Abstract
Mature dendritic cells (DCs) are potent antigen-presenting cells essential for initiating successful antiviral immune responses and would therefore serve as an ideal target for viruses seeking to evade or delay the immune response by disrupting their function. We have previously reported that VZV productively infects immature DCs (A. Abendroth, G. Morrow, A. L. Cunningham, and B. Slobedman, J. Virol. 75:6183-6192, 2001), and in the present study we assessed the ability of VZV to infect mature DCs. Mature DCs were generated from immature monocyte-derived DCs by lipopolysaccharide treatment before being exposed to VZV-infected fibroblasts. On day 4 postexposure, flow cytometry analysis revealed that 15 to 45% of mature DCs were VZV antigen positive, and immunofluorescent staining together with infectious-center assays demonstrated that these cells were fully permissive for the complete VZV replicative cycle. VZV infection of mature DCs resulted in a selective downregulation of cell surface expression of the functionally important immune molecules major histocompatibility complex (MHC) class I, CD80, CD83, and CD86 but did not alter MHC class II expression. Immunofluorescent staining showed that the downregulation of cell surface CD83 was concomitant with a retention of CD83 in cytoplasmic vesicles. Importantly, VZV infection of mature DCs significantly reduced their ability to stimulate the proliferation of allogeneic T lymphocytes. These data demonstrate that mature DCs are permissive for VZV and that infection of these cells reduces their ability to function properly. Thus, VZV has evolved yet another immune evasion strategy that would likely impair immunosurveillance and enhance the chances for lifelong persistence in the human population.
Varicella-zoster virus (VZV) is a highly species-specific herpesvirus that infects up to 90% of the human population (6). During primary infection, VZV is responsible for the predominantly childhood disease varicella (chicken pox). Following resolution of primary infection by the host immune system, the virus establishes a lifelong, latent infection in the dorsal root ganglia of the host. Reactivation from this site may occur many years later, resulting in herpes zoster (shingles), a condition which can be complicated by prolonged pain associated with postherpetic neuralgia (6, 37).
The induction of VZV-specific T-cell immunity is critical for host recovery from varicella, and both major histocompatibility complex (MHC) class I-restricted CD8+ T lymphocytes and MHC class II-restricted CD4+ T lymphocytes are sensitized to viral antigens during primary infection (5). The role of VZV-specific T lymphocytes in maintaining the equilibrium between the host and virus during latency is implied by the association between a decline in the frequency of circulating VZV-specific T lymphocytes and an increased risk of VZV reactivation, causing herpes zoster (26). However, like several other herpesviruses, VZV has the capacity to interfere with the expression of MHC class I and MHC class II molecules (2, 3). VZV-encoded immunomodulatory mechanisms that limit the presentation of VZV peptides by MHC class I or MHC class II pathways to effector T lymphocytes are likely to play an important role in the pathogenesis of VZV disease and persistence of the virus in the human population (1).
Several human viruses have evolved alternative strategies of evading immune recognition by selectively infecting and altering the function of specialized immune cells involved in host immune surveillance. For example, T lymphocytes play a critical role in adaptive immunity, and viruses such as human immunodeficiency virus (HIV) and measles virus can infect and destroy these cells, which may result in significant immunosuppression of the host (7, 16, 17).
Dendritic cells (DCs) are potent antigen-presenting cells critical for the initiation of a successful antiviral immune response through the stimulation of immunologically naïve T lymphocytes (8, 34). DCs located in the periphery exist as immature cells, expressing low levels of MHC class I and MHC class II molecules and costimulatory molecules such as CD80 and CD86. Immature DCs readily take up antigen and are induced to migrate to the secondary lymphoid organs, where they undergo maturation and present processed antigens to antigen-specific T lymphocytes (8, 34, 35). Maturation of DCs results in the downregulation of antigen uptake and processing properties and the upregulation of MHC class I and MHC class II molecules; increased surface expression of costimulatory molecules CD80, CD86, and CD40 and the maturation molecule CD83; and upregulation of adhesion molecules such as ICAM-1 (CD54) (8, 14, 36, 39-42). The ability of mature DCs to efficiently activate naïve T lymphocytes which subsequently eliminate target cells (e.g., virus-infected cells) has been attributed to their expression of these specific cell surface immune molecules (11).
It has been postulated elsewhere that DCs would be an ideal target for viruses seeking to evade or delay the immune response by disrupting their function (11). In this respect, viruses including herpes simplex virus type 1 (HSV-1) (23), human cytomegalovirus (29), human herpesvirus 6 (20), measles virus (18, 19, 32), HIV (12, 27), and lymphocytic choriomeningitis virus (33) have been shown previously to interfere with the immune function of infected DCs by a variety of mechanisms. However, not all viruses which infect DCs interfere with DC antigen-presenting function. For example, influenza virus productively infects DCs, but these cells can still function as antigen-presenting cells (10). Interestingly, we recently demonstrated that immature DCs productively infected with VZV showed little or no change in cell surface expression of MHC class I, MHC class II, CD1a, CD86, or CD40 (4). However, the ability of VZV to infect and interfere with mature DC function has yet to be elucidated.
Given the pivotal role that mature DCs play in the induction of successful antiviral immune responses, we sought to determine whether VZV could infect mature DCs and interfere with their immune function. In this study, we demonstrate that VZV can productively infect human mature DCs and produce infectious virus. VZV infection of mature DCs resulted in a specific downregulation of the functionally important immune molecules MHC class I, CD80, CD83, and CD86 and a significant reduction of T-cell stimulatory capacity.
MATERIALS AND METHODS
Virus and cells.
VZV strain Schenke, a low-passage-number clinical isolate, was propagated in human foreskin fibroblasts (HFFs). HFFs were grown in tissue culture medium Dulbecco's modified Eagle's medium (Gibco, Gaithersburg, Md.) supplemented with 10% heat-inactivated fetal calf serum (FCS) (CSL, Parkville, Australia). VZV-infected HFFs were stored at −80°C in tissue culture medium with 10% dimethyl sulfoxide (Sigma, Castle Hill, Australia).
Cell isolations and generation of DCs.
Peripheral blood mononuclear cells were isolated from healthy adult donors by density gradient sedimentation with Ficoll-Hypaque (Amersham Pharmacia Biotech, Uppsala, Sweden) and separated into monocyte- and T-lymphocyte-enriched fractions by countercurrent elutriation (29).
Cell fractions containing T lymphocytes were resuspended in RPMI (Gibco) containing 10% heat-inactivated FCS (RPMI-GM) supplemented with 10% interleukin-2 (IL-2) (Boehringer, Mannheim, Germany) at a concentration of 106 cells/ml in 12-well tissue culture plates and incubated at 37°C in a 5% CO2 atmosphere. Every 2 days cells were resuspended in fresh RPMI-GM supplemented with 10% IL-2. Typically >95% of cells when immunostained with an anti-human CD3 antibody were CD3+ by flow cytometry.
Pooled monocyte fractions were collected, resuspended at 5 × 105 cells/ml in RPMI-GM, and allowed to adhere to 24-well tissue culture plates. Immature DCs were generated by removing the nonadherent cells following a 2-h incubation at 37°C, and adherent cells were cultured for 7 days in RPMI-GM supplemented with 400 U of granulocyte-macrophage-colony-stimulating factor (GM-CSF)/ml and 500 U of IL-4/ml (Schering-Plough, Munich, Germany). Fresh medium containing cytokines was added to cells on days 2, 4, and 6 (13). Typically >90% of cells were shown by flow cytometry analysis to be of an immature DC phenotype (i.e., CD1a+ MHC class I+ MHC class II+ CD80low CD83− CD86low).
Mature DCs were generated by transferring the nonadherent immature DCs to new plates and culturing them for a further 2 days in RPMI-GM containing 2 μg of lipopolysaccharide (LPS) (Sigma, St. Louis, Mo.)/ml, 400 U of GM-CSF/ml, and 500 U of IL-4/ml for 2 days at 37°C in a 5% CO2 atmosphere (9, 31). These cultures consisted of >90% mature DCs as determined by their characteristic stellate morphology and their cell surface phenotype (i.e., CD1a+ MHC class Ihigh MHC class IIhigh CD80high CD83+ CD86high).
VZV infection of DCs.
VZV infection of mature DCs was carried out by incubating VZV-infected HFFs with DCs at a ratio of 1:2 (HFFs to DCs) in RPMI-GM supplemented with 400 U of GM-CSF/ml and 500 U of IL-4/ml. Fibroblast cultures showing 70 to 80% of cells being infected were used as the VZV-infected HFF inoculum. HFFs and DCs were mixed together in 24-well tissue culture plates, centrifuged at 150 × g for 15 min at room temperature, and then incubated at 37°C in a 5% CO2 atmosphere. Mock-infected DC cultures were set up in the same manner as described above with uninfected HFFs. Twenty-four hours postinoculation nonadherent cells were removed and reseeded into another 24-well plate with RPMI-GM containing GM-CSF and IL-4. The RPMI-GM containing cytokines was replaced every 2 days of culture.
Antibodies.
Monoclonal antibodies specific for human MHC class I (clone Tu149; phycoerythrin [PE] conjugated), human MHC class II (clone TU36; PE conjugated), human CD3 (clone S4.1; PE conjugated), and human CD14 (clone TUK4; fluorescein isothiocyanate [FITC] conjugated) and PE-conjugated goat anti-mouse immunoglobulin G (IgG), FITC-conjugated goat anti-human IgG, mouse IgG2a, PE-conjugated mouse IgG2a, and Tricolor-conjugated mouse IgG2a antibodies were obtained from Caltag Laboratories (South San Francisco, Calif.). Goat anti-mouse Alexa Fluor 594-conjugated antibody was obtained from Molecular Probes (Eugene, Oreg.). PE-conjugated monoclonal antibodies specific to human CD83 (clone HB15a) and human CD80 (clone MAB104) and unconjugated CD83 (clone HB15a) were obtained from Immunotech (Marseilles, France). PE-conjugated human CD86 (clone IT2.2) and unconjugated human HLA-DR (clone IQU9) were obtained from PharMingen (Hamburg, Germany) and Novacastra (Petersborough, United Kingdom), respectively. Monoclonal antibody specific for human CD1a (clone NA1/34) was obtained from Dako (Glostrup, Denmark). VZV nonimmune and VZV immune (immunoglobulin-purified) polyclonal human sera used for the detection of VZV-infected cells were kindly provided by A. M. Arvin (Stanford University). Rabbit polyclonal antibodies specific for VZV open reading frames 29 and 62 and glycoprotein C were kindly provided by P. R. Kinchington (University of Pittsburgh).
Flow cytometry analyses.
A quantity of 105 cells were resuspended in 100 μl of fluorescence-activated cell sorting (FACS) buffer (phosphate-buffered saline [PBS] with 1% FCS and 0.2% sodium azide). Primary antibodies, anti-human CD1a, and VZV immune polyclonal human IgG were diluted 1:50 in FACS buffer. Secondary antibodies, goat anti-human FITC-conjugated F(ab′)2 fragments, and goat anti-mouse PE-conjugated F(ab′)2 fragments were diluted 1:100 in FACS buffer. Tertiary mouse monoclonal antibodies, anti-human MHC class I, and anti-human MHC class II were diluted 1:50, and anti-human CD80, anti-human CD83, and anti-human CD86 were diluted 1:25. For all experiments, irrelevant control antibodies of the same IgG isotype were incubated with cells to control for nonspecific antibody binding. These included VZV nonimmune polyclonal human serum and mouse IgG of the same isotype. All antibodies were diluted in FACS buffer, antibody incubations were performed in the dark on ice for 30 min, and between each antibody step cells were washed in 2 ml of FACS buffer. Following immunostaining, cells were resuspended in orthofixative (PBS with 1% electron-microscopy-grade formaldehyde) and analyzed with FACSCalibur and CellQuest software (Becton Dickinson, San Jose, Calif.). Positive and negative staining of cells was decided by the level of fluorescence that exceeded, or did not exceed, the fluorescence level determined by >98% of cells from the same starting population when these cells were incubated with isotype control fluorochrome-conjugated antibodies. To calculate the fold change in cell surface immune molecule expression, the mean fluorescence intensity (MFI) values from mock-infected DCs and VZV antigen-positive DCs from the same blood donor and staining experiment were compared with each other.
Immunofluorescence microscopy.
Immunofluorescence staining was performed on cell spots and cells grown on glass coverslips. A quantity (2 × 104) of cells were centrifuged onto glass slides (Menzel-Glaser, Mainz, Germany) for 5 min at 150 × g with a Cytospin centrifuge (Shandon, Pittsburgh, Pa.) and air dried for 1 h. Cell spots and cells grown on coverslips were fixed and permeabilized in acetone for 15 min at 4°C and air dried for 1 h. To block nonspecific antibody binding, cells were incubated with blocking buffer (10% normal goat serum in PBS) for 30 min at 37°C before staining. Cells were incubated with primary antibodies (mouse anti-human MHC class II, mouse anti-human CD83, and VZV immune polyclonal human serum, all diluted 1:50) before the secondary antibodies [goat anti-human FITC-conjugated F(ab′)2 fragments diluted at 1:100 and Alexa Fluor 594 goat anti-mouse IgG conjugate diluted at 1:250] were added. All antibodies were diluted in blocking buffer and were applied to slides for 30 min at 37°C. Following each antibody incubation, slides were washed three times in PBS. Isotype control antibodies, VZV nonimmune polyclonal human serum, and mouse IgG were used to control for nonspecific antibody binding. Following immunofluorescence staining, slides and coverslips were mounted in Slow Fade antifade mounting medium (Molecular Probes), and cells were examined with an Optiscan laser scanning confocal microscope.
Infectious-center and transwell assays.
Mature DCs were separated from total cells following incubation with an antibody cocktail containing anti-CD1a, anti-CD83, and MHC class II and immunomagnetic bead separation as previously described (4). Typically, 98% mature DC purity was obtained as shown by immunostaining and flow cytometry. A quantity (104) of DCs were added to 24-well plates containing glass coverslips, preseeded with 105 HFFs. Plates were centrifuged at 150 × g for 15 min at room temperature and then incubated at 37°C in 5% CO2. Four days later, coverslips containing cell monolayers were removed, air dried, and fixed in acetone at 4°C for 15 min. Viral antigens were detected by immunofluorescence staining with a VZV immune polyclonal human serum as described above. Infectious centers were counted with a fluorescence microscope.
Supernatant assay.
Culture media from VZV-infected and mock-infected DC cultures were collected at days 2 and 4 postinfection and applied to freshly isolated mature DCs from a second donor. Two days later DCs were harvested and immunostained with antibodies to MHC class I, MHC class II, CD80, CD83, and CD86 and analyzed by flow cytometry as previously described.
Allogeneic T-lymphocyte proliferation assay.
T lymphocytes (2 × 105/well) were cocultured with mock- or VZV-infected mature DCs in RPMI-GM for 5 days in 96-well flat-bottomed tissue culture plates. Triplicate wells of T lymphocytes were incubated with mock- or VZV-infected mature DCs (day 2 postinfection) at DC/T-cell ratios of 1:100, 1:250, 1:500, and 1:1,000. After 5 days at 37°C with 5% CO2, the wells were pulse-labeled with 1 μCi of [3H]thymidine (Amersham Pharmacia Biotech) per well for 18 h. Culture supernatants were harvested onto glass fiber filters, and incorporated [3H]thymidine was measured with a microplate reader.
RESULTS
VZV productively infects human mature DCs.
Our previous demonstration that immature DCs were susceptible to VZV infection has provided a solid basis for the continued study of VZV-DC interactions (4). We therefore first sought to determine whether VZV could infect mature DCs.
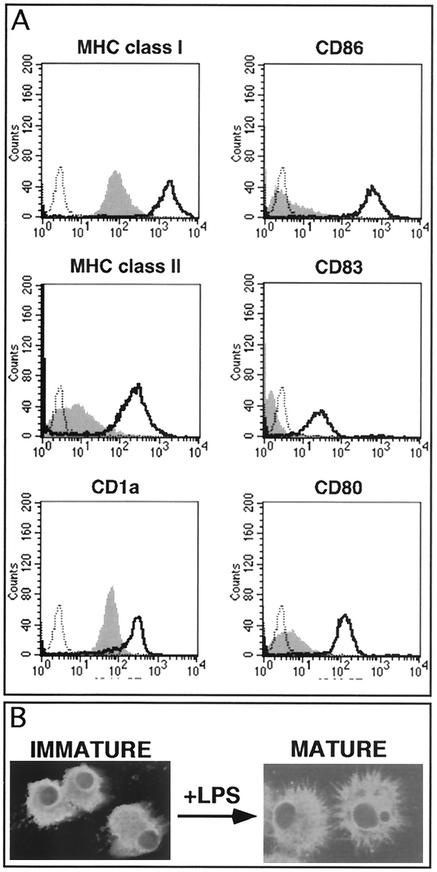
Mature human DCs were generated by a well-characterized two-step culture method (31). Immature DCs were derived from CD14+ peripheral blood human monocytes by incubation with GM-CSF and IL-4 for 7 days. These cells were immunostained for cell surface MHC class I, MHC class II, CD80, CD83, and CD86 and analyzed by flow cytometry. Cultures consisted of >90% immature DCs as defined by their cell surface staining phenotype (CD1a+ MHC I+ MHC II+ CD80low CD83− CD86low) (Fig. 1A). Immature DCs were then cultured for a further 2 days in medium containing LPS to stimulate maturation. When these cells were immunostained for the above cell surface markers and examined by flow cytometry, they displayed a characteristic mature DC surface phenotype (i.e., CD1a+ MHC class Ihigh MHC class IIhigh CD80high CD83+ CD86high) (Fig. 1A). In addition to assessment of cell surface marker expression, we also examined both immature and mature DCs by confocal microscopy after immunostaining for MHC class II. After LPS treatment the resulting cells displayed the characteristic stellate morphology of mature DCs (Fig. 1B). Based on cell surface staining and cell morphology, >90% of immature DCs were defined as being successfully converted to a mature DC phenotype.
FIG. 1.
Cell surface phenotype and morphology of human blood monocyte-derived DCs stimulated by LPS. Human monocytes were cultured in RPMI-GM containing GM-CSF and IL-4 for 7 days. These immature DCs were then incubated with LPS for a further 2 days to stimulate maturation. After culture the immature and mature DCs were stained with antibodies to MHC class I, MHC class II, CD1a, CD80, CD83, and CD86 and analyzed by flow cytometry (A) or stained with MHC class II and a fluorescent agent-conjugated secondary antibody and analyzed by confocal microscopy (B). (A) Flow cytometry histogram plots with solid gray line and black lines representing immature and mature DCs, respectively. The black dotted line represents the isotype-matched control. (B) Immunofluorescence staining for MHC class II expression in immature and mature DCs.
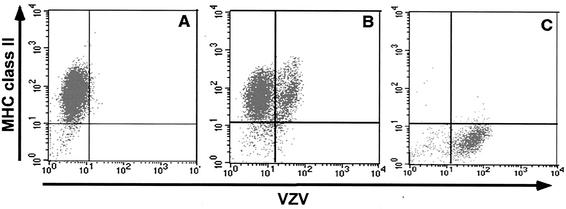
Using these mature DCs, we assessed their susceptibility to VZV infection. VZV is highly cell associated in cultured cells in vitro, and high titers of cell-free virus stocks cannot be generated (15, 38). Therefore, VZV-infected HFFs were used to inoculate mature DC cultures. Mature DCs (which are nonadherent in culture) were added directly to wells containing uninfected or VZV-infected adherent HFFs at a DC/HFF ratio of 2:1. Twenty-four hours postinfection the DCs were collected and cultured for a further 3 days. DCs were then harvested and dually stained for VZV and MHC class II antigens by using human polyclonal VZV immune serum and a mouse monoclonal antibody to MHC class II antigens, respectively. Stained cells were then subjected to flow cytometry analysis. Negative controls included mature DCs incubated with uninfected HFFs (mock infected) and incubation of mock- and VZV-infected cells with isotype control antibodies. Based upon isotype staining controls, >98% of mock-infected cells expressed MHC class II in the absence of VZV antigen expression (Fig. 2A). In VZV-infected mature DC cultures, 16% of mature MHC class II+ DCs were VZV antigen positive (Fig. 2B). In a total of five independent experiments, 15 to 45% of mature MHC class II+ DCs expressed VZV antigens at day 4 postinfection. To confirm that the MHC class II+ cells analyzed in this experiment were indeed mature DCs and not contaminating HFFs (from the inoculation procedure), we also stained VZV-infected HFFs for MHC class II expression. These cells, whether VZV+ or VZV−, remained MHC class II− (Fig. 2C). Mature DCs were also assessed by flow cytometry for VZV antigens over a time course of infection. In two replicate experiments, the mean percentage of VZV antigen-positive mature DCs at 12, 24, and 48 h postinfection was 7.4, 14.6, and 23.2%, respectively. In addition, we assessed mature DCs after exposure to cell-free VZV (multiplicity of infection = 1) and found that 31.9% of DCs were VZV antigen positive on day 4 postinfection.
FIG. 2.
Flow cytometry analysis of VZV antigen expression on human mature DCs and human fibroblasts infected with VZV. Human mature DCs were inoculated with uninfected or VZV-infected HFFs and collected 4 days postinoculation. Cells from mock-infected DC cultures (A), VZV-infected DC cultures (B), and VZV-infected HFFs (C) were stained with antibodies and fluorescent conjugates to MHC class II and VZV proteins and analyzed by flow cytometry.
The full replicative cycle of VZV in permissive cells is presumed to follow a regulated cascade of viral gene expression (15). These viral genes can be divided into three temporal classes, immediate-early (IE), early (E), and late (L) gene products, based upon their expression kinetics (23). To determine whether VZV expressed all three kinetic classes of viral proteins within mature DCs, viral antigen expression was assessed by immunofluorescence staining of mock- and VZV-infected mature DCs. On day 4 postinfection VZV- and mock-infected mature DCs were incubated with antibodies specific for either immediate-early (ORF62), early (ORF29), or late (gC) viral proteins (representative of all three kinetic classes). Cells were also stained for MHC class II.
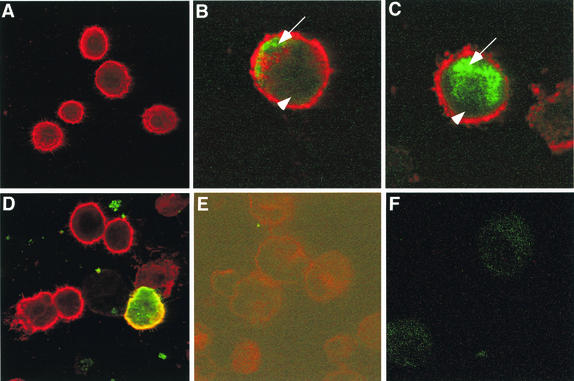
Mock-infected mature DCs stained positive for MHC class II antigens with a mouse monoclonal antibody but did not stain for VZV protein ORF62, ORF29, or gC with rabbit anti-VZV polyclonal antibodies (Fig. 3A and data not shown). In VZV-infected DC cultures, dual-stained VZV+ (green-staining) and MHC class II+ (red-staining) DCs were readily detectable (Fig. 3B to D). VZV ORF62 localized to the nucleus or cytoplasm, ORF29 localized to the nucleus, and gC localized to the cell surface and cytoplasm. The subcellular localization of these viral gene products in mature DCs was consistent with that previously reported for productive infection of permissive HFFs and immature DCs (4, 22). Isotype control antibodies did not stain either mock- or VZV-infected cells (Fig. 3E and F). In repeated experiments with mature DCs from five different donors, VZV antigens from the three kinetic classes were readily detectable. These data demonstrate that VZV could infect and synthesize IE, E, and L viral proteins in mature DCs.
FIG. 3.
Immunofluorescent staining of MHC class II and VZV antigens in VZV-infected mature DCs. Human mature DCs were inoculated with uninfected or VZV-infected HFFs and collected 4 days postinoculation. Mock-infected mature DCs (A and E) and VZV-infected mature DCs (B, C, D, and F) were incubated with a mouse monoclonal antibody to MHC class II (A to D) and rabbit polyclonal antibodies to ORF62 (B), ORF29 (C), and glycoprotein C (A and D). Anti-MHC class II (red-staining) and rabbit anti-VZV (green-staining) antibodies were detected with Alexa Fluor 594- and FITC-conjugated secondary antibodies, respectively. Negative control images (E and F) were obtained by increasing the laser voltage to enable the visualization of cells. The arrow and arrowhead indicate the nucleus and cytoplasm, respectively.
An infectious-center assay was used to determine whether new infectious virus particles were generated in VZV-infected mature DCs. Briefly, cultures of mature DCs were inoculated with VZV-infected or uninfected HFFs for a period of 24 h, and 4 days later mature DCs were harvested and incubated with an antibody cocktail consisting of anti-CD1a, anti-CD83, and anti-MHC class II antibodies. CD1a+ CD83+ MHC class II+ cells were isolated by immunomagnetic bead separation, and 10,000 cells were applied in duplicate to HFF monolayers preseeded onto glass coverslips. Four days postinoculation coverslips were harvested, fixed, and stained for VZV antigens.
VZV+ infectious centers were readily detectable in HFF monolayers incubated with infected mature DCs (193 infectious centers). Mock-infected mature DCs failed to produce any VZV-positive infectious centers. In a further two replicate experiments with mature DCs from two different donors, similar numbers of infectious centers were detected. The generation of infectious centers required cell-to-cell contact between infected mature DCs and HFFs because separation of these cells by a 1-μm-pore-size transwell filter completely abolished the appearance of infectious centers (data not shown). Taken together, these experiments demonstrate that mature DCs are permissive for VZV infection and that these cells support the full virus replicative cycle, resulting in the production of new infectious virions.
VZV downregulates cell surface immune molecules on mature DCs.
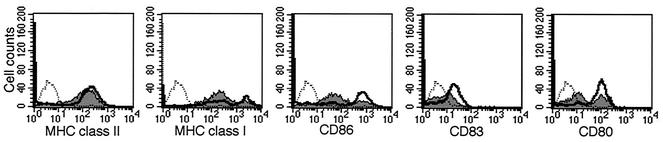
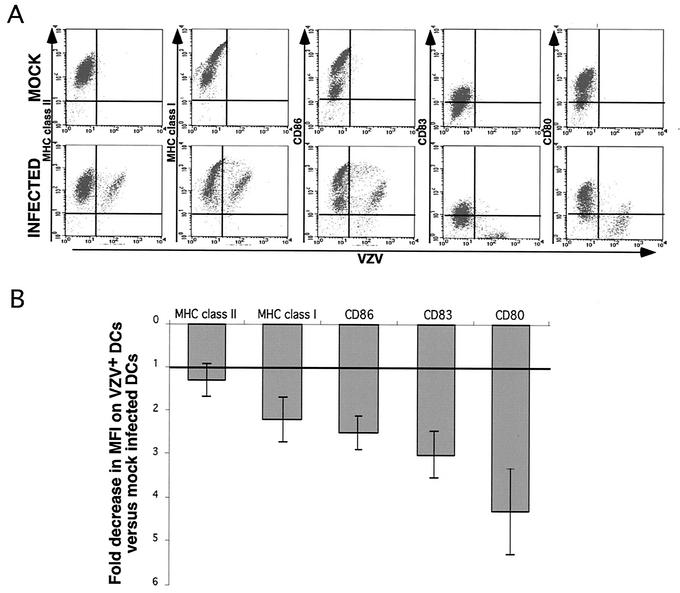
As immature DCs become mature, their ability to efficiently stimulate T lymphocytes increases as a result of an upregulation of MHC class I and MHC class II, together with costimulatory molecules CD80, CD83, and CD86 (8, 34, 35). Given the importance of immune molecule expression for mature DC function, flow cytometry was applied to assess the impact of VZV infection on cell surface MHC class I, MHC class II, CD80, CD83, and CD86 expression on cells from either mock- or VZV-infected DC cultures. Four days postinfection, cells were harvested and immunostained with antibodies specific for MHC class I, MHC class II, CD80, CD83, and CD86. In addition to the mock-infection control, DCs were also incubated with an isotype control antibody. In this experiment, 45% of mature DCs were viral antigen positive, as determined by flow cytometry. Histogram plots of cell surface immune molecule expression revealed that exposure of mature DC cultures to VZV did not significantly alter the expression of MHC class II but did cause a decrease in cell surface MHC class I, CD80, CD83, and CD86 expression on a large proportion of cells (Fig. 4).
FIG. 4.
Flow cytometry analysis of immune molecule expression on VZV-infected mature DCs. Four days postinoculation, mock- or VZV-infected mature DCs were harvested and stained for MHC class II, MHC class I, CD86, CD83, and CD80 proteins and analyzed by flow cytometry. Shown are flow cytometry histogram plots with the solid gray line and black lines representing VZV-infected and mock-infected mature DCs, respectively. The black dotted line represents the isotype-matched control.
Given that our infection protocol does not result in every mature DC becoming infected (i.e., VZV antigen positive [VZV+] by flow cytometry), we next sought to determine whether the observed downregulation of MHC class I, CD80, CD83, and CD86 occurred on DCs directly infected with VZV. Mature DCs from mock- or VZV-infected cultures were dually stained for VZV antigens and either MHC class I, MHC class II, CD80, CD83, or CD86 on day 4 postinfection (Fig. 5A). Isotype antibody was included as a control in each staining reaction. Interestingly, we observed two apparent populations of MHC class I- and CD86-expressing cells (high and low), although flow cytometry analyses demonstrated that both populations could be infected with VZV (Fig. 5A and data not shown). In five replicate experiments with mature DCs derived from five independent donors, the fold decrease in MFI of immune molecule expression on VZV+ DCs was compared to that for mock-infected DCs from the same donor (Fig. 5B). These experiments demonstrate that the cell surface expression of MHC class I, CD80, CD83, and CD86 but not MHC class II was significantly downregulated on VZV+ DCs.
FIG. 5.
Flow cytometry analysis of VZV antigen and immune molecule expression on VZV-infected mature DCs. Human mature DCs were inoculated with uninfected or VZV-infected HFFs and collected 4 days postinoculation. (A) Cells from mock-infected DC cultures (top panels) and VZV-infected DC cultures (bottom panels) were stained with antibodies and fluorescent conjugates to VZV proteins and MHC class II, MHC class I, CD86, CD83, and CD80 proteins and analyzed by flow cytometry. (B) Flow cytometry MFI analysis showing the fold decrease in immune molecule expression on VZV+ DCs compared with mock-infected DCs. This analysis was performed on data obtained from five separate VZV-infected and mock-infected mature DC cultures, and data are presented as the means (gray bars) ± standard errors of the means.
Effect of secreted factors on VZV-mediated downregulation of MHC class I, CD80, CD83, and CD86 on mature DCs.
Mature DCs were mock or VZV infected as described above. On day 4 postinfection the culture medium from these cells was collected and applied to freshly isolated uninfected mature DCs from a second donor. After 2 days of incubation together, the DCs were harvested and immunostained with mouse monoclonal antibodies specific for either MHC class I, MHC class II, CD80, CD83, or CD86 and analyzed by flow cytometry. No alteration in the expression of these cell surface molecules was observed on mature DCs inoculated with media from VZV-infected DC cultures compared with that for DCs inoculated with media from mock-infected cultures (data not shown). To confirm that the immune molecules expressed on mature DCs derived from the second donor were actually able to be downregulated by VZV, aliquots of these cells were also mock or VZV infected and assessed for the expression of cell surface VZV antigens, MHC class I, MHC class II, CD80, CD83, and CD86. As expected, the VZV-infected mature DCs showed a selective downregulation of MHC class I, CD80, CD83, and CD86 cell surface expression, whereas MHC class II expression remained unchanged (data not shown). These results demonstrate that direct virus infection is required for downregulation of MHC class I, CD80, CD83, and CD86 and that secreted factors from infected DCs do not alter the cell surface expression of these immune molecules on neighboring uninfected DCs.
Retention of CD83 molecules in VZV-infected mature DCs.
The cell surface protein CD83 represents one of the best mature DC markers, as it is not expressed on immature DCs and is induced only during DC maturation (41, 42). Although the precise function of CD83 remains to be elucidated, its expression on mature DCs along with the costimulatory molecules CD80 and CD86 suggests that it plays an important role during the immune response (25). To further assess VZV-mediated downregulation of cell surface CD83, we assessed the localization of CD83 in mature DCs exposed to VZV. Mature DCs were mock or VZV infected as described above and harvested at day 4 postinfection. Cell aliquots were spotted onto glass microscope slides before being fixed, permeabilized with acetone, and dually stained for VZV and CD83 antigens or VZV and MHC class II antigens. Included in this assay were both VZV- and mock-infected cells stained with isotype control antibodies. Following immunostaining, cells were examined by confocal microscopy.
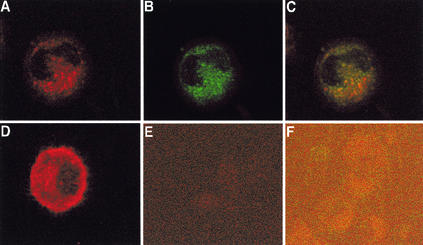
There was a significant difference in the CD83 staining patterns observed in mature DCs from mock-infected and from VZV-infected cultures (Fig. 6). In mock-infected cultures, CD83+ cells exhibited diffuse cytoplasmic and surface staining (Fig. 6D). In contrast, >90% of VZV antigen-positive cells from VZV-infected DC cultures displayed a punctate CD83 staining pattern localized to discrete cytoplasmic compartments (Fig. 6A to C). Cell surface CD83 staining was not detected on these cells. Staining was not detected in mock- or VZV-infected cell populations when isotype antibodies were used (Fig. 6E and F). There was no reduction or change in cellular localization of MHC class II in VZV-infected DCs compared with mock-infected DCs (data not shown). Similar results were obtained in a further three replicate staining experiments. These data demonstrate that VZV infection of mature DC cultures results in a loss of detectable cell surface CD83 concomitant with a retention of CD83 in cytoplasmic vesicles.
FIG. 6.
Analysis of CD83 protein localization in VZV-infected mature DCs. Shown are results of immunofluorescent staining of mock-infected (D and E) and VZV-infected (A to C and F) mature DCs at day 4 postinoculation. Anti-CD83 antibody (red staining) and anti-VZV antibody (green staining) were detected with Alexa Fluor 594- and FITC-conjugated secondary antibodies, respectively. Negative control images (E and F) were obtained by increasing the laser voltage to enable the visualization of cells. Fluorescent images A and B were overlaid to assess colocalization (yellow staining) of VZV and CD83 proteins in VZV-infected mature DCs (C).
VZV infection impairs the allogeneic T-cell-stimulatory capacity of mature DCs.
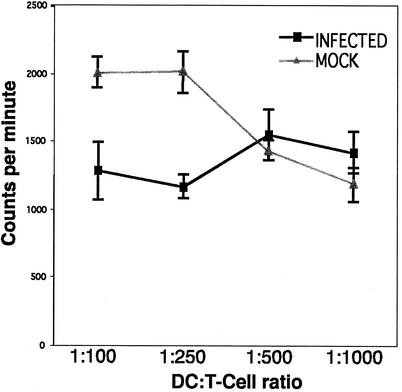
The most distinctive functional characteristic of mature DCs is their ability to stimulate T cells (8, 14, 36). Therefore, we assessed the functional consequences of the selective immune molecule alteration observed on VZV-infected mature DCs. Mock- or VZV-infected mature DCs were compared in an allogeneic mixed-lymphocyte reaction. On day 2 postinfection, various numbers of DCs from either mock-infected or VZV-infected mature DC cultures were added to allogeneic T cells, and their ability to stimulate T-cell proliferation was measured 6 days later. In this experiment, flow cytometry analysis revealed that 36% of mature DCs were VZV antigen positive.
Mature DCs from mock-infected cultures showed a high stimulatory capacity. In contrast, mature DCs inoculated with VZV-infected HFFs showed a clear reduction in their allostimulatory ability (Fig. 7). This effect was most significant at a DC/T-cell ratio of 1:250, where the percentage of inhibition was 42%. In an additional two replicate experiments, a similar inhibition in T-cell-stimulatory capacity was observed in mature DC cultures infected with VZV compared to their mock-infected counterparts. These data show that VZV infection of mature DCs inhibits their ability to stimulate the proliferation of allogeneic T cells.
FIG. 7.
Allogeneic T-cell-stimulatory capacity of mock- and VZV-infected mature DCs. Mature DCs were either VZV infected or mock infected for 2 days and then incubated with allogeneic T cells at various DC/T-cell ratios. T-cell proliferation was determined in counts per minute (± standard deviation) after cells were cocultured for 5 days and then pulsed with [3H]thymidine for 18 h.
DISCUSSION
These experiments provide the first evidence that VZV can productively infect mature human DCs and that infection results in the downregulation of specific cell surface immune molecules together with impaired T-cell-stimulatory capacity. Given the importance of mature DCs in initiating antiviral immune responses, VZV interference with mature DC function represents an immune evasion mechanism which is likely to confer a survival advantage on the virus by inhibiting or reducing the consequences of a DC-initiated immune response. It was previously demonstrated that VZV encodes two separate immune evasion strategies of specifically downregulating cell surface MHC class I (3) and inhibiting the upregulation of gamma interferon-induced MHC class II expression (2) during productive infection of primary HFFs. These VZV-encoded immunomodulatory effects are likely to delay the initial clonal amplification of VZV-specific CD8+ and CD4+ T lymphocytes and at least transiently enhance the ability of VZV to replicate at cutaneous sites. Our present report assessing mature DCs identifies a third immune evasion mechanism for VZV whereby the virus is able to productively infect a specialized immune cell (representing the most potent antigen-presenting cell type) and in doing so impairs its ability to function properly.
This study extends our previous finding that VZV can productively infect immature DCs and subsequently transfer infectious virus to autologous T lymphocytes (4). The ability of VZV to infect immature DCs and transfer virus to T lymphocytes has provided a model for virus dissemination during primary infection. In this instance, after initial infection of the human host, the virus encounters immature DCs of the respiratory mucosa which subsequently transport virus to the T-lymphocyte-rich draining lymph nodes, resulting in T-lymphocyte infection and subsequent dissemination of virus to other sites. Thus, it appears that VZV is DC tropic and can target two distinct aspects of DC function represented by immature and mature DCs. This ability to infect both DC types confers upon the virus the potential to both increase virus dissemination in the host and evade the immune response.
Mature DC function relies not only on the recognition of antigenic peptides in association with MHC molecules but also on the interaction with several other immune molecules including CD40, CD54, CD80, CD83, and CD86 (23, 28, 35). We assessed the cell surface expression of MHC class I, MHC class II, CD80, CD83, and CD86 and found that, with the exception of MHC class II, all of these molecules were downregulated on VZV-infected mature DCs. These observations indicate that the mechanism by which VZV alters immune molecule expression on mature DCs appears novel, since the pattern of immune molecule alteration differs from that of other viruses which infect and alter mature DCs. In comparison, HSV-1 infection of mature DCs results in the downregulation only of CD83 (24) and HSV-2 infection causes downregulation of MHC class I, MHC class II, CD40, CD80, and CD86 on murine DCs (C. Jones, personal communication, July 2002). Several other human viruses including measles virus (18, 19, 32) and HIV (12, 27) have been shown to infect DCs and interfere with DC antigen-presenting function. It should be noted, however, that the source of cells and/or the DC maturation stimuli may have a significant bearing on the expression of immune molecules and subsequent DC function following virus infection. In this respect, human cytomegalovirus has been shown elsewhere to downregulate MHC class I and MHC class II on monocyte-derived DCs induced to mature with LPS or tumor necrosis factor alpha (30), and yet CD34+ bone marrow-derived DCs induced to mature with CD40 display a downregulation of MHC class I, MHC class II, CD80, CD83, CD86, and CCR7 (E. S. Mocarski, personal communication, July 2002). Thus, among the human herpesviruses studied to date, there appear to be multiple strategies to interfere with DC immune molecule expression, and the present study provides evidence that VZV has done likewise.
Immunofluorescence staining and confocal microscopy demonstrated an accumulation of CD83 antigens within the cytoplasms of cells from VZV-infected mature DC cultures, and the accumulation was detected predominantly as discrete punctate foci. This staining pattern was concomitant with a significant loss of cell surface CD83 staining and was consistent with a decrease in CD83 MFI values as determined by flow cytometry. The only other published report which has examined the subcellular localization of immune molecules is that of Kruse et al., who, after showing that CD83 was the only immune molecule altered during HSV-1 infection of mature DCs, went on to further assess this molecule (24). They demonstrated that the downregulation of CD83 correlated with a redistribution of CD83 antigens to cytoplasmic lysosomes where they were degraded. Future studies will determine whether VZV and HSV-1 share a common mechanism of action with respect to CD83 downregulation during infection of mature DCs.
Although the precise molecular mechanisms responsible for VZV-mediated downregulation of cell surface MHC class I, CD80, CD83, and CD86 remain to be elucidated, our data clearly show that direct virus infection and not soluble factors are required for this phenotype. First, uninfected mature DCs cultured with conditioned media from parallel VZV-infected mature DC cultures did not cause cell surface changes in the expression of these immune molecules. Second, mature DCs separated from VZV-infected HFFs by use of a 1-μm-pore-size transwell did not show any alterations in cell surface immune molecule expression (data not shown). Third, dual staining of mature DCs for VZV antigen and immune molecule expression clearly showed that mature DCs with reduced cell surface MHC class I, CD80, CD83, and CD86 expression were VZV antigen positive. Finally, Kalinski and coworkers have reported that mature DCs are resistant to the effect of secreted cytokines (22).
The identity of the VZV gene or genes involved in the downregulation of immune molecules during productive infection of mature DCs is yet to be determined. To date, ORF66 is the only identified VZV immunomodulatory gene product. It has been shown elsewhere to downregulate MHC class I expression on HFFs transfected with an ORF66-expressing construct (3). Additional studies assessing ORF66 together with other viral open reading frames are a focus of current experiments aimed at identifying the VZV genes responsible for the inhibition of mature DC function.
In summary, this study provides the first evidence that VZV encodes an immune evasion strategy during the productive infection of mature DCs and suggests that VZV has evolved additional mechanisms to avoid immune surveillance and establish a persistent infection in the host. The selective downregulation of MHC class I, CD80, CD83, and CD86 during VZV infection of mature DCs differs from the mechanisms that other viruses employ to alter mature DC function. These findings have significant implications for our understanding of VZV pathogenesis and mechanisms of VZV-encoded immune evasion.
Acknowledgments
A.A. and B.S. contributed equally to this work.
This work was supported by NH&MRC project grant 211110 and a University of Sydney 2001 SESQUI grant. A.A. is the holder of a University of Sydney Rolf Edgar Lake Fellowship, and G.M. is the holder of an Australian Postgraduate Award and Westmead Millennium Foundation research scholarship stipend enhancement.
We thank Ann Arvin and Paul Kinchington for VZV gene-specific antibodies, Schering-Plough (Australia) for the cytokines GM-CSF and IL-4, and Stuart Turville for assistance with DC culture.
REFERENCES
- 1.Abendroth, A., and A. M. Arvin. 1999. Varicella zoster virus immune evasion. Immunol. Rev. 168:143-156. [DOI] [PubMed] [Google Scholar]
- 2.Abendroth, A., B. Slobedman, E. Lee, E. Mellins, M. Wallace, and A. M. Arvin. 2000. Modulation of major histocompatibility class II protein expression by varicella-zoster virus. J. Virol. 74:1900-1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abendroth, A., I. Lin, B. Slobedman, H. Ploegh, and A. M. Arvin. 2001. Varicella-zoster virus retains major histocompatibility complex class I proteins in the Golgi compartment of infected cells. J. Virol. 75:4878-4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abendroth, A., G. Morrow, A. L. Cunningham, and B. Slobedman. 2001. Varicella-zoster virus infection of human dendritic cells and transmission to T cells: implications for virus dissemination in the host. J. Virol. 75:6183-6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arvin, A. M. 1992. Cell-mediated immunity to varicella-zoster virus. J. Infect. Dis. 166:S35-S41. [DOI] [PubMed] [Google Scholar]
- 6.Arvin, A. M. 2001. Varicella-zoster virus, p. 2731-2768. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 7.Auwaerter, P. G., H. Kaneshima, J. M. McCune, G. Wiegand, and D. E. Griffin. 1996. Measles virus infection of thymic epithelium in the SCID-hu mouse leads to thymocyte apoptosis. J. Virol. 70:3734-3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 9.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y. J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767-811. [DOI] [PubMed] [Google Scholar]
- 10.Bhardwaj, N., A. Bender, N. Gonzalez, L. K. Bui, M. C. Garrett, and R. M. Steinman. 1994. Influenza virus-infected dendritic cells stimulate strong proliferative and cytolytic responses from human CD8+ T cells. J. Clin. Investig. 94:797-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhardwaj, N. 1997. Interactions of viruses with dendritic cells: a double-edged sword. J. Exp. Med. 186:795-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blauvelt, A., H. Asada, W. M. Saville, V. Klaus-Kovtun, D. J. Altman, R. Yarchoan, and S. I. Katz. 1997. Productive infection of dendritic cells by HIV-1 and their ability to capture virus are mediated through separate pathways. J. Clin. Investig. 100:2043-2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brossart, P., and M. J. Bevan. 1997. Presentation of exogenous protein antigens on major histocompatibility complex class I molecules by dendritic cells: pathway of presentation and regulation by cytokines. Blood 90:1594-1599. [PMC free article] [PubMed] [Google Scholar]
- 14.Cella, M., A. Engering, V. Pinet, J. Pieters, and A. Lanzavecchia. 1997. Inflammatory stimuli induce accumulation of MHC class II complexes on dendritic cells. Nature 388:782-787. [DOI] [PubMed] [Google Scholar]
- 15.Cohen, J. I., and S. E. Straus. 2001. Varicella-zoster virus and its replication, p. 2707-2730. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 16.Cohen, O. J., and A. S. Fauci. 2001. Pathogenesis and medical aspects of HIV-1 infection, p. 2043-2094. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 17.Esolen, L. M., S. W. Park, J. M. Hardwick, and D. E. Griffin. 1995. Apoptosis as a cause of death in measles virus-infected cells. J. Virol. 69:3955-3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fugier-Vivier, I., C. Servat-Delprat, P. Rivailler, M. C. Rissoan, Y. J. Liu, and C. Rabourdin-Combe. 1997. Measles virus suppresses cell mediated immunity by interfering with the survival and function of dendritic and T-cells. J. Exp. Med. 186:813-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grosjean, I., C. Caux, C. Bella, I. Berger, F. Wild, J. Banchereau, and D. Kaiserlian. 1997. Measles virus infects human dendritic cells and blocks their allostimulatory properties for CD4+ T-cells. J. Exp. Med. 186:801-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kakimoto, M., A. Hasegawa, S. Fujita, and M. Yasukawa. 2002. Phenotypic and functional alterations of dendritic cells induced by human herpesvirus 6 infection. J. Virol. 76:10338-10345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalinski, P., J. H. Schuitemaker, C. M. Hilkens, E. A. Wierenga, and M. L. Kapsenberg. 1999. Final maturation of dendritic cells is associated with impaired responsiveness to IFN-gamma and to bacterial IL-12 inducers: decreased ability of mature dendritic cells to produce IL-12 during the interaction with Th cells. J. Immunol. 162:3231-3236. [PubMed] [Google Scholar]
- 22.Kinchington, P. R., and J. I. Cohen. 2000. Varicella-zoster virus proteins, p. 74-104. In A. Gershon and A. Arvin (ed.), Varicella-zoster virus. Virology and clinical management. Cambridge University Press, Cambridge, United Kingdom.
- 23.Klagge, I. M., and S. Schneider-Schaulies. 1999. Virus interactions with dendritic cells. J. Gen. Virol. 80:823-833. [DOI] [PubMed] [Google Scholar]
- 24.Kruse, M., O. Rosorius, F. Kratzer, G. Stelz, C. Kuhnt, G. Schuler, J. Hauber, and A. Steinkasserer. 2000. Mature dendritic cells infected with herpes simplex virus type 1 exhibit inhibited T-cell stimulatory capacity. J. Virol. 74:7127-7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lechmann, M., S. Berchtold, J. Hauber, and A. Steinkasserer. 2002. CD83 on dendritic cells: more than just a marker for maturation. Trends Immunol. 23:273-275. [DOI] [PubMed] [Google Scholar]
- 26.Levin, M. J., and A. R. Hayward. 1996. The varicella vaccine. Prevention of herpes zoster. Infect. Dis. Clin. N. Am. 10:657-675. [DOI] [PubMed] [Google Scholar]
- 27.Macatonia, S. E., M. Gompels, A. J. Pinching, S. Patterson, and S. C. Knight. 1992. Antigen-presentation by macrophages but not by dendritic cells in human immunodeficiency virus (HIV) infection. Immunology 75:576-581. [PMC free article] [PubMed] [Google Scholar]
- 28.Marland, G., B. Bakker, G. J. Adema, and C. G. Figdor. 1996. Dendritic cells in immune response induction. Stem Cells 14:501.. [DOI] [PubMed] [Google Scholar]
- 29.Mason, R. R., and R. S. Weiner. 1985. Application of the Beckman JE6-B Elutriator System in the isolation of human monocyte subpopulations. Scand. J. Haematol. 34:5-8. [DOI] [PubMed] [Google Scholar]
- 30.Raftery, M. J., M. Schwab, S. M. Eibert, Y. Samstag, H. Walczak, and G. Schonrich. 2001. Targeting the function of mature dendritic cells by human cytomegalovirus: a multilayered viral defense strategy. Immunity 15:997-1009. [DOI] [PubMed] [Google Scholar]
- 31.Salio, M., M. Cella, M. Suter, and A. Lanzavecchia. 1999. Inhibition of dendritic cell maturation by herpes simplex virus. Eur. J. Immunol. 29:3245-3253. [DOI] [PubMed] [Google Scholar]
- 32.Schnorr, J. J., S. Xanthakos, P. Keikavoussi, E. Kampgen, V. ter Meulen, and S. Schneider-Schaulies. 1997. Induction of maturation of human blood dendritic cell precursors by measles virus in association with immunosuppression. Proc. Natl. Acad. Sci. USA 92:5326-5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sevilla, N., S. Kunz, A. Holz, H. Lewicki, D. Homann, H. Yamada, K. P. Campbell, J. C. de La Torre, and M. B. Oldstone. 2000. Immunosuppression and resultant viral persistence by specific viral targeting of dendritic cells. J. Exp. Med. 192:1249-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steinman, R. M. 1991. The dendritic cell system and its role in immunogenicity. Annu. Rev. Immunol. 9:271-296. [DOI] [PubMed] [Google Scholar]
- 35.Steinman, R. M., M. Pack, and K. Inaba. 1997. Dendritic cell development and maturation. Adv. Exp. Med. Biol. 417:1-6. [DOI] [PubMed] [Google Scholar]
- 36.Steinman, R. M. 1999. Dendritic cells, p. 547-573. In W. E. Paul (ed.), Fundamental immunology, 4th ed. Lippincott Raven Publishers, Philadelphia, Pa.
- 37.Watson, P. N., and R. J. Evans. 1986. Post-herpetic neuralgia: a review. Arch. Neurol. 43:836-840. [DOI] [PubMed] [Google Scholar]
- 38.Weller, T. H. 1953. Serial propagation in vitro of agents producing inclusion bodies derived from varicella and herpes zoster. Proc. Soc. Exp. Biol. 83:340-346. [DOI] [PubMed] [Google Scholar]
- 39.Young, J. W., and R. M. Steinman. 1990. Dendritic cells stimulate primary human cytolytic lymphocyte responses in the absence of CD4+ helper T cells. J. Exp. Med. 171:1315-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Young, J. W., L. Koulova, S. A. Soergel, E. A. Clark, R. M. Steinman and B. Dupont. 1992. The B7/BB1 antigen provides one of several costimulatory signals for the activation of CD4+ T lymphocytes by human blood dendritic cells in vitro. J. Clin. Investig. 90:229-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou, L. J., R. Schwarting, H. M. Smith, and T. F. Tedder. 1992. A novel cell-surface molecule expressed by human interdigitating reticulum cells, Langerhans cells, and activated lymphocytes is a new member of the Ig superfamily. J. Immunol. 149:735-742. [PubMed] [Google Scholar]
- 42.Zhou, L. J., and T. F. Tedder. 1995. Human blood dendritic cells selectively express CD83, a member of the immunoglobulin superfamily. J. Immunol. 154:3821-3835. [PubMed] [Google Scholar]