Abstract
Several reports have shown that human cytomegalovirus (HCMV)-infected cells are resistant to NK lysis. These studies have focused on receptor-ligand interactions, and different HCMV proteins have been indicated to mediate inhibitory NK signals. Here, we report that the HCMV protein UL16 is of major importance for the ability of HCMV-infected cells to resist NK cell-mediated cytotoxicity. Fibroblasts infected with the UL16 deletion mutant HCMV strain exhibited a 70% increased sensitivity to NK killing at 7 days postinfection compared to AD169-infected cells. Interestingly, HCMV-infected cells did not appear to engage inhibitory molecules on NK cells, since the levels of granzyme B were not reduced in supernatants obtained from NK cell cocultures with infected target cells compared to uninfected target cells. Furthermore, HCMV-infected cells, but not cells infected with the UL16 deletion mutant HCMV strain, exhibited a significantly increased resistance to the action of cytolytic proteins, including perforin, granzyme B, streptolysin O, and porcine NK lysin. In addition, fluorescence-activated cell sorting for UL16-positive transfected cells resulted in protection levels of 90% compared to control cells carrying the green fluorescent protein vector. Thus, the UL16 protein mediates an increased protection against the action of cytolytic proteins released by activated NK cells, possibly by a membrane-stabilizing mechanisms, rather than by delivering negative signals to NK cells.
Human cytomegalovirus (HCMV) belongs to the herpesvirus family, and a primary infection is followed by latency in myeloid-lineage cells (33). While HCMV infection usually is subclinical in immunocompetent individuals, the virus can cause severe morbidity and mortality in immunocompromised patients. Hence, the immune status of the host is of utmost importance to control HCMV infection, and the virus has developed several immune evasion strategies to be able to coexist with its host. It is a well-known phenomenon that HCMV-infected cells escape recognition by both CD4+ (23, 29, 36) and CD8+ (11, 40) T cells due to downregulation of HLA class II (25, 26, 29, 32) and class I (1, 14, 16-19, 24, 41) molecules on infected cells. A number of different HCMV proteins that affect the expression of HLA class I and class II molecules have been identified. Furthermore, HCMV-infected cells have also been shown to be protected against natural killer (NK) cell-mediated cytotoxicity (5, 7, 8, 12, 28, 39).
In vitro studies of HCMV-infected cells have suggested that protection against NK lysis may be HCMV strain specific (5, 8), and several different proteins have been postulated to be involved in the protection against NK lysis of HCMV-infected cells. These studies have focused on receptor-ligand interactions. First, the HCMV encoded HLA class I homologue UL18 was hypothesized to be involved in the protection against NK lysis, possibly by delivering inhibitory signals to NK cells through binding to LIR-1 (6) or CD94 (31). However, published data are contradictory regarding the function of UL18 in the protection against NK lysis, since infection of cells with a viral mutant strain lacking the UL18 gene still confers protection against NK lysis (22, 28). More recently, the HCMV UL16 protein was shown to mediate increased resistance to NK lysis, possibly by blocking the interaction between the NK cell and triggering receptors (UL16 binding protein) on the target cell (7). Furthermore, the HCMV protein gpUL40 has been shown to modulate HLA-E expression, which also was associated with increased NK resistance (35, 37, 39). In addition, increased HLA-G expression (30) and decreased expression of LFA-3 (8) have also been suggested to mediate a reduced sensitivity to NK killing. However, the mechanism and viral proteins responsible for this effect are unknown. Thus, although HCMV has evolved multiple mechanisms for interfering with NK killing of infected cells, the exact mechanisms responsible for the increased resistance to NK killing are still unclear.
NK cell-mediated cytotoxicity is usually mediated by degranulation resulting in apoptosis or by an interaction between Fas-Fas ligands (3). The granule-mediated apoptosis is induced by the complementary action of perforin, a pore-forming protein, and granzymes, where granzyme B plays an essential role in inducing apoptosis (15). Although the mechanism underlying granule-mediated apoptosis is unclear, accumulated data suggest that following interaction with the plasma membrane of the target cell, perforin facilitates the cytolytic delivery of granzyme, which in turn initiates caspase-dependent and -independent death pathways. Hence, HCMV-infected cells may adopt a number of strategies to avoid destruction by NK cells: (i) by inhibiting NK cell recognition, (ii) by delivering signals that inhibit activation pathways, and (iii) by blocking the action of secreted proapoptotic proteins.
Previous studies that have examined the interaction of NK cells and HCMV-infected cells have focused on how the virus inhibits lysis by engagement of inhibitory signals. We here report that HCMV-infected cells indeed trigger NK cell activation and the release of granzyme B but that HCMV-infected cells were resistant to the action of cytolytic proteins. The HCMV protein UL16 played a crucial role in maintaining membrane integrity of infected or UL16-transfected cells. These observations provide the first evidence that HCMV also can resist lysis by cytotoxic cells by a UL16-mediated general protection against the action of cytolytic proteins.
MATERIALS AND METHODS
Human fibroblast cell cultures.
Human lung fibroblasts were maintained in Dulbecco modified Eagle medium (GIBCO BRL) with 10% fetal calf serum (FCS), 2 mM l-glutamine, 100 U of penicillin per ml, and 100 μg of streptomycin per ml at 37°C and 5% CO2 and were used until passage 25.
NK cells.
Peripheral blood mononuclear cells were isolated and resuspended at a concentration of 5 × 106 cells/ml. Monocytes were removed by plastic adherence for 24 h at 37°C and 5% CO2 in RPMI medium containing 10% FCS, 2 mM l-glutamine, 100 U of penicillin per ml, and 100 μg of streptomycin per ml. On the following day, the peripheral blood lymphocytes were harvested, counted, and depleted of CD3-positive cells by incubating the cells with CD3-specific antibodies (Becton Dickinson, San Jose, Calif.) and magnetic beads (Dynal, Oslo, Norway). This step was repeated once to increase the purity of the NK cell population. The B cells were removed by the addition of CD19 magnetic beads (Dynal) to the peripheral blood lymphocytes. A fluorescence-activated cell sorter (FACSort; Becton Dickinson) was used to analyze the purity of the NK cell population. The cells were stained for the cellular markers CD3, CD45, CD14, and CD56/16. The fraction of CD3− CD56+ cells was ≥95% in each experiment. The NK cells were cultured at 37°C and 5% CO2 in RPMI with 10% FCS, 2 mM l-glutamine, 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 1,000 U of interleukin-2 per ml for 2 to 4 days.
Treatment of NK cells with strontium (Sr2+).
To examine the importance of the granule release in the NK killing of uninfected and HCMV-infected HL cells, NK cells were treated with Sr2+ as previously described (27) to induce degranulation. Briefly, 10 μl of 2.5 M SrCl2 was added per ml of effector cells at a concentration of 5 × 106 cells/ml. As control, the same volume of distilled water (dH2O) was added to control NK cells. After 18 h of incubation at 37°C, the cells were washed before being used in the cytotoxicity assay.
HCMV infection of fibroblasts.
Fibroblasts were infected with HCMV strains AD169 and Towne (both laboratory strains) and HA (a clinical isolate) and with three mutant HCMV strains: RV670, which lacks the genes US1 to -9 and US11; a UL18 knockout HCMV strain, UL18ΔHCMV (4); and a UL16 knockout strain, UL16ΔHCMV (20). The fibroblasts were cultured in Dulbecco modified Eagle medium (GIBCO BRL) with 5% FCS, 2 mM l-glutamine, 100 U of penicillin per ml, and 100 μg of streptomycin per ml during the infection. All of the mutant HCMV strains used are derived from the AD169 strain.
Transfection of fibroblasts.
Cells were transfected either with a pDC409 vector or with a pEGFP-C2 vector containing the UL16 gene (termed UL16:pDC409 and UL16:pEGFP-C2, respectively) by using a Duofect kit according to the instructions of the manufacturer (QUANTUM, Appligene) or were transfected by using the polyethyleneimine (PEI) method, as follows. A mixture of 5 μg of DNA and 1.2 μl of PEI (PEI stock of 22.5 mg of 25-kDa PEI [Aldrich] and 10 ml of dH2O [pH 7]) was diluted with dH2O to 20 μl. The mixture was left for 10 min at room temperature before being added to the cell cultures. Transfected cells were analyzed for their sensitivity to NK lysis and to treatment with perforin and streptolysin O (SLO).
Cytotoxicity test.
The NK cell-mediated cytotoxicity was measured by using a cyto96 cytotoxicity kit (Promega, Madison, Wis.), and the specific lysis was calculated by using the following formula: % cytoxicity = 100 × [(total release in experimental wells − effector spontaneous release − target spontaneous release)/(target maximum release − target spontaneous release)]. The spontaneous release from uninfected or HCMV-infected target cells never exceeded 20% of the total release. A paired t test was used to calculate P values, and differences were considered to be statistically significant at a P value of ≤0.05.
Measurement of the release of granzyme B from NK cells.
NK cells were incubated with uninfected or HCMV-infected fibroblasts, and supernatants were collected at 4 h after coincubation of target cells with NK cells. The granzyme B levels in the supernatants were measured by using an enzyme-linked immunosorbent assay method as previously described (34). Positive and negative controls were included as described elsewhere (34).
Lysis of cells with SLO, perforin (10), granzyme B (13), and NK lysin (2). (i) SLO.
Fifty microliters of target cells at 106 cells/ml was incubated with 100 U of SLO per ml for 30 min at 37°C in a total volume of 100 μl. The percentage of dead cells was measured by using the cyto96 cytotoxicity kit as described above.
(ii) Perforin and granzyme B.
Perforin and granzyme B were used as previously described (9). Briefly, for perforin, uninfected and HCMV-infected cells were incubated with different concentrations of perforin for 45 min at 37°C, and the percentage of dead cells was analyzed by staining the cells with propidium iodide or trypan blue. For perforin and granzyme B, 0.5 permeabilizing unit (1 permeabilizing units is the amount of perforin which permeabilizes 50% of the fibroblasts after 20 min of incubation) of perforin and 1 μg of granzyme B per ml were added to the cells (106/ml) in Eppendorf tubes in a total volume of 500 μl and left for 3 h at 37°C. Thereafter, the percentage of dead cells was analyzed by staining cells with propidium iodide or trypan blue.
(iii) NK lysin.
Fifty microliters of target cells at 106 cells/ml was incubated with 100 μg of NK lysin per ml for 30 min at 37°C in a total volume of 100 μl. The percentage of dead cells was measured by using the cyto96 cytotoxicity kit as described above.
Sorting of transfected cells.
Fibroblasts were transfected with the vector pEGFP-C2 or UL16:pEGFP-C2 by using Lipofectamine 2000 according to instructions of the manufacturer (Invitrogen Life Technologies). Green fluorescent protein (GFP)-expressing cells were sorted and collected by using a MoFLo cytometer and sorter (Dakocytomation, Glostrup, Denmark). Collected cells (GFP-expressing cells) were counted and used in NK assays and SLO experiments as described above.
RESULTS
The HCMV protein UL16 mediates protection against NK-mediated cytotoxicity.
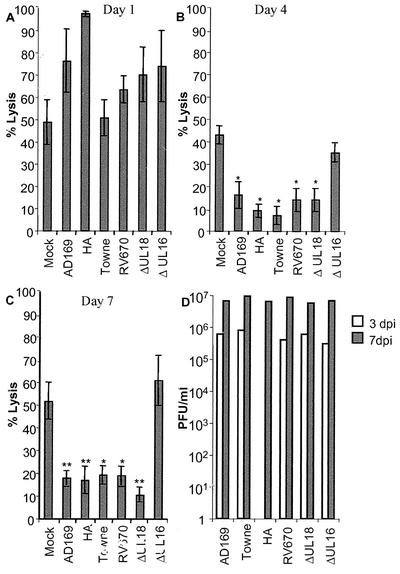
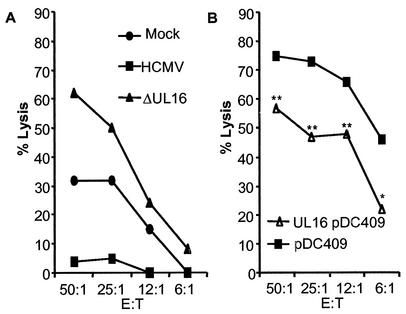
Previous studies have suggested that fibroblasts infected with certain HCMV strains, not including AD169, are resistant to killing by NK cells (5, 8, 38), possibly by the engagement of inhibitory molecules on the NK cells. To examine whether the expression of HLA class I molecules, the HCMV HLA class I homologue UL18, or the HCMV UL16 protein plays a role in mediating inhibitory signals to NK cells, fibroblasts were infected with different laboratory strains of HCMV (AD169 and Towne), a clinical isolate (HA), and the following mutant strains: (i) RV670 (a recombinant AD169 HCMV strain lacking the genes US1 to -9 and US11, which include four genes known to downregulate HLA class I molecule expression), (ii) UL16ΔHCMV (a recombinant AD169 HCMV strain lacking the UL16 gene), and (iii) UL18ΔHCMV (a recombinant AD169 HCMV strain lacking the HCMV class I homologue gene UL18). The cells were examined for their susceptibility to NK lysis at different time points after infection. At 1 day postinfection (dpi), fibroblasts infected with these different HCMV strains (multiplicity of infection [MOI], 1 to 10) did not demonstrate a difference in susceptibility to NK lysis compared to uninfected cells (Fig. 1A). However, at 4 and 7 dpi, fibroblasts infected with the HCMV strains AD169, Towne, HA, RV670, and UL18ΔHCMV exhibited an increased protection against NK lysis (Fig. 1B and C, respectively). In contrast, cells infected with the UL16ΔHCMV strain did not show a reduced susceptibility to NK lysis compared to uninfected cells (Fig. 1A to C and 2A) and showed a 70% increased sensitivity to NK cytotoxicity compared to AD169-infected cells. Complementing this result, transfecting fibroblasts with the UL16 gene (UL16-pDC409) resulted in a significantly increased protection against NK killing (Fig. 2B). Although the transfection level generally was low (8 to 35%), a 22 to 58% increased level of protection was observed, where paired t test analysis revealed P values of <0.005 at effector/target cell ratios of 50:1, 25:1, and 12,5:1 (n = 5). Furthermore, examination of fibroblasts infected with the different HCMV strains at 1, 4, and 7 dpi did not reveal a significant difference in infection level as analyzed by immediate-early staining (100% immediate-early-positive cells at 4 and 7 dpi [data not shown]), and cells infected with HCMV strains AD169, Towne, HA, RV670, UL18ΔHCMV, and UL16ΔHCMV did not display a difference in viral growth (Fig. 1D). These results suggest that UL16 is one of the major HCMV proteins that mediate resistance to NK killing of infected cells.
FIG. 1.
HCMV- but not UL16ΔHCMV-infected cells are resistant to NK lysis. Fibroblasts uninfected or infected with AD169, HA, Towne, UL18ΔHCMV, RV670, or UL16ΔHCMV were analyzed for their susceptibility to NK killing at different time points after infection. (A to C) Sensitivity of infected cells to NK killing at 1 (MOI, 10), 4 (MOI, 5), and 7 (MOI, 0.5) dpi, respectively (n = 5; mean values ± standard errors of the means at an effector/target cell ratio of 50:1 are shown). (D) Cells infected with the different HCMV strains demonstrate similar levels of viral growth at 3 and 7 dpi (n = 3; mean values ± standard errors of the means are shown). Statistical analysis was done by the paired t test (**, P ≤ 0.005; *, P ≤ 0.05).
FIG. 2.
UL16-transfected cells exhibit increased protection against NK lysis. UL16-transfected cells were analyzed for their sensitivity to NK lysis. (A) Results from a representative experiment showing susceptibility of HCMV AD169- and UL16ΔHCMV-infected cells to NK killing at 7 dpi, compared to uninfected cells. (B) One representative example of the sensitivity of UL16-transfected cells to NK lysis. E:T, effector/target cell ratio. **, P ≤ 0.005; *, P ≤ 0.05.
HCMV-infected cells trigger NK cell activation and the release of granzyme B.
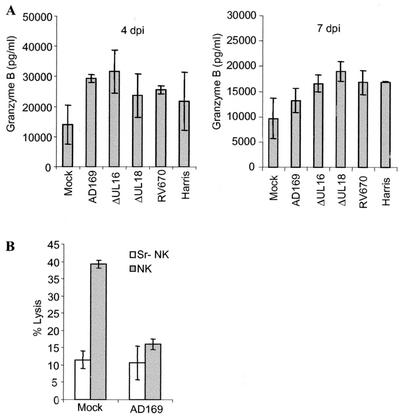
In order to verify whether HCMV-infected cells delivered inhibitory signals to NK cells, the amount of granzyme B released from activated NK cells was measured in supernatants obtained from NK cells incubated with uninfected or HCMV (AD169, HA, RV670, ΔUL18, and ΔUL16)-infected fibroblasts that were cocultured with NK cells for 4 h. Interestingly, the levels of granzyme B were not decreased in supernatants from NK cells cocultured with infected cells (at 4 or 7 dpi) compared to supernatants from NK cells cocultured with uninfected cells (Fig. 3). These results clearly suggest that HCMV-infected cells trigger NK cells to release their granule content of granzyme B and suggest that virus-infected cells may not be protected against NK lysis mainly through an engagement of negative signals to NK cells. To further examine the importance of the granule pathway in lysis of fibroblasts, we also analyzed the susceptibility of uninfected and HCMV-infected fibroblasts to NK cells treated with strontium (Sr), which is an agent that induces degranulation of NK cells. Degranulation of NK cells prior to the NK assay resulted in a significant reduction in the ability of NK cells to kill uninfected cells, and the percent lysis reached levels similar to those observed for HCMV-infected cells. A decreased susceptibility of HCMV-infected cells to untreated or Sr-treated NK cells was not demonstrated at 7 dpi (Fig. 3B). Hence, uninfected cells were mainly lysed by the cytolytic proteins released from the NK granules and not by a Fas-Fas ligand interaction pathway.
FIG. 3.
HCMV-infected cells trigger the release of granzyme B. (A) To analyze whether HCMV-infected cells delivered inhibitory signals to NK cells, the amount of granzyme B was measured in the supernatant after cocultivation of NK cells with uninfected or infected target cells. The presence of granzyme B in the supernatants obtained from NK cells incubated with target cells infected with the different HCMV strains compared to uninfected cells is shown for 4 and 7 dpi. The bars represent the mean values ± standard errors of the means. (B) To examine whether NK cells lyse fibroblast mainly by using the granule pathway and not the Fas-FasL interaction, NK cells were treated with strontium (Sr) (which causes degranulation of the NK cells) prior to the NK cell lysis test. While Sr-treated NK cells exhibited a reduced ability to kill uninfected cells compared to untreated NK cells, the susceptibility of HCMV-infected cells to NK lysis was not affected. Mean values ± standard deviations (n = 4) at an effector/target cell ratio of 50:1 are shown.
HCMV-infected cells are protected against the action of cytolytic proteins.
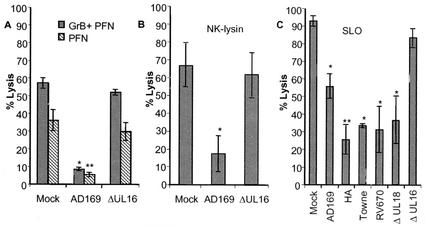
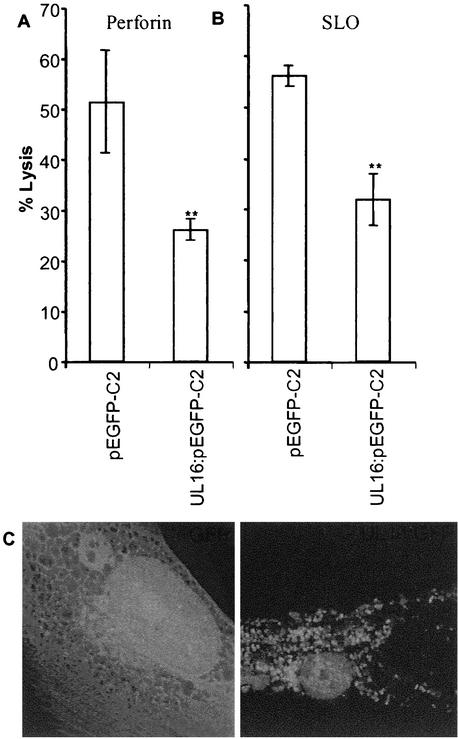
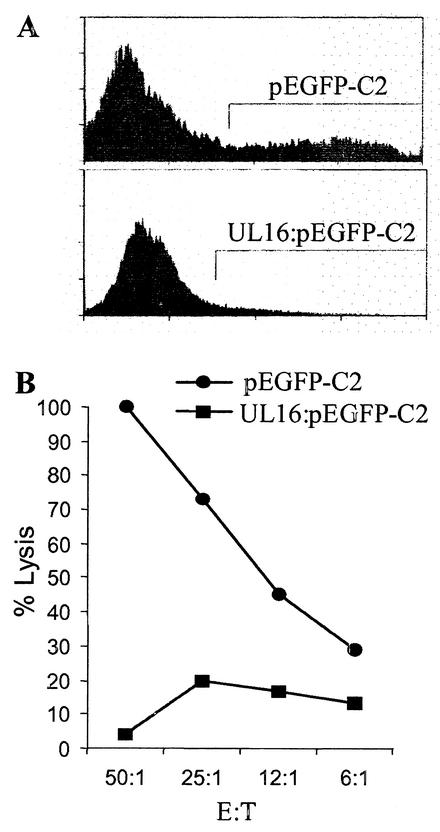
Due to the observation that the levels of granzyme B were not decreased in cell supernatants obtained from NK cells incubated with HCMV-infected cells, we examined whether HCMV-infected cells were less susceptible to the action of cytotoxic proteins delivered by activated NK cells. Uninfected and HCMV-infected cells were analyzed for their sensitivity to cytolytic proteins, including perforin, granzyme B, the bacterial toxin SLO, and porcine NK lysin (a porcine antibacterial peptide), which utilize different mechanisms to induce pore formation and to induce apoptosis of target cells. Interestingly, while AD169-infected cells exhibited a >80% increased resistance to the action of perforin in the presence or absence of granzyme B (Fig. 4A), UL16ΔHCMV-infected cells were not protected against membrane damage at 7 dpi (Fig. 4A). Similar results were demonstrated for NK lysin (Fig. 4B). In addition, while fibroblasts infected with AD169, Towne, HA, or the two mutant AD169 strains RV670 and ΔUL18 showed an increased resistance to SLO treatment at 7 dpi (Fig. 4C), cells infected with the UL16ΔHCMV strain were not protected against SLO treatment (Fig. 4C). Remarkably, transient transfection of fibroblasts with a vector containing a GFP-tagged UL16 gene resulted in 50% increased resistance to perforin (P < 0.001) and 43% increased resistance to SLO (P < 0.001) (Fig. 5A and B, respectively), despite a general low transfection level (8 to 30%). To confirm that the protection also was valid for NK lysis, we sorted and collected UL16-transfected cells or vector-positive cells and analyzed the two cell population for their sensitivity to NK killing. The cells that were selected for experimental analysis are shown within the bars in Fig. 6A. While GFP-transfected cells (pEGFP-C2) were highly susceptible to NK killing (Fig. 6B), UL16-GFP-expressing cells (UL16:pEGFP-C2) exhibited a very high increased resistance to NK lysis (Fig. 6B). In addition, sorted UL16-GFP-expressing cells were also significantly less susceptible to SLO treatment than pEGFP-C2-positive cells (data not shown). Hence, these results clearly suggest that the UL16 protein protects target cells from NK cell lysis and also from damage mediated by membranolytic proteins with entirely different mechanisms of action.
FIG. 4.
HCMV-infected cells exhibit an increased resistance to perforin, granzyme B, NK lysin, and SLO. (A and B) AD169-infected (A) and UL16ΔHCMV-infected (B) fibroblasts were examined for their sensitivities to perforin (PFN), granzyme B (GrB), and NK lysin, and the percentage of dead cells is shown (n = 4). (C) Cells infected with AD169, HA, Towne, RV670, UL18ΔHCMV, or UL16ΔHCMV were also analyzed for their susceptibility to SLO treatment at 7 dpi (n = 5). Mean values ± standard errors of the means are shown. Statistical analysis was by the paired t test (**, P ≤ 0.005; *, P ≤ 0.05).
FIG. 5.
UL16-transfected cells exhibit increased resistance to the action of perforin and SLO. (A and B) Cells transiently transfected with a vector containing the UL16 gene (UL16:pEGFP-C2), were examined for susceptibility to perforin (A) and SLO (B) treatment compared to uninfected cells. The mean percentages of dead cells (± standard errors of the means) are shown. **, P ≤ 0.005; *, P ≤ 0.05. (C) The intracellular distributions of UL16-GFP (right) and a GFP control (left) were analyzed by confocal immunofluorescence.
FIG. 6.
The UL16 protein confers protection against NK lysis. GFP-expressing cells (pEGFP-C2 and UL16: pEGFP-C2) were sorted with a MoFLo sorter and collected to be used in an NK assay. (A) The cells that were selected for sorting are shown within the bars. (B) Susceptibility of GFP-expressing cells to NK killing. E:T, effector/target cell ratio.
DISCUSSION
NK cells are generally believed to play an important role in the first line of defense against virus infections. Previous studies have shown that HCMV-infected fibroblasts become less sensitive to NK lysis, but the mechanisms responsible for this protection are not clearly understood. In this study, we report that fibroblasts infected with three different strains of HCMV exhibited an increased resistance to NK lysis at 4 and 7 dpi. This protection from NK cell lysis was also observed when cells were infected with the HCMV AD169 mutant strains UL18ΔHCMV and RV670, which suggests that NK resistance is not mainly dependent on the virus-encoded HLA class I homologue UL18 or the expression of HLA class I molecules on infected cells. However, infection with UL16ΔHCMV did not offer protection, suggesting that the HCMV UL16 protein provides a major inhibitory effect on NK lysis of infected cells. Since granzyme B secretion from NK cells was not decreased after incubation with HCMV-infected fibroblasts compared to uninfected fibroblasts, HCMV-infected cells did not appear to deliver significant inhibitory signals to NK cells. Instead, we found that HCMV-infected cells were resistant to the cytolytic effects of perforin, porcine NK lysin, and the bacterial toxin SLO. This resistance was mediated by the HCMV protein UL16, since UL16ΔHCMV-infected fibroblasts were not protected and since transient expression of UL16 in uninfected fibroblasts conferred an increased resistance against both NK-mediated permeabilization and the effects of isolated perforin and SLO.
For the in vivo situation, uninfected cells, such as macrophages, endothelial cells, and fibroblasts, are generally not believed to be susceptible to NK lysis. Therefore, these in vitro experimental systems must unfortunately be considered artificial, in the sense that NK cells are stimulated with cytokines and hence are lymphokine-activated killer cells. However, these cells are generally referred to as NK cells. NK cells are not believed to normally require exogenous cytokine stimulation to perform their functions. However, this experimental system is widely used to examine the function of NK cells in the killing of virus-infected cells. Using this system, we found not only a clear protective effect against NK lysis by the HCMV protein UL16 but also that the UL16 protein appears to mediate a membrane stabilization of target cells that was unrelated to NK cell activation.
Previous attempts to understand the protective effect of HCMV infection have focused on the role of inhibitory NK signals, including HLA-E (35, 39), HLA-G (30), adhesion molecules (8), and the class I homologue UL18 (31). While the UL18 protein initially appeared to mediate protection against NK lysis (31), this finding remains uncertain due to conflicting evidence presented by other investigators (22, 28). Furthermore, more recent evidence suggests that the UL40-mediated induction of HLA-E expression may confer protection against CD94/NKG2A NK cells (35, 39). In addition, the UL16 protein has been suggested to prevent the NK-triggering molecules ULBP-1, -2, and -3 from interfering with NK cells and thereby to increase protection of infected cells against NK lysis (7). Our results offer the unanticipated explanation that a virus-encoded protein mediates a generalized protection against the action of cytolytic proteins. Resistance of HCMV-infected cells to the action of SLO and NK lysin would indeed provide a very powerful tactic to evade membrane damage caused by both NK and cytotoxic T cells. We speculate that the HCMV UL16 protein, either by interaction with additional proteins or by itself, can neutralize the action of cytolytic peptides in several different ways: (i) by preventing pore formation in the cell membrane (a membrane stabilization function), (ii) by blocking the binding of these proteins to the cell membrane, (iii) by degrading the cytolytic proteins inside the cells, or (iv) by inhibiting intracellular signaling pathways induced by cytolytic proteins that cause apoptosis of the target cell. Interestingly, tumor cells have been reported to exhibit increased resistance against perforin but not against SLO or the action of complement, possibly because of impaired binding of perforin to the tumor cell membrane (21). Hence, further studies that aim to clarify the role of UL16 in mediating increased cellular membrane integrity are ongoing in our laboratory. It is hoped that such studies will give further insights into the poorly understood ability of cytolytic proteins to kill their target cells, and they may have important consequences far beyond the area of infectious diseases.
Acknowledgments
We thank Mats Andersson for providing the NK lysin, Giannis Spyrou for initial discussion regarding this project, and Erna Möller for critical comments and discussions.
This work was supported by grants from the Swedish Medical Research Council (12615-01A, 12615-04A, and 00793-36B), The Swedish Society of Medicine (98020633 and 1999-02-0347), the Tobias Foundation (1313/98, 33/02 20/01), the Swedish Childrens Cancer Research Foundation (98/065 and 01/046,), the Emil and Wera Cornells Foundation, and the Heart and Lung Foundation (1999-41-305 and 200141486). C.S.-N. is a fellow of the Wenner-Gren Foundation, Sweden. H.B. was supported by Wellcome Trust. C.J.F. was supported by NIH grant AI/GM44941 and a National Arthritis Foundation Biomedical Grant.
REFERENCES
- 1.Ahn, K., A. Gruhler, B. Galocha, T. R. Jones, E. J. Wiertz, H. L. Ploegh, P. A. Peterson, Y. Yang, and K. Fruh. 1997. The ER-luminal domain of the HCMV glycoprotein US6 inhibits peptide translocation by TAP. Immunity 6:613-621. [DOI] [PubMed] [Google Scholar]
- 2.Andersson, M., H. Gunne, B. Agerberth, A. Boman, T. Bergman, B. Olsson, A. Dagerlind, H. Wigzell, H. G. Boman, and G. H. Gudmundsson. 1996. NK-lysin, structure and function of a novel effector molecule of porcine T and NK cells. Vet. Immunol. Immunopathol. 54:123-126. [DOI] [PubMed] [Google Scholar]
- 3.Arase, H., N. Arase, and T. Saito. 1995. Fas-mediated cytotoxicity by freshly isolated natural killer cells. J. Exp. Med. 181:1235-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Browne, H., M. Churcher, and T. Minson. 1992. Construction and characterization of a human cytomegalovirus mutant with the UL18 (class I homolog) gene deleted. J. Virol. 66:6784-6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cerboni, C., M. Mousavi-Jazi, A. Linde, K. Soderstrom, M. Brytting, B. Wahren, K. Karre, and E. Carbone. 2000. Human cytomegalovirus strain-dependent changes in NK cell recognition of infected fibroblasts. J. Immunol. 164:4775-4782. [DOI] [PubMed] [Google Scholar]
- 6.Cosman, D., N. Fanger, L. Borges, M. Kubin, W. Chin, L. Peterson, and M. L. Hsu. 1997. A novel immunoglobulin superfamily receptor for cellular and viral MHC class I molecules. Immunity 7:273-282. [DOI] [PubMed] [Google Scholar]
- 7.Cosman, D., J. Mullberg, C. L. Sutherland, W. Chin, R. Armitage, W. Fanslow, M. Kubin, and N. J. Chalupny. 2001. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity 14:123-133. [DOI] [PubMed] [Google Scholar]
- 8.Fletcher, J. M., H. G. Prentice, and J. E. Grundy. 1998. Natural killer cell lysis of cytomegalovirus (CMV)-infected cells correlates with virally induced changes in cell surface lymphocyte function-associated antigen-3 (LFA-3) expression and not with the CMV-induced down-regulation of cell surface class I HLA. J. Immunol. 161:2365-2374. [PubMed] [Google Scholar]
- 9.Froelich, C. J., K. Orth, J. Turbov, P. Seth, R. Gottlieb, B. Babior, G. M. Shah, R. C. Bleackley, V. M. Dixit, and W. Hanna. 1996. New paradigm for lymphocyte granule-mediated cytotoxicity. Target cells bind and internalize granzyme B, but an endosomolytic agent is necessary for cytosolic delivery and subsequent apoptosis. J. Biol. Chem. 271:29073-29079. [DOI] [PubMed] [Google Scholar]
- 10.Froelich, C. J., J. Turbov, and W. Hanna. 1996. Human perforin: rapid enrichment by immobilized metal affinity chromatography (IMAC) for whole cell cytotoxicity assays. Biochem. Biophys. Res. Commun. 229:44-49. [DOI] [PubMed] [Google Scholar]
- 11.Gilbert, M. J., S. R. Riddell, B. Plachter, and P. D. Greenberg. 1996. Cytomegalovirus selectively blocks antigen processing and presentation of its immediate-early gene product. Nature 383:720-722. [DOI] [PubMed] [Google Scholar]
- 12.Groh, V. V., R. Rhinehart, J. Randolph-Habecker, M. S. Topp, S. R. Riddell, and T. Spies. 2001. Costimulation of CD8alphabeta T cells by NKG2D via engagement by MIC induced on virus-infected cells. Nat. Immunol. 2:255-260. [DOI] [PubMed] [Google Scholar]
- 13.Hanna, W. L., X. Zhang, J. Turbov, U. Winkler, D. Hudig, and C. J. Froelich. 1993. Rapid purification of cationic granule proteases: application to human granzymes. Protein Expr. Purif. 4:398-404. [DOI] [PubMed] [Google Scholar]
- 14.Hengel, H., J. O. Koopmann, T. Flohr, W. Muranyi, E. Goulmy, G. J. Hammerling, U. H. Koszinowski, and F. Momburg. 1997. A viral ER-resident glycoprotein inactivates the MHC-encoded peptide transporter. Immunity 6:623-632. [DOI] [PubMed] [Google Scholar]
- 15.Heusel, J. W., R. L. Wesselschmidt, S. Shresta, J. H. Russell, and T. J. Ley. 1994. Cytotoxic lymphocytes require granzyme B for the rapid induction of DNA fragmentation and apoptosis in allogeneic target cells. Cell 76:977-987. [DOI] [PubMed] [Google Scholar]
- 16.Hewitt, E. W., S. S. Gupta, and P. J. Lehner. 2001. The human cytomegalovirus gene product US6 inhibits ATP binding by TAP. EMBO J. 20:387-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones, T. R., and L. Sun. 1997. Human cytomegalovirus Us2 destabilizes major histocompatibility complex class I heavy chains. J. Virol. 71:2970-2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones, T. R., E. J. Wiertz, L. Sun, K. N. Fish, J. A. Nelson, and H. L. Ploegh. 1996. Human cytomegalovirus US3 impairs transport and maturation of major histocompatibility complex class I heavy chains. Proc. Natl. Acad. Sci. USA 93:11327-11333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jun, Y., E. Kim, M. Jin, H. C. Sung, H. Han, D. E. Geraghty, and K. Ahn. 2000. Human cytomegalovirus gene products US3 and US6 down-regulate trophoblast class I MHC molecules. J. Immunol. 164:805-811. [DOI] [PubMed] [Google Scholar]
- 20.Kaye, J., H. Browne, M. Stoffel, and T. Minson. 1992. The UL16 gene of human cytomegalovirus encodes a glycoprotein that is dispensable for growth in vitro. J. Virol. 66:6609-6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lehmann, C., M. Zeis, N. Schmitz, and L. Uharek. 2000. Impaired binding of perforin on the surface of tumor cells is a cause of target cell resistance against cytotoxic effector cells. Blood 96:594-600. [PubMed] [Google Scholar]
- 22.Leong, C. C., T. L. Chapman, P. J. Bjorkman, D. Formankova, E. S. Mocarski, J. H. Phillips, and L. L. Lanier. 1998. Modulation of natural killer cell cytotoxicity in human cytomegalovirus infection: the role of endogenous class I major histocompatibility complex and a viral class I homolog. J. Exp. Med. 187:1681-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le Roy, E., A. Muhlethaler-Mottet, C. Davrinche, B. Mach, and J. L. Davignon. 1999. Escape of human cytomegalovirus from HLA-DR-restricted CD4+ T-cell response is mediated by repression of gamma interferon-induced class II transactivator expression. J. Virol. 73:6582-6589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Machold, R. P., E. Wiertz, T. R. Jones, and H. L. Ploegh. 1997. The HCMV gene products Us11 and Us2 differ in their ability to attack allelic forms of murine major histocompatibility complex (MHC) class I heavy chains. J. Exp. Med. 185:363-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller, D. M., B. M. Rahill, J. M. Boss, M. D. Lairmore, J. E. Durbin, J. W. Waldman, and D. D. Sedmak. 1998. Human cytomegalovirus inhibits major histocompatibility complex class II expression by disruption of the Jak/Stat pathway. J. Exp. Med. 187:675-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller, D. M., Y. Zhang, B. M. Rahill, W. J. Waldman, and D. D. Sedmak. 1999. Human cytomegalovirus inhibits IFN-α stimulated antiviral and immunoregulatory responses by blocking multiple levels of IFN-α signal transduction. J. Immunol. 162:6107-6113. [PubMed] [Google Scholar]
- 27.Neighbour, P. A., H. S. Huberman, and Y. Kress. 1982. Human large granular lymphocytes and natural killing: ultrastructural studies of strontium-induced degranulation. Eur. J. Immunol. 12:588-595. [DOI] [PubMed] [Google Scholar]
- 28.Odeberg, J., C. Cerboni, H. Browne, K. Kärre, E. Möller, E. Carbone, and C. Söderberg-Nauclér. 2001. Human cytomegalovirus (HCMV) infected endothelial cells and macrophages are less susceptible to NK lysis Independently of downregulation of classical HLA class I molecules, or expression of the HCMV class I homologue, UL18. Scand J. Immunol. 55:149-161. [DOI] [PubMed] [Google Scholar]
- 29.Odeberg, J., and C. Soderberg-Naucler. 2001. Reduced expression of HLA class II molecules and interleukin-10- and transforming growth factor β1-independent suppression of T-cell proliferation in human cytomegalovirus-infected macrophage cultures. J. Virol. 75:5174-5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Onno, M., C. Pangault, G. Le Friec, V. Guilloux, P. Andre, and R. Fauchet. 2000. Modulation of HLA-G antigen expression by human cytomegalovirus: specific induction in activated macrophages harboring human cytomegalovirus infection. J. Immunol. 164:6426-6434. [DOI] [PubMed] [Google Scholar]
- 31.Reyburn, H. T., O. Mandelboim, M. Vales-Gomez, D. M. Davis, L. Pazmany, and J. L. Strominger. 1997. The class I MHC homologue of human cytomegalovirus inhibits attack by natural killer cells. Nature 386:514-517. [DOI] [PubMed] [Google Scholar]
- 32.Sedmak, D. D., A. M. Guglielmo, D. A. Knight, D. J. Birmingham, E. H. Huang, and W. J. Waldman. 1994. Cytomegalovirus inhibits major histocompatibility class II expression on infected endothelial cells. Am. J. Pathol. 144:683-692. [PMC free article] [PubMed] [Google Scholar]
- 33.Söderberg-Naucler, C., and J. Y. Nelson. 1999. Human cytomegalovirus latency and reactivation—a delicate balance between the virus and its host's immune system. Intervirology 42:314-321. [DOI] [PubMed] [Google Scholar]
- 34.Spaeny-Dekking, E. H., W. L. Hanna, A. M. Wolbink, P. C. Wever, A. J. Kummer, A. J. Swaak, J. M. Middeldorp, H. G. Huisman, C. J. Froelich, and C. E. Hack. 1998. Extracellular granzymes A and B in humans: detection of native species during CTL responses in vitro and in vivo. J. Immunol. 160:3610-3616. [PubMed] [Google Scholar]
- 35.Tomasec, P., V. M. Braud, C. Rickards, M. B. Powell, P. McSharry, S. Gadola, V. Cerundolo, L. K. Borysiewicz, A. McMichael, and G. W. G. Wilkinson. 2000. Surface expression of HLA-E, an inhibitor of natural killer cells, enhanced by human cytomegalovirus gpUL40. Science 287:1031-1033. [DOI] [PubMed] [Google Scholar]
- 36.Tomazin, R., J. Boname, N. R. Hegde, D. M. Lewinsohn, Y. Altschuler, T. R. Jones, P. Cresswell, J. A. Nelson, S. R. Ridell, and D. C. Johnson. 1999. Cytomegalovirus US2 destroys two compartment of the MHC class II pathway, preventing recognition by CD4+ T cells. Nat. Med. 5:1039-1043. [DOI] [PubMed] [Google Scholar]
- 37.Ulbrecht, M., S. Martinozzi, M. Grzeschik, H. Hengel, J. W. Ellwart, M. Pla, and E. H. Weiss. 2000. Cutting edge: the human cytomegalovirus UL40 gene product contains a ligand for HLA-E and prevents NK cell-mediated lysis. J. Immunol. 164:5019-5022. [DOI] [PubMed] [Google Scholar]
- 38.Waner, J. L., and J. A. Nierenberg. 1985. Natural killing (NK) of cytomegalovirus (CMV)-infected fibroblasts: a comparison between two strains of CMV, uninfected fibroblasts, and K562 cells. J. Med. Virol. 16:233-244. [DOI] [PubMed] [Google Scholar]
- 39.Wang, E. C., B. McSharry, C. Retiere, P. Tomasec, S. Williams, L. K. Borysiewicz, V. M. Braud, and G. W. Wilkinson. 2002. UL40-mediated NK evasion during productive infection with human cytomegalovirus. Proc. Natl. Acad. Sci. USA 99:7570-7575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Warren, A. P., D. H. Ducroq, P. J. Lehner, and L. K. Borysiewicz. 1994. Human cytomegalovirus-infected cells have unstable assembly of major histocompatibility complex class I complexes and are resistant to lysis by cytotoxic T lymphocytes. J. Virol. 68:2822-2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wiertz, E. J., T. R. Jones, L. Sun, M. Bogyo, H. J. Geuze, and H. L. Ploegh. 1996. The human cytomegalovirus US11 gene product dislocates MHC class I heavy chains from the endoplasmic reticulum to the cytosol. Cell 84:769-779. [DOI] [PubMed] [Google Scholar]