Abstract
After intraocular injection of the virulent pseudorabies virus (PRV) strain Becker into late-stage chicken embryos, the virus spreads and replicates in the brain, where severe edema and hemorrhaging follow. By contrast, the attenuated Bartha strain does not cause severe brain pathology despite viral replication and spread throughout the brain (B. W. Banfield, G. S. Yap, A. C. Knapp, and L. W. Enquist, J. Virol. 72:4580-4588, 1998). These observations prompted us to explore the mechanism by which the virulent Becker strain mediates pathology in the chicken embryo central nervous system (CNS). To test the hypothesis that Becker infection induced an inflammatory response in the developing CNS, we examined the ability of the anti-inflammatory corticosteroid dexamethasone (Dex) to protect chicken embryos from PRV-induced brain damage. We found that Dex is not sufficient to protect the chicken embryo CNS from damage due to Becker infection. Surprisingly, systemic Dex delivery appeared to potentiate CNS damage, which was preceded by petechial hemorrhaging in the optic lobes. Taken together, these data suggest that the severe pathology elicited during the Becker infection is due not to immunopathology but to damage by the virus itself, possibly through the damage to or destruction of endothelial cells.
The alphaherpesvirus subfamily contains the neurotropic human pathogens herpes simplex virus type 1 (HSV-1) and HSV-2 as well as the swine pathogen pseudorabies virus (PRV). Alphaherpesviruses enter their hosts through the mucosal epithelium and then spread to the peripheral nervous system where the viruses establish a lifelong latent infection in the sensory ganglia. Upon reactivation, newly synthesized virus is able to reinfect the mucosal epithelium. In healthy adults, infection, establishment of latency, and reactivation generally do not cause serious health problems. In neonatal and immunocompromised individuals, however, the replication and spread of virus in the peripheral tissues are not controlled by the immune system, and the virus can spread systemically, often infecting the central nervous system (CNS) with lethal consequences. We have taken advantage of the chicken embryo eye model to examine the roles of individual alphaherpesvirus genes in acute infection of the developing CNS.
In this model, the virulent PRV strain Becker elicits frank tissue pathology that is characterized by edema, hemorrhage, and ultimately liquefaction of brain tissue. By contrast, the attenuated Bartha strain replicates and spreads throughout the brain efficiently without causing significant tissue damage (1). These data indicated that the host responds differently to brain infections by virulent and attenuated strains of PRV. Studies of various viruses that cause acute, lethal neurological diseases have shown that viruses can cause neuronal damage, either directly from damage due to the viral infection itself or indirectly from damage caused by resident or invading immune cells responding to viral antigens. For example, it has been shown that infection with rabies virus does not produce a strong inflammatory response (7). However, rabies virus-infected cells do show a strong downregulation of late-host-response gene expression and cell death apparently due to apoptosis. The highly virulent street strains of rabies virus do not induce apoptosis until very late in infection (7), which allows the virus to replicate and spread throughout the brain of the host. By contrast, experiments using the anti-inflammatory corticosteroid dexamethasone (Dex) showed that the neuronal damage in Borna virus disease is due to the destructiveness of the adaptive immune response to viral antigens (5).
How then does PRV cause tissue damage in the brain, and why do the virulent virus strains cause this neuropathology but the attenuated strains do not? One clue to the answer to this question comes from the observation that 36 to 48 h after inoculation, prior to the appearance of hemorrhage, Becker- but not Bartha-infected embryos develop edema, a hallmark of inflammation (1). This observation suggests that Becker induces an acute inflammatory response in the brains of infected embryos but that Bartha does not. In addition, studies of the closely related human pathogen, HSV, have shown that during herpes simplex encephalitis there is a strong acute inflammatory response as well as a long-term cellular and humoral immune activation (14, 23, 26). Herpes simplex encephalitis is characterized by many of the same pathologies that we observe after intraocular (IO) infection of chicken embryos with virulent PRV strains, including swelling of brain tissue and petechial hemorrhaging (3, 12). These similarities may suggest that, as with Borna disease virus, it is the destructiveness of the immune response to virulent PRV strains that causes the severe brain pathology. Thus, we anticipated that inhibitors of the inflammatory response, such as Dex, would protect against the neuronal damage caused by PRV. The present study demonstrates that Dex is unable to protect against the severe neuropathology that is elicited by the virulent PRV Becker strain, indicating that the host immune response is not responsible for the observed tissue damage. Furthermore, our data suggest that the virulent Becker strain infects brain endothelial cells efficiently but that the attenuated Bartha strain does not. These data may suggest that differences in cell tropism influence the degree of tissue pathology after acute infection of the CNS by alphaherpesviruses.
IO injection of PRV.
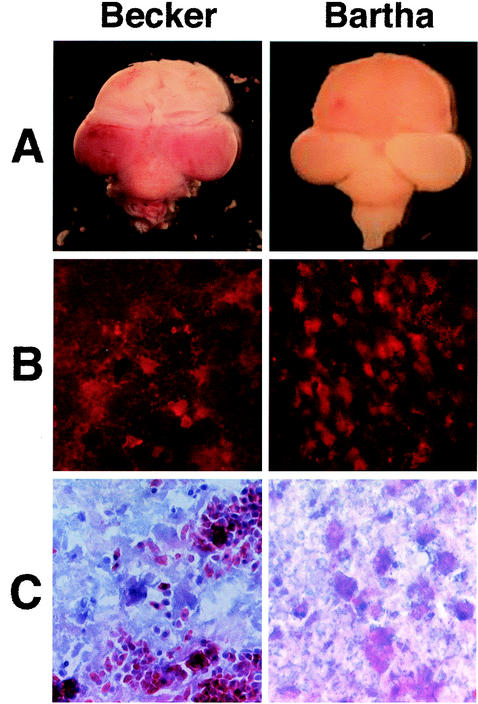
For these investigations, viruses were propagated and their titers were determined on PK15 cells, and 1 μl of inoculum containing 105 PFU of virus was injected IO into the chicken embryo as described previously (1). Previously, the PRV Becker strain was shown to be nearly 100-fold more virulent than the Bartha strain, as demonstrated by determining the 50% lethal doses after IO injection into the chicken embryos (1). Gross morphological examination of the brains of embryos infected with either Becker or Bartha showed a marked difference in pathologies (Fig. 1A). By 72 h after injection, the Becker-infected embryo showed severe hemorrhaging in the brain stem and both optic lobes (Fig. 1A). By contrast, the Bartha-infected embryo showed no signs of hemorrhaging or tissue damage in the brain despite the ability of the virus to replicate and spread efficiently in the brain (Fig. 1A). Brains from Becker- and Bartha-infected embryos were harvested, fixed in 4% paraformaldehyde for 16 h, transferred to 20% sucrose for a minimum of 16 h, and stored at 4°C until frozen at −80°C in Histo-prep embedding medium (Fisher Scientific Co., Pittsburgh, Pa.). Frozen tissue was sectioned in the coronal plane into 14-μm-thick sections at −20°C with a cryostat. Figure 1B and C show serial sections through the isthmo-optic nucleus (ION) of Becker- or Bartha-infected brains that were either stained for viral antigen by using a monoclonal antibody specific for the membrane glycoprotein B (gB) (Fig. 1B) or stained with hematoxylin and eosin (H&E) (Fig. 1C). The ION was examined because it is the first site of the brain to be infected after IO inoculation (B. W. Banfield, N. Brecha, and L. W. Enquist, unpublished observations). At 72 h after injection, the Becker-infected brain showed significant viral infection through the ION (Fig. 1B). Similarly, the Bartha-infected brain also showed significant infection in the ION (Fig. 1B). Despite the fact that both viruses caused significant infection, only the Becker virus caused extensive hemorrhaging and tissue damage in the ION, as seen in the H&E-stained sections (Fig. 1C). Four Becker-infected animals were examined. With H&E staining, the Bartha-infected brain tissue showed normal structure and no signs of hemorrhaging (Fig. 1C). The brains from four Bartha-infected embryos were sectioned, stained with H&E, and examined. There was no indication of hemorrhaging in the Bartha-infected brain sections despite extensive infection throughout the brains.
FIG. 1.
PRV infection in the chicken embryo eye model. (A) Dorsal view of whole brains 72 h after IO injection with 105 PFU in 1 μl of either the virulent Becker (left) or the attenuated Bartha (right) strain of PRV. (B) Serial cryostat sections (14-μm thick) of Becker-infected (left) or Bartha-infected (right) brains 72 h after inoculation. Sections were stained with an antibody specific to gB, followed by an Alexa 568 conjugated secondary antibody. The red fluorescence indicates virus infection. (C) Serial sections corresponding to sections shown in panel B were stained with H&E.
Dex is capable of protecting against LPS-induced inflammation.
Lipopolysaccharide (LPS) from the cell walls of gram-negative bacteria is a useful reagent for eliciting an acute inflammatory response. Corticosteroids are potent inhibitors of inflammation, and Dex has been shown to inhibit the inflammatory response in many animal models, including that of the chorioallantoic membrane of chicken embryos (2, 4, 8). We first wanted to show that Dex was capable of inhibiting an inflammatory response in the brains of chicken embryos. We began by injecting 1 μl of LPS in a range of concentrations (from 5 to 150 ng/ml) intracranially (i.c.) into 75 embryos at embryonic day 13 (E13) to determine the level of LPS necessary to elicit an inflammatory response in this model. Because IO injections of virus are performed at E12, and the virus requires 18 to 24 h to reach the brain, the LPS was injected i.c. at E13 so that both LPS- and Becker-induced brain pathologies would occur at the same developmental stage. A total of 24 embryos were sacrificed at 6, 24, and 48 h after injection, and their brains were examined for indications of inflammation. All 24 of the LPS-treated embryos developed edema and hemorrhaging throughout the brain. However, the brains of five embryos treated with 100 ng of LPS per ml developed gross pathologies by 24 h similar to those seen in Becker-infected brains by 48 h after injection.
We next determined that Dex was able to inhibit the LPS-induced inflammatory response. At E12, we injected 20 embryos with 100 μl of 100 μM Dex in sterile saline intravenously (i.v.). Briefly, eggs were candled to locate a suitable blood vessel for injection. A small section of shell was removed from above the blood vessel in each egg, and a drop of sterile mineral oil was applied to the dry membrane to visualize the chorioallantoic membrane beneath it. A 28-gauge needle attached to a syringe loaded with Dex was inserted into the vein, and the Dex was slowly injected into the bloodstream. This was followed on E13 by i.c. injection of 100 ng of LPS per ml. Forty control animals were injected either with Dex i.v. and sterile saline i.c. (n = 20) or with sterile saline i.v. and i.c. (n = 20). Of the nine Dex-treated embryos examined, none developed edema or hemorrhaging in the brain other than minor tissue damage at the LPS injection site (data not shown). These data indicated that treatment with 100 μM Dex 24 h prior to LPS challenge protected the embryos from LPS-induced hemorrhaging in the brain. In addition to providing protection against the LPS-induced brain pathology, treatment with Dex also increased the mean survival time of the embryos from 36 h post-LPS challenge to 62 h post-LPS challenge (15 embryos per group).
Dex is unable to protect against Becker-induced pathology.
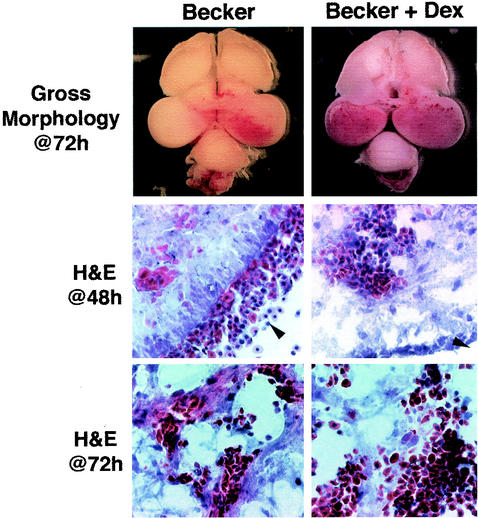
Once we determined that a 100-μl dose of a 100 μM solution of Dex was effective in preventing LPS-induced inflammation, we wanted to determine whether Dex was capable of preventing the severe brain pathology induced by Becker infection. On E12, 31 embryos were injected intravenously with Dex and then briefly returned to the incubator to recover. Four to 6 h after the i.v. injections, the embryos were injected IO with 105 PFU of Becker. At 24, 48, 72, and 96 h after the Becker injections, six embryos, one or two per time point, were sacrificed and the brains were removed for examination. Gross morphological examination of each of the embryos showed increasingly severe tissue pathologies over time. This pattern of progressive hemorrhage and tissue damage over time is characteristic of Becker infection in this model (1). Figure 2 shows the gross morphology of representative brains 72 h after the inoculation of a Becker-infected embryo and a Becker-infected embryo treated with Dex. Surprisingly, we found that Dex was ineffective in preventing the virus-induced hemorrhaging and edema of infected chicken embryos. In fact, the damage appeared more severe in the six Dex-treated embryos than in the three embryos examined that received no steroid treatment. These data suggested that the Becker-induced brain pathology was not due to an inflammatory response. Moreover, it appeared that the host inflammatory response was protective against the virus-induced tissue damage. Figure 2 shows H&E-stained sections of untreated and Dex-treated Becker-infected brain tissues 48 h after injection. These sections showed a large amount of blood throughout the brain tissue. H&E-stained sections 72 h after injection showed an increased amount of blood in the tissue as well as large areas of tissue destruction (Fig. 2). Based on these observations, we investigated whether Dex treatment would have an effect on the inability of Bartha to cause tissue damage. Gross morphological examination of whole brains and H&E-stained sections of four Bartha-infected, Dex-treated embryos indicated that steroid treatment did not enable Bartha to cause tissue damage in infected brains (data not shown).
FIG. 2.
Dex does not protect against Becker-induced brain pathology. At E12, embryos were injected i.v. with 100 μl of either sterile saline (left) or 100 μM Dex (right), followed by 105 PFU in 1 μl of Becker at E12.(Upper panels) Dorsal view of whole brains 72 h after Becker inoculation; (middle panels) H&E-stained, 14-μm-thick sections from the brains of Becker-infected embryos 48 h after IO injection; (lower panels) H&E-stained, 14-μm-thick sections from the brains of Becker-infected embryos 72 h after IO injection.
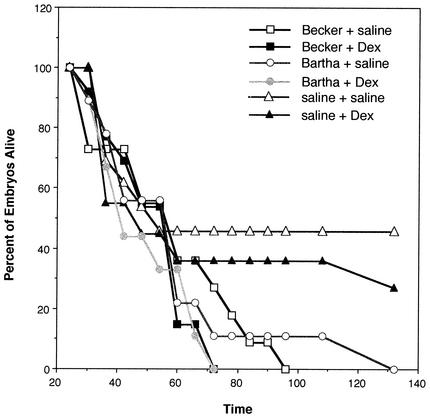
We next assessed the effects of the Dex treatment on the survival of the embryos by measuring time to death (Fig. 3). The high mortality of the control groups illustrates the severity of the injection procedure. By 72 h after IO injection of the Becker virus, 100% of the embryos treated with Dex died (n = 13) whereas 28% of the infected embryos not treated with steroid survived (n = 11). Similarly, 100% of the nine Bartha-infected embryos treated with steroid had died by 72 h after infection, whereas 11% of the nine untreated Bartha-infected embryos survived to 132 h after infection. These data support our morphological analysis in that not only did the Dex treatment not protect the embryos against the Becker-induced pathologies, but, in fact, it also slightly decreased the embryos' survival times. Taken together, these data suggest that not only is the inflammatory response of the chicken embryo not the causative agent of the Becker-induced pathology but also suppression of inflammation increases the virulence of the virus, indicating that the damage to the brain is not caused by inflammatory cells but is a result of the viral infection itself.
FIG. 3.
Dex reduces the mean time to death of Becker- and Bartha-infected embryos. Chicken embryos were injected i.v. with 100 μl of either 100 μM Dex or sterile saline, followed by IO injection with 105 PFU in 1 μl of Becker (n = 11) or Bartha (n = 9). The y axis shows the percentage of embryos surviving. The x axis shows the time in hours after virus injection.
Becker, but not Bartha, is able to infect endothelial cells.
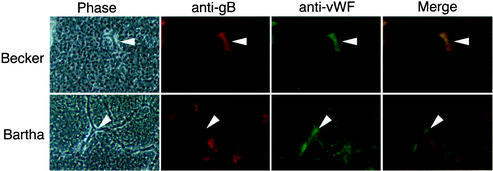
During Becker infection in the presence or absence of Dex treatment, we often observed petechial hemorrhaging (Fig. 2). Petechial hemorrhaging is characteristic of endothelial cell damage. We therefore hypothesized that Becker infection was causing damage to the endothelial cells of the brain. We proposed that Becker, but not Bartha, was able to infect the endothelial cells of the brain, causing damage or death that would lead to an increase in vascular permeability or disruption of the vascular integrity. To test this hypothesis we stained Becker- or Bartha-infected brain sections with antisera against PRV gB and von Willebrand factor (vWF), an endothelial cell marker. A minimum of 12 coronal sections throughout the brains of Becker- and Bartha-infected embryos were examined. Figure 4 shows representative sections. An Alexa 568-conjugated secondary antibody (anti-gB) was used to detect the viral infection (red), while an Alexa 48-conjugated secondary antibody (anti-vWF) was used to detect the endothelial cells (green) in the tissue. Merged images of the fluorescent images are shown in Fig. 4. The Becker-infected tissues show precise colocalization of the infected cells with the vWF-expressing endothelial cells, indicating that there is viral infection in these cells. However, the Bartha-infected cells do not colocalize with the endothelial cells, indicating that there is no endothelial cell infection. We were unable to detect Bartha infection of endothelial cells in any sections, whereas Becker infection of endothelial cells was readily observed. In sections where endothelial cells could be precisely identified, the only cells infected with Becker were endothelial cells. This was likely due to the extensive tissue damage caused by the Becker strain; once the Becker virus invades any area of the brain, the tissue destruction of that area proceeds very quickly. It is therefore difficult to locate areas of richly infected brain tissue that maintain enough structural integrity to identify distinct cell types. By contrast, Bartha infection does not cause this tissue destruction so that tissue architecture is maintained in the presence of extensive viral infection.
FIG. 4.
Becker, but not Bartha, is able to infect endothelial cells. Becker- and Bartha-infected brains were harvested and sectioned 72 h after infection. Sections were stained with antibodies specific to gB and vWF, an endothelial cell marker, followed by Alexa 568 and Alexa 488 conjugated secondary antibodies. The panels show phase-contrast images of the brain sections (Phase), red fluorescence indicating virus-infected cells (anti-gB), green fluorescence indicating endothelial cells (anti-vWF), and merged images of the red- and green-fluorescent panels (Merge). Arrowheads indicate blood vessels within the brain tissue.
In this report, we provide evidence that the tissue pathology induced by the virulent Becker strain in the chicken embryo brain is not a result of an inflammatory response by the host. We also show that this virus-induced damage is somewhat inhibited by the host's innate immune response. Initial infection by most viruses causes a rapid, nonspecific immune response by the host (24, 25). This response includes the rapid secretion of pro- and anti-inflammatory cytokines and chemokines in a complex, tightly regulated cascade that makes up an acute inflammatory response (9). It is not surprising then that viruses have evolved a variety of ways of interfering with this response. Once disregulated, this cascade of events in the inflammatory process can cause serious harm to the host, such as the overproduction of interleukin-1 and tumor necrosis factor alpha that leads to septic shock (11).
Leshchinsky and Klasing showed that LPS induces the production of the proinflammatory cytokines interleukin-1β, myelomonocytic growth factor, and gamma interferon as well as the anti-inflammatory cytokine transforming growth factor β in day-old chickens (15). We have shown, at a gross morphological level, that LPS-induced inflammation produces characteristic edema and causes hemorrhaging in the brains of late-stage embryos and that immunosuppression by Dex inhibits this inflammatory response. Additionally, Dex-treated animals had a greater rate of survival than did the untreated animals. Taken together, these data indicate that Dex is a potent inhibitor of inflammation in the chicken embryo model.
After IO injection in the chicken embryo, the virulent Becker strain of PRV spreads to well-defined areas of the brain. This infection of the chicken embryo brain results in a severe pathology characterized by edema, hemorrhaging, and ultimately liquefaction of brain tissue and embryo death (1). Interestingly, the attenuated Bartha strain of PRV is also capable of infecting the brain of chicken embryos and will ultimately cause the death of the animals but does not cause any of the edema, hemorrhaging, or tissue damage seen with Becker infection (reference 1 this report). This difference in pathology is not related to the spread of the viruses in the brain because the Bartha strain is able to spread throughout the midbrain and brain stem prior to death (1). Presumably, the lethality of Bartha is due to the extensive spread of virus throughout the brain so that areas critical to survival are ultimately destroyed. However, death occurs much sooner in Becker-infected animals and prior to extensive viral spread in the brain. This would suggest that it is the severe pathology elicited by the Becker virus rather than the spread of the viral infection itself that is the cause of death in these animals. The attenuated Bartha strain is a vaccine strain that has several well-characterized mutations in its genome. The genome of PRV is separated into two distinct regions, designated the unique long region (UL) and the unique short region (US), with the US flanked by inverted repeat sequences. Within the UL of the Bartha genome are several point mutations in the gC (22), UL21 (13, 17), and gM genes (6). Mutations in the US of Bartha include the gene encoding US3 (18) and a large deletion that eliminates the expression of gI, gE, US9, and US2 (16, 19, 20). We have shown that by repairing the large deletion in Bartha with Becker sequences, we restored the ability of the Bartha virus to cause tissue damage and hemorrhaging in the chicken embryo brain (A. C. Clase and B. W. Banfield, unpublished observations). This finding suggests that of gE, gI, US9, and US2, one or more play a role in the development of this pathology.
The edema and hemorrhaging seen in Becker-infected animals are likely caused by an increase in vascular permeability within the brain. Vascular permeability can be affected by viral infection in a number of ways. One possibility is that the Becker infection induces the production of inflammatory cytokines. It has been demonstrated that astrocytes and brain macrophages in PRV-infected rodents contain viral antigens (21). It follows that these phagocytic cells may become activated and begin secreting inflammatory products in an effort to combat the viral infection. However, here we show that Dex is unable to inhibit the Becker-induced pathology. Additionally, pretreatment with Dex appears to have a deleterious effect on Becker-infected embryos, causing a more severe tissue pathology (Fig. 2) and decreasing the mean time to death in treated embryos infected with Becker or Bartha (Fig. 3). Taken together, these data suggest that the host inflammatory response is not responsible for the death of Becker-infected embryos and may be impeding the viral infection. Alternatively, Dex treatment of infected embryos may enhance the replication of virus in the brain, resulting in increased tissue damage as well as a reduced mean time to death. Regardless of the precise mechanism leading to tissue damage, suppression of the inflammatory response by Dex does not protect chicken embryos from virus challenge.
It is also possible that the Becker strain of PRV is able to infect endothelial cells in the brain, damaging them and leading to vascular leakage. It has been shown that endothelial cells begin forming the blood-brain barrier by E10 during chicken embryonic development (10). Damage to these cells could disrupt this protective function, leading to local vascular leakage as well as damage to distant areas through an interruption in the blood supply. Petechial hemorrhaging like that seen in both the Dex-treated and untreated Becker-infected embryos is often associated with damage to endothelial cells (Fig. 2). Experiments indicated that Becker, but not Bartha, was able to infect endothelial cells in the chicken embryo CNS after IO infection (Fig. 4). We believe that of gE, gI, US9, and US2, one or more may be involved in this altered cell tropism.
Collectively, the results presented here suggest that the severe tissue pathologies seen in the brains of Becker-infected chickens are not the results of uncontrolled inflammatory responses by the hosts. Thus, infection and destruction of a critical cell type such as endothelial cells would be predicted to result in extensive tissue damage. Experiments to define the role of endothelial cell infection in this severe pathology are under way.
Acknowledgments
We thank Christine Calton and Jessica Randall for expert technical assistance and all the members of the Banfield laboratory for helpful discussions.
This work was supported in part by research grant number 5-FY00-631 from the March of Dimes Birth Defects Foundation and by NIAID grant AI48626 to B.W.B.
REFERENCES
- 1.Banfield, B. W., G. S. Yap, A. C. Knapp, and L. W. Enquist. 1998. A chicken embryo eye model for the analysis of alphaherpesvirus neuronal spread and virulence. J. Virol. 72:4580-4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beckers, M., M. Gladis-Villanueva, W. Hamann, W. Schmutzler, and G. Zwadlo-Klarwasser. 1997. The use of the chorio-allantoic membrane of the chick embryo as test for anti-inflammatory activity. Inflamm. Res. 46(Suppl. 1):S29-S30. [PubMed] [Google Scholar]
- 3.Boos, J., and J. H. Kim. 1986. Sporadic encephalitis, p. 55-93. Viral encephalitis pathology, diagnosis and management. Blackwell Scientific Publications, London, United Kingdom.
- 4.Cronstein, B. N., S. C. Kimmel, R. I. Levin, F. Martiniuk, and G. Weissmann. 1992. A mechanism for the antiinflammatory effects of corticosteroids: the glucocorticoid receptor regulates leukocyte adhesion to endothelial cells and expression of endothelial-leukocyte adhesion molecule 1 and intercellular adhesion molecule 1. Proc. Natl. Acad. Sci. USA 89:9991-9995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dietzschold, B., K. Morimoto, and D. C. Hooper. 2001. Mechanisms of virus-induced neuronal damage and the clearance of viruses from the CNS. Curr. Top. Microbiol. Immunol. 253:145-155. [DOI] [PubMed] [Google Scholar]
- 6.Dijkstra, J. M., T. C. Mettenleiter, and B. G. Klupp. 1997. Intracellular processing of pseudorabies virus glycoprotein M (gM): gM of strain Bartha lacks N-glycosylation. Virology 237:113-122. [DOI] [PubMed] [Google Scholar]
- 7.Gourmelon, P., D. Briet, L. Court, and H. Tsiang. 1986. Electrophysiological and sleep alterations in experimental mouse rabies. Brain Res. 398:128-140. [DOI] [PubMed] [Google Scholar]
- 8.Gow, R. M., M. K. O'Bryan, B. J. Canny, G. T. Ooi, and M. P. Hedger. 2001. Differential effects of dexamethasone treatment on lipopolysaccharide-induced testicular inflammation and reproductive hormone inhibition in adult rats. J. Endocrinol. 168:193-201. [DOI] [PubMed] [Google Scholar]
- 9.Guidotti, L. G., and F. V. Chisari. 2001. Noncytolytic control of viral infections by the innate and adaptive immune response. Annu. Rev. Immunol. 19:65-91. [DOI] [PubMed] [Google Scholar]
- 10.Ikeda, E., I. Flamme, and W. Risau. 1996. Developing brain cells produce factors capable of inducing the HT7 antigen, a blood-brain barrier-specific molecule, in chick endothelial cells. Neurosci. Lett. 209:149-152. [DOI] [PubMed] [Google Scholar]
- 11.Karin, M., and L. Chang. 2001. AP-1—glucocorticoid receptor crosstalk taken to a higher level. J. Endocrinol. 169:447-451. [DOI] [PubMed] [Google Scholar]
- 12.Kennedy, P. G., J. H. Adams, D. I. Graham, and G. B. Clements. 1988. A clinico-pathological study of herpes simplex encephalitis. Neuropathol. Appl. Neurobiol. 14:395-415. [DOI] [PubMed] [Google Scholar]
- 13.Klupp, B. G., B. Lomniczi, N. Visser, W. Fuchs, and T. C. Mettenleiter. 1995. Mutations affecting the UL21 gene contribute to avirulence of pseudorabies virus vaccine strain Bartha. Virology 212:466-473. [DOI] [PubMed] [Google Scholar]
- 14.Lellouch-Tubiana, A., M. Fohlen, O. Robain, and F. Rozenberg. 2000. Immunocytochemical characterization of long-term persistent immune activation in human brain after herpes simplex encephalitis. Neuropathol. Appl. Neurobiol. 26:285-294. [DOI] [PubMed] [Google Scholar]
- 15.Leshchinsky, T. V., and K. C. Klasing. 2001. Divergence of the inflammatory response in two types of chickens. Dev. Comp. Immunol. 25:629-638. [DOI] [PubMed] [Google Scholar]
- 16.Lomniczi, B., M. L. Blankenship, and T. Ben-Porat. 1984. Deletions in the genomes of pseudorabies virus vaccine strains and existence of four isomers of the genomes. J. Virol. 49:970-979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lomniczi, B., S. Watanabe, T. Ben-Porat, and A. S. Kaplan. 1987. Genome location and identification of functions defective in the Bartha vaccine strain of pseudorabies virus. J. Virol. 61:796-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lyman, M. G., G. L. Demmin, and B. W. Banfield. 2003. The attenuated pseudorabies virus strain Bartha fails to package the tegument proteins Us3 and VP22. J. Virol. 77:1403-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mettenleiter, T. C., N. Lùkacs, and H.-J. Rziha. 1985. Pseudorabies virus avirulent strains fail to express a major glycoprotein. J. Virol. 56:307-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petrovskis, E. A., J. G. Timmins, T. M. Gierman, and L. E. Post. 1986. Deletions in vaccine strains of pseudorabies virus and their effect on synthesis of glycoprotein gp63. J. Virol. 60:1166-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rinaman, L., J. P. Card, and L. W. Enquist. 1993. Spatiotemporal responses of astrocytes, ramified microglia, and brain macrophages to central neuronal infection with pseudorabies virus. J. Neurosci. 13:685-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robbins, A. K., J. P. Ryan, M. E. Whealy, and L. W. Enquist. 1989. The gene encoding the gIII envelope protein of pseudorabies virus vaccine strain Bartha contains a mutation affecting protein localization. J. Virol. 63:250-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skoldenberg, B. 1996. Herpes simplex encephalitis. Scand. J. Infect. Dis. Suppl. 100:8-13. [PubMed] [Google Scholar]
- 24.Thomson, A. 1998. The cytokine handbook, 3rd ed. Academic Press, San Diego, Calif.
- 25.Vilcek, J., and G. C. Sen. 1996. Interferons and other cytokines, p. 375-399. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 26.Whitley, R. J., and B. Roizman. 2002. Herpes simplex viruses, p. 375-401. In D. D. Richman, R. J. Whitley, and F. G. Hayden (ed.), Clinical virology, 2nd ed. ASM Press, Washington, D.C.