing apoptosis. An important element in understanding viral pathogenesis is determining which products of viral infection induce apoptosis in host cells and their mechanisms of action. Vesicular stomatitis virus (VSV), the prototype rhabdovirus, was one of the early viruses shown to induce apoptosis (25). Our laboratory has recently demonstrated that at least two products of viral infection contribute to the induction of apoptosis in VSV-infected cells (24). One of these products is the viral matrix (M) protein, and the other product has yet to be identified. M protein induces apoptosis in transfected cells in the absence of other viral products. However, in the context of a virus infection, M protein has contrasting effects on VSV-induced apoptosis, depending on the cell type. This was shown by using isogenic recombinant viruses containing either wild-type (wt) or mutant M proteins. In HeLa cells, the M protein mutant virus induces apoptosis more slowly than the recombinant wt virus (rwt virus) indicating that wt M protein accelerates apoptosis induced by VSV in HeLa cells. In contrast, the M protein mutant virus induces apoptosis more rapidly than rwt virus in BHK cells, indicating that a product of viral infection other than M protein contributes to the induction of apoptosis in BHK cells and that wt M protein delays apoptosis in BHK cells infected with VSV (24). The goal of the experiments presented here was to determine how M protein has these contrasting effects in HeLa and BHK cells.
M protein is a structural component of the virion and has multiple roles in viral assembly and cytopathogenesis. Expression of M protein in the absence of other products of viral infection causes many of the same cellular effects as infection with VSV. For example, the inhibition of host gene expression, which is characteristic of VSV infection, has been shown to be caused by M protein (9, 10, 16, 31). M protein inhibits transcription by all three host RNA polymerases in the absence of other viral products (2). In the case of host RNA polymerase II, the target of the inhibition was identified as the transcription factor TFIID (39, 40). M protein also blocks nucleocytoplasmic transport of RNAs and proteins (21). M protein-induced inhibition of nucleocytoplasmic transport appears to be caused by its interaction with one or more nuclear pore components, including the nucleoporin Nup98 (32, 37).
M protein mutants demonstrate that the viral assembly functions of M protein are genetically separable from its cytopathic functions (10, 28). For example, an M protein mutant in which methionine 51 is substituted for by arginine (M51R) is defective in the inhibition of host gene expression but is fully functional in viral assembly. In contrast, other M protein mutants are defective in the viral assembly functions but inhibit host gene expression as effectively as wt M protein. The ability of M protein to induce apoptosis is genetically correlated with the ability to inhibit host gene expression (24). This genetic correlation suggests that apoptosis induced by M protein may be caused by the inhibition of host gene expression.
The goal of the experiments presented here was to test the hypothesis that both the M protein-induced acceleration in HeLa cells and delay in BHK cells during VSV-induced apoptosis are due to the inhibition of host gene expression. According to this hypothesis, there is a fundamental difference in the induction of apoptosis in HeLa cells compared with that in BHK cells. In our hypothesis, HeLa cells do not require new host gene expression for the induction of apoptosis. In contrast, BHK cells do require new host gene expression for the induction of apoptosis, and the inhibition of host gene expression would be expected to delay VSV-induced apoptosis. Thus, the inhibition of host gene expression by M protein has different effects on VSV-induced apoptosis in the two different cell types, depending on the requirement for host gene expression in apoptosis. This hypothesis was tested by determining whether two pharmacologic inhibitors of host gene expression, actinomycin D and 5,6-dichlorobenzimidazole riboside (DRB), affect the induction of apoptosis by VSV similar to wt M protein. HeLa cells infected with an M51R M protein mutant virus in the presence of actinomycin D entered apoptosis at a rate similar to that of cells infected with rwt virus, supporting our hypothesis that the inhibition of host gene expression accelerates apoptosis in HeLa cells infected with VSV. In BHK cells infected with M51R M protein mutant virus in the presence of actinomycin D, apoptosis was delayed, supporting our hypothesis that the inhibition of host gene expression delays apoptosis in BHK cells infected with VSV. It was also determined that the caspase-9 pathway is more important than the caspase-8 pathway for apoptosis induced by M protein, similar to the pharmacologic inhibitors of host gene expression, providing further support for the hypothesis that the induction of apoptosis by M protein is caused by the inhibition of host gene expression.
MATERIALS AND METHODS
Viruses and cells.
The recombinant viruses rwt and r-M51R-M were isolated from cDNA clones and grown as previously described (24). BHK cells and HeLa cells were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS). For individual experiments, infections were performed with a multiplicity of infection of 20 PFU per cell in DMEM containing 2% FBS. Stable HeLa-Bcl-2 cells were generated by transfecting HeLa cells with the h-Bcl-2 pcDNA3 plasmid (gift of Zheng Cui), which contains a human Bcl-2 gene driven by the cytomegalovirus (CMV) immediate-early promoter and a neomycin drug resistance marker. After 24 h, the transfected cells were split at low densities in selection medium containing G418 to promote the formation of colonies that contained the h-Bcl-2 pcDNA3 plasmid. Individual colonies selected for G418 resistance were harvested and screened for overexpression of Bcl-2 by Western blotting. HeLa empty vector cells were generated by transfection with pcDNA3 plasmid, and individual colonies were screened for drug resistance.
Expression of M protein in transfected cells.
M mRNA was generated by in vitro transcription with a plasmid DNA template encoding M protein as previously described by using a commercial kit (Message Machine, Ambion, Inc.) (8). The resulting mRNAs contained 5′ caps and 3′ poly(A) sequences that enhance expression in transfected cells. Cells were grown in 24-well plates to about 50% confluence and transfected with 30 ng of M mRNA or with yeast RNA as a negative control. The total amount of RNA transfected was held constant at 300 ng in all samples with the addition of yeast RNA. The transfections were performed with Lipofectin reagent as previously described (8).
The transfection efficiencies of the HeLa-Bcl-2 cells and the HeLa-empty vector cells were compared to that of HeLa cells by transfecting enhanced green fluorescence protein mRNA and analyzing the cells by flow cytometry as described previously (24). The transfection efficiencies were nearly identical for all of the cell lines. The amount of M protein expression after transfection with M mRNA was determined by Western blotting with the 23H12 anti-M protein monoclonal antibody as described previously (34). The M protein expression levels were nearly identical for all of the cell lines.
Time-lapse microscopy.
BHK and HeLa cells were grown to about 50% confluency in 25-cm-diameter flasks, infected with rwt virus or rM51R-M virus, and/or treated with 5 μg of actinomycin D per ml (Sigma-Aldrich Co.). The flask was placed on a rocker for 30 min at room temperature and then placed on the stage of a Zeiss Axiovert inverted phase-contrast time-lapse microscopy system equipped with an incubator containing an atmosphere of 5% CO2 and at a temperature of 37°C as previously described (12). The progression of the cells was observed by phase-contrast time-lapse microscopy with a Dage MTI-100 video camera affixed to the microscope at a time-lapse ratio of 600:1. The time that each of 80 to 120 cells entered apoptosis was determined from a time-date generator record on the videotape.
Caspase activity assays.
HeLa or BHK cells were grown in 24-well plates to about 50% confluency. Cells were either infected with recombinant viruses, transfected with M mRNA, or treated with actinomycin D, 25 μg of DRB per ml (Sigma-Aldrich Co.), or 50 ng of TRAIL per ml (R&D Systems, Inc.) as indicated in the figure legends. Duplicate wells were lysed, and caspase activity was determined with fluorogenic substrates for caspase-3 (DVED-AFC; R&D Systems, Inc.), caspase-8 (IETD-AFC; R&D Systems, Inc.), and caspase-9 (LEHD-AFC; R&D Systems, Inc.) according to the protocol supplied by the manufacturer. Each sample was incubated for 2 h with the peptide substrate, and the reaction was stopped by the addition of 900 μl of 10 mM Tris-10 mM NaCl (pH 8.1). Fluorescence intensities were measured at excitation and emission wavelengths of 400 and 490 nm, respectively. Duplicate samples were used for a Lowry assay to determine protein concentration. The cells were lysed with phosphate-buffered saline containing 0.1% sodium dodecyl sulfate (SDS), and a Lowry assay was performed.
MTT assay.
The HeLa cell lines were grown in 96-well plates to about 50% confluency. Cells were either transfected with M mRNA or treated with actinomycin D or DRB for 30 h. Samples were prepared in triplicate according to the protocol supplied by the manufacturer. MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide) labeling reagent (Roche) was added to each well for 4 h, and the reaction was stopped with the addition of a solubilization solution supplied by the manufacturer. The plates were incubated overnight at 37°C. The absorbance of the samples was measured with an enzyme-linked immunosorbent assay (ELISA) plate reader at a wavelength of 540 nm.
Quantitation of M protein levels.
The indicated HeLa cell lines were infected with rwt virus or rM51R-M virus for 12 h. At the start of infection, cells were labeled continuously throughout infection with DMEM containing 100 μCi of [35S]methionine per ml. Cells were solubilized with 0.5 ml of 2% SDS disruption buffer. The DNA in the samples was sheared in a syringe with a 26-gauge needle, and equal amount of proteins were resolved by SDS-polyacrylamide gel electrophoresis (PAGE) on 10% polyacrylamide gels. The gels were fixed, dried, and analyzed by phosphorescence imaging. The radioactivity of the M protein bands was quantified with ImageQuant software (Molecular Dynamics, Inc.) as described previously (24).
Caspase-8 inhibitor.
HeLa cells were grown in 24-well plates to about 50% confluency. Cells were pretreated for 1 h with a synthetic inhibitor of caspase-8 (Z-IETD-FMK; R&D Systems, Inc.) at the indicated concentrations. Cells were either transfected with M mRNA for 30 h, treated with actinomycin D or DRB for 30 h, or treated with TRAIL (R&D Systems, Inc.) for 6 h. Cells that were transfected with M mRNA were treated with the caspase-8 inhibitor at the time of the addition of the mRNA-Lipofectin mixture. Images of the cells were captured by phase-contrast microscopy with a ×20 objective. Between 300 and 700 cells in five representative images for each sample were scored as being either round or flat for each experiment. The criteria for evaluation of cell rounding were described previously (28).
RESULTS
Actinomycin D compensates for effects of M protein mutation on VSV-induced apoptosis.
Our previous results indicated that the effects of M protein mutation on the induction of apoptosis by VSV differ markedly between HeLa cells and BHK cells (24). Our hypothesis is that both the delay in apoptosis in HeLa cells and the acceleration of apoptosis in BHK cells infected with M protein mutant virus are due to the inability of the mutant M protein to inhibit host gene expression. The requirement for new host gene expression in order to undergo apoptosis in response to VSV infection in HeLa versus BHK cells was tested by infecting cells with an M protein mutant virus (rM51R-M virus) in the presence or absence of actinomycin D (Fig. 1). The rM51R-M virus is a recombinant virus that contains a substitution of arginine for methionine at position 51 of the 229-amino-acid M protein, and the rwt virus is the isogenic recombinant wild-type virus control. The M51R mutation renders the M protein defective in its ability to inhibit host gene expression, but the mutant M protein is fully functional in viral assembly. Host RNA synthesis was inhibited after infection with rwt virus or treatment with actinomycin D as measured by [3H]uridine incorporation (2a). However, actinomycin D inhibited host RNA synthesis more quickly and to a greater extent than rwt virus. After treatment for 2 h with actinomycin D, host RNA synthesis was less than 1% of mock-treated cells, while host RNA synthesis was 12% of mock-infected control cells after infection with rwt virus for 4 h. In contrast, host RNA synthesis was still 80% of mock-infected control cells after infection with rM51R-M virus for 4 h.
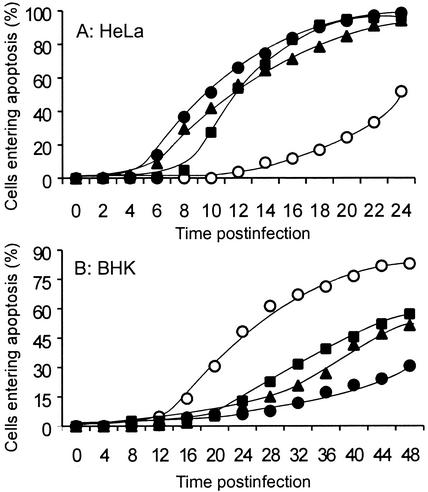
FIG. 1.
M protein and actinomycin D have similar effects on VSV-induced apoptosis. HeLa cells (A) or BHK cells (B) were infected with rwt virus (solid squares), rM51R-M virus (open circles), or rM51R-M virus and treated with actinomycin D (solid circles) or were treated with actinomycin D alone (solid triangles) and then analyzed by phase-contrast time-lapse microscopy. The time that each of 80 to 120 cells entered apoptosis was determined by the onset of membrane blebbing from the time-date record on the videotape. The data shown are the cumulative percentage of cells entering apoptosis as a function of time (hours) postinfection. The data represent an average of two experiments.
HeLa cells (Fig. 1A) or BHK cells (Fig. 1B) were infected with rM51R-M virus in the presence or absence of 5 μg of actinomycin D per ml. Cells were infected with rwt virus or treated with 5 μg of actinomycin D per ml alone as controls. This concentration of actinomycin D was used because it had previously been shown to effectively inhibit host RNA synthesis while having little if any effect on viral RNA synthesis (1). Time-lapse microscopy was used to determine the timing of morphological changes associated with the induction of apoptosis. Cells undergoing apoptosis proceed through a characteristic sequence of morphological changes beginning with cell rounding, followed by membrane blebbing and cell shrinkage, and ultimately concluding in cell membrane rupture. The time that each of 80 to 120 cells in the field began membrane blebbing was determined from the time-date record on the videotape. The onset of membrane blebbing was chosen as the criterion for the time of entry into apoptosis, because membrane blebbing occurs in cells undergoing apoptosis but not necrosis. The data were expressed as the cumulative percentage of cells that had entered apoptosis as a function of time postinfection.
HeLa cells infected with rM51R-M virus (Fig. 1A) entered apoptosis more slowly than cells infected with rwt virus as reported previously. It was also shown previously that the M51R mutation does not decrease M protein expression. In fact, rM51R-M virus synthesizes more M protein than rwt virus at later times postinfection (24). Therefore, the reduction in apoptosis observed in cells infected with rM51R-M virus is not due to differences in levels of M protein expression. HeLa cells infected with rM51R-M virus in the presence of actinomycin D (Fig. 1A) entered apoptosis at a rate similar to that of cells infected with rwt virus or treated with actinomycin D alone (Fig. 1A). These results support our hypothesis that the inhibition of host gene expression accelerates apoptosis in HeLa cells infected with VSV.
Results from similar experiments performed with BHK cells contrast markedly with those obtained with HeLa cells. BHK cells infected with rM51R-M virus (Fig. 1B) entered apoptosis more rapidly than cells infected with rwt virus. Since the M51R M protein is defective in the ability to induce apoptosis, these results indicate that another product of viral infection is the main inducer of apoptosis in BHK cells and that the effect of wt M protein is to delay apoptosis induced by this other viral product in most BHK cells. These results were also similar to our previously published results (24). However, when BHK cells were infected with rM51R-M virus in the presence of actinomycin D (Fig. 1B), apoptosis was delayed, similar to the induction of apoptosis by rwt virus or actinomycin D alone. This result supports our hypothesis that the inhibition of host gene expression delays apoptosis in BHK cells infected with VSV.
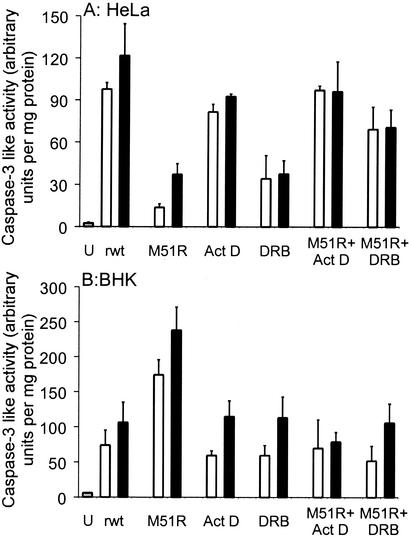
The differences in the timing of induction of apoptosis induced by rM51R-M virus with or without the addition of actinomycin D were confirmed by assaying the activity of caspase-3 (Fig. 2). HeLa cells (Fig. 2A) or BHK cells (Fig. 2B) were either uninfected (U) or were infected with rwt or rM51R-M viruses in the presence or absence of actinomycin D. Another inhibitor of host gene expression, DRB, a nucleoside analog, was also tested to determine whether it would also influence the induction of apoptosis by VSV similar to actinomycin D. DRB was used at a concentration of 25 μg/ml because this concentration had previously been shown to effectively inhibit host gene expression (26, 41). HeLa cells were infected for 12 or 18 h (Fig. 2A), while BHK cells were infected for 20 or 30 h (Fig. 2B) to reflect the difference in the timing of apoptosis in the two cell types. Cell lysates were prepared, and caspase-3 activity was determined by adding a fluorogenic substrate.
FIG. 2.
M protein and other inhibitors of host gene expression have similar effects on VSV-induced caspase-3 activation. HeLa cells (A) or BHK cells (B) were untreated (U), infected with rwt virus (rwt), infected with r-M51R-M virus (M51R), treated with actinomycin D (Act D), treated with DRB, infected with rM51R-M virus and treated with actinomycin D (M51R + Act D), or infected with rM51R-M virus and treated with DRB (M51R + DRB). HeLa cell lysates (A) were harvested at 12 h (open bars) and 18 h (solid bars), while BHK cell lysates (B) were harvested at 20 h (open bars) and 30 h (solid bars). Caspase-3-like activity was measured with a fluorogenic substrate. Protein concentrations were determined with a Lowry assay. The amount of caspase-3 activity is expressed in arbitrary fluorescence units per milligram of protein. The data represent the average ± standard deviation of three experiments.
In HeLa cells, rwt virus induced more caspase-3 activation than rM51R-M virus at both times postinfection (Fig. 2A). These results support the conclusion that wt M protein accelerates apoptosis in HeLa cells, similar to the results obtained with time-lapse microscopy (Fig. 1). HeLa cells infected with rM51R-M virus in the presence of actinomycin D or DRB activated more caspase-3 than HeLa cells infected with rM51R-M virus alone. These results are similar to the time-lapse microscopy results (Fig. 1) and support the conclusion that the inhibition of host gene expression accelerates apoptosis in HeLa cells infected with VSV.
In BHK cells, rM51R-M virus induced more caspase-3 activation than rwt virus at both times postinfection (Fig. 2B). These results support the conclusion that wt M protein delays apoptosis in BHK cells, similar to the results obtained with time-lapse microscopy (Fig. 1). BHK cells infected with rM51R-M virus in the presence of actinomycin D or DRB activated less caspase-3 than BHK cells infected with rM51R-M alone, similar to the time-lapse microscopy results (Fig. 1). This result supports our hypothesis that the inhibition of host gene expression delays apoptosis in BHK cells infected with VSV. Taken together, the results in Fig. 1 and 2 show that pharmacologic inhibitors of host RNA synthesis enhance apoptosis in HeLa cells and delay apoptosis in BHK cells in a manner similar to wt M protein.
Bcl-2 inhibits the induction of apoptosis by M protein, actinomycin D, or DRB.
We had previously shown that wt M protein induces apoptosis in the absence of other products of viral infection in both BHK and HeLa cells. The M51R M protein mutant is defective in the inhibition of host gene expression and is also defective in the induction of apoptosis. This result supports the idea that the induction of apoptosis by M protein is due to its ability to inhibit host gene expression. This hypothesis was further tested by comparing the caspase pathways activated by M protein to the caspase pathways activated by the pharmacologic inhibitors of host gene expression, actinomycin D and DRB. Inhibitors of the upstream caspases, caspase-8 and -9, were used to determine which caspase pathways were important for the induction of apoptosis by M protein and other inhibitors of host gene expression. Even though the molecular targets for actinomycin D and DRB are different, it was expected that these inhibitors of host RNA synthesis would induce apoptosis through the activation of the caspase-9 pathway, since that pathway is typically activated by internal cellular damage or stress. Therefore, if M protein induces apoptosis by inhibiting host gene expression, the same caspase pathways should be activated as those in cells treated with actinomycin D or DRB.
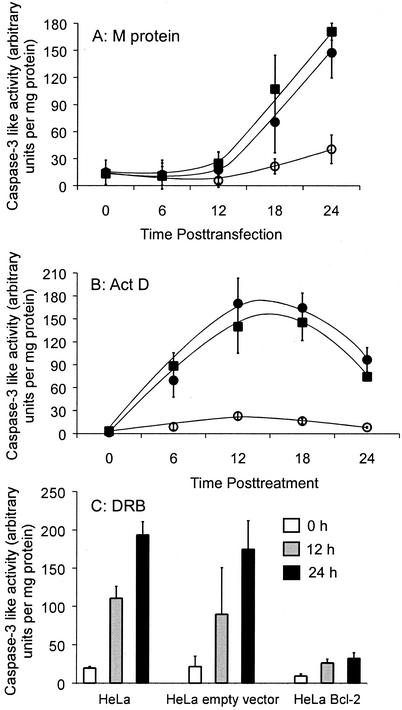
To determine whether the caspase-9 pathway is important for apoptosis induced by M protein, actinomycin D, or DRB, HeLa cell lines that stably overexpressed the antiapoptotic host protein Bcl-2 were isolated. Bcl-2 has been shown to preferentially inhibit the caspase-9 pathway (4). The HeLa-Bcl-2 cell lines were generated by transfection with plasmid DNA encoding Bcl-2 under control of the CMV immediate-early promoter, followed by antibiotic selection. The overexpression of Bcl-2 in these cell lines was confirmed by Western blot analysis (data not shown). The original HeLa cells, control HeLa cells stably transfected with the empty vector, or HeLa-Bcl-2 cells were either transfected with M mRNA (Fig. 3A) or treated with actinomycin D (Fig. 3B) or DRB (Fig. 3C). The induction of apoptosis was quantified by assaying the activity of the downstream caspase, caspase-3. At the indicated times, cell lysates were prepared, and the amount of apoptosis was determined by assaying caspase activity with a fluorogenic caspase-3 substrate. Expression of M protein induced a substantial amount of caspase-3 activity in HeLa cells (Fig. 3A) and HeLa-empty vector cells, indicating that M protein induced apoptosis in both cell types. However, expression of M protein induced much less caspase-3 activation in HeLa-Bcl-2 cells. These results were not due to effects of Bcl-2 on transfection efficiency or M protein expression, since the percentage of cells transfected and the levels of M protein expression were nearly identical for all of these cell lines (not shown).
FIG. 3.
Apoptosis induced by M protein, actinomycin D, or DRB is reduced by overexpression of Bcl-2. HeLa cells (solid squares), HeLa-empty vector cells (solid circles), and HeLa-Bcl-2 cells (open circles) were either transfected with M mRNA (A), treated with actinomycin D (B), or treated with DRB (C) for the indicated times and analyzed for caspase-3 activity and protein concentrations as described in the legend to Fig. 2. The amount of caspase-3 activated is expressed in arbitrary fluorescence units per milligram of protein. The data represent the average ± standard deviation of three experiments.
The results obtained after actinomycin D treatment were similar to those obtained by M protein expression. After treatment with actinomycin D, a significant amount of caspase-3 was activated in HeLa cells and HeLa-empty vector cells but not in HeLa-Bcl-2 cells (Fig. 3B). The time course of caspase-3 activation by actinomycin D treatment of HeLa cells and HeLa-empty vector cells differed from that induced by M protein. In particular, the increase in caspase-3 activity was more rapid than that induced by transfection with M mRNA, reflecting the more rapid inhibition of host gene expression by actinomycin D. Furthermore, there was a decrease in caspase-3 activity in samples treated with actinomycin D for more than 18 h. This decrease was caused by the late stages of cell death in which membrane permeability causes the loss of caspase-3 from the cell.
Similar to the results from M protein and actinomycin D, overexpression of Bcl-2 inhibited activation of caspase-3 after treatment with DRB (Fig. 3C). Collectively, these data in Fig. 3 indicate that Bcl-2 inhibits induction of apoptosis by M protein, similar to that of pharmacologic inhibitors of host gene expression (actinomycin D and DRB). The time course of caspase-3 activation by DRB treatment was slower than that induced by actinomycin D treatment in that activity continued to increase between 12 and 24 h. This difference reflects the fact that DRB is a less potent inducer of apoptosis than actinomycin D.
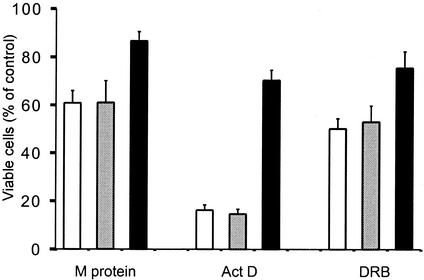
The inhibition by Bcl-2 of apoptosis induced by M protein and other inhibitors of host gene expression was confirmed by determining whether overexpression of Bcl-2 reduced cell death, as measured with MTT, a tetrazolium salt that is cleaved to form a formazan dye by metabolically active cells but not dead cells. Thus, the amount of dye is proportional to the number of viable cells present. This experiment tested the possibility that overexpression of Bcl-2 may have inhibited activation of caspase-3 without preventing cell death by other mechanisms. Cells were transfected with M mRNA or treated with actinomycin or DRB for 30 h, and then MTT was added to each sample. The reaction was stopped by the addition of a solubilization solution, and the absorbance of each sample was analyzed with an ELISA plate reader. Data are expressed as a percentage of the level in an untreated control (Fig. 4). The viability of HeLa cells or HeLa-empty vector cells transfected with M mRNA was reduced to about 60% of the control viability. Since the transfection efficiency was approximately 40 to 60% (unpublished data), this is the expected result if most of the transfected cells die. Overexpression of Bcl-2 increased cell viability after expression of M protein. This confirms the results obtained by analyzing caspase activation (Fig. 3). Overexpression of Bcl-2 also increased cell viability after treatment with actinomycin D and DRB, further supporting the results obtained by analyzing caspase activation (Fig. 3). Thus, the results in Fig. 3 and 4 support the conclusion that Bcl-2 inhibits the induction of apoptosis by M protein, actinomycin D, and DRB (Fig. 4). These results suggest that the caspase-9 pathway is important for the induction of apoptosis by M protein similar to other inhibitors of host gene expression.
FIG. 4.
Overexpression of Bcl-2 increases cell viability after transfection with M mRNA or treatment with inhibitors of host gene expression. HeLa cells (open bars), HeLa-empty vector cells (shaded bars), and HeLa-Bcl-2 cells (solid bars) were transfected with M mRNA (M protein) or treated with actinomycin D (Act D) or DRB for 30 h and analyzed for cell viability with an MTT assay. MTT was added to each sample and metabolized by living cells for 4 h. A solubilization solution was added to each sample. The samples were analyzed with an ELISA plate reader. For each sample, cell viability was determined as a percentage of that of a control. The control for the M mRNA transfection was transfection with yeast RNA, while the control for the samples treated with actinomycin D and DRB was untreated cells. The data represent the average ± standard deviation of three experiments.
Bcl-2 inhibits induction of apoptosis by VSV.
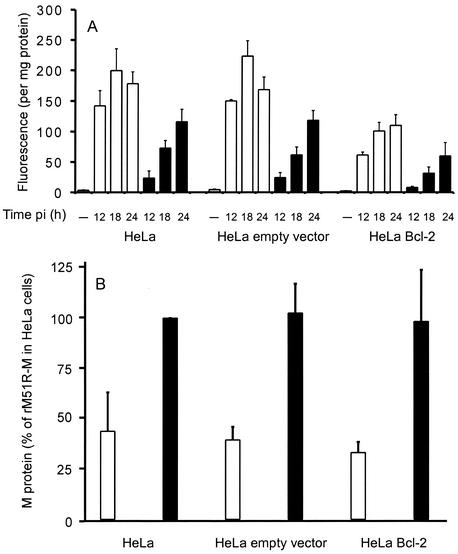
Since wt M protein accelerates apoptosis induced by VSV in HeLa cells (Fig. 1 and 2), and Bcl-2 inhibits apoptosis induced by M protein (Fig. 3 and 4), Bcl-2 should decrease apoptosis induced by VSV. To test this hypothesis, the amount of caspase-3 activity was determined after infection with rwt and rM51R-M viruses in the HeLa cells that overexpress Bcl-2. Figure 5 shows the level of caspase-3 activity in HeLa cells, HeLa-empty vector cells, and HeLa-Bcl-2 cells after infection with rwt virus or rM51R-M virus for the times indicated.
FIG. 5.
Overexpression of Bcl-2 reduces apoptosis induced by VSV in HeLa cells. (A) Quantitation of apoptosis by caspase-3 activity. The indicated HeLa cell lines were infected with rwt virus (open bars) or rM51R-M virus (solid bars) for the indicated times postinfection (pi). Uninfected samples denoted by the dashes were negative controls. Cell lysates were analyzed for caspase-3-like activity and protein concentrations as described in the legend to Fig. 2. The amount of caspase-3 activity is expressed in arbitrary fluorescence units per milligram of protein. The data represent the average ± standard deviation of three experiments. (B) Quantitation of M protein by [35S]methionine labeling. The indicated HeLa cell lines were infected with rwt virus (open bars) or rM51R-M virus (solid bars) for 12 h. Cells were labeled with [35S]methionine continuously throughout infection. Equal amounts of cellular proteins were analyzed by SDS-PAGE and phosphorescence imaging. Figure 5B shows quantitation of the radioactivity of the M protein bands from three separate experiments expressed as a percentage of the amount of M protein synthesis with rM51R-M in HeLa cells. The data represent the average ± standard deviation of three experiments.
Higher levels of caspase-3 were activated in HeLa cells and HeLa-empty vector cells infected with rwt virus than in cells infected with rM51R-M virus (Fig. 5), similar to the results in Fig. 2. However, there was a reduction in the amount of caspase-3 activated in the HeLa-Bcl-2 cells after infection with rwt virus compared to the untransfected HeLa cells or the empty vector control. This supports our hypothesis that M protein is a major contributor to apoptosis induced by VSV in HeLa cells and also indicates that activation of the caspase-9 pathway is important for VSV-induced apoptosis in HeLa cells. Bcl-2 was less effective at reducing VSV-induced apoptosis than apoptosis induced by M protein alone (Fig. 3 and 4). This is probably due to the fact that there are multiple inducers of apoptosis in VSV-infected cells. Interestingly, overexpression of Bcl-2 also decreased the amount of caspase-3 activated by rM51R-M virus, which contains an M protein that is defective in the induction of apoptosis (23). Thus, Bcl-2 may also inhibit induction of apoptosis by a viral product other than M protein, which contributes to apoptosis induced by rM51R-M virus.
To determine whether the differences in the induction of apoptosis in HeLa-Bcl-2 cells could be attributed to differences in M protein levels, the levels of M protein expression were determined. HeLa cells, HeLa-empty vector cells, and HeLa-Bcl-2 cells were infected with rwt or rM51R-M viruses for 12 h. Cells were labeled continuously throughout infection with [35S]methionine. Equal amounts of cellular proteins were analyzed by SDS-PAGE and phosphorescence imaging. Figure 5B shows quantitation of the radioactivity of the M protein bands from three separate experiments expressed as a percentage of the amount of M protein synthesis with the rM51R-M in HeLa cells, which was near the maximum rate. Similar to our previously published results, by 12 h postinfection, there was less M protein synthesis in HeLa cells and HeLa-empty vector cells infected with rwt virus than in cells infected with rM51R-M virus (24). The reduction in M protein levels in cells infected with rwt virus (Fig. 5B) is caused by the dramatic inhibition of both viral and host protein syntheses in cells infected with rwt virus. The rM51R-M virus is defective in the inhibition of protein synthesis, and thus more viral proteins are synthesized. Importantly, there was little if any difference in M protein synthesis in HeLa-Bcl-2 cells that were infected with rwt virus or rM51R-M virus compared to the level in control HeLa or HeLa-empty vector cells. This result indicates that the differences in the induction of apoptosis in HeLa-Bcl-2 cells were not due to the differences in M protein levels.
Bcl-2 reduces both caspase-8- and -9-like activities induced by M protein and actinomycin D.
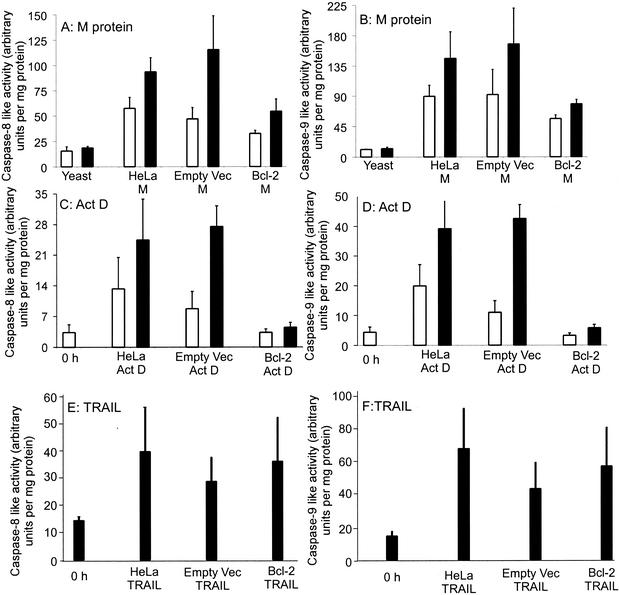
The inhibition of apoptosis by overexpression of Bcl-2 suggests that M protein induces apoptosis by the activation of caspase-9. However, it is not clear whether caspase-9 is activated directly or indirectly through cross talk. There are some reports that Bcl-2 reduces apoptosis by inducers that are known to directly activate the caspase-8 pathway, such as Fas and tumor necrosis factor (TNF) in certain cell types (3, 18, 22). This inhibition by Bcl-2 is presumably due the prevention of the indirect effect, in which activation of the caspase-9 pathway occurs following activation of caspase-8 by TNF or Fas. The indirect effects of the two upstream caspases on each other are commonly referred to as cross talk between the two pathways. Caspase-9 can be activated as an indirect effect of activation of caspase-8, through cleavage of Bid (4). Likewise, caspase-8 can be activated as an indirect effect of activation of caspase-9, following activation of caspase-3 (38). The cross talk between the pathways serves to amplify caspase activation and occurs in apoptosis by many inducers. If the caspase-8 pathway is activated by M protein and the caspase-9 pathway is activated by cross talk, then we would expect Bcl-2 to prevent the activation of caspase-9 but not caspase-8. However, if the caspase-9 pathway is activated by M protein, we would expect Bcl-2 to prevent the activation of both caspase-8 and capsase-9. To determine whether the upstream caspases, caspase-8 and -9, were both inhibited by Bcl-2 after transfection with M mRNA or treatment with actinomycin D, cell lysates were assayed for caspase activity by using substrates preferentially cleaved by caspase-8 and -9. The activities detected by these two substrates will be referred to as “caspase-8-like” and “caspase-9-like,” since there are other caspases that have detectable activity with these substrates.
Figure 6 shows the results of the caspase-8 (Fig. 6A, C, and E) and caspase-9 (Fig. 6B, D, and F) activity assays performed after transfection with M mRNA (Fig. 6A and B), treatment with actinomycin D (Fig. 6C and D), or treatment with TRAIL or TNF-related apoptosis-inducing ligand (Fig. 6E and F). TRAIL was used as a control and has previously been demonstrated to induce apoptosis through the activation of the caspase-8 pathway (35). In preliminary experiments, it was determined that treatment with TRAIL for 6 h induced the maximal amount of apoptosis. In Fig. 6A and B, HeLa cells were transfected with yeast RNA as a negative control or with M mRNA for 18 or 24 h. Expression of M protein in HeLa cells and HeLa-empty vector cells activated considerable amounts of caspase-8-like (Fig. 6A) and caspase-9-like (Fig. 6B) activities compared to those in controls transfected with yeast RNA. However, transfection of HeLa-Bcl-2 cells with M mRNA induced less activation of both caspase-8 and caspase-9. Bcl-2 has been shown to inhibit the caspase-9 pathway and was predicted to inhibit caspase-9 activation after transfection with M mRNA. However, because Bcl-2 also inhibits induction of caspase-8-like activity, this indicates that the caspase-8 activation by M protein involves cross talk between the two apoptotic pathways.
FIG. 6.
Overexpression of Bcl-2 reduces M protein and actinomycin D-induced activation of caspase-8 and -9. The HeLa cell lines were either transfected with M mRNA (A and B), treated with actinomycin D (C and D), or treated with TRAIL (E and F) and analyzed for the activation of caspase-8-like activity (A, C, and E) or caspase-9-like activity (B, D, and F) with fluorogenic substrates preferentially cleaved by each caspase. The HeLa cell lines were transfected with M mRNA for 18 (open bars) or 24 (solid bars) h. Cells were transfected with yeast RNA as negative controls. Treatment with actinomycin D was for 6 (open bars) or 12 (solid bars) h, and cells harvested at 0 h were negative controls. Treatment with TRAIL was for 6 h, and cells harvested at 0 h were negative controls. Cell lysates were analyzed for caspase-8- or -9-like activities and protein concentrations as described in the legend to Fig. 2. The amount of caspase-8 and -9 activity is expressed in arbitrary fluorescence units per milligram of protein. The data represent the average ± standard deviation of three experiments.
In Fig. 6C and D, cells were treated with actinomycin D for 0 h as a negative control or for 6 or 12 h. Similar to the results of M protein expression, HeLa cells and HeLa-empty vector cells had considerable amounts of both caspase-8-like and caspase-9-like activities after treatment with actinomycin D (Fig. 6C and D). However, overexpression of Bcl-2 in HeLa-Bcl-2 cells inhibited activation of both caspases-8 and -9 after treatment with actinomycin D (Fig. 6C and D). These results indicate that the caspase-9 pathway is important for actinomycin D-induced apoptosis and that the caspase-8 activation by actinomycin D involves cross talk between the two apoptotic pathways. The results of the experiments with actinomycin D were similar to the results with M protein, supporting the hypothesis that actinomycin D and M protein activate the same caspase pathways.
In Fig. 6E and F, cells were treated with TRAIL for 0 h as a negative control or for 6 h. Both HeLa cells and HeLa-empty vector cells had considerable amounts of both caspase-8-like and caspase-9-like activities after treatment with TRAIL (Fig. 6E and F). Unlike the results for M protein and actinomycin D, there was no decrease in caspase-8 or -9 activation in HeLa-Bcl-2 cells. This is the expected result for TRAIL, since it is known to induce apoptosis through the caspase-8 pathway but not the caspase-9 pathway. Thus, an inhibitor of the caspase-9 pathway would not be expected to inhibit apoptosis induced by TRAIL.
Caspase-8 inhibitor does not inhibit apoptosis induced by M protein, actinomycin D, or DRB.
The observation that caspase-8-like activity was induced by M protein and actinomycin D raises the question of whether the caspase-8 pathway, as well as the caspase-9 pathway, plays an important role in apoptosis by these inducers. To address this question, cells were pretreated with a synthetic inhibitor of caspase-8, Z-IETD-FMK, and then were either transfected with M mRNA or treated with TRAIL, actinomycin D, or DRB for 30 h. Cells were then analyzed for the morphological changes associated with apoptosis. Representative phase-contrast images from these experiments are shown in Fig. 7. In order to quantify the results obtained with the caspase-8 inhibitor, the numbers of round and flat cells were counted in images taken from three experiments. The percentage of round cells for each sample is shown in Fig. 8. Cell rounding was chosen as the criterion for cells that have entered into apoptosis, because it is the longest-lasting morphological change observed in apoptotic cells at this magnification.
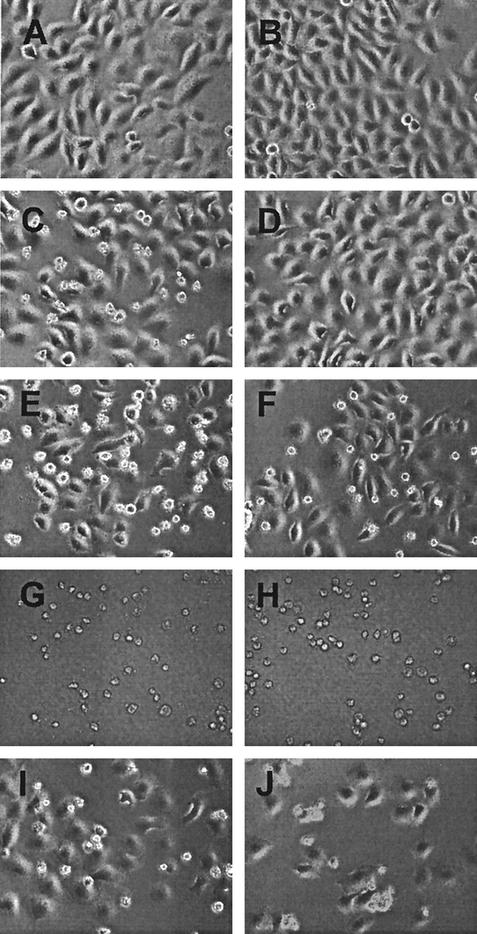
FIG. 7.
Images of cells transfected with M mRNA or treated with inhibitors of host gene expression in the presence of a caspase-8 inhibitor. HeLa cells were untreated (A) or transfected with yeast RNA (B) as negative controls. HeLa cells were treated with TRAIL as a positive control (C and D), transfected with M mRNA (E and F), treated with actinomycin D (G and H), or treated with DRB (I and J). Cells shown in panels D, F, H, and J were pretreated with 100 μM synthetic caspase-8 inhibitor, Z-IETD-FMK. Phase-contrast images were captured with a ×20 objective. Representative images were chosen from three separate experiments.
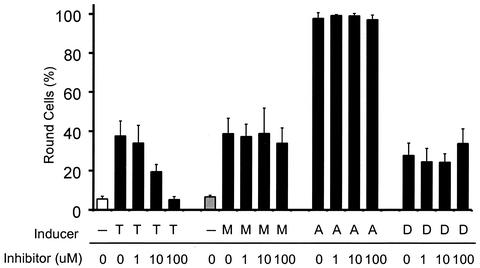
FIG. 8.
The caspase-8 inhibitor does not prevent the morphological changes associated with apoptosis induced by M protein, actinomycin D, or DRB. Phase-contrast images were captured of HeLa cells transfected with M mRNA (M), or treated with TRAIL (T), actinomycin D (A), or DRB (D) in the presence of the indicated concentrations of the caspase-8 inhibitor as described in the legend to Fig. 7. HeLa cells were untreated (open bar) or transfected with yeast RNA (shaded bar) as negative controls. Between 300 and 700 cells in five representative images for each sample were scored as either flat or round. The data are expressed as the percentage of round cells and represent the average ± standard deviation of three experiments.
HeLa cells were either untreated (Fig. 7A) or transfected with yeast RNA (Fig. 7B) as negative controls. Most of the cells in Fig. 7A and B are flat, but there are a few round cells, which are cells undergoing cell division. The percentages of round cells in these samples were approximately 5 and 6%, respectively (Fig. 8). HeLa cells treated with the positive control, TRAIL, for 6 h (Fig. 7C) display an increase in the percentage of round cells to approximately 38% (Fig. 7C and 8). Many of the round cells in Fig. 7C display cell shrinkage, and some also appear to be undergoing membrane blebbing at higher magnifications (data not shown), which are characteristic morphological changes associated with apoptosis. As expected, there was a measurable decrease in the percentage of round cells after treatment with TRAIL in the presence of increasing concentrations of caspase-8 inhibitor (Fig. 8). This indicates that the caspase-8 inhibitor prevents apoptosis induced by TRAIL. These are the expected results for TRAIL, since it is known to inhibit apoptosis through the activation of the caspase-8 pathway.
Approximately 38% of HeLa cells transfected with M mRNA for 30 h were round and were also undergoing other morphological changes associated with apoptosis (Fig. 7E and 8). The remaining cells that were still flattened were cells that were not transfected. Unlike the result for cells treated with TRAIL, the caspase-8 inhibitor did not decrease the percentage of cells undergoing cell rounding or other morphological changes associated with apoptosis. After transfection with M mRNA, even in the presence of the highest concentration of inhibitor (100 μM), the amount of round cells (34%) was similar to that in the sample without caspase-8 inhibitor (Fig. 7F and 8). Thus, the caspase-8 inhibitor did not decrease the amount of round cells and did not prevent M protein-induced apoptosis. This indicates that the activation of the caspase-8 pathway is not important for the induction of apoptosis by M protein.
Similarly, the presence of the caspase-8 inhibitor did not decrease the amount of apoptotic cells treated with actinomycin D, because 97% of cells were round in the samples treated with actinomycin D in the absence or presence of the caspase-8 inhibitor (Fig. 7G and H and 8). In the case of cells treated with DRB, 28% of the cells were round (Fig. 7I and 8), indicating that DRB is a less-potent inducer of cell death than actinomycin D. The presence of the caspase-8 inhibitor did not decrease the percentage of apoptotic cells in samples treated with DRB (Fig. 7J and 8), indicating that the caspase-8 pathway is less important than the caspase-9 pathway for the induction of apoptosis by DRB, similar to the results obtained with actinomycin D and M protein.
DISCUSSION
It had previously been determined that at least two products of viral infection contribute to the induction of apoptosis in VSV-infected cells (24). One of these viral products is M protein, and the other product has yet to be identified. Our data indicated that wt M protein accelerates VSV-induced apoptosis in HeLa cells and delays VSV-induced apoptosis in BHK cells. We have also determined that M protein delays VSV-induced apoptosis in other cell lines such as mouse L cells and mouse prostate epithelial cell lines (unpublished data). The goal of the experiments presented here was to test the hypothesis that both the M protein-induced acceleration in HeLa cells and delay in BHK cells during VSV-induced apoptosis are due to the inhibition of host gene expression. In our hypothesis, the fundamental difference between the induction of apoptosis in HeLa cells and that in BHK cells is that HeLa cells do not require new host gene expression for induction of apoptosis, whereas BHK cells do require new host gene expression. This hypothesis was tested by infecting cells with an M protein mutant virus (rM51R-M virus) in the presence or absence of the pharmacologic inhibitors of host gene expression actinomycin D and DRB. Actinomycin D and DRB accelerated induction of apoptosis of HeLa cells infected with rM51R-M virus and delayed apoptosis in BHK cells infected with rM51R-M virus, similar to the effects of wt M protein (Fig. 1 and 2). Thus, pharmacologic inhibitors of host gene expression can compensate for the inability of rM51R-M virus to induce apoptosis at the same rate as rwt virus.
The hypothesis that the induction of apoptosis by M protein is due to its ability to inhibit host gene expression is further supported by the shared properties of apoptosis induced by M protein to apoptosis induced by other inhibitors of host gene expression. The caspase-9 pathway is more often activated by internal cellular damage, in contrast to the caspase-8 pathway, which is more often activated by external stimuli binding to cell death receptors. Thus, we expected that the caspase-9 pathway would be important for apoptosis induced by pharmacologic inhibitors of host gene expression. Caspases similar to both caspase-8 and caspase-9 were activated by M protein, actinomycin D, and DRB (Fig. 6) (data not shown). In most cases of apoptosis, both upstream caspases are activated, but often an inducer will activate one caspase pathway, and the other pathway will be activated by cross talk. Caspase-9 can be activated as an indirect effect of activation of caspase-8, through cleavage of Bid (4). Likewise, caspase-8 can be activated as an indirect effect of activation of caspase-9 following cleavage of caspase-3 (38). To differentiate whether activation of one or both caspase pathways is necessary for apoptosis induced by M protein or other inhibitors of host gene expression, inhibitors of the two main caspase pathways were used. Inhibition of the caspase-9 pathway was achieved by overexpression of Bcl-2, while inhibition of the caspase-8 pathway was achieved by using a synthetic caspase-8 inhibitor. Our results indicate that the caspase-9 pathway is more important than the caspase-8 pathway for apoptosis induced by M protein, actinomycin D, and DRB, suggesting that M protein and the pharmacologic inhibitors of host gene expression induce apoptosis through the activation of the caspase-9 pathway and the caspase-8 pathway is activated by cross talk (Fig. 3, 4, 7, and 8). The fact that M protein activates the caspase-9 pathway, the pathway more often activated by internal cellular damage, also supports the hypothesis that induction of apoptosis by M protein is caused by damage or stress to the cell caused by the inhibition of host gene expression.
It was also determined that Bcl-2 reduces apoptosis induced by both rwt and rM51R-M viruses (Fig. 5). It was anticipated that Bcl-2 would decrease apoptosis induced by VSV, since wt M protein accelerates apoptosis induced by VSV in HeLa cells (Fig. 1 and 2), and Bcl-2 inhibits apoptosis induced by M protein (Fig. 3 and 4). This result is consistent with another report that Bcl-2 and Bcl-XL partially protect neuronal cells from apoptosis induced by other strains of VSV (14). Similarly, caspase-9 is activated to a greater extent than caspase-8 during VSV infection of mouse 3T3 cells, indicating the importance of the caspase-9 pathway in VSV-induced apoptosis (7). This is corroborated by the results that a synthetic inhibitor of caspase-9 reduces apoptosis by VSV (6). All of these data emphasize the importance of the activation of caspase-9 during VSV-induced apoptosis.
While M protein contributes to induction of apoptosis by VSV, there is clearly another viral product that induces apoptosis in VSV-infected cells. The presence of another viral inducer of apoptosis was most apparent in BHK cells when comparing apoptosis induced by rwt virus and that induced by r-M51R-M virus, which contains a mutant M protein that is defective in induction of apoptosis. BHK cells infected with r-M51R-M virus enter apoptosis more rapidly than cells infected with rwt virus, indicating that a product of viral infection other than M protein is contributing to apoptosis of BHK cells infected with VSV and that the inhibition of host gene expression by M protein delays apoptosis induced by VSV (Fig. 1 and 2). This result raises two important issues: the identity of other products of viral infection that are contributing to apoptosis induced by VSV and the identity of the host proteins whose expression is necessary for apoptosis induced by VSV.
The other products of viral infection may either be a viral protein or combination of viral proteins other than M protein, a viral RNA, or other viral products. Perhaps the most likely candidate is viral double-stranded RNA (dsRNA) (13, 27). dsRNA is a byproduct of viral replication and is a major activator of protein kinase R (PKR), an antiviral product that is known to induce apoptosis (5, 36). The ability of a dominant-negative mutant of PKR to suppress VSV-induced apoptosis is consistent with this hypothesis (7). However, treatment with dsRNA primarily activates caspase-8 rather than caspase-9 (7). Leader RNA is another viral product that may contribute to induction of apoptosis of VSV-infected cells. Leader RNA is a small (50 nucleotides) transcript from the 3′ end of the genome, which does not encode a protein. Leader RNA has been implicated in the cytopathic effects of VSV infection and could play a role in the induction of apoptosis (15, 20, 29, 33). The viral glycoprotein (G protein) is another candidate that may contribute to induction of apoptosis of VSV-infected cells. For example, G protein might bind to cellular receptors that activate pathways involved in induction of apoptosis. Stable cell lines have been made that express the VSV G protein, indicating that expression of G protein is not inherently toxic to cells (17). Thus, G protein probably cannot induce apoptosis in the absence of other products of viral infection. However, it is possible that during a viral infection, G protein may interact with other viral products to activate cell death receptors. Our future experiments will be to identify the viral products other than M protein that contribute to the induction of apoptosis by VSV.
The observation that new host gene expression is necessary for VSV-infected BHK cells to undergo apoptosis rapidly (Fig. 1 and 2) is consistent with data in other systems. For example, actinomycin D has been shown to delay apoptosis induced by coxsackievirus B4 in neurons (23), and actinomycin D reduces apoptosis induced by other agents, such as sodium arsenite, also in neurons (30). This indicates that there are other cell types that also require new host gene expression in order to undergo apoptosis.
There are several examples of proapoptotic gene products whose synthesis is stimulated by inducers of apoptosis. For example, microarray analysis has shown that treatment of glioma cells with dsRNA induces the expression of many genes encoding proteins involved in apoptosis, such as proteins of the TNF signaling pathway (19). Similarly, measles virus infection induces expression of proapoptotic proteins that are involved in the host cell stress response, including growth arrest and DNA damage-inducible protein 153 (GADD153), a transcription factor associated with apoptosis, and Herp, an endoplasmic reticulum stress-inducible protein (11). Our future experiments will address which proapoptotic host gene products are induced by VSV and which are suppressed by M protein.
Acknowledgments
We acknowledge the Microscopy Core Laboratory at Wake Forest University, especially Mark Willingham for critical advice. We thank Griffith Parks for helpful advice and comments on the manuscript.
This work was supported by Public Health Service grants AI 32983 and AI 15892 from the National Institute of Allergy and Infectious Diseases. S.A.K. was supported by the National Institute of Health Training grant T32 A107401. The microscopy Core Laboratory was supported in part by the core grant for the Comprehensive Cancer Center of Wake Forest University CA12197 for the National Cancer Institute.
REFERENCES
- 1.Ahmed, M., and D. S. Lyles. 1997. Identification of a consensus mutation in M protein of vesicular stomatitis virus from persistently infected cells that affects inhibition of host-directed gene expression. Virology 237:378-388. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed, M., and D. S. Lyles. 1998. Effect of vesicular stomatitis virus matrix protein on transcription directed by host RNA polymerases I, II, and III. J. Virol. 72:8413-8419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2a.Ahmed, M., M. O. McKenzie, S. Puckett, M. Hojnacki, L. Poliquin, and D. S. Lyles. 2003. Ability of the matrix protein of vesicular stomatitis virus to suppress beta interferon gene expression is genetically correlated with the inhibition of host RNA and protein synthesis. J. Virol. 77:4646-4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albrecht, H., J. Tschopp, and C. V. Jongeneel. 1994. Bcl-2 protects from oxidative damage and apoptotic cell death without interfering with activation of NF-kappa B by TNF. FEBS Lett. 351:45-48. [DOI] [PubMed] [Google Scholar]
- 4.Antonsson, B., and J. C. Martinou. 2000. The Bcl-2 protein family. Exp. Cell Res. 256:50-57. [DOI] [PubMed] [Google Scholar]
- 5.Balachandran, S., C. N. Kim, W. C. Yeh, T. W. Mak, K. Bhalla, and G. N. Barber. 1998. Activation of the dsRNA-dependent protein kinase, PKR, induces apoptosis through FADD-mediated death signaling. EMBO J. 17:6888-6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balachandran, S., M. Porosnicu, and G. N. Barber. 2001. Oncolytic activity of vesicular stomatitis virus is effective against tumors exhibiting aberrant p53, Ras, or Myc function and involves the induction of apoptosis. J. Virol. 75:3474-3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balachandran, S., P. C. Roberts, T. Kipperman, K. N. Bhalla, R. W. Compans, D. R. Archer, and G. N. Barber. 2000. Alpha/beta interferons potentiate virus-induced apoptosis through activation of the FADD/caspase-8 death signaling pathway. J. Virol. 74:1513-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Black, B. L., G. Brewer, and D. S. Lyles. 1994. Effect of vesicular stomatitis virus matrix protein on host-directed translation in vivo. J. Virol. 68:555-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Black, B. L., and D. S. Lyles. 1992. Vesicular stomatitis virus matrix protein inhibits host cell-directed transcription of target genes in vivo. J. Virol. 66:4058-4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Black, B. L., R. B. Rhodes, M. McKenzie, and D. S. Lyles. 1993. The role of vesicular stomatitis virus matrix protein in inhibition of host-directed gene expression is genetically separable from its function in virus assembly. J. Virol. 67:4814-4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bolt, G., K. Berg, and M. Blixenkrone-Moller. 2002. Measles virus-induced modulation of host-cell gene expression. J. Gen. Virol. 83:1157-1165. [DOI] [PubMed] [Google Scholar]
- 12.Collins, J. A., C. A. Schandi, K. K. Young, J. Vesely, and M. C. Willingham. 1997. Major DNA fragmentation is a late event in apoptosis. J. Histochem. Cytochem. 45:923-934. [DOI] [PubMed] [Google Scholar]
- 13.Der, S. D., Y. L. Yang, C. Weissmann, and B. R. Williams. 1997. A double-stranded RNA-activated protein kinase-dependent pathway mediating stress-induced apoptosis. Proc. Natl. Acad. Sci. USA 94:3279-3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desforges, M., G. Despars, S. Berard, M. Gosselin, M. O. McKenzie, D. S. Lyles, P. J. Talbot, and L. Poliquin. 2002. Matrix protein mutations contribute to inefficient induction of apoptosis leading to persistent infection of human neural cells by vesicular stomatitis virus. Virology 295:63-73. [DOI] [PubMed] [Google Scholar]
- 15.Dunigan, D. D., S. Baird, and J. Lucas-Lenard. 1986. Lack of correlation between the accumulation of plus-strand leader RNA and the inhibition of protein and RNA synthesis in vesicular stomatitis virus infected mouse L cells. Virology 150:231-246. [DOI] [PubMed] [Google Scholar]
- 16.Ferran, M. C., and J. M. Lucas-Lenard. 1997. The vesicular stomatitis virus matrix protein inhibits transcription from the human beta interferon promoter. J. Virol. 71:371-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Florkiewicz, R. Z., A. Smith, J. E. Bergmann, and J. K. Rose. 1983. Isolation of stable mouse cell lines that express cell surface and secreted forms of the vesicular stomatitis virus glycoprotein. J. Cell Biol. 97:1381-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fulda, S., E. Meyer, and K. M. Debatin. 2002. Inhibition of TRAIL-induced apoptosis by Bcl-2 overexpression. Oncogene 21:2283-2294. [DOI] [PubMed] [Google Scholar]
- 19.Geiss, G., G. Jin, J. Guo, R. Bumgarner, M. G. Katze, and G. C. Sen. 2001. A comprehensive view of regulation of gene expression by double-stranded RNA-mediated cell signaling. J. Biol. Chem. 276:30178-30182. [DOI] [PubMed] [Google Scholar]
- 20.Grinnell, B. W., and R. R. Wagner. 1983. Comparative inhibition of cellular transcription by vesicular stomatitis virus serotypes New Jersey and Indiana: role of each viral leader RNA. J. Virol. 48:88-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Her, L. S., E. Lund, and J. E. Dahlberg. 1997. Inhibition of Ran guanosine triphosphatase-dependent nuclear transport by the matrix protein of vesicular stomatitis virus. Science 276:1845-1848. [DOI] [PubMed] [Google Scholar]
- 22.Jaattela, M., M. Benedict, M. Tewari, J. A. Shayman, and V. M. Dixit. 1995. Bcl-x and Bcl-2 inhibit TNF and Fas-induced apoptosis and activation of phospholipase A2 in breast carcinoma cells. Oncogene 10:2297-2305. [PubMed] [Google Scholar]
- 23.Joo, C. H., Y. K. Kim, H. Lee, H. Hong, S. Y. Yoon, and D. Kim. 2002. Coxsackievirus B4-induced neuronal apoptosis in rat cortical cultures. Neurosci. Lett. 326:175-178. [DOI] [PubMed] [Google Scholar]
- 24.Kopecky, S. A., M. C. Willingham, and D. S. Lyles. 2001. Matrix protein and another viral component contribute to induction of apoptosis in cells infected with vesicular stomatitis virus. J. Virol. 75:12169-12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koyama, A. H. 1995. Induction of apoptotic DNA fragmentation by the infection of vesicular stomatitis virus. Virus Res. 37:285-290. [DOI] [PubMed] [Google Scholar]
- 26.Laub, O., E. B. Jakobovits, and Y. Aloni. 1980. 5,6-Dichloro-1-B-ribofuranosylbenzimidazole enhances premature termination of late transcription of simian virus 40 DNA. Proc. Natl. Acad. Sci. USA 77:3297-3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee, S. B., and M. Esteban. 1994. The interferon-induced double-stranded RNA-activated protein kinase induces apoptosis. Virology 199:491-496. [DOI] [PubMed] [Google Scholar]
- 28.Lyles, D. S., and M. O. McKenzie. 1997. Activity of vesicular stomatitis virus M protein mutants in cell rounding is correlated with the ability to inhibit host gene expression and is not correlated with virus assembly function. Virology 299:77-89. [DOI] [PubMed] [Google Scholar]
- 29.McGowan, J. J., S. U. Emerson, and R. R. Wagner. 1982. The plus-strand leader RNA of VSV inhibits DNA-dependent transcription of adenovirus and SV40 genes in a soluble whole-cell extract. Cell 28:325-333. [DOI] [PubMed] [Google Scholar]
- 30.Namgung, U., and Z. Xia. 2001. Arsenic induces apoptosis in rat cerebellar neurons via activation of JNK3 and p38 MAP kinases. Toxicol. Appl. Pharmacol. 174:130-138. [DOI] [PubMed] [Google Scholar]
- 31.Paik, S.-Y., A. C. Banerjea, G. G. Harmison, C.-J. Chen, and M. Schubert. 1995. Inducible and conditional inhibition of human immunodeficiency virus proviral expression by vesicular stomatitis virus matrix protein. J. Virol. 69:3529-3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petersen, J. M., L.-S. Her, V. Varvel, E. Lund, and J. E. Dahlberg. 2000. The matrix protein of vesicular stomatitis virus inhibits nucleocytoplasmic transport when it is in the nucleus and associated with nuclear pore complexes. Mol. Cell. Biol. 20:8590-8601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poirot, M. K., W. M. Schnitzlein, and M. E. Reichmann. 1985. The requirement of protein synthesis for VSV inhibition of host cell RNA synthesis. Virology 140:91-101. [DOI] [PubMed] [Google Scholar]
- 34.Re, G. G., D. J. Hazen-Martin, R. El Bahtimi, N. A. Brownlee, M. C. Willingham, and A. J. Garvin. 1999. Prognostic significance of Bcl-2 in Wilms' tumor and oncogenic potential of Bcl-X(L) in rare tumor cases. Int. J. Cancer 84:192-200. [DOI] [PubMed] [Google Scholar]
- 35.Seol, D. W., J. Li, M. H. Seol, S. Y. Park, R. V. Talanian, and T. R. Billiar. 2001. Signaling events triggered by tumor necrosis factor-related apoptosis-inducing ligand (TRAIL): caspase-8 is required for TRAIL-induced apoptosis. Cancer Res. 61:1138-1143. [PubMed] [Google Scholar]
- 36.Thomis, D. C., and C. E. Samuel. 1995. Mechanism of interferon action: characterization of the intermolecular autophosphorylation of PKR, the interferon-inducible, RNA-dependent protein kinase. J. Virol. 69:5195-5198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.von Kobbe, C., J. M. van Deursen, J. P. Rodrigues, D. Sitterlin, A. Bachi, X. Wu, M. Wilm, M. Carmo-Fonseca, and E. Izaurralde. 2000. Vesicular stomatitis virus matrix protein inhibits host cell gene expression by targeting the nucleoporin nup98. Mol. Cell 6:1243-1252. [DOI] [PubMed] [Google Scholar]
- 38.Wieder, T., F. Essmann, A. Prokop, K. Schmelz, K. Schulze-Osthoff, R. Beyaert, B. Dorken, and P. T. Daniel. 2001. Activation of caspase-8 in drug-induced apoptosis of B-lymphoid cells is independent of CD95/Fas receptor-ligand interaction and occurs downstream of caspase-3. Blood 97:1378-1387. [DOI] [PubMed] [Google Scholar]
- 39.Yuan, H., S. Puckett, and D. S. Lyles. 2001. Inhibition of host transcription by vesicular stomatitis virus involves a novel mechanism that is independent of phosphorylation of TATA-binding protein (TBP) or association of TBP with TBP-associated factor subunits. J. Virol. 75:4453-4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuan, H., B. K. Yoza, and D. S. Lyles. 1998. Inhibition of host RNA polymerase II-dependent transcription by vesicular stomatitis virus results from inactivation of TFIID. Virology 251:383-392. [DOI] [PubMed] [Google Scholar]
- 41.Zandomeni, R., M. C. Zandomeni, D. Shugar, and R. Weinmann. 1986. Casein kinase type II is involved in the inhibition by 5,6-dichloro-1-β-d-ribofuranosylbenzimidazole of specific RNA polymerase II transcription. J. Biol. Chem. 261:3414-3419. [PubMed] [Google Scholar]