Abstract
Epstein-Barr virus (EBV) is a potent growth-transforming agent of human B cells. It has previously been shown that viral latent membrane protein 1 (LMP1) is essential for EBV-induced transformation of normal B cells and contributes to maintenance of latency in vitro. Using the EBV-positive Burkitt's lymphoma line P3HR1-c16, which lacks LMP1 during latency and which can readily be activated into virus-productive lytic cycle, we found that LMP1 inhibits lytic cycle induction via the transcription factor NF-κB. In addition, LMP1 inhibits lytic cycle progress via two distinct NF-κB-independent mechanisms: one involving the cytosolic C-terminal activating regions and the other involving the transmembrane region of LMP1. These findings indicate that in B cells EBV self-limits its lytic cycle via three distinct LMP1-mediated mechanisms.
Epstein-Barr virus (EBV) is a gammaherpesvirus infecting more than 90% of adults worldwide. Following infection in immunocompetent individuals, the virus generally establishes asymptomatic lifelong persistence in B lymphocytes (53). Most EBV-infected B cells in the healthy host show a resting phenotype (38) with limited expression of viral genes (42). However, some infected cells express several latent viral genes (15, 54) that lead to growth transformation of B cells (44, 49). This is thought to be one mechanism for expansion of the EBV-infected B-cell population (44). In addition, the replicative lytic cycle, which produces infectious virus particles, is also thought to help expand the pool of infected cells (41). For transmission of the virus to new hosts, nonpersistent lytic infection of epithelial cells may be important (5).
EBV growth transformation of primary B cells requires at least five key latent viral genes (33). One of these genes encodes latent membrane protein 1 (LMP1) (34). This enhances cell survival through upregulation of antiapoptotic genes (12, 24, 35, 56). LMP1 has six hydrophobic transmembrane domains, facilitating spontaneous self-oligomerization and creating a complex that constitutively activates cell signaling pathways via a cytoplasmic C terminus that mimics tumor necrosis factor (TNF) receptor superfamily members (22). The C-terminal activating region 1 (CTAR1) of LMP1 is located proximal to the membrane and binds the TNF receptor-associated factors (TRAFs) (10, 26). CTAR2 is located toward the far C terminus of LMP1 and binds the TNF receptor-associated death domain protein (TRADD) and receptor-interacting protein (RIP) (13, 26, 30). Consequently, LMP1 triggers several signaling pathways that lead to the activation of several transcription factors, including NF-κB, STATs, AP-1, and ATF2 (6, 13, 14, 21, 26). Of all the transcription factors targeted by LMP1, NF-κB attains the highest level of activity (6) and is essential for most LMP1-stimulated gene expression (9, 23, 37, 40, 59).
Recently, it was reported that both LMP1 and activated CD40, a TNF receptor superfamily member upregulated by LMP1, impaired induction of the lytic cycle of EBV (2). Both LMP1 and CD40 are potent activators of NF-κB (20), although whether this is how they inhibit lytic cycle induction was not tested. Paradoxically, it has been shown that full-length LMP1 is expressed during the lytic cycle in EBV-positive B cells (48). Very little is known about the functions of LMP1 during the lytic cycle (32).
It is difficult to study the lytic cycle of EBV because of the lack of a fully permissive culture system (32, 33). Normal B cells transformed with EBV in vitro give rise to lymphoblastoid cell lines displaying a latency III phenotype, where about 11 transformation-associated genes are expressed (44, 49). A small percentage of these cells (usually less than 1%) may spontaneously enter the lytic cycle (32, 33, 46, 49). EBV-positive Burkitt's lymphoma (BL) cell lines generally show a more restricted, LMP1-deficient, latency I pattern of EBV latent gene expression, where EBNA1 is the only viral protein expressed (48, 49), and do not enter the lytic cycle in vitro. Some BL cell lines can be induced to enter the lytic cycle with variable efficiency (typically 5 to 40%) by treatment with chemicals such as phorbol esters or N-butyrate, by ligation of surface immunoglobulin, or by transfection with plasmid expressing a lytic cycle transactivator (1, 11, 48, 50). The P3HR1-c16 subclone of the Jijoye BL cell line is particularly easy to work with, as it is highly inducible (32, 43) and is transfectable (32).
The exact signal that triggers the lytic cycle of EBV in vivo remains unknown (28, 33), but once triggered, the lytic cycle gains impetus from the transactivators Zta and Rta, which drive their own and each other's expression via their respective promoters, Zp and Rp (7, 16, 19, 28). These transcription factors then drive expression of early lytic cycle gene subsets (16), and a cascade is initiated. Plasmid-like replication of the EBV genome (from OriP) is followed by rolling-circle replication (from OriLyt) (18). From this point (12 to 24 h postinduction), late lytic cycle antigens, including many structural proteins, are produced (16, 48). In vitro, cells remain viable for a further 2 to 6 days until the lytic cycle culminates in the release of infectious virions (33). This prolongation of the lytic cycle is intriguing, and in this context LMP1 is of particular interest, as LMP1 promotes cell survival during latency (12, 24, 35, 56), impairs lytic cycle induction via an unidentified mechanism (2), and is expressed in those B cells that succeed in entering the lytic cycle (46, 48). We therefore postulated that LMP1 might prolong the lytic cycle. Using the inducible P3HR1-c16 cell line as a model, we have obtained data suggesting that this is indeed the case. Furthermore, we demonstrate that the maintenance of latency and the inhibition of lytic cycle progress are due to three different LMP1-mediated mechanisms.
LMP1 inhibits EBV lytic cycle induction via NF-κB.
In order to determine how LMP1 inhibits lytic cycle induction, we developed a model for the measurement of lytic cycle induction in cotransfected P3HR1-c16 cells, as shown in Fig. 1. Cultured cells were cotransfected with an expression plasmid for an inert green fluorescent marker (rCD2-EGFP) and either a control empty vector or the pSG5-LMP1 expression plasmid (26). Plasmid rCD2-EGFP was created by in-frame insertion of rat CD2 transmembrane region cDNA into pEGFP-N1 (Clontech). The cells were cultured for 48 h and induced to enter the lytic cycle with sodium butyrate, a histone deacetylase inhibitor that triggers broad-ranging gene expression (29). The cells were then fixed, permeabilized, and analyzed for Zta expression by flow cytometry. Figure 1A shows representative dot plots (originally two-color plots but shown here in black and white), which demonstrate that 10.4% of the control, yet only 2.1% of the LMP1-transfected cells, were induced to enter the lytic cycle. This represents a 77% inhibition of lytic cycle induction by LMP1. Without the addition of sodium butyrate, cells were not induced to enter the lytic cycle (data not shown).
FIG. 1.
LMP1-mediated inhibition of EBV lytic cycle induction. (A) Zta expression in cells induced to lytic cycle with sodium butyrate. P3HR1-c16 cells were cultured and electroporated, using methods described previously (32), with 6 μg of plasmid rCD2-EGFP and either 3 μg of empty pSG5 vector (control) or 3 μg of the B95.8 LMP1 expression plasmid, pSG5-LMP1 (26). Electroporation was performed using a Bio-Rad Genepulser II electroporator set at 950 μF and 240 V. The cells were seeded in 4 ml of fresh medium and cultured for 48 h before induction to the lytic cycle by resuspension in fresh medium containing 3 mM sodium butyrate. The cells were cultured for a further 24 h, fixed, permeabilized, and stained for Zta expression as described previously (32) using 1 μg of murine monoclonal antibody BZ.1 (58) per ml, and red phycoerythrin (RPE)- conjugated anti-mouse immunoglobulin G. The cells were analyzed by flow cytometry (40,000 events) using a FACScalibur flow cytometer (Becton Dickinson Co., San Jose, Calif.). The left panel shows a dot plot of the data obtained for cells cotransfected with empty pSG5 vector; the right panel shows data for the cells cotransfected with LMP1 expression plasmid. (B) Mechanism of LMP1-mediated inhibition of EBV lytic cycle induction. The experiment described above was repeated in triplicate and extended to include cotransfections of 6 μg of rCD2-EGFP expression plasmid with 3 μg of pSG5-LMP1AAAG expression plasmid (6) or 3 μg of pSG5-LMP1 plus 0.2 μg of IκBαΔN expression plasmids. Lytic cycle induction was calculated as the percentage of EGFP-positive cells that expressed Zta. The histogram shown displays means and standard errors (indicated by the error bars). One-way ANOVA P values and pooled-variance 95% confidence intervals were calculated using Minitab software (Minitab Inc., Pennsylvania State University). (C) Western blot analysis of LMP1 expression. Protein extracts of the cotransfections from panel B were analyzed for LMP1 expression by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting with murine monoclonal antibodies CS.1 to CS.4 (46) as described previously (17, 47). The positions of molecular size standards (in kilodaltons) are shown to the left of the gel.
Given that both LMP1 and CD40 can inhibit EBV lytic cycle induction (2) and that both are potent activators of NF-κB (20), we hypothesized that LMP1 might inhibit lytic cycle induction via NF-κB. We tested this by cotransfecting IκBαΔN expression plasmid (kindly provided by Dean W. Ballard, Howard Hughes Medical Institute, Nashville, Tenn.) to block LMP1-mediated NF-κB activation. Cytoplasmic IκB proteins, of which IκBαΔN is a degradation-resistant variant, inhibit NF-κB (31). We also cotransfected plasmid expressing the nonsignaling mutant of LMP1, LMP1AAAG, which is mutated at the CTAR1 and CTAR2 domains and does not bind TRAFs or TRADD (6). Experiments utilizing the procedure outlined in the legend to Fig. 1A were performed in triplicate, and lytic cycle induction was calculated. The results, shown in Fig. 1B, were analyzed by analysis of variance (ANOVA) (P < 0.001), and the corresponding 95% confidence intervals confirmed that the induction of LMP1 transfectants was significantly lower than that for the control by approximately 68%. Furthermore, induction of cells cotransfected with LMP1AAAG or with LMP1 plus IκBαΔN expression plasmids was not significantly different from that of the control. Thus, IκBαΔN abolished LMP1-mediated inhibition of lytic cycle induction, and LMP1AAAG, which cannot induce NF-κB (6), did not inhibit lytic cycle induction. A representative immunoblot probed with LMP1-specific antibodies (Fig. 1C) confirmed that IκBαΔN did not affect LMP1 expression and that LMP1AAAG was expressed at a level similar to that of wild-type LMP1. From the data in Fig. 1, we deduce that LMP1 inhibits lytic cycle induction via NF-κB.
Basal NF-κB activity inhibits lytic cycle induction.
Having demonstrated that LMP1 can inhibit lytic cycle induction via NF-κB, we tested whether basal NF-κB activity also modulates lytic cycle induction. The experimental procedure described in the legend to Fig. 1A was repeated for triplicate cotransfections of rCD2-EGFP expression plasmid with increasing doses of IκBαΔN expression plasmid (Fig. 2A). ANOVA analysis of the results (P < 0.002) revealed that induction of Zta-positive cells significantly increased with IκBαΔN dose. Figure 2B shows the results of luciferase reporter assays for NF-κB activity in similar cotransfections. The results confirm that basal NF-κB activity decreased with increasing IκBαΔN dose. We therefore conclude that basal NF-κB activity contributes to the inhibition of lytic cycle induction.
FIG. 2.
Effect of basal NF-κB activity on EBV lytic cycle induction. (A) Effect of increasing IκBαΔN expression plasmid dose on lytic cycle induction. The induction and staining procedures described in the legend to Fig. 1A were performed on triplicate cotransfections of P3HR-c16 cells with 6 μg of rCD2-EGFP expression plasmid and 0-, 0.2-, and 2-μg doses of IκBαΔN expression plasmid. The total amount of DNA per cotransfection was kept constant by the addition of an appropriate amount of empty pSG5. Flow cytometry data were processed as described in the legend to Fig. 1B. (B) Effect of increasing IκBαΔN expression plasmid dose on basal NF-κB activity. The cotransfections for panel A were repeated to include 3 μg of the NF-κB luciferase reporter plasmid 3Enh.κB-ConALuc (3). The cells were assayed for luciferase activity 24 h later as described previously (37). The histogram displays means and standard errors (indicated by the error bars).
LMP1 inhibits expression of early lytic cycle antigens via an NF-κB-independent mechanism.
It was of interest to examine whether the presence of LMP1 during the lytic cycle could hinder progress of the lytic cycle beyond the expression of Zta. To investigate this, P3HR1-c16 cells were cotransfected with an amount of the Zta expression plasmid p509 (45) optimum to induce the lytic cycle (data not shown) together with empty pSG5 vector (control), LMP1 expression plasmid, LMP1 plus IκBαΔN expression plasmids, or LMP1AAAG expression plasmid. In luciferase reporter assays, IκBαΔN was found to reduce lytic cycle NF-κB activity exactly as seen in Fig. 2 (data not shown). Transfection efficiencies were similar, ranging from 1.8 to 2.1% Zta-positive cells as determined by flow cytometry (data not shown). The cells were harvested 24 h later and immunoblotted for the expression of early lytic cycle antigens using a human serum, EE, from a chronic infectious mononucleosis patient with high titers of antibodies to lytic cycle antigens (48). Stripping and reprobing the blots with antibodies for the cellular protein, actin, confirmed equal loading. Representative immunoblots (Fig. 3A) clearly demonstrate that LMP1 expression plasmid reduced expression of Zta and other early lytic cycle antigens (EA). Intriguingly, IκBαΔN did not eliminate this effect. Furthermore, LMP1AAAG expression plasmid did not noticeably affect early lytic cycle antigen levels.
FIG. 3.
Effect of LMP1 on early EBV lytic cycle. (A) Effect of LMP1 on the expression of early lytic cycle antigens. P3HR1-c16 cells were cotransfected with 8 μg of p509 Zta expression plasmid and either 3 μg of empty pSG5 vector (control), 3 μg of LMP1 expression plasmid, 3 μg of LMP1 plus 0.2 μg of IκBαΔN expression plasmids, or 3 μg of LMP1AAAG expression plasmid, as described in the legend to Fig. 1. The total amount of DNA per cotransfection was kept constant by the addition of an appropriate amount of empty pSG5. After 24 h, the cells were probed for expression of early lytic cycle antigens by immunoblotting with EE serum as described previously (32). To confirm equal loads in the lanes, the blot was stripped and reprobed for actin using antiactin antibodies as described previously (47). (B) Expression of exogenous Zta and LMP1. The cotransfections and immunoblots described above were repeated in EBV-negative DG75 cells electroporated at 270 V. Immunoblots were probed with BZ.1 antibody to Zta, CS.1 to CS.4 antibodies to LMP1, or antiactin antibodies, as described previously (32, 47). The positions of molecular size markers (in kilodaltons) are shown to the left of the blots.
To rule out the possibility that the decrease in Zta expression observed in Fig. 3A was due to repression of the heterologous plasmid promoter in p509, the cotransfections were repeated in an EBV-negative B-cell line, DG75. Figure 3B shows representative immunoblots probed for Zta, LMP1, and actin. The uniformity of Zta expression, irrespective of the coexpression of LMP1, IκBαΔN, or LMP1AAAG, indicates that the different Zta levels observed in Fig. 3A were not due to repression of the heterologous promoter.
We next considered whether LMP1 might repress transcription of the endogenous Zta gene. The cotransfection experiment described in the legend to Fig. 3A was repeated (in triplicate) and modified to include the pHEBO:Zp-wt-luc reporter plasmid, in which luciferase gene expression is regulated by the +12 to −552 region of the EBV Zp promoter (kindly provided by Paul Farrell, Ludwig Institute, London, United Kingdom). Flow cytometric analysis of cells immunostained for Zta showed essentially the same percentage of transfected cells in all samples (1.9% ± 0.1%), and measurement of the mean fluorescence intensity of Zta-positive cells (Fig. 4A) showed a significant (P < 0.001 by ANOVA) decrease of Zta expression in samples containing LMP1. However, no significant difference in Zp promoter activity (P < 0.16 by ANOVA) was observed among samples cotransfected with p509, irrespective of LMP1 (Fig. 4B).
FIG. 4.
Effect of LMP1 on Zta expression. The cotransfections described in the legend to Fig. 3A were repeated in triplicate to include 4 μg of the luciferase reporter plasmid for Zta activity, pHEBO:Zp-wt-luc. (A) Flow cytometric analysis of Zta expression. After 24 h, samples of cells were fixed, permeabilized, and analyzed for Zta expression by flow cytometry, as described in the legend to Fig. 1. (B) Zta transcriptional activity in cotransfected cells. Samples of cells were assayed for luciferase activity as described previously (37). Cells cotransfected with empty pSG5 in place of the p509 Zta expression plasmid were included as a negative control. Both histograms shown display means and standard errors (indicated by the error bars).
Figures 3 and 4 showed that LMP1 can impair Zta and EA expression during lytic cycle forcibly induced with exogenous Zta. This is apparently mediated by a CTAR1- or CTAR2-dependent, yet NF-κB-independent, function of LMP1, possibly involving other transcription factors such as AP-1 or ATF-2. Within the lytic cycle, this function of LMP1 reduces Zta expression without affecting the overall level of transcription from Zp (Fig. 4). Together, these results indicate that the observed impairment of Zta (and EA) expression occurs after initiation of the lytic cycle and via a posttranscriptional mechanism.
Both wild-type LMP1 and LMP1AAAG inhibit progress to late lytic cycle.
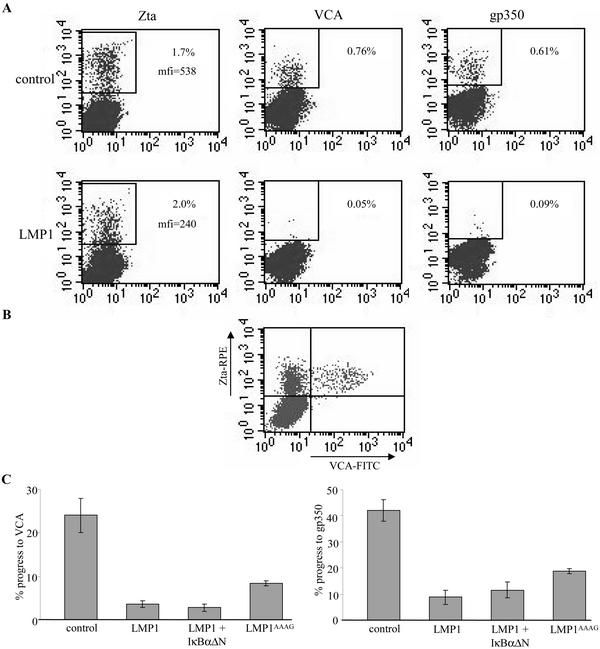
Figures 3 and 4 demonstrated that expression of exogenous LMP1 during the lytic cycle inhibits stages of the early lytic cycle postinduction independently of NF-κB. As a follow-up, we investigated whether progress to late lytic cycle is also inhibited. The cotransfections described in the legend to Fig. 3A were repeated (in triplicate), and 48 h later, the cells were fixed and permeabilized. The expression of Zta and two late lytic cycle antigens, an envelope glycoprotein (gp350) and viral capsid antigen (VCA), was analyzed by flow cytometry. Representative dot plots of the data obtained are displayed in Fig. 5A. In all samples, the percentages of cells expressing Zta were similar (1.94% ± 0.13%). However, exogenous LMP1 approximately halved the level of Zta expression and reduced the percentage of cells expressing VCA or gp350 by 85 to 95%.
FIG. 5.
Effect of LMP1 on progress to late EBV lytic cycle. The cotransfections described in the legend to Fig. 3A were repeated in triplicate. After 48 h, the cells were fixed, permeabilized, and stained for Zta as described in the legend to Fig. 1. (A) Expression of late lytic cycle antigens. VCA was detected using V3 murine ascitic fluid (diluted 1:200) (55) (provided by Gary Pearson, Georgetown University, Washington, D.C.), and gp350 was detected using 1 μg of murine monoclonal antibody 72A1 (25) per ml. Primary antibody staining was detected by subsequent staining with red phycoerythrin (RPE)-conjugated anti-mouse immunoglobulin G (IgG) F(ab′)2 fragments (DAKO) (diluted 1:50). Antibody incubations were for 30 min at 37°C. The cells were analyzed by flow cytometry (as described in the legend to Fig. 1). The top row shows dot plots of data for Zta, VCA, and gp350 staining from a representative cotransfection with empty pSG5 vector; the bottom row shows the same for a pSG5-LMP1 cotransfection. mfi, mean fluorescence intensity. (B) Expression of Zta in late lytic cycle. P3HR1-c16 cells were transfected with 8 μg of Zta expression plasmid, and 48 h later, the cells were fixed and permeabilized (32). The cells were stained for simultaneous expression of Zta and VCA by use of antibodies BZ.1 and V3, respectively (32). BZ.1 staining was detected with RPE-conjugated anti-mouse IgG1 monoclonal antibody, and V3 staining was detected with fluorescein isothiocyanate (FITC)-conjugated anti-mouse IgG2a monoclonal antibody. The cells were analyzed by two-color flow cytometry (as described in the legend to Fig. 1), and a dot plot of the data was obtained. (C) Effect of LMP1 on progress to late lytic cycle. The total flow cytometry data obtained in panel A were processed to represent lytic cycle progress. For a given cotransfection, the percentage of VCA- or gp350-positive cells was divided by the percentage of Zta-positive cells and multiplied by 100. The histograms shown display means and standard errors (indicated by the error bars).
Two-color immunofluorescence staining confirmed that all VCA-positive cells also coexpress Zta (Fig. 5B). This meant the previous flow cytometry data could be processed to quantify lytic cycle progress, i.e., the number of cells in late lytic cycle was expressed as a percentage of Zta-positive cells. The results from triplicate samples (Fig. 5C) revealed that progress from induction to VCA expression (P < 0.001 by ANOVA) and gp350 expression (P < 0.001 by ANOVA) was substantially inhibited by exogenous LMP1. Cotransfection of IκBαΔN expression plasmid did not abolish this inhibition. Surprisingly, LMP1AAAG inhibited lytic cycle progress almost as efficiently as wild-type LMP1 (Fig. 5C). These findings suggest that LMP1 inhibits progress to late lytic cycle independently of NF-κB, and that in contrast to the inhibition of early lytic cycle (Fig. 3A), the LMP1 CTAR regions are not critical.
The transmembrane region of LMP1 inhibits progress to late lytic cycle.
The data in Fig. 5 indicated that the CTAR regions of LMP1 were not critical for the inhibition of progress to late lytic cycle. We speculated that the transmembrane region of LMP1 might be responsible. To investigate this, we constructed a plasmid (EGFP-stop195LMP1) encoding an N-terminal enhanced green fluorescent protein (EGFP)-tagged LMP1 with the cytosolic C terminus deleted by replacing His195 with a stop codon. This mutagenesis was performed in plasmid pEGFP-LMP1, which was created by in-frame insertion of B95.8 LMP1 cDNA into pEGFP-C1 (Clontech). The EGFP tags enabled detection, since all available LMP1 antibodies target the cytoplasmic C terminus. The rCD2-EGFP expression plasmid was included as a negative control, as it encodes an EGFP-tagged nonsignaling transmembrane protein. The aforementioned plasmids were cotransfected into P3HR1-c16 cells with the p509 Zta expression plasmid, and progress to late lytic cycle was quantified as described in the legend to Fig. 5. Flow cytometry confirmed expression of the EGFP constructs, which indicated similar mean transfection efficiencies for all samples (1.5% ± 0.1%). The results (Fig. 6) show that both the full-length LMP1 protein and the LMP1 protein with the C terminus deleted inhibited progress to VCA (P < 0.004 by ANOVA) and gp350 (P < 0.007 by ANOVA) expression. These results suggest that the LMP1 transmembrane region plays a role in impeding progress to the late lytic cycle.
FIG. 6.
The LMP1 transmembrane region impedes progress to late EBV lytic cycle. The experimental procedures described in the legend to Fig. 5A and C were repeated for triplicate cotransfections of 8 μg of Zta expression plasmid with either 3 μg of empty pEGFP-N1 vector, 3 μg of EGFP-LMP1, 3 μg of EGFP-stop195LMP1, or 3 μg of rCD2-EGFP expression plasmids. The histograms display means and standard errors (indicated by the error bars).
Implications of EBV lytic cycle inhibition by LMP1.
It has previously been shown that LMP1 inhibits EBV lytic cycle induction (2). The present investigation has identified the probable mechanism by which this occurs and revealed a surprising complexity to the ways in which LMP1 can affect the lytic cycle. Using the P3HR1-c16 cell line as a model, we have found that LMP1 interferes with EBV lytic cycle in three distinct ways. First, the LMP1 CTAR regions inhibit lytic cycle induction via activation of the transcription factor NF-κB (Fig. 1). Second, the LMP1 CTAR regions inhibit stages of early lytic cycle postinduction independently of NF-κB (Fig. 3 and 4). Third, LMP1 inhibits progress to late lytic cycle via its transmembrane domains and independently of NF-κB (Fig. 5 and 6).
Consistent with the observation that LMP1 blocks induction of the lytic cycle via NF-κB, we also demonstrated that basal NF-κB activity contributes to the inhibition of lytic cycle induction (Fig. 2). It is therefore probable that the previously reported inhibition of lytic cycle induction by the LMP1 analogue, CD40 (2), is also mediated via NF-κB. We were unable to test whether basal NF-κB activity alone prevents spontaneous lytic cycle induction, as complete inhibition of NF-κB was not achieved by transfection with IκBαΔN. Chemical inhibitors that completely inhibit NF-κB activity in B cells could have been used, but these induce apoptosis (our unpublished data), which may concomitantly induce Zta expression (28). It is noteworthy that active NF-κB is present in resting B cells, and activity greatly increases with B-cell activation (4; our unpublished data). This may explain why the lytic cycle is rare both in vivo (8) and in vitro (32).
In contrast to B cells, the lytic cycle can frequently be activated in EBV-infected epithelial cells (5, 36, 51, 58). While experimentally infected epithelial cell lines do not usually express LMP1 (27, 36), there is some evidence that LMP1 may be weakly expressed upon activation to the lytic cycle (36). In vivo, oral hairy leukoplakia (HLP) in AIDS patients represents a nonmalignant lesion of EBV-infected epithelial cells undergoing active EBV replication. In HLP, some studies indicate that the lytically infected cells are LMP1 negative (39), while other studies have reported detection of LMP1 (52, 57). At this stage, it is not possible to conclude whether LMP1 functions similarly during the lytic cycle in both epithelial cells and B cells.
The expression of LMP1 during EBV lytic cycle in various B-cell lines has been reported previously, and it should be noted that the levels of LMP1 in the lytic cycle are variable (32, 48). The P3HR1-c16 line was useful for the present study, since the level of LMP1 expressed following induction of lytic cycle is comparatively low, which allowed the limited functional analysis with exogenous LMP1 mutants. The unexpected conclusion of these experiments was that LMP1 impedes progress of the lytic cycle via two distinct mechanisms that are both independent of NF-κB activation. These additional functions of LMP1, together with the variable level of LMP1 expression during the lytic cycle, may explain why different cell lines show different degrees of progress when induced into the lytic cycle.
There is evidence suggesting that lytic cycle progress is inhibited in vivo. Prang et al. (41) used a powerful combination of reverse transcription-PCR, nested PCR, and probe hybridization to analyze gene expression in B cells from peripheral blood. They found that lytic cycle gene expression occurs in healthy EBV-infected individuals and that it is higher in infectious mononucleosis patients but that only activity from early lytic cycle genes was readily detectable. The results suggest that the lytic cycle in vivo encounters a bottleneck which limits late gene expression. This observation could be explained by immune system targeting of late lytic cycle cells, although our recent observations on the antigen-presenting function of lytic cycle B cells cast doubts on this possibility (32). Furthermore, a bottleneck is seen in vitro without any immunosurveillance or cell lysis (Fig. 5B) (32, 48). Alternatively, a role for LMP1 in impeding lytic cycle progress would be consistent with both the in vitro and in vivo observations.
The benefit of impeded lytic cycle progress in vivo could be that damage to the host is limited and, at any given time, viral antigen release may be below a threshold for antigen-presenting cell stimulation of an efficient immune response. Together, these factors could aid viral persistence and transmission to new hosts.
Acknowledgments
We gratefully acknowledge the financial support of the Medical Research Council and the Wellcome Trust. In addition, C.F. was supported by the Leverhulme Trust and P.B. was supported by the Leukemia Research Fund, London.
We thank Ciaran Richardson for technical assistance.
REFERENCES
- 1.Adamson, A. L., and S. Kenney. 2001. Epstein-Barr virus immediate-early protein BZLF1 is SUMO-1 modified and disrupts promyelocytic leukemia bodies. J. Virol. 75:2388-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adler, B., E. Schaadt, B. Kempkes, U. Zimber-Strobl, B. Baier, and G. W. Bornkamm. 2002. Control of Epstein-Barr virus reactivation by activated CD40 and viral latent membrane protein 1. Proc. Natl. Acad. Sci. USA 99:437-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arenzana-Seisdedos, F., B. Fernandez, I. Dominguez, J. M. Jacque, D. Thomas, M. T. Diaz-Meco, J. Moscat, and J. L. Virelizier. 1993. Phosphatidylcholine hydrolysis activates NF-κB and increases human immunodeficiency virus replication in human monocytes and T lymphocytes. J. Virol. 67:6596-6604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berberich, I., G. L. Shu, and E. A. Clark. 1994. Cross-linking CD40 on B cells rapidly activates nuclear factor-kappa B. J. Immunol. 153:4357-4366. [PubMed] [Google Scholar]
- 5.Borza, C. M., and L. M. Hutt-Fletcher. 2002. Alternate replication in B cells and epithelial cells switches tropism of Epstein-Barr virus. Nat. Med. 8:594-599. [DOI] [PubMed] [Google Scholar]
- 6.Brennan, P., J. E. Floettmann, A. Mehl, M. Jones, and M. Rowe. 2001. Mechanism of action of a novel latent membrane protein-1 dominant negative. J. Biol. Chem. 276:1195-1203. [DOI] [PubMed] [Google Scholar]
- 7.Darr, C. D., A. Mauser, and S. Kenney. 2001. Epstein-Barr virus immediate-early protein BRLF1 induces the lytic form of viral replication through a mechanism involving phosphatidylinositol 3-kinase activation. J. Virol. 75:6135-6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Decker, L. L., L. D. Klaman, and D. A. Thorley-Lawson. 1996. Detection of the latent form of Epstein-Barr virus DNA in the peripheral blood of healthy individuals. J. Virol. 70:3286-3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Devergne, O., E. D. Cahir McFarland, G. Mosialos, K. M. Izumi, C. F. Ware, and E. Kieff. 1998. Role of the TRAF binding site and NF-κB activation in Epstein-Barr virus latent membrane protein 1-induced cell gene expression. J. Virol. 72:7900-7908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devergne, O., E. Hatzivassiliou, K. M. Izumi, K. M. Kaye, M. F. Kleijnen, E. Kieff, and G. Mosialos. 1996. Association of TRAF1, TRAF2, and TRAF3 with an Epstein-Barr virus LMP1 domain important for B-lymphocyte transformation: role in NF-κB activation. Mol. Cell. Biol. 16:7098-7108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Renzo, L., J. Avila-Carino, and E. Klein. 1993. Induction of the lytic viral cycle in Epstein Barr virus carrying Burkitt lymphoma lines is accompanied by increased expression of major histocompatibility complex molecules. Immunol. Lett. 38:207-214. [DOI] [PubMed] [Google Scholar]
- 12.D'Souza, B., M. Rowe, and D. Walls. 2000. The bfl-1 gene is transcriptionally upregulated by the Epstein-Barr virus LMP1, and its expression promotes the survival of a Burkitt's lymphoma cell line. J. Virol. 74:6652-6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eliopoulos, A. G., S. M. Blake, J. E. Floettmann, M. Rowe, and L. S. Young. 1999. Epstein-Barr virus-encoded latent membrane protein 1 activates the JNK pathway through its extreme C terminus via a mechanism involving TRADD and TRAF2. J. Virol. 73:1023-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eliopoulos, A. G., N. J. Gallagher, S. M. Blake, C. W. Dawson, and L. S. Young. 1999. Activation of the p38 mitogen-activated protein kinase pathway by Epstein-Barr virus-encoded latent membrane protein 1 coregulates interleukin-6 and interleukin-8 production. J. Biol. Chem. 274:16085-16096. [DOI] [PubMed] [Google Scholar]
- 15.Falk, K., I. Ernberg, R. Sakthivel, J. Davis, B. Christensson, J. Luka, M. Okano, H. L. Grierson, G. Klein, and D. T. Purtilo. 1990. Expression of Epstein-Barr virus-encoded proteins and B-cell markers in fatal infectious mononucleosis. Int. J. Cancer 46:976-984. [DOI] [PubMed] [Google Scholar]
- 16.Feederle, R., M. Kost, M. Baumann, A. Janz, E. Drouet, W. Hammerschmidt, and H. J. Delecluse. 2000. The Epstein-Barr virus lytic program is controlled by the co-operative functions of two transactivators. EMBO J. 19:3080-3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fielding, C. A., K. Sandvej, A. Mehl, P. Brennan, M. Jones, and M. Rowe. 2001. Epstein-Barr virus LMP-1 natural sequence variants differ in their potential to activate cellular signaling pathways. J. Virol. 75:9129-9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fixman, E. D., G. S. Hayward, and S. D. Hayward. 1992. trans-Acting requirements for replication of Epstein-Barr virus ori-Lyt. J. Virol. 66:5030-5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flemington, E. K., A. E. Goldfeld, and S. H. Speck. 1991. Efficient transcription of the Epstein-Barr virus immediate-early BZLF1 and BRLF1 genes requires protein synthesis. J. Virol. 65:7073-7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Floettmann, J. E., A. G. Eliopoulos, M. Jones, L. S. Young, and M. Rowe. 1998. Epstein-Barr virus latent membrane protein-1 (LMP1) signalling is distinct from CD40 and involves physical cooperation of its two C-terminus functional regions. Oncogene 17:2383-2392. [DOI] [PubMed] [Google Scholar]
- 21.Gires, O., F. Kohlhuber, E. Kilger, M. Baumann, A. Kieser, C. Kaiser, R. Zeidler, B. Scheffer, M. Ueffing, and W. Hammerschmidt. 1999. Latent membrane protein 1 of Epstein-Barr virus interacts with JAK3 and activates STAT proteins. EMBO J. 18:3064-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gires, O., U. Zimber-Strobl, R. Gonnella, M. Ueffing, G. Marschall, R. Zeidler, D. Pich, and W. Hammerschmidt. 1997. Latent membrane protein 1 of Epstein-Barr virus mimics a constitutively active receptor molecule. EMBO J. 16:6131-6140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He, Z., B. Xin, X. Yang, C. Chan, and L. Cao. 2000. Nuclear factor-kappaB activation is involved in LMP1-mediated transformation and tumorigenesis of rat-1 fibroblasts. Cancer Res. 60:1845-1848. [PubMed] [Google Scholar]
- 24.Henderson, S., M. Rowe, C. Gregory, D. Croom-Carter, F. Wang, R. Longnecker, E. Kieff, and A. Rickinson. 1991. Induction of bcl-2 expression by Epstein-Barr virus latent membrane protein 1 protects infected B cells from programmed cell death. Cell 65:1107-1115. [DOI] [PubMed] [Google Scholar]
- 25.Hoffman, G. J., S. G. Lazarowitz, and S. D. Hayward. 1980. Monoclonal antibody against a 250,000-dalton glycoprotein of Epstein-Barr virus identifies a membrane antigen and a neutralizing antigen. Proc. Natl. Acad. Sci. USA 77:2979-2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huen, D. S., S. A. Henderson, D. Croom-Carter, and M. Rowe. 1995. The Epstein-Barr virus latent membrane protein-1 (LMP1) mediates activation of NF-κB and cell surface phenotype via two effector regions in its carboxy-terminal cytoplasmic domain. Oncogene 10:549-560. [PubMed] [Google Scholar]
- 27.Imai, S., J. Nishikawa, and K. Takada. 1998. Cell-to-cell contact as an efficient mode of Epstein-Barr virus infection of diverse human epithelial cells. J. Virol. 72:4371-4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inman, G. J., U. K. Binne, G. A. Parker, P. J. Farrell, and M. J. Allday. 2001. Activators of the Epstein-Barr virus lytic program concomitantly induce apoptosis, but lytic gene expression protects from cell death. J. Virol. 75:2400-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inoue, H., K. Shiraki, S. Ohmori, T. Sakai, M. Deguchi, T. Yamanaka, H. Okano, and T. Nakano. 2002. Histone deacetylase inhibitors sensitize human colonic adenocarcinoma cell lines to TNF-related apoptosis inducing ligand-mediated apoptosis. Int. J. Mol. Med. 9:521-525. [PubMed] [Google Scholar]
- 30.Izumi, K. M., E. D. Cahir McFarland, A. T. Ting, E. A. Riley, B. Seed, and E. D. Kieff. 1999. The Epstein-Barr virus oncoprotein latent membrane protein 1 engages the tumor necrosis factor receptor-associated proteins TRADD and receptor-interacting protein (RIP) but does not induce apoptosis or require RIP for NF-κB activation. Mol. Cell. Biol. 19:5759-5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karin, M., and Y. Ben-Neriah. 2000. Phosphorylation meets ubiquitination: the control of NF-[κ]B activity. Annu. Rev. Immunol. 18:621-663. [DOI] [PubMed] [Google Scholar]
- 32.Keating, S., S. Prince, M. Jones, and M. Rowe. 2002. The lytic cycle of Epstein-Barr virus is associated with decreased expression of cell surface major histocompatibility complex class I and class II molecules. J. Virol. 76:8179-8188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kieff, E., and A. B. Rickinson. 2001. Epstein-Barr virus and its replication, p. 2511-2573. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Strauss (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 34.Kilger, E., A. Kieser, M. Baumann, and W. Hammerschmidt. 1998. Epstein-Barr virus-mediated B-cell proliferation is dependent upon latent membrane protein 1, which simulates an activated CD40 receptor. EMBO J. 17:1700-1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laherty, C. D., H. M. Hu, A. W. Opipari, F. Wang, and V. M. Dixit. 1992. The Epstein-Barr virus LMP1 gene product induces A20 zinc finger protein expression by activating nuclear factor kappa B. J. Biol. Chem. 267:24157-24160. [PubMed] [Google Scholar]
- 36.Li, Q. X., L. S. Young, G. Niedobitek, C. W. Dawson, M. Birkenbach, F. Wang, and A. B. Rickinson. 1992. Epstein-Barr virus infection and replication in a human epithelial cell system. Nature 356:347-350. [DOI] [PubMed] [Google Scholar]
- 37.Mehl, A. M., J. E. Floettmann, M. Jones, P. Brennan, and M. Rowe. 2001. Characterization of intercellular adhesion molecule-1 regulation by Epstein-Barr virus-encoded latent membrane protein-1 identifies pathways that cooperate with nuclear factor kappa B to activate transcription. J. Biol. Chem. 276:984-992. [DOI] [PubMed] [Google Scholar]
- 38.Miyashita, E. M., B. Yang, G. J. Babcock, and D. A. Thorley-Lawson. 1997. Identification of the site of Epstein-Barr virus persistence in vivo as a resting B cell. J. Virol. 71:4882-4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niedobitek, G., L. S. Young, R. Lau, L. Brooks, D. Greenspan, J. S. Greenspan, and A. B. Rickinson. 1991. Epstein-Barr virus infection in oral hairy leukoplakia: virus replication in the absence of a detectable latent phase. J. Gen. Virol. 72:3035-3046. [DOI] [PubMed] [Google Scholar]
- 40.Pai, S., B. J. O'Sullivan, L. Cooper, R. Thomas, and R. Khanna. 2002. RelB nuclear translocation mediated by C-terminal activator regions of Epstein-Barr virus-encoded latent membrane protein 1 and its effect on antigen-presenting function in B cells. J. Virol. 76:1914-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prang, N. S., M. W. Hornef, M. Jager, H. J. Wagner, H. Wolf, and F. M. Schwarzmann. 1997. Lytic replication of Epstein-Barr virus in the peripheral blood: analysis of viral gene expression in B lymphocytes during infectious mononucleosis and in the normal carrier state. Blood 89:1665-1677. [PubMed] [Google Scholar]
- 42.Qu, L., and D. T. Rowe. 1992. Epstein-Barr virus latent gene expression in uncultured peripheral blood lymphocytes. J. Virol. 66:3715-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rabson, M., L. Heston, and G. Miller. 1983. Identification of a rare Epstein-Barr virus variant that enhances early antigen expression in Raji cells. Proc. Natl. Acad. Sci. USA 80:2762-2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rickinson, A. B., and E. Kieff. 2001. Epstein-Barr virus, p. 2575-2627. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Strauss (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 45.Rooney, C. M., D. T. Rowe, T. Ragot, and P. J. Farrell. 1989. The spliced BZLF1 gene of Epstein-Barr virus (EBV) transactivates an early EBV promoter and induces the virus productive cycle. J. Virol. 63:3109-3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rowe, M., H. S. Evans, L. S. Young, K. Hennessy, E. Kieff, and A. B. Rickinson. 1987. Monoclonal antibodies to the latent membrane protein of Epstein-Barr virus reveal heterogeneity of the protein and inducible expression in virus-transformed cells. J. Gen. Virol. 68:1575-1586. [DOI] [PubMed] [Google Scholar]
- 47.Rowe, M., and M. Jones. 2001. Detection of EBV latent proteins by Western blotting. Methods Mol. Biol. 174:229-242. [DOI] [PubMed] [Google Scholar]
- 48.Rowe, M., A. L. Lear, D. Croom-Carter, A. H. Davies, and A. B. Rickinson. 1992. Three pathways of Epstein-Barr virus gene activation from EBNA1-positive latency in B lymphocytes. J. Virol. 66:122-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rowe, M., D. T. Rowe, C. D. Gregory, L. S. Young, P. J. Farrell, H. Rupani, and A. B. Rickinson. 1987. Differences in B cell growth phenotype reflect novel patterns of Epstein-Barr virus latent gene expression in Burkitt's lymphoma cells. EMBO J. 6:2743-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takada, K., and Y. Ono. 1989. Synchronous and sequential activation of latently infected Epstein-Barr virus genomes. J. Virol. 63:445-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takasaka, N., M. Tajima, K. Okinaga, Y. Satoh, Y. Hoshikawa, T. Katsumoto, T. Kurata, and T. Sairenji. 1998. Productive infection of Epstein-Barr virus (EBV) in EBV-genome-positive epithelial cell lines (GT38 and GT39) derived from gastric tissues. Virology 247:152-159. [DOI] [PubMed] [Google Scholar]
- 52.Thomas, J. A., D. H. Felix, D. Wray, J. C. Southam, H. A. Cubie, and D. H. Crawford. 1991. Epstein-Barr virus gene expression and epithelial cell differentiation in oral hairy leukoplakia. Am. J. Pathol. 139:1369-1380. [PMC free article] [PubMed] [Google Scholar]
- 53.Thorley-Lawson, D. A., and G. J. Babcock. 1999. A model for persistent infection with Epstein-Barr virus: the stealth virus of human B cells. Life Sci. 65:1433-1453. [DOI] [PubMed] [Google Scholar]
- 54.Tierney, R. J., N. Steven, L. S. Young, and A. B. Rickinson. 1994. Epstein-Barr virus latency in blood mononuclear cells: analysis of viral gene transcription during primary infection and in the carrier state. J. Virol. 68:7374-7385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vroman, B., J. Luka, M. Rodriguez, and G. R. Pearson. 1985. Characterization of a major protein with a molecular weight of 160,000 associated with the viral capsid of Epstein-Barr virus. J. Virol. 53:107-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang, S., M. Rowe, and E. Lundgren. 1996. Expression of the Epstein Barr virus transforming protein LMP1 causes a rapid and transient stimulation of the Bcl-2 homologue Mcl-1 levels in B-cell lines. Cancer Res. 56:4610-4613. [PubMed] [Google Scholar]
- 57.Webster-Cyriaque, J., J. Middeldorp, and N. Raab-Traub. 2000. Hairy leukoplakia: an unusual combination of transforming and permissive Epstein-Barr virus infections. J. Virol. 74:7610-7618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Young, L. S., R. Lau, M. Rowe, G. Niedobitek, G. Packham, F. Shanahan, D. T. Rowe, D. Greenspan, J. S. Greenspan, A. B. Rickinson, et al. 1991. Differentiation-associated expression of the Epstein-Barr virus BZLF1 transactivator protein in oral hairy leukoplakia. J. Virol. 65:2868-2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang, L., L. Wu, K. Hong, and J. S. Pagano. 2001. Intracellular signaling molecules activated by Epstein-Barr virus for induction of interferon regulatory factor 7. J. Virol. 75:12393-12401. [DOI] [PMC free article] [PubMed] [Google Scholar]