Abstract
CD8+-T-cell (TCD8+) responses to infectious viruses are characterized by an immunodominance hierarchy in which the majority of TCD8+ respond to one or a few immunodominant determinants, with a minority of TCD8+ responding to a number of subdominant determinants. It is now well established that exogenous antigens are capable of inducing TCD8+ to such immunodominant determinants, but the diversity of the response and the nature of the immunodominance hierarchy have not been examined. We addressed this issue by characterizing TCD8+ responses to influenza virus preparations rendered inert by incubation for 10 min at 100°C, as first reported by Speidel et al. (Eur. J. Immunol. 27:2391-2399, 1997). Extending these findings, we show that the primary TCD8+ response to boiled virus can be sufficiently robust to be detected ex vivo by intracellular cytokine staining and that the response encompasses many of the peptides recognized by TCD8+ induced by infectious virus. Importantly, the immunodominance hierarchy elicited was leveled, and we were unable to detect TCD8+ that were specific for boiled virus. We used peritoneal exudate cells as antigen-presenting cells in vitro, and a number of observations indicated that boiled virus is processed via a phagocytic route that is likely to be endosomal in nature. These findings suggest that the repertoires of immunogenic peptides generated by endosomes and cytosolic processes overlap to a surprising degree. Furthermore, they demonstrate that the form of antigen administered can influence immunodominance hierarchies and that exogenous-antigen vaccines can induce broad and balanced TCD8+ responses.
Nearly all paradigms are short lived, at least in their most absolute sense. For nearly 2 decades, immunology gospel dictated that protein antigens were incapable of inducing CD8+-T-cell (TCD8+) responses, which required immunization with either antigens capable of inducing biosynthesis of “endogenous antigens” in host cells or whole cells expressing the nominal antigen (the latter phenomenon is known as cross-priming [4]). Nevertheless, paradigm be damned, reports kept surfacing that protein antigens could induce memory TCD8+ responses (reviewed in reference 40). Remarkably, Speidel and colleagues found that protein antigens could become TCD8+ immunogens, albeit weak ones, simply through boiling (at the same time, their abilities to elicit antibodies plummet) (30).
The widening discrepancy between paradigm and observation seemed to be reconciled by the finding that the artificial delivery of exogenous antigens to the cytosol enabled their entry to the classical pathway trod by endogenous antigens (15, 39). Indeed, the discovery that professional antigen-presenting cells (pAPCs) can transfer endocytosed antigens to the cytosol (17, 23) provides a cellular biological basis for the presentation of exogenous antigens to TCD8+.
Nature often does not favor simplicity, however, and the existence of the endosome-to-cytosol pathway does not preclude the endosomal generation of peptide class I complexes à la the major histocompatibility complex (MHC) class II antigen-processing system. Indeed, solid evidence has accumulated for the MHC class I processing of particulate antigens in endosomal compartments of pAPCs (21, 22, 36).
The endosomal processing of antigens raises a serious issue of specificity. Given the complexity of the classical cytosolic pathway, which imposes at least two filters (in the forms of the proteasome and the transporter associated with antigen processing [TAP]) on the peptides provided to class I molecules, how can the action of endosomal proteases result in the generation of the same determinants? Biologically, and pragmatically for those intent on raising TCD8+ by endosomally processed vaccines, this is a crucial question. There is little point in making use of endosomal antigen processing to induce TCD8+ that cannot recognize target cells expressing determinants produced by the cytosolic pathway. By the same token, if exogenous immunogens can elicit TCD8+ that recognize endogenously processed antigens, then the advantages of using non-biosynthetic-based immunogens are obvious.
To date, studies of exogenous antigen processing have been limited to one or two determinants in a given system. Here, we take advantage of the influenza virus (IV) system to study the immunogenicity of multiple determinants in a complex antigen, in this case virus preparations rendered completely noninfectious by boiling. Numerous IV determinants recognized by H-2b and H-2d mice have been defined through the combined efforts of a number of laboratories (2, 3, 11, 14, 19, 27, 33, 35). These determinants can be placed into a stable immunodominance hierarchy that varies little between inbred individuals immunized under a given set of conditions (1, 6). In this study, we compared the immunodominance hierarchies induced by infectious and boiled virus and examined the mechanism of presentation of boiled virus.
MATERIALS AND METHODS
Cell lines.
The dendritic cell line DC2.4 (H-2b; provided by K. Rock, University of Massachusetts Medical School, Worcester, Mass. [26]), the thymoma cell line EL-4 (H-2b), TAP2-deficient lymphoma cell line RMA/S (H-2b), and P815 (H-2d) mastocytoma cells were maintained in RPMI 1640 containing 10% fetal calf serum, 5 × 10−5 M β-mercaptoethanol, and 2 mM glutamine (RP-10).
Mice and cytotoxic-T-lymphocyte priming in vivo.
For in vivo priming, 8- to 10-week-old female BALB/c mice and C57BL/J6 (B6) mice (Taconic, Germantown, N.Y.), were injected intraperitoneally (i.p.) with ∼600 hemagglutinating units of chicken egg allantoic fluid containing infectious or boiled (100°C for 10 min) IV Puerto Rico/8/34 (H1N1) (PR8) or A/NT/60/68 (H3N2) (NT60 virus). Splenocytes and peritoneal exudate cells collected by balanced salt solution-bovine serum albumin lavage were directly assessed after 7 days for intracellular cytokine staining (ICS). Alternatively, splenocytes were cultured for 7 days in vitro with IV-infected autologous spleen cells with I+ medium (Iscove’s modified Dulbecco’s modified essential medium [Iscove’s MDEM], 10% fetal calf serum, 5 × 10−5 M β-mercaptoethanol, 50 μg of gentamicin sulfate/ml).
Generation of specific TCD8+ lines.
For generation of TCD8+ lines, animals were generally used at more than 30 days after priming. TCD8+ stimulation was always carried out in RP-10 with 10 U of recombinant human interleukin-2/ml. In brief, 3 × 107 splenocytes were stimulated with 1/40 to 1/50 the number of NT60-infected or peptide-pulsed EL-4 cells, which were irradiated with 220 Gy. Stimulated live T cells were harvested through Ficoll-Hypaque gradient and enriched for CD8+ cells by depletion of B220+ and CD4+ cells by using monoclonal antibody (MAb)-coated M-450 Dynal beads.
TCD8+ functional assays.
For ICS, following lysis of erythrocytes, splenocytes were resuspended at a concentration of 107/ml in I+, and 200 μl was added per well to round-bottom 96-well plates. Synthetic peptides (>95% purity) were added to a concentration of 0.5 μM. In the case of cysteine-containing peptides, the medium was supplemented with 200 μM TCEP [tris (2-carboxyethyl) phosphine hydrochloride] (Pierce, Rockford, Ill.) to prevent sulfhydryl modification (8). After 2 h of incubation at 37°C, brefeldin A (BFA) was added, and cells were incubated for a further 4 h to accumulate gamma interferon (IFN-γ) in the endoplasmic reticulum of activated cells. Cells were then incubated on ice for 1 h with a Cy-Chrome-conjugated anti-CD8 α MAb (BD PharMingen, San Diego, Calif.) at a 1:50 dilution in phosphate-buffered saline (PBS), washed, fixed with 1% paraformaldehyde in PBS at room temperature for 20 min, and then incubated with fluorescein isothiocyanate-conjugated anti-IFN-γ MAb (PharMingen) at a 1:100 dilution in PBS containing 0.3% saponin (Calbiochem, San Diego, Calif.). Stained cells were analyzed by flow cytometry gated on the CD8+ cells.
51Cr-release assays were performed as follows. A total of 106 target cells were labeled with 100 μCi of Na51CrO4 (Perkin-Elmer, Boston, Mass.) in a minimum volume of medium at 37°C for 60 min. After two washes, 104 cells were aliquoted into round-bottom 96-well plates containing serial dilutions of effector TCD8+. In some experiments, TCEP was freshly dissolved in H2O and used at a concentration of 200 μM in microcytotoxicity assay wells. The radioactivity in supernatants collected after 6 h of incubation at 37°C was determined by using a gamma counter. The percentage of specific release was then determined as follows: percentage of specific release = [(TCD8+-induced release − spontaneous release)/(release by detergent − spontaneous release)] × 100.
Antigen presentation assay.
Thioglycollate-induced peritoneal exudate cells (tPEC) were harvested from C57BL/J6 (B6) and B6 TAP1 knockout mice (TAP−/−; obtained from Taconic) 3 days after i.p. injection with 1 ml of thioglycolate (BD Biosciences, Franklin Lakes, N.J.). Harvested tPEC were pooled and resuspended with I+ at a concentration of 2 × 106/ml. One hundred microliters of the tPEC suspension was plated into a 96-well U microplate, and then 50 μl of boiled NT60 was added. For inhibitor studies, tPEC in suspension were pretreated for 30 min with 2 μM cytochalasin D (Calbiochem), the lowest concentration that inhibits uptake of fluorescent Saccharomyces cerevisiae, (Molecular Probes, Eugene, Oreg.), or 25 mM NH4Cl (Sigma, St. Louis, Mo.). After 3 to 6 h of incubation, 100 μl of specific TCD8+ at a concentration of 106/ml was added with BFA (Sigma) to give a final concentration of 7 μg/ml. ICS was performed 3 h later. Viability was confirmed by either trypan blue exclusion visually or ethidium homodimer (Molecular Probes) exclusion by flow cytometry.
β2m addition assay.
tPEC were harvested from B6 and TAP−/− mice and washed five times with I− medium (Iscove's MDEM, 5 × 10−5 M β-mercaptoethanol, 0.1% bovine serum albumin). tPEC (4 × 105/ml) were cultured overnight in 96-well round-bottom plates in 200 μl of I− with or without 5 μg of human β2 microglobulin (β2m)/ml (Fitzgerald Industries International, Inc., Concord, Mass.). Cells were then incubated with 50 μl of boiled-NT60 (bNT60)- or infectious-NT60-containing allantoic fluid for 3 h. One hundred microliters of TCD8+ at a concentration of 106/ml was then added into each well with BFA at a final concentration of 5 μg/ml. ICS was performed after a 3-h incubation.
Peptide binding assay.
Freshly harvested tPEC were washed with PBS and suspended at a concentration of 4 × 105/ml in 200 μl of PBS supplemented with 0.1% NaN3. Cells were incubated on ice for 20 min with or without 10 μg of synthetic peptide FAPGNYPAL, which binds both Kb and Db with high affinity (9). Cells were washed three times and then incubated for 20 min on ice in 100 μl of PBS containing a 5 μM concentration of synthetic peptide SIINFEKL coupled to fluorescein via the ɛ-amino group of the Lys residue (10). Cells were washed, and the amount of peptide bound by live cells (determined by ethidium homodimer exclusion) was determined by flow cytometry. Alternatively, fresh tPEC were incubated similarly with synthetic peptide SIINFEKL on ice, and the amounts of cell surface peptide class I complexes were enumerated by flow cytometry following staining with the 25-D1.16 MAb coupled to Cy5.
RESULTS
Boiled virus induces a balanced TCD8+ response against determinants generated by virus-infected cells.
Speidel et al. (30) reported that boiled IV is capable of priming for secondary in vitro responses to the nucleoprotein (NP) determinant NP366-374 as measured by cytotoxicity. Boiling the virus is expected to destroy all viral functions, and as expected, heating virus-containing allantoic fluid for 10 min at 100°C reduced viral infectivity, hemagglutination, and fusion activity to undetectable levels (data not shown).
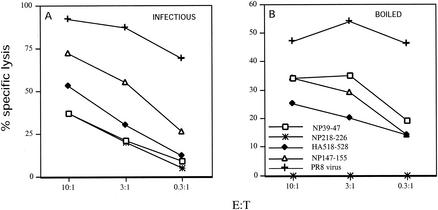
We first compared the immunogenicities of infectious and boiled PR8 (bPR8) in BALB/c mice by immunizing mice by i.p. injection and stimulating memory TCD8+ in vitro with virus-infected APCs. After 7 days of culture, cells were tested for their capacity to lyse histocompatible target cells sensitized with Kd-restricted peptides. As seen in Fig. 1A, infectious virus induces TCD8+ that can be ranked according to their lytic activity as follows: NP147-155 > HA518-526 > NP39-47 = NP218-226. bPR8 elicited an easily detected secondary response that was approximately half the magnitude of that elicited by PR8 as detected on PR8-infected cells (Fig. 1B). The dose used was close to optimal since increasing or decreasing the amount of bPR8 by fivefold decreased immunogenicity (data not shown). The immunogenicity of bPR8 could not be attributed solely to contamination with free protein or peptides present in allantoic fluid, since purified virus was also immunogenic upon boiling (data not shown). Importantly, the spectrum of specificities elicited by bPR8 was similar to but clearly different from that of PR8: NP218-226-specific TCD8+ were not primed, and the activities of the other specificities were rearranged (NP39-47 = NP147-155 > HA518-526).
FIG. 1.
TCD8+ response of BALB/c mice to boiled virus. Splenocytes from BALB/c mice immunized with infectious (A) or boiled (B) PR8 were restimulated in vitro for 7 days and then used in a 51Cr-release assay at the indicated effector-to-target cell ratio (E:T). P815 target cells were infected with PR8 or sensitized with synthetic peptides as indicated. Similar results were obtained in an additional experiment.
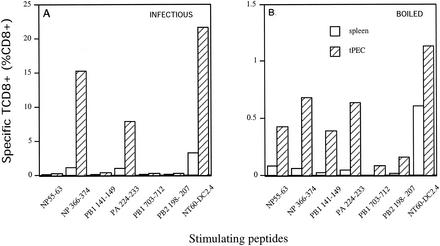
We extended these findings by immunizing B6 mice with infectious NT60 or bNT60. bNT60 proved to be more immunogenic than bPR8. This allowed us to quantitate both local (peritoneal exudate) and splenic primary responses at 7 days postinfection by ICS, which provides a much more direct measure of TCD8+ priming than does measuring of lytic activities of secondary in vitro cultures (Fig. 2). The total number of responding TCD8+ elicited by bNT60 was approximately 5% of that elicited by NT60, as assessed on NT60-infected APCs. Importantly, the typical immunodominance hierarchy induced by NT60 introduced by i.p. injection, NP366-374 ≥ PA224-233 > PB2198-207, NP55-63, and PA141-149 (7), was altered, with relative decreases in TCD8+ responding to immunodominant determinants NP366-374 and PA224-233 and relative increases in TCD8+ responses to subdominant determinants, particularly NP55-63 and PA141-149.
FIG. 2.
TCD8+ response of B6 mice to boiled virus. Splenocytes or tPEC from B6 mice immunized with infectious (A) or boiled (B) NT60 were tested ex vivo by ICS for recognition of DC2.4 cells sensitized with the indicated peptides or with infectious NT60. Note that the response elicited by infectious NT60 was ∼20-fold higher than that elicited by bNT60. Similar results were obtained in four additional experiments.
The major portion of TCD8+ induced by boiled virus recognizes virus-infected cells.
These data make the important point that boiled IV induces TCD8+ that are specific for the same peptides generated from endogenous IV proteins. To address the critical question of whether TCD8+ against novel determinants are also induced, we immunized mice with NT60 or bNT60 and determined the percentage of TCD8+ activated by APCs sensitized with infectious versus boiled virus. For reasons described below, we used thioglycolate tPEC as APCs. As shown in Table 1, bNT60 was less efficient than NT60 as both an immunogen and an antigen. The crucial finding of this experiment was that, using TCD8+ induced by bNT60, bNT60 was proportionally worse as an antigen than NT60. Thus, relative to NT60, bNT60 displayed a 12-fold decrease in the percentage of NT60-induced TCD8+ activated and a 23-fold decrease in the percentage of bNT60-induced TCD8+ activated. While this experiment does not eliminate the possibility that novel specificities arise in response to bNT60, it indicates that the major portion of the response is directed against peptides generated by the cytosolic pathway.
TABLE 1.
NT60 and bNT60 elicit highly overlapping sets of TCD8+a
| Immunogen | % of TCD8+ (mean ± SD) respondingb to antigen:
|
|
|---|---|---|
| NT60 | bNT60 | |
| NT60 | 36.7 ± 5.6 | 3.0 ± 0.8 |
| bNT60 | 7.04 ± 0.61 | 0.3 ± 0.4 |
pTEC from B6 mice primed 7 days previously with either infectious NT60 or bNT60 were harvested and incubated with B6 tPEC exposed to infectious NT60 or bNT60 for 3 h. TCD8+ were obtained from four mice immunized with NT60 and three mice immunized with bNT60.
As determined by ICS.
Characterization of presentation of bNT60 in vitro.
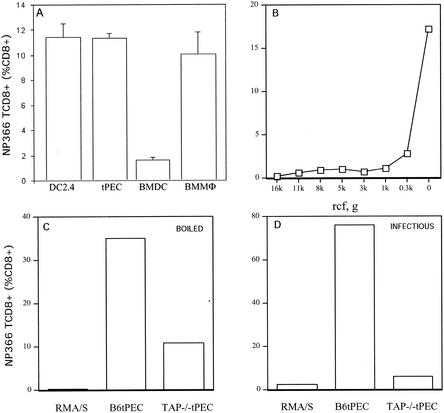
To explore the extent to which antigens from boiled IV are presented by cytosolic versus endosomal processing, we generated a TCD8+ line specific for NP366-374. We first determined which cells were able to present bNT60 to these cells. Primary bone marrow-derived dendritic cells and bone marrow macrophages were cultured as described previously (16, 17). tPEC were harvested from mice 3 days after i.p. injection of thioglycolate. We also used the DC-like cell line DC2.4. Cells were incubated with bNT60 for 6 h and then with TCD8+ for 3 h in the presence of BFA to accumulate intracellular IFN-γ (Fig. 3A). All of the pAPCs were able to generate NP366-374 from bNT60. Bone marrow-derived dendritic cells were actually the least effective APCs. We chose tPEC for further study based on their high capacity to present bNT60 to TCD8+ and ease of preparation from both wild-type and knockout mice.
FIG. 3.
Presentation of bNT60 to NP366-374-specific TCD8+ in vitro. (A) The indicated cells were incubated with bNT60 for 6 h prior to addition of an NP366-374-specific TCD8+ line. After 3 h, the activation of TCD8+ was assessed by ICS. BMDC, bone marrow-derived dendritic cells; BMMΦ, bone marrow macrophages. (B) tPEC incubated as in panel A with supernatants from bNT60 after centrifugation for 10 min at the indicated relative centrifugal force (rcf) were used to stimulate NP366-374-specific TCD8+. (C and D) Cells were incubated with bNT60 or infectious NT60 as indicated and used to stimulate NP366-374-specific TCD8+. Similar results were obtained in an additional experiment.
To demonstrate that presentation was not based on the presence of peptides in the virus preparation, we centrifuged virus at various g forces for 10 min and tested the capacity of tPEC to present material present in the supernatant. As seen in Fig. 3B, antigenic material was completely depleted by low-speed centrifugation, demonstrating that NP366-374 is generated from highly aggregated material. Furthermore, TAP-deficient RMA/S cells, which present antigenic peptides at extremely low concentrations, were unable to present bNT60 to TCD8+ (Fig. 3C).
The presentation of NP366-374 by IV-infected cells via the standard cytosolic route is largely TAP dependent (13, 32). We compared the capacity of tPEC from B6 and TAP1−/− mice to present bNT60 to NP366-374-specific TCD8+. In the absence of TAP, activation by boiled virus was reduced by approximately threefold while activation by infectious virus was reduced by approximately 15-fold (Fig. 3C). The absence of TAP is accompanied by a decrease in the number of peptide-receptive (PR) class I molecules on the cell surface (10) and in endosomal compartments (5, 28, 29). We extended these observations to tPEC by measuring the binding of a fluorescent version of SIINFEKL, which associates with both Kb and Db (10). Specific binding to class I molecules was determined by subtracting background binding values obtained when cells were first incubated with an unlabeled peptide that also binds Db and Kb. As shown in Table 2, tPEC from B6 mice possess ∼40% more PR class I molecules than do tPEC from TAP−/− mice, as determined by the difference in peptide binding. Similarly enhanced binding of nonmodified SIINFEKL to Kb on B6 tPEC was demonstrated by staining of cells with Cy5-conjugated 25-D1.16 MAb (20), which is specific for Kb-Ova257-264 complexes (Table 2). These findings are consistent with the idea that the decreased presentation of boiled virus by TAP1−/− cells is due to a decrease in PR class I molecules in post-Golgi complex compartments.
TABLE 2.
TAP knockout decreases PR molecules on tPECa
| Peptide | Mice
|
|
|---|---|---|
| B6 | TAP−/− | |
| SIINFEKL-Fl | 88 | 63 |
| SIINFEKL | 106 | 71 |
Peptide binding to tPEC was determined by flow cytometry. Values represent mean channel fluorescence of viable cells. SIINFEKL-Fl (SIINFEKL coupled to fluorescein) binding to cells was measured directly. Data represent specific binding to class I molecules as determined by subtracting background binding values obtained when cells were incubated with a competitor peptide prior to the addition of the labeled peptide. Binding of SIINFEKL to Kb was determined by using Cy5-conjugated 25-D1.16. Background binding to cells not exposed to peptide was subtracted from values shown. Similar results were obtained in two additional experiments.
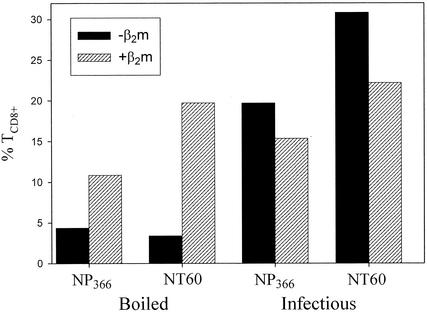
β2m acquired from the media is responsible for upwards of 90% of PR cell surface class I molecules. β2m dependence provides another measure of presentation by the exogenous route, though for the effect to be absolute, cells must be incubated for 5 to 10 days in β2m-free media (24, 34). Primary cells cannot survive for such long periods, but we were able to incubate tPEC overnight in serum-free medium with or without β2m. As shown in Fig. 4A, presentation of bNT60 by B6 tPEC to either NP366-374-specific TCD8+ or polyclonal anti-NT60 TCD8+ was enhanced by β2m. Similar findings were made using tPEC from TAP−/− mice (data not shown). Importantly, presentation of infectious NT60 by B6 tPEC demonstrated the opposite dependence on exogenous β2m, which decreased presentation (Fig. 4A).
FIG. 4.
Presentation of boiled virus is enhanced by β2m. tPEC from B6 mice were incubated overnight with or without human β2m and exposed to either infectious NT60 or bNT60. Cells were tested for their capacity to activate NP366-374- or NT60-specific TCD8+ as described in the legend to Fig. 3. Similar, though less dramatic, results were obtained in two additional experiments.
We further examined the mechanism of presentation of bNT60 to NP366-374- and NT60-specific TCD8+ by treating B6 and TAP1−/− tPEC with agents that affect endocytosis or exocytosis (Table 3). If presentation is based on the processing of aggregated antigen, as suggested by the depletion of antigenicity by low-speed centrifugation, then it should be blocked by prevention of phagocytosis. This can be accomplished by treating cells with the actin-polymerized drug cytochalasin D. Since cytochalasin D is more selective for phagocytosis at lower doses, in a preliminary experiment we established a concentration (2 μg/ml) that blocked uptake of fluorescent zymosan A (S. cerevisiae) BioParticles in 95% of cells (data not shown). This dose was sufficient to completely block presentation by TAP1−/− tPEC and reduce by threefold the number of TCD8+ activated by B6 tPEC. Presentation was completely blocked by the lysosomotropic agent NH4Cl, which interferes with endosomal trafficking and the activity of acid proteases by increasing the pH of acidic compartments. These findings strongly suggest that presentation of bNT60 to NP366-374- and NT60-specific TCD8+ occurs predominantly, if not exclusively, in a phagosomal compartment.
TABLE 3.
Presentation of bNT60 to NP366-374-specific TCD8+ is blocked by endosomal and phagosomal inhibitors
| Treatmenta | % of TCD8+ activated/% inhibitionb
|
|
|---|---|---|
| B6 mice | TAP−/− mice | |
| Control | 6.62 (100%) | 2.01 (100%) |
| CD | 2.43/64 | 0/100 |
| NH4Cl | 0.08/99 | 0/100 |
tPEC from B6 or TAP−/− mice were exposed to cytochalasin D (CD) or NH4Cl for 30 min prior to and during incubation with bNT60. In control experiments, inhibitors had only minor effects on the TCD8+ activation induced by limiting amounts of peptides.
Data show the effect of inhibitors on presentation of bNT60 and are relative to control values. Similar results were obtained in two additional experiments.
DISCUSSION
Recent interest in the processing of exogenous antigens has been stoked by three factors. First is the need to develop effective vaccines for eliciting TCD8+ responses to pathogens and tumors. Second are the findings that molecular chaperones bound to some yet-to-be-defined form of nominal antigen can induce TCD8+ responses (31). Third is the recognition that Bevan's cross-priming phenomenon may account for a considerable amount of priming to infectious organisms (12), though the importance of cross-priming is not universally acknowledged (18).
Here we demonstrate that the specificities of TCD8+ responding to boiled IV overlap considerably with those elicited by infectious virus. A mountain of evidence indicates that these determinants are generated from infectious virus via the standard cytosolic pathway (though the involvement of the proteasome in the generation of many of the determinants has not been definitively established), at least in the nonprofessional APCs studied in vitro (37). While we cannot eliminate the possibility that boiled virus is presented via a cytosolic route, our findings suggest that it is presented via endosomal processing. If this is true, then the data shown in Table 1 indicate that the sets of peptides generated by endosomal and cytosolic processing overlap to a surprisingly high degree.
How is this possible? Studies of immunodominance indicate that perhaps only one in five peptides capable of high-affinity binding to class I molecules is generated from its source proteins in sufficient amounts by the cytosolic pathway to be immunogenic (38). It is not difficult to see how the endosomal route could generate a high percentage of the peptides generated by the cytosolic route. The trouble comes from achieving this without generating additional determinants that are generated in limiting amounts by the cytosolic route. Since the major filter in cytosolic processing is proteolysis (not TAP), this suggests an unexpected overlap in the specificities of endosomal and cytosolic proteases.
We do not mean to imply that the overlap is seamless. Indeed, Schirmbeck, Wild, and Reimann have reported that distinct determinants are generated by endosomal and cytosolic processing of hepatitis B virus surface antigen (25). It is possible, indeed even likely, that we have missed some determinants uniquely immunogenic in boiled virus. The point is rather that such determinants do not dominate the response to boiled virus.
Overall, the TCD8+ response to boiled virus is more balanced than the response to infectious virus. This may be due to a more balanced generation of peptides. Alternatively, it could reflect differences in the nature of the APC or the conditions of antigen presentation. If nothing else, interferons and other cytokines induced by virus infection will alter the conditions during antigen presentation. Additional potential factors include the cytopathogenicity of the virus for APCs and the continued synthesis of viral antigens until the virus is cleared.
From the standpoint of vaccine development, the present results potentially represent welcome news. They suggest that it may be possible to obtain a balanced, reasonably robust immune response with an extremely simple antigen preparation that avoids the unavoidable side effects of live viral vaccines or the potential dangers of DNA vaccines. It may be possible to boost immunogenicity further by using adjuvants. Importantly, TCD8+ induced by this noninfectious preparation recognize determinants generated by virus-infected cells.
Acknowledgments
We are grateful to Bethany Buschling and James Gibbs for providing outstanding technical assistance.
REFERENCES
- 1.Belz, G. T., P. G. Stevenson, and P. C. Doherty. 2000. Contemporary analysis of MHC-related immunodominance hierarchies in the CD8+ T cell response to influenza A viruses. J. Immunol. 165:2404-2409. [DOI] [PubMed] [Google Scholar]
- 2.Belz, G. T., W. Xie, J. D. Altman, and P. C. Doherty. 2000. A previously unrecognized H-2Db-restricted peptide prominent in the primary influenza A virus-specific CD8+ T-cell response is much less apparent following secondary challenge. J. Virol. 74:3486-3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belz, G. T., W. Xie, and P. C. Doherty. 2001. Diversity of epitope and cytokine profiles for primary and secondary influenza A virus-specific CD8+ T cell responses. J. Immunol. 166:4627-4633. [DOI] [PubMed] [Google Scholar]
- 4.Bevan, M. J. 1976. Minor H antigens introduced on H-2 different stimulating cells cross-react at the cytotoxic T cell level during in vivo priming. J. Immunol. 117:2233-2238. [PubMed] [Google Scholar]
- 5.Chefalo, P. J., and C. V. Harding. 2001. Processing of exogenous antigens for presentation by class I MHC molecules involves post-Golgi peptide exchange influenced by peptide-MHC complex stability and acidic pH. J. Immunol. 167:1274-1282. [DOI] [PubMed] [Google Scholar]
- 6.Chen, W., L. C. Anton, J. R. Bennink, and J. W. Yewdell. 2000. Dissecting the multifactorial causes of immunodominance in class I-restricted T cell responses to viruses. Immunity 12:83-93. [DOI] [PubMed] [Google Scholar]
- 7.Chen, W., C. C. Norbury, Y. Cho, J. W. Yewdell, and J. R. Bennink. 2001. Immunoproteasomes shape immunodominance hierarchies of antiviral CD8+ T cells at the levels of T cell repertoire and presentation of viral antigens. J. Exp. Med. 193:1319-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, W., J. W. Yewdell, R. L. Levine, and J. R. Bennink. 1999. Modification of cysteine residues in vitro and in vivo affects the immunogenicity and antigenicity of major histocompatibility complex class I-restricted viral determinants. J. Exp. Med. 189:1757-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cole, G. A., T. L. Hogg, M. A. Coppola, and D. L. Woodland. 1997. Efficient priming of CD8+ memory T cells specific for a subdominant epitope following Sendai virus infection. J. Immunol. 158:4301-4309. [PubMed] [Google Scholar]
- 10.Day, P. M., F. Esquivel, J. Lukszo, J. R. Bennink, and J. W. Yewdell. 1995. Effect of TAP on the generation and intracellular trafficking of peptide-receptive major histocompatibility complex class I molecules. Immunity 2:137-147. [DOI] [PubMed] [Google Scholar]
- 11.Deng, Y., J. W. Yewdell, L. C. Eisenlohr, and J. R. Bennink. 1997. MHC affinity, peptide liberation, T cell repertoire, and immunodominance all contribute to the paucity of MHC class I-restricted peptides recognized by antiviral CTL. J. Immunol. 158:1507-1515. [PubMed] [Google Scholar]
- 12.den Haan, J. M., and M. J. Bevan. 2001. Antigen presentation to CD8+ T cells: cross-priming in infectious diseases. Curr. Opin. Immunol. 13:437-441. [DOI] [PubMed] [Google Scholar]
- 13.Esquivel, F., J. W. Yewdell, and J. R. Bennink. 1992. RMA/S cells present endogenously synthesized cytosolic proteins to class I-restricted cytotoxic T lymphocytes. J. Exp. Med. 175:163-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falk, K., O. Rötzschke, K. Deres, J. Metzger, G. Jung, and H.-G. Rammensee. 1991. Identification of naturally processed viral nonapeptides allows their quantification in infected cells and suggests an allele-specific T cell epitope forecast. J. Exp. Med. 174:425-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore, M. W., F. R. Carbone, and M. J. Bevan. 1988. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell 54:777-785. [DOI] [PubMed] [Google Scholar]
- 16.Norbury, C. C., B. J. Chambers, A. R. Prescott, H. G. Ljunggren, and C. Watts. 1997. Constitutive macropinocytosis allows TAP-dependent major histocompatibility complex class I presentation of exogenous soluble antigen by bone marrow-derived dendritic cells. Eur. J. Immunol. 27:280-288. [DOI] [PubMed] [Google Scholar]
- 17.Norbury, C. C., L. J. Hewlett, A. R. Prescott, N. Shastri, and C. Watts. 1995. Class I MHC presentation of exogenous soluble antigen via macropinocytosis in bone marrow macrophages. Immunity 3:783-791. [DOI] [PubMed] [Google Scholar]
- 18.Ochsenbein, A. F., S. Sierro, B. Odermatt, M. Pericin, U. Karrer, J. Hermans, S. Hemmi, H. Hengartner, and R. M. Zinkernagel. 2001. Roles of tumour localization, second signals and cross priming in cytotoxic T-cell induction. Nature 411:1058-1064. [DOI] [PubMed] [Google Scholar]
- 19.Oukka, M., N. Riché, and K. Kosmatopoulos. 1994. A nonimmunodominant nucleoprotein-derived peptide is presented by influenza A virus-infected H-2b cells. J. Immunol. 152:4843-4851. [PubMed] [Google Scholar]
- 20.Porgador, A., J. W. Yewdell, Y. Deng, J. R. Bennink, and R. N. Germain. 1997. Localization, quantitation, and in situ detection of specific peptide-MHC class I complexes using a monoclonal antibody. Immunity 6:715-726. [DOI] [PubMed] [Google Scholar]
- 21.Ramachandra, L., E. Noss, W. H. Boom, and C. V. Harding. 1999. Phagocytic processing of antigens for presentation by class II major histocompatibility complex molecules. Cell. Microbiol. 1:205-214. [DOI] [PubMed] [Google Scholar]
- 22.Reimann, J., and R. Schirmbeck. 1999. Alternative pathways for processing exogenous and endogenous antigens that can generate peptides for MHC class I-restricted presentation. Immunol. Rev. 172:131-152. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez, A., A. Regnault, M. Kleijmeer, P. Ricciardi-Castagnoli, and S. Amigorena. 1999. Selective transport of internalized antigens to the cytosol for MHC class I presentation in dendritic cells. Nat. Cell Biol. 1:362-368. [DOI] [PubMed] [Google Scholar]
- 24.Schirmbeck, R., S. Thoma, and J. Reimann. 1997. Processing of exogenous hepatitis B surface antigen particles for Ld-restricted epitope presentation depends on exogenous β2-microglobulin. Eur. J. Immunol. 27:3471-3484. [DOI] [PubMed] [Google Scholar]
- 25.Schirmbeck, R., J. Wild, and J. Reimann. 1998. Similar as well as distinct MHC class I-binding peptides are generated by exogenous and endogenous processing of hepatitis B virus surface antigen. Eur. J. Immunol. 28:4149-4161. [DOI] [PubMed] [Google Scholar]
- 26.Shen, Z., G. Reznikoff, G. Dranoff, and K. L. Rock. 1997. Cloned dendritic cells can present exogenous antigens on both MHC class I and class II molecules. J. Immunol. 158:2723-2730. [PubMed] [Google Scholar]
- 27.Sherman, L. A., T. A. Burke, and J. A. Biggs. 1992. Extracellular processing of antigens that bind class I major histocompatibility molecules. J. Exp. Med. 175:1221-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song, R., and C. V. Harding. 1996. Roles of proteasomes, transporter for antigen presentation (TAP), and β2-microglobulin in the processing of bacterial or particulate antigens via an alternate class I MHC processing pathway. J. Immunol. 156:4182-4190. [PubMed] [Google Scholar]
- 29.Song, R., A. Porgador, and C. V. Harding. 1999. Peptide-receptive class I major histocompatibility complex molecules on TAP-deficient and wild-type cells and their roles in the processing of exogenous antigens. Immunology 97:316-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Speidel, K., W. Osen, S. Faath, I. Hilgert, R. Obst, J. Braspenning, F. Momburg, G. J. Hammerling, and H. G. Rammensee. 1997. Priming of cytotoxic T lymphocytes by five heat-aggregated antigens in vivo: conditions, efficiency, and relation to antibody responses. Eur. J. Immunol. 27:2391-2399. [DOI] [PubMed] [Google Scholar]
- 31.Srivastava, P. K., A. Menoret, S. Basu, R. J. Binder, and K. L. McQuade. 1998. Heat shock proteins come of age: primitive functions acquire new roles in an adaptive world. Immunity 8:657-665. [DOI] [PubMed] [Google Scholar]
- 32.Townsend, A., C. Öhlen, J. Bastin, H.-G. Ljunggren, L. Foster, and K. Kärre. 1989. Association of class I major histocompatibility heavy and light chains induced by viral peptides. Nature 340:443-448. [DOI] [PubMed] [Google Scholar]
- 33.Townsend, A. R. M., J. Rothbard, F. M. Gotch, G. Bahadur, D. Wraith, and A. J. McMichael. 1986. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell 44:959-968. [DOI] [PubMed] [Google Scholar]
- 34.Vitiello, A., T. A. Potter, and L. A. Sherman. 1990. The role of β2-microglobulin in peptide binding by class I molecules. Science 250:1423-1426. [DOI] [PubMed] [Google Scholar]
- 35.Vitiello, A., L. Yuan, R. W. Chesnut, J. Sidney, S. Southwood, P. Farness, M. R. Jackson, P. A. Peterson, and A. Sette. 1996. Immunodominance analysis of CTL responses to influenza PR8 virus reveals two new dominant and subdominant Kb-restricted epitopes. J. Immunol. 157:5555-5562. [PubMed] [Google Scholar]
- 36.Wick, M. J., and H. G. Ljunggren. 1999. Processing of bacterial antigens for peptide presentation on MHC class I molecules. Immunol. Rev. 172:153-162. [DOI] [PubMed] [Google Scholar]
- 37.Yewdell, J., L. C. Anton, I. Bacik, U. Schubert, H. L. Snyder, and J. R. Bennink. 1999. Generating MHC class I ligands from viral gene products. Immunol. Rev. 172:97-108. [DOI] [PubMed] [Google Scholar]
- 38.Yewdell, J. W., and J. R. Bennink. 1999. Immunodominance in major histocompatibility complex class I-restricted T lymphocyte responses. Annu. Rev. Immunol. 17:51-88. [DOI] [PubMed] [Google Scholar]
- 39.Yewdell, J. W., J. R. Bennink, and Y. Hosaka. 1988. Cells process exogenous proteins for recognition by cytotoxic T lymphocytes. Science 239:637-640. [DOI] [PubMed] [Google Scholar]
- 40.Yewdell, J. W., C. C. Norbury, and J. R. Bennink. 1999. Mechanisms of exogenous antigen presentation by MHC class I molecules in vitro and in vivo: implications for generating CD8+ T cell responses to infectious agents, tumors, transplants, and vaccines. Adv. Immunol. 73:1-77. [DOI] [PubMed] [Google Scholar]