Abstract
Retroviruses can be used to accelerate hematopoietic cancers predisposed to neoplastic disease by prior genetic manipulations such as in transgenic or knockout mice. The virus imparts a second neoplastic “hit,” providing evidence that the initial hit is transforming. In the present study, a unique retrovirus was developed that can induce a high incidence of myeloid disease and has a broad host range. This agent is a Moloney murine leukemia virus (Mo-MuLV)-based virus that has most of the U3 region of the long terminal repeat (LTR) replaced with that of retrovirus 4070A. Like Mo-MuLV, this virus, called MOL4070LTR, is NB-tropic and not restricted by Fv1 allelles. MOL4070LTR causes myeloid leukemias in ca. 50% of mice, a finding in contrast to Mo-MuLV, which induces almost exclusively lymphoid disease. The data suggest that the LTR of the 4070A virus expands the tissue tropism of the disease to the myeloid lineage. Interesting, MCF recombinant envelope was expressed in the lymphoid but not the myeloid neoplasms of BALB/c mice. This retrovirus has the potential for accelerating myeloid disease in genetically engineered mice.
Retroviruses have been widely used in vivo as insertional mutagens to identify oncogenes and tumor suppressors that are involved in the development of hematopoietic neoplasms (reviewed in references 1 and 18). During the infectious process viral RNA is copied into DNA, which is imported into the nucleus and inserted into the host genome. The viral long terminal repeat (LTR) can activate transcription of flanking oncogenes or, more rarely, inactivate a tumor suppressor (7, 19). The mutagenic effects of the retrovirus are clonally selected because of a resulting growth advantage conferred by the virus. In addition, genes or sequences flanking the virus can easily be identified and isolated by using retroviral probes or inverse PCR with virus-specific primers.
Retroviral insertional mutagenesis has been successfully utilized in transgenic or gene knockout mice to identify cooperating oncogenes. This approach was used as a second hit in several studies aimed at determining the genes involved in T-cell lymphomagenesis (2, 6, 14, 29). For example, oncogenes that synergize with a c-myc transgene were found after infection with Moloney murine leukemia virus (Mo-MuLV). Several collaborating genes were identified, including Pim-1, Tic-1, Ahi-1, and Bmi-1. In all of these experiments, mice were exogenously infected with retroviruses. Using a related strategy, Blaydes et al. (5) looked for somatic mutations that collaborate with the loss of Nf1 in the induction of myeloid leukemia. The virus in the investigation, rather than being introduced by inoculation, was present as a result of transplacental transmission, as described elsewhere (3). Blaydes et al. (5) backcrossed C57BL/6J mice, containing a loss-of-function mutant of Nf1, to BXH-2 mice that express the horizontally transmitted ecotropic MuLV. Using the virus as a tag, they identified the Epi1 locus as a collaborator with Nf1 loss in the induction of acute myeloid leukemia.
We were interested in developing an MuLV that (i) could be inoculated into newborn mice to produce early myeloid cell-derived leukemias typical of those found in humans (e.g., myelomonocytic, monoblastic, monocytic, and myeloid with granulocyte maturation) and (ii) would have a wide tropism for the purpose of accelerating disease and identifying collaborating genetic alterations in transgenic and knockout mice. Although Mo-MuLV has wide tropism, it induces almost exclusively monocytic disease in pristane-treated young adults after intravenous inoculation (30, 33) and lymphomas when inoculated into newborn mice (12, 25).
There are several examples to suggest that amphotropic virus 4070A may have more propensity for inducing myeloid disease than Mo-MuLV. Although it induces in newborn mice predominantly T-cell neoplasms, it can also induce, at a very low incidence, myeloid-like tumors and B-cell tumors (22). Interestingly, we found that, in pristane-treated young adult DBA/2 mice, virus 4070A induces exclusively myeloid (monocytic) disease (31) compared to Mo-MuLV, which induces both monocytic and lymphoid disease under the same experimental conditions (33). We also found that 4070A virus can, when inoculated into newborn mice, accelerate myeloid disease in 129sv mice that express myeloid-specific oncogenes (unpublished data).
Based on the evidence presented above, one would predict that 4070A virus could be used to accelerate myeloid disease. However, the virus has a limited host range caused by the Fv1b allele and is, therefore, N-tropic (24). It is restricted in many strains used by investigators for transgenic and knockout experiments (e.g., FVB and C57BL/6). Fv1 is a gene that controls susceptibility to infection after the virus has entered the cell but before the viral DNA is integrated into the host genome (4, 16). The Fv1 gene was cloned and found to encode an endogenous retrovirus capsid antigen-like protein, and the tropism of the virus is determined by the capsid antigen protein encoded by gag (11, 23). This region of the virus is presumed to be involved in the integration process.
In order to prepare an NB-tropic virus (not restricted in Fv1b or Fv1n mice) that would retain the myeloid disease inducing sequences of the 4070A, we decided to construct a recombinant between Mo-MuLV and 4070A. One would predict that the disease tissue tropism of 4070A would be due to either its unique envelop region, which provides expanded tropism to other mammals, or to the LTR, which has been previously found to confer lineage specificity to other viruses (8, 9). Ott et al. (22) found that the capacity of 4070A for broadened target cell specificity was not provided to Mo-MuLV by the replacement of its carboxy-terminal region of pol and most of env gene by analogous sequences from 4070A. The recombinant virus still produced exclusively lymphoblastic lymphomas. Therefore, we reasoned that the myeloid-inducing capacity of 4070A might be contained in the LTR. We constructed a recombinant that has most of the U3 region of the 4070A LTR and the gag, pol, and env sequences of Mo-MuLV. The Fv1 tropism would, therefore, be conferred by the Mo-MuLV sequences.
Construction of a recombinant virus NB-tropic retrovirus.
To construct this virus, we started with permuted clones of Mo-MuLV (C53) (28) and 4070A (10, 21), which contain only one LTR. In the final recombinant virus, the NheI(71)-SstI(386) LTR fragment from 4070A was substituted for the NheI(7583)-SstI(7970) LTR fragment of Mo-MuLV. The accession numbers for Mo-MuLV and the 4070A virus are AF033811 and M55597, respectively. A plasmid was prepared by subcloning the HindIII-BclI fragment of Mo-MuLV, including the LTR, into a Litmus 29 plasmid engineered to have a BclI site. A large SstI-NheI fragment containing Mo-MuLV and Litmus 29 plasmid sequences was purified and recombined with the NheI-SstI LTR fragment from 4070A. The recombinant virus was than reassembled in two cloning steps as a permuted clone in the HindIII site of pBluescript II KS. The resulting plasmid, pMOL4070LTR, was digested with HindIII and transfected into NIH 3T3 cells by using the calcium phosphate transfection system from Invitrogen. After subculture of the cells over a period of 2 weeks, the culture supernatant was collected, filtered, and tested in an XC assay as previously described (26).
Leukemogenicity of MOL4070LTR recombinant virus and Mo-MuLV in FVB and BALB/c mice.
For the production of virus for the infection of mice, NIH 3T3 cells chronically infected with Mo-MuLV or the recombinant MOL4070LTR virus were mixed with equal numbers of unfected NIH 3T3 cells (105 cells of each type were seeded in 100-mm dishes). The cells were propagated for 4 days, the medium was replaced with fresh medium and, on day 5, the virus-containing medium was harvested and assayed in the XC assay. For leukemogenesis studies, newborn FVB/NCr or BALB/cAnNCr mice were injected intraperitoneally with 1 × 105 to 2 × 105 PFU in 0.1 ml of medium. Mice were housed and cared for in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals. The animals were routinely monitored for evidence of disease, and moribund mice were euthanized by cervical dislocation. Cytospins were prepared from enlarged spleens, and the cells were stained in Diff-Quik (Dade Behring AG, Newark, Del.). Tissues were fixed in Fekete's modification of Tellyesniczky's fluid (13), processed into paraffin, and stained with hematoxylin and eosin. Immunohistochemistry was also performed with the following antibodies: rabbit anti-CD3 (Dako), rat anti-mouse F4/80 (Serotec), and rabbit anti-human myeloperoxidase (Dako).
Characterization of hematopoietic neoplasms.
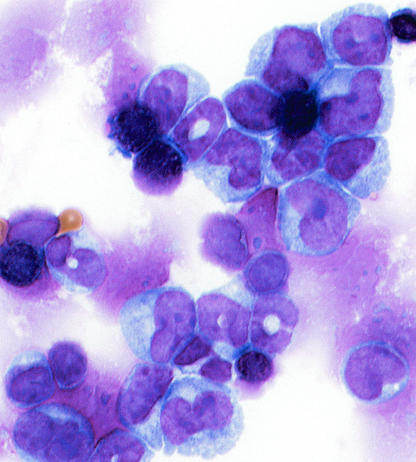
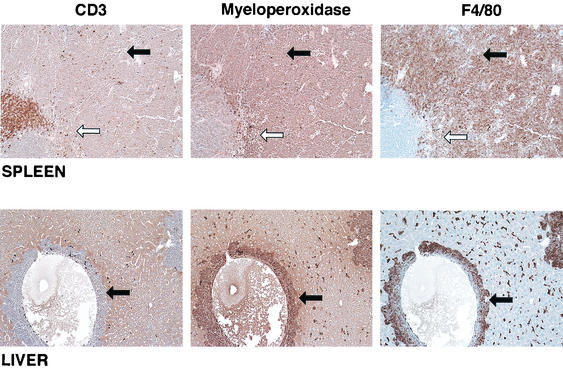
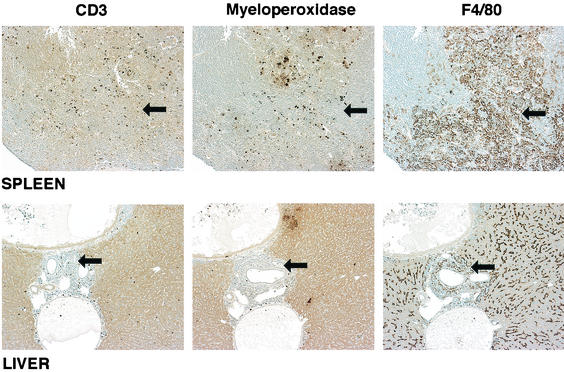
Myeloid disease was induced in approximately half of the mice that had been inoculated as newborns with the recombinant MOL4070LTR virus (Table 1). This incidence was extremely high compared to the incidence of myeloid disease reported for mice infected with 4070A virus (22), and myeloid disease was not produced by Mo-MuLV in newborn mice here or in previous studies in the absence of pristane. These data are particularly interesting and suggest that the capacity to induce a high incidence of myeloid disease under these experimental conditions requires the collaboration of genes from both Mo-MuLV and 4070A. The myeloid diseases consisted of various subtypes. The most prominent phenotype was myelomonocytic, but there were rare occurences of the monocytic, monoblastic, and granulocytic-with-maturation phenotypes. A typical example of a myelomonocytic leukemia in a BALB/c mouse is shown in Fig. 1 and 2. The cytospin preparation from the spleen depicts fairly large cells with a couple of different nuclear morphologies (Fig. 1). Some had donut-shaped nuclei, whereas others had nuclei that were elongated and bean shaped. Most cells had copious cytoplasm. From the immunohistochemistry in Fig. 2, it appears that all of the leukemic cells stained positive for both myeloperoxidase and F4/80 but were negative for CD3. The periarteriolar lymphoid sheath (PALS) is evident by the CD3 staining of the spleen in the top left panel. In the middle and right panels, which depict the same area of the spleen, one can see that outside of the PALS most of the cells stained for both the granulocytic and the monocytic markers. Infiltrating leukemic cells are evident in the liver in the bottom panels near a blood vessel and again are myeloperoxidase and F4/80 positive. Figure 3 shows a monocytic leukemia from in the spleen and liver of an FVB mouse. In this case, the tumor cells are stained predominantly with F4/80. In the liver, both positively staining normal Kupffer cells and infiltrating perivascular leukemia cells in the central portion of the photograph can be seen.
TABLE 1.
Incidence and phenotypes of hematopoietic neoplasms
| Virus | Mouse strain | Tumor diagnosis | Latency period (wk) | Incidence (no. of mice with neo- plasms/total no. infected [%]) |
|---|---|---|---|---|
| MOL4070LTR | FVB | Leukemia, myeloid | 32.3 | 12/22 (55) |
| Leukemia, myelomonocytic | 29.5 | 8/22 (36) | ||
| Leukemia, monoblastic or monocytic | 31.5 | 3/22 (14) | ||
| Leukemia, granulocyte with maturation | 42 | 1/22 (5) | ||
| Hematopoietic neoplasm, undifferentiated, blastic | 17 | 1/22 (5) | ||
| Mixed T-cell lymphoma, lymphoblastic; leukemia, myeloid | 16.7 | 3/22 (14) | ||
| T-cell lymphoma, lymphoblastic | 35.8 | 5/22 (23) | ||
| BALB/c | Leukemia, myeloid | 29.4 | 10/28 (36) | |
| Leukemia, myelomonocytic | 28.7 | 9/28 (32) | ||
| Leukemia, monoblastic | 36.0 | 1/28 (4) | ||
| Mixed, myeloid and lymphoid | 32 | 3/28 (1) | ||
| Lymphoma lymphoblastic | 28.9 | 13/28 (46) | ||
| Mo-MuLV | FVB | T-cell lymphoma, lymphoblastic | 31.8 | 12/12 (100) |
| BALB/c | T-cell lymphoma, lymphoblastic | 16.2 | 7/7 (100) |
FIG.1.
Cytospin of spleen cells from mouse 58B-2, which was diagnosed with myelomonocytic leukemia. Magnification, ×240.
FIG. 2.
Immunohistochemistry of spleen and liver tissue from BALB/c mouse 58B-2 inoculated with MOL4070ALTR and diagnosed with myelomonocytic leukemia. Staining was performed with antibody to myeloperoxidase, F4/80, and CD3 antibody. Magnification, ×60. The white arrow indicates the location of the PALS; the black arrows point to neoplastic cells.
FIG. 3.
Immunohistochemistry of spleen and liver tissue from FVB mouse 57-10 inoculated with MOL4070ALTR and diagnosed with monocytic leukemia. Staining was performed with antibody to myeloperoxidase, F4/80, and CD3 antibody. Magnification, ×60. The white arrow indicates the PALS; the black arrows point to neoplastic cells.
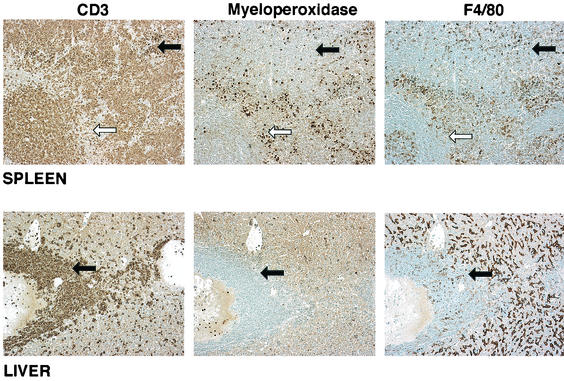
T-cell lymphomas occurred in 23% of the FVB mice and in 46% of the BALB/c mice (Table 1). An example from an FVB mouse is shown in Fig. 4. These lymphomas were mostly lymphoblastic in nature. A mixed disease was also found in both FVB and BALB/c mice, with incidences of 14 and 1%, respectively. These animals had evidence of both a T-cell lymphoma and myeloid leukemia. Interestingly, both the myeloid and lymphoid neoplasms had the capacity to metastasize to the liver, the kidney, lung and, less often, the heart.
FIG. 4.
Immunohistochemistry of spleen and liver tissue from FVB mouse 57-11 inoculated with MOL4070ALTR and diagnosed with a lymphoblastic T-cell lymphoma. Staining was performed with antibodies to myeloperoxidase, F4/80, and CD3 antibody. Magnification, ×60. The white arrow indicates the PALS; the black arrows point to neoplastic cells.
Clonality and high proviral copy number in neoplasms induced by MOL4070LTR.
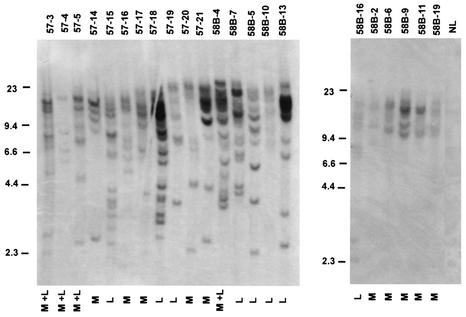
In order to examine the clonality of the leukemias and lymphomas induced in MOL4070LTR mice, we prepared genomic DNA from spleen tissue and digested it with EcoRI, which does not cut within the virus. A Southern analysis of this DNA was carried out with a 4070A-specific LTR probe (as described in reference 31). There appeared to be numerous clonal integrations in most neoplasms (Fig. 5). However, in certain cases, such as BALB/c T-cell lymphomas (58B-6 and 58B-10), myeloid leukemia (58B-2), and a spleen with both myeloid and lymphoid neoplasms (57-4), there was evidence of oligoclonality (Fig. 5). Although the same amount of DNA was electrophoresed in these instances, the bands were much lighter, suggesting that the sequences were found less frequently than one copy per cell. However, we cannot rule out in these cases that there was significant contamination by normal tissue.
FIG. 5.
Proviral integrations in MOL4070ALTR-induced neoplasms. Genomic DNA was isolated from the spleens, which were enlarged in each case and shown to contain hematopoietic neoplasms. The DNA (15 μg) was digested with EcoRI and separated on 7% agarose gels. After being blotted onto Nytran (Schleicher & Schuell), hybridization was performed with a 4070A-specific LTR probe (31). M, myeloid; L, lymphoid.
MCF virus induction in MOL4070LTR induced neoplasms.
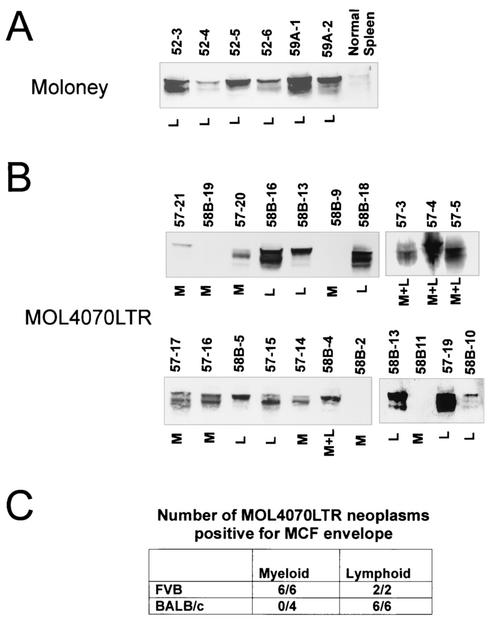
A common feature of Mo-MuLV-induced neoplasms is the expression of env gene recombinant viruses called mink cell focus-inducing (MCF) viruses. Although the generation of MCF viruses is not absolutely required for leukemogenesis, they are believed to make the disease process more efficient (12). Because they have been associated primarily with erythroid and lymphoid disease, we thought it would be interesting to determine whether these viruses are associated with myeloid disease induced by MOL4070LTR. Neoplasms were examined for the presence of MCF envelope protein. As shown in Fig. 6, myeloid leukemias, in contrast to lymphoid neoplasms, induced in BALB/c mice were not associated with the expression of MCF envelope protein (Fig. 6B and C). We conclude that these myeloid leukemias can develop efficiently without forming an MCF recombinant virus. All neoplasms in FVB mice, both lymphoid and myeloid, were associated with expression of the MCF envelope. In these cases, the presence of MCF could be a product of efficient recombination to form MCF virus in tissues before they become transformed. However, we looked at MCF protein expression, with the same antibody, in spleen tissue in FVB and BALB/c mice 3.5 weeks after infection, and we could not detect expression (data not shown). Alternatively, production of MCF virus may be required for myeloid disease in this particular strain of mouse, and these MCFs are observed after selection of clonal neoplasms. In any case, our study shows that the formation of MCF recombinants is not required for the induction of myeloid disease by ecotropic virus in at least some strains of mice.
FIG. 6.
Detection of MCF viral envelope in hematopoeitic neoplasms. Western blot analyses were performed on spleen tissues with monoclonal antibody 7C10, which specifically recognizes MCF viral envelope protein (32). A total of 50 μg of protein was electrophoresed and analyzed as reported elsewhere (20). (A) Mo-MuLV-induced T-cell lymphoblastic lymphomas were used as a positive control, and a spleen from a normal BALB/c mouse was used as a negative control. Neoplasms whose designations begin with “52” are from FVB mice. Neoplasms whose designations begin with “59A” are from BALB/c mice. (B) MOL4070LTR-induced neoplasms. Neoplasms whose designations begin with “57” are from FVB mice, and those whose designations begin with “58B” are from BALB/c mice. M, myeloid; L, lymphoid. (C) Table summarizing the results from panel B.
In summary, our data show that the MOL4070LTR virus can infect a wide range of mice due to its NB tropism and induce almost equally tumors of the myeloid and the T-cell lineages. Substitution of the LTR of the 4070A virus for the Mo-MuLV LTR expanded the phenotype of leukemias induced by Mo-MuLV. This range of phenotypes is similar to that found by Ott et al. to be induced by the 4070A virus in newborn NIH/Swiss mice (22). These authors reported that the virus induced, in addition to T-cell tumors, a very low incidence of myeloid cell-like tumors and B-cell tumors. Although we did not find any B-cell tumors in the experiments presented here, a B-cell tumor was induced in a pristane-treated FVB mouse infected with the MOL4070LTR. The observed increase in incidence of myeloid leukemias induced by this virus might indicate that the Mo-MuLV sequences provide a more efficient infection of target cells. It is conceivable that the envelope of Mo-MuLV, which is very different from that of the 4070A virus, might account for this difference.
Other viruses that have been shown to induce myeloid leukemia are Cas-Br-E and the Graffi MuLVs. Although Cas-Br-E MuLV induces a wide range of disease types, including T- and B-cell lymphomas, myelogenous leukemias, and erythroleukemias in NFS/N mice (15, 17), it is N-tropic and therefore useful in only a limited number of mouse strains. Graffi MuLV is very effective in inducing myeloid disease and is NB-tropic (27). Although the Graffi virus was originally reported to induce exclusively myeloid disease, it has recently been shown to also induce lymphoid disease (Eric Rassart, unpublished data). The fact that the LTR of Graffi MuLV, compared to other retroviruses, was most homologous to the LTRs of 4070A and Cas-Br-E MuLV correlates with our observation that the LTR of 4070A can facilitate a high incidence of myeloid disease.
In conclusion, MOL4070LTR has allowed us to demonstrate the myeloid-inducing capacity of the 4070A LTR. With this virus we have been able to show that myeloid leukemias, in contrast to T-cell lymphomas, do not have to depend on the generation of MCF viruses for efficient induction by ectropic retroviruses. MOL4070LTR is predicted to be a useful virus for the acceleration of leukemias in transgenic and knockout mice that are partially susceptible to neoplastic disease. It may have great utility when one does not know to which leukemic phenotype the animal will be more susceptible (e.g., lymphoid or myeloid). We are now using it in mice that have a targeted deletion of the proposed tumor suppressor p15INK4b.
Acknowledgments
We thank Sandra Ruscetti for supplying 7C10 antibody and for helpful discussions. We also thank Martina Schmidt for critical reading of the manuscript.
This project has been funded in part with funds from the National Cancer Institute, National Institutes of Health (N01-C0-12400).
The content of this article does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does the mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
REFERENCES
- 1.Athias, G. B., C. R. Starkey, and L. S. Levy. 1994. Retroviral determinants of leukemogenesis. Crit. Rev. Oncog. 5:169-199. [DOI] [PubMed] [Google Scholar]
- 2.Baxter, E. W., K. Blyth, E. R. Cameron, and J. C. Neil. 2001. Selection for loss of p53 function in T-cell lymphomagenesis is alleviated by Moloney murine leukemia virus infection in myc transgenic mice. J. Virol. 75:9790-9798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bedigian, H. G., L. A. Shepel, and P. C. Hoppe. 1993. Transplacental transmission of a leukemogenic murine leukemia virus. J. Virol. 67:6105-6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bishop, K. N., M. Bock, G. Towers, and J. P. Stoye. 2001. Identification of the regions of Fv1 necessary for murine leukemia virus restriction. J. Virol. 75:5182-5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blaydes, S. M., S. C. Kogan, B. T. Truong, D. J. Gilbert, N. A. Jenkins, N. G. Copeland, D. A. Largaespada, and C. I. Brannan. 2001. Retroviral integration at the Epi1 locus cooperates with Nf1 gene loss in the progression to acute myeloid leukemia. J. Virol. 75:9427-9434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blyth, K., A. Terry, N. Mackay, F. Vaillant, M. Bell, E. R. Cameron, J. C. Neil, and M. Stewart. 2001. Runx2: a novel oncogenic effector revealed by in vivo complementation and retroviral tagging. Oncogene 20:295-302. [DOI] [PubMed] [Google Scholar]
- 7.Buchberg, A. M., H. G. Bedigian, N. A. Jenkins, and N. G. Copeland. 1990. Evi-2, a common integration site involved in murine myeloid leukemogenesis. Mol. Cell. Biol. 10:4658-4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chatis, P. A., C. A. Holland, J. W. Hartley, W. P. Rowe, and N. Hopkins. 1983. Role for the 3′ end of the genome in determining disease specificity of Friend and Moloney murine leukemia viruses. Proc. Natl. Acad. Sci. USA 80:4408-4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chatis, P. A., C. A. Holland, J. E. Silver, T. N. Frederickson, N. Hopkins, and J. W. Hartley. 1984. A 3′ end fragment encompassing the transcriptional enhancers of nondefective Friend virus confers erythroleukemogenicity on Moloney leukemia virus. J. Virol. 52:248-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chattopadhyay, S. K., A. I. Oliff, D. L. Linemeyer, M. R. Lander, and D. R. Lowy. 1981. Genomes of murine leukemia viruses isolated from wild mice. J. Virol. 39:777-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DesGroseillers, L., and P. Jolicoeur. 1983. Physical mapping of the Fv-1 tropism host range determinant of BALB/c murine leukemia viruses. J. Virol. 48:685-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan, H. 1997. Leukemogenesis by Moloney murine leukemia virus: a multistep process. Trends Microbiol. 5:74-82. [DOI] [PubMed] [Google Scholar]
- 13.Fekete, E. 1938. A comparative study of the mammary gland in a high and a low tumor strain of mice. Am. J. Pathol. 14:557-578. [PMC free article] [PubMed] [Google Scholar]
- 14.Haupt, Y., W. S. Alexander, G. Barri, S. P. Klinken, and J. M. Adams. 1991. Novel zinc finger gene implicated as myc collaborator by retrovirally accelerated lymphomagenesis in E mu-myc transgenic mice. Cell 65:753-763. [DOI] [PubMed] [Google Scholar]
- 15.Holmes, K., W. Y. Langdon, T. N. Fredrickson, R. L. Coffmann, P. M. Hoffman, J. Hartley, and H. C. Morse III. 1986. Analysis of neoplasms induced by Cas-Br-M MuLV tumor extracts. J. Immunol. 137:679-688. [PubMed] [Google Scholar]
- 16.Jolicoeur, P., and D. Baltimore. 1976. Effect of Fv-1 gene product on proviral DNA formation and integration in cells infected with murine leukemia viruses. Proc. Natl. Acad. Sci. USA 73:2236-2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jolicoeur, P., N. Nicolaiew, L. DesGroseillers, and E. Rassart. 1983. Molecular cloning of infectious viral DNA from ecotropic neurotropic wild mouse retrovirus. J. Virol. 45:1159-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jonkers, J., and A. Berns. 1996. Retroviral insertional mutagenesis as a strategy to identify cancer genes. Biochem. Biophys. Acta 1287:29-57. [DOI] [PubMed] [Google Scholar]
- 19.Largaespada, D. A., J. D. Shaughnessy, Jr., N. A. Jenkins, and N. G. Copeland. 1995. Retroviral integration at the Evi-2 locus in BXH-2 myeloid leukemia cell lines disrupts Nf1 expression without changes in steady-state Ras-GTP levels. J. Virol. 69:5095-5102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishigaki, K., C. Hanson, D. Thompson, T. Yugawa, M. Hisasue, H. Tsujimoto, and S. Ruscetti. 2002. Analysis of the disease potential of a recombinant retrovirus containing Friend murine leukemia virus sequences and a unique long terminal repeat from feline leukemia virus. J. Virol. 76:1527-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oliff, A., M. D. McKinney, and O. Agranovsky. 1985. Contribution of the gag and pol sequences to the leukemogenicity of Friend murine leukemia virus. J. Virol. 54:864-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ott, D. E., J. Keller, K. Sill, and A. Rein. 1992. Phenotypes of murine leukemia virus-induced tumors: influence of 3′ viral coding sequences. J. Virol. 66:6107-6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ou, C. Y., L. R. Boone, C. K. Koh, R. W. Tennant, and W. K. Yang. 1983. Nucleotide sequences of gag-pol regions that determine the Fv-1 host range property of BALB/c N-tropic and B-tropic murine leukemia viruses. J. Virol. 48:779-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pincus, T., W. P. Rowe, and F. Lilly. 1971. A major genetic locus affecting resistance to infection with murine leukemia viruses. II. Apparent identity to a major locus described for resistance to friend murine leukemia virus. J. Exp. Med. 133:1234-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenberg, N., and P. Jolicoeur. 2002. Retroviral pathogenesis, p. 475-586. In J. M. Coffin, Hughes S.H., and Varmus H.E. (ed.), Retroviruses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y. [PubMed]
- 26.Rowe, W. P., W. E. Pugh, and J. W. Hartley. 1970. Plaque assay techniques for murine leukemia viruses. Virology 42:1136-1139. [DOI] [PubMed] [Google Scholar]
- 27.Ru, M., C. Shustik, and E. Rassart. 1993. Graffi murine leukemia virus: molecular cloning and characterization of the myeloid leukemia-inducing agent. J. Virol. 67:4722-4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shoemaker, C., S. Goff, E. Gilboa, M. Paskind, S. W. Mitra, and D. Baltimore. 1980. Structure of a cloned circular Moloney murine leukemia virus DNA molecule containing an inverted segment: implications for retrovirus integration. Proc. Natl. Acad. Sci. USA 77:3932-3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Lohuizen, M., S. Verbeek, B. Scheijen, E. Wientjens, G. H. van der, and A. Berns. 1991. Identification of cooperating oncogenes in E mu-myc transgenic mice by provirus tagging. Cell 65:737-752. [DOI] [PubMed] [Google Scholar]
- 30.Wolff, L., and R. Koller. 1990. Regions of the Moloney murine leukemia virus genome specifically related to induction of promonocytic tumors. J. Virol. 64:155-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolff, L., R. Koller, and W. Davidson. 1991. Acute myeloid leukemia induction by amphotropic murine retrovirus (4070A): clonal integrations involve c-myb in some but not all leukemias. J. Virol. 65:3607-3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolff, L., R. Koller, and S. Ruscetti. 1982. Monoclonal antibody to spleen focus-forming virus-encoded gp52 provides a probe for the amino-terminal region of retroviral envelope proteins that confers dual tropism and xenotropism. J. Virol. 43:472-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolff, L., J. F. Mushinski, G. L. Shen-Ong, and H. C. Morse III 1988. A chronic inflammatory response. Its role in supporting the development of c-myb and c-myc related promonocytic and monocytic tumors in BALB/c mice. J. Immunol. 141:681-689. [PubMed] [Google Scholar]