Abstract
Replication of the neurotropic JHM strain of mouse hepatitis virus within the central nervous system is controlled by cellular immunity. However, following initial clearance, virus reactivates in the absence of humoral immunity. Viral recrudescence is prevented by the transfer of antiviral antibody (Ab). To characterize the specificity and biological functions of Ab critical for maintaining viral persistence, monoclonal Abs specific for the viral spike, matrix, and nucleocapsid proteins were transferred into infected B-cell-deficient mice following initial virus clearance. Neutralizing immunoglobulin G (IgG) but not IgA anti-spike Ab suppressed virus recrudescence, reduced viral antigen in most cell types except oligodendroglia, and was associated with reduced demyelination. Nonneutralizing monoclonal Abs specific for the spike, matrix, and nucleocapsid proteins did not prevent recrudescence, demonstrating that neutralization is critical for maintaining JHM mouse hepatitis virus persistence within the central nervous system. Ab-mediated protection was not associated with alterations in virus-specific T-cell function or inflammation. Furthermore, neutralizing Ab delayed but did not prevent virus recrudescence. These data indicate that following acute viral clearance cellular immunity is ineffective in controlling virus recrudescence and suggest that the continued presence of neutralizing Ab is the essential effector in maintaining viral persistence within the central nervous system.
Viruses use various strategies to evade immunosurveillance and thereby establish persistence (1). The lytic phenotype of cytopathic viruses presents a potential barrier to persistence, as a critical number of infected cells must survive; however, this may be subverted by virus persistence in semipermissive cells or in cells refractory to immune surveillance (1). Control of cytopathic viral infections is predominantly mediated by humoral immunity (41). Antibodies (Ab) contribute to virus control by neutralizing cell-free virus, thereby limiting spread, and also by eliminating virus-infected cells, via either complement-mediated cytotoxicity or Ab-dependent cell-mediated cytotoxicity (15, 16, 27). Ab-mediated virus control generally targets domains exposed on the virion surface, such as surface glycoproteins or external capsid proteins. By contrast, Ab specific for internal or nonstructural proteins are nonneutralizing and, in general, not protective (1). However, Ab may also neutralize virus inside infected cells or inhibit viral RNA transcription (1, 8,16). In addition, Ab recognition of cell surface viral glycoproteins influences viral gene expression (16, 21), possibly providing an important mechanism by which viruses evade Ab detection, thereby facilitating viral persistence. A variety of viruses persist in both the human and rodent central nervous system (CNS) despite high levels of neutralizing antibody in serum and/or cerebrospinal fluid (1, 6,9, 15, 33). Virus variants arising due to alterations in either cellular tropism (1, 4) or antigenic determinants (21) also facilitate persistence. Furthermore, the unique nature of the CNS itself may contribute to viral persistence (1). Low levels of major histocompatibility complex (MHC) expression, limited antigen (Ag) sampling by peripheral immune cells, and relative inaccessibility due to the blood-brain barrier predispose the CNS as a favored site for viral persistence (1, 7).
Immunological defense to noncytopathic viruses is primarily mediated by cellular immunity, with Ab appearing later during infection (41). In its primary host the mouse, the neurotropic JHM strain of mouse hepatitis virus (JHMV) infects various CNS cell types and is noncytopathic in vivo (38). Acute infection resolves into a nonproductive persistent infection accompanied by chronic ongoing demyelination (38). Although CD8+ T cells are the primary effectors of virus clearance, separate effector mechanisms control viral replication in distinct CNS cell types. During acute infection, virus-specific CD8+ T cells eliminate virus from astrocytes and microglia via a perforin-dependent mechanism, whereas virus infection of oligodendroglia is controlled by gamma interferon (IFN-γ) (22, 28, 38). CD4+ T cells help expand CD8+ T cells and maintain their effector function and survival within the CNS parenchyma (3, 37). They may also contribute directly or indirectly to virus control via secretion of IFN-γ (3, 40). Ab appears to play a redundant role during acute JHMV infection due to both its delayed appearance relative to virus decline (28, 39) and clearance from the CNS of infected Ab-deficient mice (23, 31). Nevertheless, despite the noncytopathic nature of JHMV in vivo, a humoral immune component has been implicated in various animal challenge models (4, 13, 23, 29, 31, 33). Ab transfer prior to, or concomitant with, viral infection increases survival (4, 13, 19, 26). In addition, JHMV-resistant Norway rats exhibit a more rapid and robust neutralizing Ab response than do susceptible Lewis rats (33). Finally, protection of JHMV-infected suckling mice by neutralizing Ab present in milk further suggests a protective role for neutralizing Ab (17, 29). The possibility that Ab may also act in concert with cellular immunity to completely eliminate infectious JHMV during acute infection is supported by incomplete virus clearance from the CNS of B-cell-deficient mice at times when virus can no longer be recovered from immunocompetent mice. A crucial role for Ab in regulating viral persistence became evident from JHMV recrudescence in the CNS of mice either devoid of B cells or mice containing B cells unable to secrete antiviral Ab (23, 31). Moreover, transfer of polyclonal Ab after initial clearance inhibits viral recrudescence in B-cell-deficient mice (23). The delayed accumulation but subsequent retention of Ab-secreting cells in the CNS harboring persisting JHMV supports a role for sustained Ab secretion in regulating viral persistence (39).
To determine the role and specificities of Ab in regulating JHMV persistence, monoclonal Ab (MAb) with different biological activities were transferred into infected B-cell-deficient μMT mice prior to virus recrudescence. The data indicate that neutralization is the most crucial biological activity in controlling virus persistence within the CNS. However, the ability to inhibit cell-cell fusion also appears to play a supporting role by limiting viral spread. Although MAb with other specificities protect when transferred prior to infection (4, 13, 19, 26), no evidence was found to support their contribution to the control of viral persistence. The inability of passively transferred Ab to sustain viral persistence without reemergence of infectious virus in B-cell-deficient mice suggests that maintenance of JHMV persistence requires continued local Ab secretion.
MATERIALS AND METHODS
Mice.
Homozygous μMT mice (C57BL/6-Igh-6tm1Cgn) were obtained from the Jackson Laboratory (Bar Harbor, Maine) and bred under pathogen-free conditions at the University of Southern California Keck School of Medicine. The absence of Ab was confirmed by testing for serum immunoglobulin G (IgG) by enzyme-linked immunosorbent assay (ELISA) as previously described (23). Syngeneic wild-type (wt) C57BL/6 mice were obtained from the National Cancer Institute (Frederick, Md.). Mice of both sexes were used between 7 and 8 weeks of age.
Viral infection, passive transfers, and titer determination.
Mice were infected intracerebrally with 250 PFU of the 2.2v-1 MAb-derived variant of JHMV in 30 μl of Dulbecco's phosphate-buffered saline (PBS), pH 7.4 (11, 12). Mice received polyclonal Ab (500 μl) or MAb (500 μg) intraperitoneally (i.p.) on days 9, 12, and 17 postinfection (p.i). In some experiments, an additional injection of polyclonal Ab was administered at day 24 p.i. Tissue levels of infectious JHMV were determined from clarified homogenates prepared from one-half of the brain by plaque assay on monolayers of DBT cells as previously described (12, 13, 36). Plaque numbers were determined following 48 h of incubation at 37°C. Data represent the average of duplicate determinations from four or more individual mice per group.
Clinical disease.
Clinical disease was graded as previously described (11, 12): 0, healthy; 1, ruffled fur and hunchbacked appearance; 2, low mobility and inability to stand upright; 3, paralysis and wasting; 4, moribundity and death. Data represent the average for four or more mice per group from three or more experiments.
Ab preparations.
Anti-JHMV polyclonal Ab was obtained from JHMV-hyperimmunized mice (23). Isolation and biological characterization of anti-JHMV MAb have been previously described (10, 13). The isotype, neutralizing, and fusion inhibition activities of the anti-spike (S) protein MAb used are listed in Table 1. Characteristics of the anti-nucleocapsid and anti-matrix protein MAb are described in Table 2. Control mice were injected i.p. with either an equal volume of sterile PBS or an equivalent concentration of an IgG1 control MAb (designated 902), specific for human immunodeficiency virus gp120 (30).
TABLE 1.
Properties of the Anti-S protein specific MAb
| Antibody | Isotype | Titera | Neutralization | Neutralization activity (μg)c | Fusion inhibition | Fusion inhibition activity (μg)c | Reference |
|---|---|---|---|---|---|---|---|
| pAb-1 | Polyclonal | 4.9 | + | 80 | + | 80 | 23 |
| J.7.2 | IgG2b | 6.3 | + | 2.5 | + | 50 | 10 |
| J.2.6 | IgG2b | 7.7 | + | 0.065 | + | 26 | 10 |
| J.7.5 | IgG2b | 4.9 | + | 3 | ND | ND | 10 |
| 5B1 | IgG1 | 4.9 | + | 150 | ND | ND | 4 |
| 5B9 | IgA | 7.7 | + | 200 | ND | ND | 4 |
| J.2.5 | IgG2a | 4.9 | NDb | ND | + | 30 | 10 |
| J.1.2 | IgG2b | 4.9 | ND | ND | ND | ND | 10 |
| J.1.16 | IgG2b | 6.3 | ND | ND | ND | ND | 10 |
| J.7.1 | IgG2a | 5.6 | ND | ND | ND | ND | 10 |
Titer (log10) determined by ELISA.
ND, not detectable.
Activity determined by calculating the neutralization or fusion inhibition titer based on Ab concentration.
TABLE 2.
Properties of non-S protein specific MAb
| MAb | Specificitya | Isotype | Titerb | Reference(s) |
|---|---|---|---|---|
| J.3.3 | Nc | IgG2a | 3.5 | 10, 35 |
| J.3.5 | N | IgG2a | 5.6 | 10, 35 |
| J.1.3 | M | IgG2b | 5.6 | 10, 13 |
| J.3.9 | M | IgG2a | 4.9 | 13 |
| J.2.7 | M | IgG2a | 4.9 | 10, 13 |
| pAb-2 | Polyclonal | 4.9 | 23 |
No detectable neutralizing or fusion-inhibiting activities.
Titer determined by ELISA as described in Materials and Methods.
N, anti-nucleocapsid protein; M, anti-matrix protein.
Protein concentrations were determined by ELISA with anti-mouse IgG-coated plates as previously described (35). Briefly, 10 μg of anti-mouse IgG (ICN Pharmaceuticals, Aurora, Ohio) was adsorbed overnight at 4°C. Serial dilutions of purified IgG2a, IgG2b (Zymed, South San Francisco, Calif.), or JHMV-specific MAb were adsorbed for 4 h at 37°C. After three washings with PBS containing 0.05% Tween 20, color was developed by consecutive incubations with horseradish peroxidase anti-mouse IgG (Zymed) and 1 mg of 2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid) (ABTS) (Roche Diagnostics, Indianapolis, Ind.)/ml in PBS containing 0.001% H2O2. Color intensity was determined at 405 nm by using a microplate autoreader (BioTek Instruments, Winooski, Vt.). Protein concentrations were determined from standard curves obtained with purified IgG isotypes.
Anti-JHMV titers were determined by ELISA as described previously (39). Briefly, JHMV-coated plates were adsorbed with serial fivefold dilutions of MAb overnight at 4°C. Following consecutive incubations with biotinylated anti-mouse IgG Ab (BD PharMingen, San Diego, Calif.), streptavidin peroxidase (Sigma-Aldrich, St. Louis, Mo.), and ABTS substrate, optical densities were determined at 405 nm. Titers were calculated as the reciprocal of the highest dilution. Positive values exceeded 2 standard deviations over mean negative controls.
Neutralization and fusion inhibition titers were determined as previously described (10). Briefly, following heat inactivation (56°C for 30 min), serial twofold dilutions were incubated with 200 PFU of JHMV in 96-well tissue culture plates for 90 min at 37°C. DBT cells (9 × 104 cells/well) were added prior to incubation at 37°C for 48 h. Neutralization titers represent the highest dilution providing complete inhibition of the JHMV-induced cytopathic effect. Fusion inhibition activity was determined similarly, except that confluent monolayers of DBT cells in 96-well plates were infected with 200 PFU and incubated for 4 h at 37°C prior to addition of serial dilutions of heat-inactivated MAb. The cytopathic effect was determined after 48 h of incubation at 37°C by comparison to wells with virus only. Neutralization and fusion inhibition activities were determined by calculating the neutralizing or fusion-inhibiting titers corresponding to protein concentrations.
Isolation of CMC.
CNS mononuclear cells (CMC) were isolated from the CNS of JHMV-infected mice as described previously (2). Briefly, brains and spinal cords were removed, homogenized in RPMI 1640 medium supplemented with 25 mM HEPES by using Ten Broeck tissue homogenizers, and adjusted to 30% Percoll (Pharmacia, Piscataway, N.J.). Following centrifugation at 800 × g for 20 min at 4°C onto a 1-ml cushion of 70% Percoll, CMC were collected from the 30%-70% interphase and were washed in RPMI 1640-HEPES medium prior to analysis.
Flow cytometry.
Cells (n = 5 × 105) were preincubated with a mixture of polyclonal mouse and human serum (10%) and rat anti-mouse FcγIII/IIR MAb (2.4G2) (BD PharMingen) for 20 min on ice to inhibit nonspecific binding. Phycoerythrin, fluorescein isothiocyanate, or cytochrome c-coupled MAb, including anti-CD4 (GK1.5), anti-CD8 (53.67), anti-Ig (polyclonal), and anti-CD19 (1D3), were obtained from BD PharMingen. The Db MHC class I tetramer complexed with the JHMV H-2b immunodominant S510 peptide (amino acids 510 to 539) has been described previously (2). Cells were stained for expression of cell surface markers in 1% bovine serum albumin in PBS and were analyzed with a FACSCalibur flow cytometer (Becton Dickinson, San Jose, Calif.) by using Cellquest Pro software.
ELISPOTs.
IFN-γ enzyme-linked immunospot (ELISPOT) assays were used to measure the frequency of Ag-specific IFN-γ-secreting cells within CMC as previously described (2). Briefly, serial dilutions of CMC were plated in triplicate into 96-well plates (Millipore Co., Bedford, Mass.) coated with 10 μg of anti-IFN-γ MAb (R4.6A2; BD PharMingen) and were stimulated with irradiated naïve splenocytes (5 × 105/well) in the presence or absence of the MHC class I-restricted 1 μM S510 peptide for CD8+ T cells or the MHC class II-restricted M133 peptide (amino acids 133 to -147) for CD4+ T cells (2, 39). EL-4 supernatant (2.5%) was added as a source of interleukin 2, and the plate contents were incubated for 36 h at 37°C. IFN-γ secretion was detected following consecutive incubation with biotinylated anti-IFN-γ MAb (XMG1.2; BD PharMingen), strepatavidin peroxidase (Sigma, St. Louis, Mo.), and 3,3-dimanobenzidine (Sigma) in PBS containing 0.001% H2O2. Spots were counted using a Microplate Autoreader (BioTek Instruments). Data from two dilutions (n = 6) are presented.
Histopathology.
Brains bisected in the mid-coronal plane and spinal cords were examined for inflammation, distribution of Ag, and myelin loss. Tissues were fixed for 3 h in Clark's solution (75% ethanol and 25% glacial acetic acid) prior to embedding in paraffin. Sections were stained with either hematoxylin and eosin or luxol fast blue to determine inflammation and demyelination. Distribution of JHMV Ag was determined by immunoperoxidase staining (Vectastain-ABC kit; Vector Laboratories, Burlingame, Calif.) with the anti-JHMV MAb J.3.3 specific for the carboxyl terminus of the N protein as primary Ab (10, 35) and horse anti-mouse MAb as secondary Ab (Vector Laboratories). Sections were scored in a blinded fashion for inflammation, viral Ag, and demyelination. Representative fields were identified based on the average score of all sections in each experimental group.
Statistical analysis.
Results presented as mean plus or minus standard deviation were analyzed by using Student's paired t test, analysis of variance, or the chi- square test. A P of ≤0.05 was taken as statistically significant.
RESULTS
Neutralizing anti-S protein Ab prevents CNS viral recrudescence.
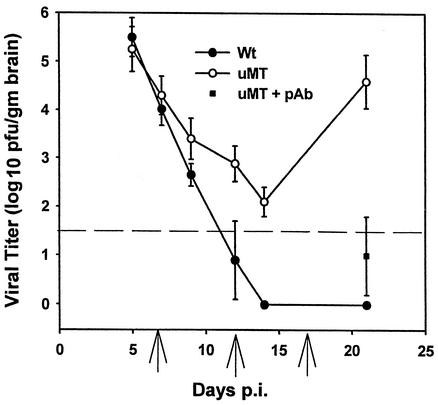
JHMV replication in the CNS of B-cell-deficient μMT mice was examined to establish an Ab treatment schedule for effective virus control. Replication in both wt and μMT mice peaked at day 5 p.i. and was reduced in both groups with similar kinetics to day 10 p.i. (Fig. 1). Following initial control of virus replication, JHMV reactivated in the CNS of μMT mice and approached levels found during acute infection by day 21 p.i. Passive transfer of polyclonal Ab with neutralizing/fusion-inhibiting activity (pAb-1) into infected μMT mice at 9, 12, and 17 days p.i. significantly decreased both virus replication (Fig. 1 and 2A) and clinical scores: 3.4 ± 0.4 in controls versus 1.9 ± 0.6 in pAb-1 recipient mice at 21 days p.i., consistent with previous data (23). Injection of PBS (Fig. 2A) or a control MAb (30; data not shown) had no effect on viral recrudescence.
FIG. 1.
JHMV recrudescence in the CNS of B-cell-deficient mice. Virus replication in the CNS of JHMV-infected C57BL/6 and μMT mice. The dashed line indicates the limit of viral detection. One group of infected μMT mice received polyclonal Ab (pAb) at the times indicated by the arrows. Data represent the average of at least three separate experiments with four or more mice per time point. Error bars indicate standard errors of the means. gm brain, gram of brain.
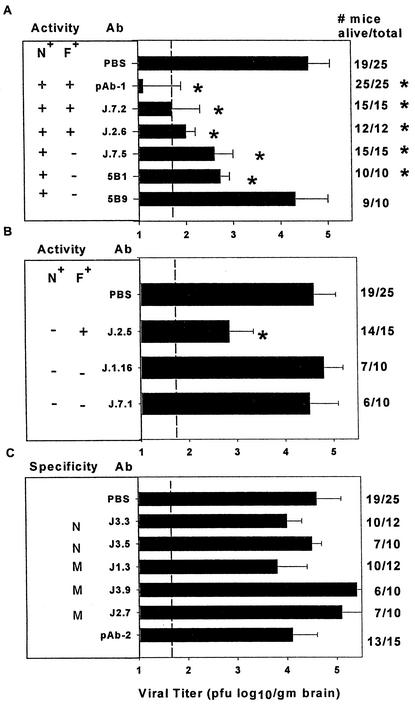
FIG. 2.
Analysis of anti-S protein MAb for inhibition of viral recrudescence. N+, neutralizing; F+, fusion inhibiting. Ab was transferred i.p. on days 9, 12, and 17 p.i., and virus was assayed at 21 days p.i. Control groups were treated with PBS only. Virus titers in recipients of N+ anti-S protein MAb (A), N− anti-S protein MAb (B), and N− anti-M and anti-N protein MAb (C). Error bars indicate standard errors of the means. The dashed line indicates the limit of viral detection. *, P < 0.05. Survival rate and number of mice per group are shown on the right. Data represent the average of at least three separate experiments with four or more mice per group.
To determine the specificity and mechanism of Ab-mediated protection, anti-JHMV S protein MAb expressing different biological activities, neutralizing and/or fusion inhibiting (Table 1), were transferred to infected μMT mice by using the protocol effective with pAb-1 (23) (Fig. 2A). Similar to mice that received pAb-1, recipients of anti-S protein MAb expressing both neutralizing and fusion-inhibiting activities, i.e., MAb J.7.2 and J.2.6 (Table 1), exhibited reduced clinical signs compared to controls. In addition, all anti-S protein MAb-treated recipients survived until day 21 p.i. compared to 76% of control mice. Consistent with reduced morbidity and mortality, infectious virus was reduced in the CNS of neutralizing/fusion-inhibiting MAb recipients (Fig. 2A). Although MAb with both neutralizing and fusion-inhibiting activities were clearly effective in inhibiting virus recrudescence, neither was as effective as pAb-1 (Fig. 2A), despite higher relative in vitro neutralizing and fusion-inhibiting activities (Table 1). To determine if neutralizing activity alone prevented recrudescence, protection was also examined following transfer of MAb J.7.5, which has approximately the same neutralizing activity as MAb J.7.2 but lacks fusion-inhibiting activity (Table 1). Similar to neutralizing/fusion-inhibiting MAb recipients, J.7.5 MAb recipients also exhibited reduced morbidity and mortality compared to controls; all survived and showed reduced virus recrudescence at day 21 p.i. (Fig. 2A). No evidence for viral escape mutants was detected by in vitro neutralization (data not shown), indicating that the residual virus present in the CNS at day 21 p.i. did not result from selection of Ab neutralization escape variants.
To determine if reduced virus recrudescence was restricted to IgG2 isotypes (J.7.2, J.2.6, and J.7.5 [Table 1]) and to confirm that neutralizing activity alone prevented recrudescence, neutralizing/non-fusion-inhibiting MAb of the IgG1 (5B1) or IgA (5B9) isotypes were tested (Table 1). Both MAb increased survival at day 21 p.i. compared to controls (Fig. 2A); however, only MAb 5B1 decreased morbidity. Furthermore, similar to the IgG2 isotype MAb, only IgG1 MAb 5B1 inhibited virus recrudescence. The IgA isotype MAb (5B9) was unable to prevent virus recrudescence (Fig. 2A) despite exhibiting similar neutralizing activity (Table 1). These data suggest that MAb with neutralizing activity of both the IgG1 and IgG2a/b isotypes are more efficient at preventing recrudescence than neutralizing MAb of the IgA isotype, irrespective of in vitro neutralizing activity.
MHV infects adjacent cells via cell-cell fusion in vitro (5, 10). Neutralizing/fusion-inhibiting MAb inhibited viral recrudescence slightly better than neutralizing/non-fusion-inhibiting MAb (Fig. 2A), suggesting a possible role for fusion-inhibiting activity in suppressing virus recrudescence. To discriminate between neutralizing and fusion-inhibiting activities, an IgG2a nonneutralizing/fusion-inhibiting anti-S protein MAb (J.2.5) was compared to IgG2a and IgG2b anti-S protein specific MAb with no in vitro biological activity (J.7.1 and J.1.16 [Table 1]). Survival was increased only slightly (Fig. 2B) in MAb J.2.5 recipients, although clinical disease was similar to that found in PBS controls. However, JHMV recrudescence was suppressed by MAb J.2.5 (Fig. 2B), although not as efficiently as by neutralizing/non-fusion-inhibiting MAb (Fig. 2A). Passive transfer of either nonneutralizing/non-fusion-inhibiting anti-S protein MAb failed to affect clinical disease, mortality rates, or virus recrudescence, despite being of the IgG isotype (Fig. 2B). These data suggest that both fusion inhibition and neutralization activities contribute to preventing viral recrudescence. Furthermore, these antiviral effects appear to be more effective in concert.
Role of non-S protein specific Ab during viral recrudescence.
Anti-M and anti-N protein specific MAb administered prior to, or concomitant with, infection protect mice from death, although inhibition of virus replication was not a constant finding (4, 13, 19, 26, 35). Infected μMT mice were therefore treated with MAb specific for the JHMV M or N protein (Table 2) to determine their potential contribution(s) to preventing virus recrudescence. Neither of the two MAb specific for the N protein, nor any of the three MAb specific for the M protein, afforded clinical protection, decreased mortality, or inhibited virus recrudescence compared to controls (Fig. 2C). These data contrast with the ability of anti-M protein specific MAb J.1.3 and anti-N protein specific MAb J.3.3 to protect during acute infection (13, 35). Finally, a polyclonal Ab with no neutralizing or fusion-inhibiting activity (pAb-2) was also unable to decrease mortality or prevent virus recrudescence (Fig. 2C). These data support the primary role(s) of neutralization and fusion inhibition activities in suppressing virus recrudescence.
Neutralizing Ab limits virus replication to oligodendrocytes during recrudescence.
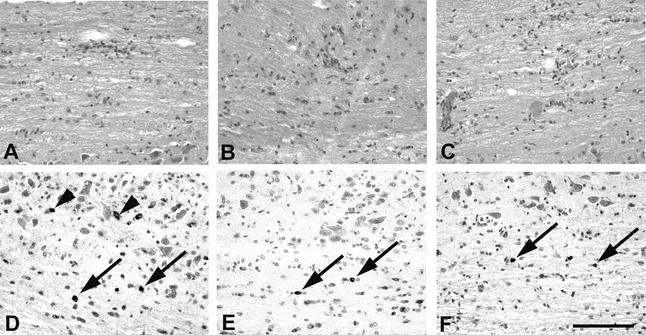
Polyclonal Ab-mediated suppression of virus recrudescence in the CNS of JHMV-infected μMT mice reduced virus replication and viral Ag from the brain (23). By contrast, viral Ag was retained in spinal cord oligodendroglia and was associated with extensive demyelination (23). To determine if elimination of viral Ag from the brain but not from the spinal cord was due to neutralizing activity and to examine the effect of reducing viral Ag on both the inflammatory response and demyelination, infected μMT recipients of neutralizing/fusion-inhibiting MAb were compared to controls. No difference in the extent of demyelination was observed in any group at day 21 p.i., irrespective of whether or not virus recrudescence had been suppressed (data not shown), consistent with previous data on protection mediated by polyclonal Ab (23). The extent and distribution of inflammatory cells within the CNS was also similar in all recipient groups compared to that in the PBS-treated controls during recrudescence (Fig. 3). Numerous Ag-positive astrocytes, microglia/macrophages, and oligodendroglia were found throughout the neuroaxis in PBS-treated mice, consistent with the presence of infectious virus (Fig. 3). Ag-positive cells were increased in spinal cords relative to brains and only very rarely were infected neurons noted at day 21 p.i. (data not shown). Consistent with previous data (23), viral Ag was reduced in all groups in which viral recrudescence was suppressed via MAb transfer (Fig. 3); however, residual viral Ag localized predominantly to oligodendroglia. The ability of neutralizing MAb to selectively reduce viral Ag in astrocytes and microglia/macrophages suggests that oligodendroglia either do not express the viral S protein on their cell surface or may, in some other way, be partially refractory to MAb-mediated inhibition of viral recrudescence.
FIG. 3.
Reductions in viral Ag in MAb recipients. N+, neutralizing; F+, fusion inhibiting. Inflammation within the spinal cords of infected μMT recipients of PBS (A), N−/F+ (B), or N+/F+ MAb (C) at day 21 p.i. (hematoxylin and eosin). Note comparable distribution of infiltration in the spinal cords of all groups of mice. Viral Ag in spinal cords of infected μMT recipients of either PBS (D), N−/F+ (E), or N+/F+ MAb (F) at 21 days p.i. (immunoperoxidase with hematoxylin counterstain). Bar = 1,200 μm. Arrows indicate infected oligodendrocytes. Arrowheads indicate infected astrocytes.
T cells are not altered by Ab-mediated inhibition of JHMV recrudescence.
Passive Ab-mediated protection of wt mice prior to, or concomitant with, infection is associated with increased CNS inflammation (13). However, CNS inflammation is similar in infected μMT mice undergoing virus recrudescence and in wt mice at day 21 p.i. (23). Nevertheless, flow cytometry analysis revealed fewer virus-specific CD8+ T cells in the CNS of μMT mice than in wt mice, suggesting limited T-cell responsiveness as a factor contributing to virus recrudescence (3). To determine if Ab-mediated control of virus recrudescence influenced the composition or extent of CNS inflammation, CMC were compared during recrudescence and Ab-mediated suppression. No significant differences in the percentage of total CD8+ T cells were detected comparing either μMT mice treated with PBS, J.2.5, or J.7.2 recipients and wt mice at 21 days p.i. (Table 3). Although fewer tetramer+ and IFN-γ-secreting virus-specific CD8+ T cells were found in the CNS of infected μMT mice than in wt controls, there was no difference between MAb-protected and PBS-treated control groups (Table 3). Similar percentages of CD4+ T cells were also detected (Table 3). Although the frequency of virus-specific CD4+ T cells in infected μMT mice was reduced compared to that in wt mice at day 21 p.i., no difference was found between PBS-treated and MAb-protected groups (Table 3). These data demonstrate that Ab-mediated suppression of virus recrudescence does not influence either the frequency of T cells within the CNS or alter the percentage of virus-specific T cells. These data are consistent with the absence of a role for T-cell-mediated virus suppression in the CNS as a mechanism of controlling persistence and support the concept that virus recrudescence is regulated by Ab with neutralizing and/or fusion-inhibiting biological activities.
TABLE 3.
Effect of MAb on CNS T-cell recruitment
| Mouse typea | % CD8 T cellsb | Results for virus-specific CD8 cells
|
% CD4 cellsb | IFN-γ content in virus-specific CD4 cells | |
|---|---|---|---|---|---|
| Tetramer contentc | IFN-γ contentd | ||||
| wt C57BL/6 | 15 | 40 | 1,600 ± 160 | 14 | 800 ± 60 |
| μMT + PBS | 16 | 13 | 380 ± 80 | 10 | 200 ± 36 |
| μMT + J.2.5 | 17 | 12 | 460 ± 80 | 15 | 260 ± 40 |
| μMT + J.7.2 | 14 | 15 | 320 ± 50 | 12 | 120 ± 50 |
Mice were analyzed at day 21 p.i. Data are representative of at least three separate experiments.
Percentage within total CNS-derived cells.
Percentage of tetramer+ within the CD8+-T-cell population.
Virus-specific IFN-γ-secreting CD8+ (S510 peptide) and CD4+ (M133 peptide) cells per 106 total cells detected by ELISPOT.
Maintenance of viral persistence.
These data demonstrate that neutralizing Ab plays a critical role in establishing and/or maintaining persistent infection following initial cell-mediated clearance of JHMV from the CNS. Furthermore, analysis of CNS virus-specific plasma cells during JHMV infection revealed increased accumulation and retention after infectious virus is cleared (39). To determine if constitutive Ab was required to maintain viral persistence, monolayers of DBT cells were infected with JHMV and neutralizing/fusion-inhibiting pAb-1 or nonneutralizing/non-fusion-inhibiting pAb-2 was added at 6 h p.i. Control cultures containing nonneutralizing/non-fusion-inhibiting pAb-2 showed extensive cytopathology and complete loss of viable cells by 72 h p.i. By contrast, infected cells treated with neutralizing/fusion-inhibiting pAb-1 showed no evidence of cytopathology or loss of viability at 72 h p.i. However, removal of the neutralizing/fusion-inhibiting Ab at 72 h p.i. resulted in extensive cytopathology within 24 h and complete loss of viable cells within 36 h after Ab removal. These data suggested a necessity for the continued presence of Ab to suppress cytopathology and maintain the viability of infected cells.
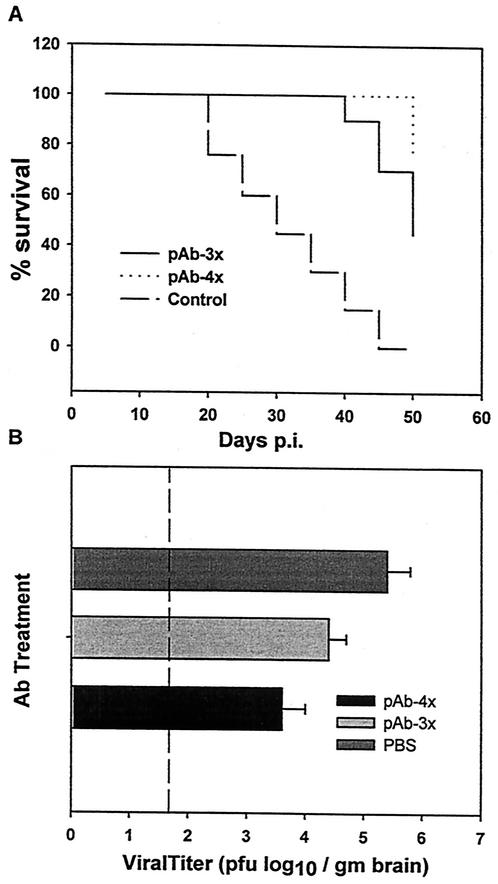
To determine if neutralizing Ab sufficed not only to inhibit virus replication but also to maintain persistence, Ab-protected infected μMT mice were examined for subsequent evidence of virus recrudescence. In contrast to the high mortality in PBS (Fig. 4A) or pAb-2 (data not shown) recipients, the majority (90%) of neutralizing/fusion-inhibiting pAb-1 recipients survived to 40 days p.i. (Fig. 4A). PBS-treated infected μMT mice showed a progressive increase in demyelination from days 21 to 30 p.i. Neutralizing/fusion-inhibiting pAb-1 recipient mice had reduced myelin loss at day 30 p.i. compared to both PBS-treated mice at day 30 p.i. (Fig. 5) and pAb-1 recipients examined at day 21 p.i. (data not shown). Nevertheless, following day 40 p.i., pAb-1- treated recipients exhibited increasing morbidity with only a cumulative 50% survival at 50 days p.i. (Fig. 4A). Consistent with increasing morbidity and mortality in the neutralizing/fusion-inhibiting Ab-protected group, infectious virus was detected in the CNS at 40 days p.i. in neutralizing/fusion-inhibiting pAb-1 recipients and, as expected, in the limited number of control group survivors (Fig. 4B). Virus recovered from the CNS of both groups was compared for susceptibility to pAb-1-mediated neutralization to determine if the virus emerging in the CNS contained neutralization escape variants. No evidence for neutralizing escape mutants in the CNS of either group was found (data not shown), suggesting that delayed recrudescence following Ab-mediated protection was not due to selection of neutralization escape variants.
FIG. 4.
Long-term effect of polyclonal Ab (pAb) transfer on JHMV pathogenesis. Mortality of B-cell-deficient mice following transfer of neutralizing or nonneutralizing pAb at days 9, 12, and 17 p.i. (A). Virus titers were determined at 40 days p.i. in the brains of μMT mice receiving three doses (pAb-3x) or at 45 days p.i. in mice that received a fourth dose (pAb-4x) of the neutralizing polyclonal Ab on day 24 p.i. (B). The dashed line indicates the limit of viral detection. Data represent the average of at least three separate experiments. Error bars indicate standard errors of the means. gm brain, gram of brain.
FIG. 5.
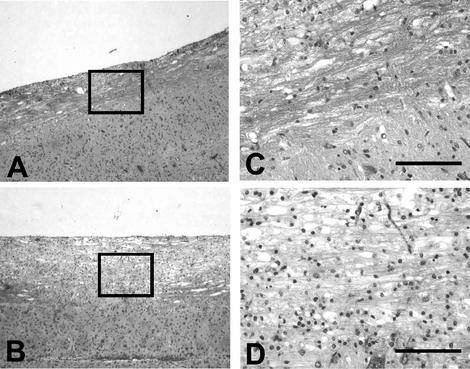
Decreased demyelination in Ab recipients. Spinal cords of JHMV-infected μMT mouse recipients of PBS or polyclonal anti-JHMV Ab at 30 days p.i. (luxol fast blue). Increased demyelination in spinal cords of control PBS recipients (A and C), compared to that in spinal cords of Ab recipients (B and D). Bar = 400 μm (C and D).
To determine if the waning suppression of infectious virus could be prevented, mice protected via transfer of neutralizing/fusion-inhibiting Ab-1 at days 9, 12, and 17 p.i. were divided into two groups. One received an additional injection of neutralizing/fusion-inhibiting pAb-1 at 24 days p.i., while the other received nonneutralizing/non-fusion-inhibiting pAb-2. At day 45 p.i., 50% of the mice receiving three injections of the neutralizing/fusion-inhibiting pAb-1, followed by a single injection of the nonneutralizing/non-fusion-inhibiting pAb-2, had succumbed to infection (Fig. 4A). By contrast, no mortality was found in mice that received neutralizing/fusion-inhibiting pAb-1 at days 9, 12, 17, and 24 p.i. Although infections virus was recovered from the CNS of both groups, mice receiving the additional injection of neutralizing/fusion-inhibiting Ab had reduced CNS virus levels at 45 days p.i. compared to survivors of the group that received three injections of neutralizing/fusion-inhibiting Ab followed by nonneutralizing/non-fusion-inhibiting Ab (Fig. 4B). These data support the notion that the maintenance of JHMV persistence within the CNS is regulated by neutralizing Ab and suggest the possibility that cells secreting anti-JHMV Ab are required within the CNS to effectively control persistent infection.
DISCUSSION
JHMV infection of the CNS provides an excellent model to study mechanisms of encephalomyelitis and demyelination and the dynamics of host-virus interactions (38). The outcome of viral infection depends on the competition between viral replication and the host's ability to initiate and sustain an antiviral but nondeleterious immune response. Consistent with this notion, a vigorous CD8+-T-cell response eliminates JHMV from infected CNS cell types during acute infection (38). However, CD8+ T cells lose their cytolytic activity concomitant with viral clearance and do not reexpress this effector function during virus recrudescence in B-cell-deficient mice, despite retaining some capability to secrete cytokines (3, 23, 31). The absence of a recovery of cytolytic function or enhanced T-cell recruitment, despite increasing Ag in the CNS of recrudescing B-cell-deficient mice, suggested that an alternative effector mechanism regulates persistent CNS infection (3, 16, 31). Consistent with the concept that humoral immunity contributes to controlling JHMV persistence, there is a distinct delay in the recruitment of plasma cells secreting JHMV-specific Ab until after the majority of infectious virus has been eliminated from the CNS of wt mice (39).
Passive transfer of MAb with different specificities and biological activities into infected μMT mice after initial virus clearance demonstrates that the ability to neutralize infectious virus is the most critical component for suppression of CNS viral recrudescence. This finding is reinforced by several reports demonstrating reduction in virus replication during acute peripheral and CNS infections via transfer of neutralizing Ab (6, 8, 16, 34). Previous studies in mice that received MAb prior to infection have demonstrated that Ab of either IgG or IgM isotypes provide protection (4, 6, 8, 13, 14, 15). Transfer of different Ab isotypes with neutralizing activity revealed that only neutralizing IgG (IgG2 > IgG1) suppressed JHMV recrudescence in infected μMT mice. A single in vitro neutralizing IgA MAb examined was unable to suppress virus recrudescence, despite its detection by ELISA in perfused mouse brains at day 21 p.i. (data not shown). A limited antiviral role for IgA, rather than an inability to enter the CNS, is further supported by the low number of virus-specific IgA-secreting plasma cells in the CNS of infected wt mice (39). Ab specific for structural proteins with no neutralizing activity played no detectable role during virus recrudescence and/or persistence, despite the ability to increase survival rates when transferred prior to or concomitant with viral infection (4, 13, 19, 26). In contrast to the majority of MAb tested that lacked neutralizing activity, S protein specific nonneutralizing/fusion-inhibiting MAb also provided slight protection from virus recrudescence in infected μMT mice. However, the mechanism of protection afforded by the nonneutralizing/fusion-inhibiting Ab is unclear. Although JHMV spreads from cell to cell via a fusion-mediated process in vitro (5, 10), JHMV-induced fusion is not detected in vivo (23, 31). These data suggest that a process similar to cell-cell fusion may be involved in the spread of JHMV within the CNS, which may not be apparent due to the paucity of viral receptors expressed by CNS cells.
Antiviral Ab control virus infections via several mechanisms. The most direct mechanism is Ab binding to virus, rendering it noninfectious. In addition, Ab-virus complexes may activate the complement pathway. However, analysis of MHV infection in mice deficient in complement indicated no role for complement (13, 25). The absence of natural killer cells and low levels of macrophages retained in the CNS following acute JHMV infection (24) also suggest that Ab-dependent cell-mediated cytotoxicity does not contribute to maintaining JHMV persistence. Finally, the observation that nonneutralizing/non-fusion-inhibiting polyclonal Ab failed to suppress virus recrudescence is consistent with the absence of an Fcγ-R-mediated mechanism of IgG protection (25) and the ability of F(ab′)2 fragments with neutralizing activity to afford protection (18).
Increased viral Ag was found in all cell types in the CNS of μMT mice during JHMV recrudescence compared to the level found in wt mice (23), and a similar pattern was detected in infected μMT mice treated with PBS, the control MAb, and all viral MAb unable to inhibit virus replication during recrudescence. Examination of the CNS of the neutralizing MAb and polyclonal Ab-1 recipients and to a limited extent of the CNS of recipients of the nonneutralizing/fusion-inhibiting MAb, which were partially protected from viral recrudescence, demonstrated that virus replication was inhibited in most CNS cell types. The ability of these Ab to limit viral replication was greatest in microglia and astrocytes. However, the number of infected oligodendrocytes, especially in spinal cord, was only reduced slightly, suggesting that this CNS cell type may be refractory to Ab-mediated suppressive mechanisms. Similar to these observations, transfer of neutralizing MAb prior to JHMV infection also inhibited viral replication in most CNS cell types including neurons, with the exception of oligodendrocytes (4). These observations may provide an explanation for the inability of the neutralizing Ab to completely eliminate infectious virus from the CNS during recrudescence, similar to the observation of enhanced viral replication in oligodendroglia in IFN-γ-deficient mice (28). These data suggest that the transfer of neutralizing Ab is more effective in regulating virus replication in astrocytes and microglia/macrophages. Neither enhanced recruitment nor reexpression of effector function by CD8+ T cells occurs following virus recrudescence in JHMV-infected μMT mice (3). However, during acute infection regulation of JHMV replication in these cell types is dependent upon CD8+-T-cell-mediated perforin-dependent cytolysis (22). No increase in total or virus-specific CD8+ T cells was observed following Ab-mediated suppression of viral recrudescence. These data suggest that Ab does not enhance T-cell-mediated clearance from astrocytes and microglia (22, 28), consistent with the concept that Ab mediates a T-cell-independent suppression of persistence in specific CNS cell types.
Infectious JHMV is cleared from immunocompetent hosts; however, virus persists for prolonged periods and is associated with ongoing demyelination (2, 24). Although neutralizing Ab reduced virus to undetectable levels in the majority of recipients, control of virus replication was only transient. Additional Ab prolonged survival but was still unable to prevent reemergence of infectious virus. The frequency of infected oligodendroglia was not dramatically decreased via Ab-mediated suppression of JHMV replication; however, it was associated with reduced myelin loss between days 21 and 30 p.i. The ability of Ab to provide only transient suppression has also been observed following peripheral virus or bacterial infections of immunocompromised mice (16, 20, 34). Analysis of these models suggests that continued control was related to levels of Ab in serum. Preliminary analysis suggests that JHMV recrudescence does not compromise the integrity of the blood-brain barrier, consistent with the absence of increased inflammation, but that levels of Ab in serum decline prior to recrudescence. Although selection of quasispecies has been suggested to contribute to JHMV persistence (32), transient control, followed by subsequent recrudescence of infectious virus, indicates that replication and/or assembly is not compromised in this model due to accumulation of defective viral quasispecies.
The process by which neutralizing Ab maintains JHMV persistence within the CNS is unclear. Analysis of the cell types infected suggests that oligodendroglia represent the reservoir of persistence and that astrocytes and microglia/macrophages are secondary targets of replication. Although neutralization of infectious particles is most likely, it does not explain preferential persistence in oligodendrocytes. One potential mechanism is differential regulation of receptor expression of these cell types during the course of infection. The receptor may be down regulated on astrocytes and microglia but not on the oligodendrocytes, making the latter more prone to persistent infection. Alternatively, an intracellular blockade of virus replication, which is relatively ineffective in oligodendrocytes, may be involved, resulting in the requirement for continued high levels of Ab. Irrespective of the mechanism, data indicate that the presence of neutralizing Ab, possibly in conjunction with Ab that express fusion-inhibiting activity, is essential to suppress virus recrudescence and maintain a persistent infection in the absence of detectable infectious virus. The concept that the continued presence of Ab within the CNS plays little or no role in initial control of viral replication but is subsequently required to maintain JHMV persistence is consistent with the delayed accumulation of virus-specific plasma cells in the CNS of wt mice (39), concomitant with or following virus clearance. Sustained local antiviral Ab levels within the CNS, provided by retention of plasma cells, thus appears to be a vital component in preventing virus recrudescence and controlling JHMV persistence.
Acknowledgments
We thank Wen Wei for assistance with the histopathology, Maria R. Ramirez for breeding mice, David Hinton for helpful discussions, and Michael J. Buchmeier, Scripps Research Institute (La Jolla, Calif.) for kindly providing the 5B170 (IgG1) and 5B93.9 (IgA) hybridomas. The control hybridoma 902 (anti-gp120) was obtained from Bruce Chesebro through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
This work was supported by National Institutes of Health grants AI 47249, AI 40667, and NS18146.
REFERENCES
- 1.Ahmed, R., L. A. Morrison, and D. M. Knipe. 1997. Viral persistence, p. 181-205. In N. Nathanson, R. Ahmed, F. Gonzalez-Scarano, D. E. Griffin, K. V. Holmes, F. A. Murphy, and H. L. Robinson (ed.), Viral pathogenesis. Lippincott-Raven Publishers, Philadelphia, Pa.
- 2.Bergmann, C. C., J. D. Altman, D. Hinton, and S. A. Stohlman. 1999. Inverted immunodominance and impaired cytolytic function of CD8+ T cells during viral persistence in the CNS. J. Immunol. 163:3379-3387. [PubMed] [Google Scholar]
- 3.Bergmann, C. C., C. Ramakrishna, M. Kornacki, and S. A. Stohlman. 2001. Impaired T cell immunity in B cell-deficient mice following viral central nervous system infection. J. Immunol. 167:1575-1583. [DOI] [PubMed] [Google Scholar]
- 4.Buchmeier, M. J., H. A. Lewicki, P. J. Talbot, and R. L. Knobler. 1984. Murine hepatitis virus-4 (strain JHM)-induced neurologic disease is modulated in vivo by monoclonal antibody. Virology 132:261-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins, A. R., R. L. Knobler, H. Powell, and M. J. Buchmeier. 1982. Monoclonal antibodies to murine hepatitis virus-4 (strain JHM) define the viral glycoproteins responsible for attachment and cell-cell fusion. Virology 119:358-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coutelier, J. P., J. T. van der Logt, F. W. Heessen, G. Warnier, and J. Van Snick. 1987. IgG2a restriction of murine antibodies elicited by viral infections. J. Exp. Med. 165:64-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cserr, H. F., and P. M. Knopf. 1992. Cervical lymphatics, the blood-brain barrier and the immunoreactivity of the brain: a new view. Immunol. Today 13:507-512. [DOI] [PubMed] [Google Scholar]
- 8.Dietzschold, B., M. Kao, Y. M. Zheng, Z. Y. Chen, G. Maul, Z. F. Fu, C. E. Rupprecht, and H. Koprowski. 1992. Delineation of putative mechanisms involved in antibody-mediated clearance of rabies virus from the central nervous system. Proc. Natl. Acad. Sci. USA 89:7252-7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drescher, K. M., L. R. Pease, and M. Rodriguez. 1997. Antiviral immune responses modulate the nature of central nervous system (CNS) disease in a murine model of multiple sclerosis. Immunol. Rev. 159:177-193. [DOI] [PubMed] [Google Scholar]
- 10.Fleming, J. O., S. A. Stohlman, R. C. Harmon, M. M. C. Lai, J. A. Frelinger, and L. P. Weiner. 1983. Antigenic relationships of murine coronaviruses: analysis using monoclonal antibodies to JHM (MHV-4) virus. Virology 131:296-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleming, J. O., M. D. Trousdale, F. A. el-Zaatari, S. A. Stohlman, and L. P. Weiner. 1986. Pathogenicity of antigenic variants of murine coronavirus JHM selected with monoclonal antibodies. J. Virol. 58:869-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fleming, J. O., M. D. Trousdale, J. Bradbury, S. A. Stohlman, and L. P. Weiner. 1987. Experimental demyelination induced by coronavirus JHM (MHV-4): molecular identification of a viral determinant of paralytic disease. Microb. Pathog. 3:9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleming, J. O., R. A. Shubin, M. A. Sussman, N. Casteel, and S. A. Stohlman. 1989. Monoclonal antibodies to the matrix (E1) glycoprotein of mouse hepatitis virus protect mice from encephalitis. Virology 168:162-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furrer, E., T. Bilzer, L. Stitz, and O. Planz. 2001. Neutralizing antibodies in persistent Borna disease virus infection: prophylactic effect of gp94-specific monoclonal antibodies in preventing encephalitis. J. Virol. 75:943-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerhard, W., K. Mozdzanowska, M. Furchner, G. Washko, and K. Maiese. 1997. Role of the B-cell response in recovery of mice from primary influenza virus infection. Immunol. Rev. 159:95-103. [DOI] [PubMed] [Google Scholar]
- 16.Griffin, D., B. Levine, W. Tyor, S. Ubol, and P. Despres. 1997. The role of antibody in recovery from alphavirus encephalitis. Immunol. Rev. 159:155-161. [DOI] [PubMed] [Google Scholar]
- 17.Kolb, A. J., L. Pewe, J. Webster, S. Perlman, C. B. Whitelaw, and S. G. Siddel. 2001. Virus-neutralizing monoclonal antibody expressed in milk of transgenic mice provides full protection against virus-induced encephalitis. J. Virol. 75:2803-2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lamarre, A., and P. J. Talbot. 1995. Protection from lethal coronavirus infection by immunoglobulin fragments. J. Immunol. 154:3975-3984. [PubMed] [Google Scholar]
- 19.Lecomte, J., V. Cainelli-Gebara, G. Mercier, S. Mansour, P. J. Talbot, G. Lussier, and D. Oth. 1987. Protection from mouse hepatitis virus type 3-induced acute disease by an anti-nucleoprotein monoclonal antibody. Brief report. Arch. Virol. 97:123-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li, J. S., E. Yager, M. Reilly, C. Freeman, G. R. Reddy, A. A. Reilly, F. K. Chu, and G. M. Winslow. 2001. Outer membrane protein-specific monoclonal antibodies protect SCID mice from fatal infection by the obligate intracellular bacterial pathogen Ehrlichia chaffeensis. J. Immunol. 166:1855-1862. [DOI] [PubMed] [Google Scholar]
- 21.Liebert, U. G., S. Schneider-Schaulies, K. Baczko, and V. ter Meulen. 1990. Antibody-induced restriction of viral gene expression in measles encephalitis in rats. J. Virol. 64:706-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin, M. T., S. A. Stohlman, and D. R. Hinton. 1997. Mouse hepatitis virus is cleared from the central nervous systems of mice lacking perforin-mediated cytolysis. J. Virol. 71:383-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin, M. T., D. R. Hinton, N. W. Marten, C. C. Bergmann, and S. A. Stohlman. 1999. Antibody prevents virus reactivation within the central nervous system. J. Immunol. 162:7358-7368. [PubMed] [Google Scholar]
- 24.Marten, N. W., S. A. Stohlman, and C. C. Bergmann. 2001. MHV infection of the CNS: mechanisms of immune-mediated control. Viral Immunol. 14:1-18. [DOI] [PubMed] [Google Scholar]
- 25.Matthews, A. E., S. R. Weiss, M. J. Shlomchik, L. G. Hannum, J. L. Gombold, and Y. Paterson. 2001. Antibody is required for clearance of infectious murine hepatitis virus A59 from the central nervous system, but not the liver. J. Immunol. 167:5254-5263. [DOI] [PubMed] [Google Scholar]
- 26.Nakanaga, K., K. Yamanouchi, and K. Fujiwara. 1986. Protective effect of monoclonal antibodies on lethal mouse hepatitis virus infection in mice. J. Virol. 59:168-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ochsenbein, A. F., D. D. Pinschewer, B. Odermatt, M. C. Carroll, H. Hengartner, and R. M. Zinkernagel. 1999. Protective T cell-independent antiviral antibody responses are dependent on complement. J. Exp. Med. 190:1165-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parra, B., D. R. Hinton, N. W. Marten, C. C. Bergmann, M. T. Lin, C. S. Yang, and S. A. Stohlman. 1999. IFN-gamma is required for viral clearance from central nervous system oligodendroglia. J. Immunol. 162:1641-1647. [PubMed] [Google Scholar]
- 29.Perlman, S., R. Schelper, E. Bolger, and D. Ries. 1987. Late onset, symptomatic, demyelinating encephalomyelitis in mice infected with MHV-JHM in the presence of maternal antibody. Microb. Pathog. 2:185-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pincus, S., K. Weherly, and B. Chesebro. 1989. Treatment of HIV tissue culture infection with monoclonal antibody-ricin A chanin conjugates. J. Immunol. 142:3070-3075. [PubMed] [Google Scholar]
- 31.Ramakrishna, C., S. A. Stohlman, R. D. Atkinson, M. J. Shlomchik, and C. C. Bergmann. 2002. Mechanisms of central nervous system viral persistence: the critical role of antibody and B cells. J. Immunol. 168:1204-1211. [DOI] [PubMed] [Google Scholar]
- 32.Rowe, C. L., S. C. Baker, M. J. Nathan, and J. O. Fleming. 1997. Evolution of mouse hepatitis virus: detection and characterization of spike deletion variants during persistent infection. J. Virol. 71:2959-2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sedgwick, J. D., and R. Dorries. 1991. The immune system response to viral infection of the CNS. Neurosciences 3:93. [Google Scholar]
- 34.Stitz, L., K. Noske, O. Planz, E. Furrer, W. I. Lipkin, and T. Bilzer. 1998. A functional role for neutralizing antibodies in Borna disease: influence on virus tropism outside the central nervous system. J. Virol. 72:8884-8892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stohlman, S. A., C. Bergmann, D. Cua, H. Wege, and R. van der Veen. 1994. Location of antibody epitopes within the mouse hepatitis virus nucleocapsid protein. Virology 202:146-153. [DOI] [PubMed] [Google Scholar]
- 36.Stohlman, S. A., C. C. Bergmann, R. C. van der Veen, and D. R. Hinton. 1995. Mouse hepatitis virus-specific cytotoxic T lymphocytes protect from lethal infection without eliminating virus from the central nervous system. J. Virol. 69:684-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stohlman, S. A., C. C. Bergmann, M. T. Lin, D. J. Cua, and D. R. Hinton. 1998. CTL effector function within the central nervous system requires CD4+ T cells. J. Immunol. 160:2896-2904. [PubMed] [Google Scholar]
- 38.Stohlman, S. A., C. C. Bergmann, and S. Perlman. 1999. Mouse hepatitis virus, p. 537-557. In R. Ahmed and I. Chen (ed.), Persistent viral infections. Wiley, New York, N.Y.
- 39.Tschen, S., C. C. Bergmann, C. Ramakrishna, S. Morales, R. Atkinson, and S. A. Stohlman. 2002. Recruitment kinetics and composition of antibody secreting cells within the central nervous system following viral encephalomyelitis. J. Immunol. 168:2922-2929. [DOI] [PubMed] [Google Scholar]
- 40.Xue, S., and S. Perlman. 1997. Antigen specificity of CD4 T cell response in the central nervous system of mice infected with mouse hepatitis virus. Virology 238:68-78. [DOI] [PubMed] [Google Scholar]
- 41.Zinkernagel, R. M. 1997. Virus-induced immunopathology, p. 163-179. In N. Nathanson, R. Ahmed, F. Gonzalez-Scarano, D. E. Griffin, K. V. Holmes, F. A. Murphy, and H. L. Robinson (ed.), Viral pathogenesis. Lippincott-Raven Publishers, Philadelphia, Pa.